Abstract
Supplements added to the culture media (e.g., growth factors and dexamethasone) have been successful in improving mechanical and biochemical properties of engineered cartilage towards native values. Trimethylamine N-oxide (TMAO), a natural osmolyte found in shark cartilage, is thought to induce protein folding, and counteracts the destabilizing effect of the high concentrations of urea stored by sharks. The objective of this study was to investigate the use of TMAO as a media supplement for promoting growth of functional engineered cartilage in culture. In the first study, TMAO was added to the culture media for the first 14 days in culture and concentrations of 0–200 mM were evaluated. In the second study, TMAO was supplemented to the culture media following chondroitinase ABC digestion, which has been previously shown to mediate an increased collagen content in engineered cartilage. A dose-dependent response was observed with improved mechanical and biochemical properties for engineered constructs cultured with TMAO at concentrations of 5–100 mM. The Young's modulus of digested constructs cultured in TMAO was 2× greater than digested constructs cultured in the control medium and recovered to undigested control levels by day 42. In conclusion, these initial studies with TMAO as a media supplement show promise for improving the compressive mechanical properties, increasing extracellular matrix production, and increasing the recovery time following chABC digestion.
Keywords: cartilage tissue engineering, trimethylamine N-oxide (TMAO), chondroitinase ABC (chABC), culture media supplement
Articular cartilage is the soft tissue lining the bones of diarthrodial joints and functions as the weight-bearing cushion of the joint.1,2 The harsh loading environment and the avascular nature of cartilage lead to poor healing after injury. As a result, cell-based therapies, including tissue engineering strategies for growing clinically relevant grafts, are being intensively researched.3–5 An autologous cell source, such as chondrocytes from healthier cartilage tissue, would be ideal for growing clinically relevant engineered cartilage, because of the cell's pre-differentiated state. However, achieving mechanical and biochemical properties similar to healthy native tissue poses several challenges.6,7 Supplements added to the culture media (e.g., growth factors and dexamethasone) have been successful in improving mechanical and biochemical properties towards native values.8,9
Various hydrogel systems have been introduced for tissue engineering of articular cartilage (e.g., agarose, alginate, PEG, and hyaluronan). Hydrogel scaffolds act to encapsulate cells, prevent leakage of elaborated extracellular matrix, stabilize the chondrocyte phenotype, and provide a biocompatible scaffold system for tissue regeneration.10 Currently, agarose hydrogels seeded with autologous and juvenile allogeneic chondrocytes are being used for cartilage repair (e.g., Genzyme's Carticel® and Zimmer/Isto's DeNovo®).11,12 Our laboratory has extensive experience using agarose as a scaffold for long-term culture of engineered constructs seeded with juvenile bovine and mature canine chondrocytes. These engineered cartilage tissues typically exhibit native Young's modulus and glycoaminoglycan (GAG) content, but sub-native levels of collagen content.6,13,14
This study investigates the effect of trimethylamine N-oxide (TMAO), a natural osmolyte found in shark tissues, as a culture media supplement for cartilage tissue engineering. Sharks have a skeletal structure of cartilage instead of bone. Sharks store a high concentration of urea in their tissues to maintain an osmotic balance with their external environment; however, urea causes protein destabilization.15 TMAO induces protein folding and counteracts the destabilizing effects of urea.15–18 Yancey and Siebenaller19 demonstrated that TMAO is effective in reducing denaturation and proteolysis of tissues from fish and mammalian species (bovine). The TMAO concentration measured in shallow water sharks is approximately 200 mM, while the concentration of urea is 2× greater than the TMAO concentration (400 mM).15,20 The extracellular matrix of shark cartilage, mostly water (40–45%), proteoglycans (20–25% by dry weight) and collagen (20–25% by dry weight), is comprised of similar components as articular cartilage found in land vertebrates.21
Inspired by the potential role that TMAO plays in supporting the cartilaginous skeleton of the shark, we speculate that TMAO may be useful as a supplement for promoting growth of functional engineered cartilage in culture. In the current study, we test the hypothesis that the stabilizing properties of TMAO will improve cartilage growth and collagen content of engineered cartilage. To test this hypothesis we added TMAO as a media supplement to (1) cultures of chondrocyte-seeded hydrogel constructs6,13,14 and (2) to cultures of engineered constructs following chondroitinase ABC (chABC) digestion to further enhance collagen production.6,22
MATERIALS AND METHODS
Prior to cell culture studies, the effect of TMAO on pH and osmolarity was evaluated over 10 days. Dishes were prepared with a final volume of 10 ml of saline bath solution (PBS) at TMAO (Sigma-Aldrich, St. Louis, MO) concentrations of 0, 5, 50, 100, 200, 500, or 1000 mM TMAO and incubated in sterile conditions at 37°C with 5% CO2. At days 0, 1, 3, 7, and 10 a 1 ml aliquot was removed to measure the pH and the osmolarity of the solution using a freezing point osmometer. TMAO concentrations that remained below physiological levels (400 mOsm)23 were chosen for tissue culture studies.
Articular chondrocytes were harvested from juvenile bovine wrist joints (3–6 weeks old), and digested with Collagenase IV (Worthington, Lakewood, NJ) for 11 h at 37°C with stirring. Cells were expanded for one passage (plating density = 45,000 cells/cm2) in high-glucose Dulbecco's modified Eagle's medium (DMEM) media containing 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin and amphotericin B, 0.5 ng/ml bFGF, 0.5 ng/ml PDGF, and 1 ng/ml of TGF-β1 (Invitrogen Co., Carlsbad, CA).24,25 Once the cells were confluent, they were detached through trypsinization (Trypsin EDTA; Cellgro Mediatech, Inc., Manassas, VA) and suspended in serum media.
Primary (Study 1) or passaged (Study 2) cells were cast into a slab with a concentration of 30 million cells/ml in 2% weight per volume agarose (Type VII, Sigma-Aldrich). Samples were cored from the slab (construct dimensions: ϕ = 4 mm; thickness = 2.34 mm) and cultured in a chemically defined media (CM: DMEM with 0.1 uM dexamethasone, 40 mg/ml l-proline, 50 mg/ml ascorbate 2-phosphate, 100 mg/ml sodium pyruvate, 1× ITS + premix, 100 U/ml penicillin, and 100 mg/ml streptomycin and amphotericin B (Invitrogen Co.)) supplemented with 10 ng/ml of TGF-β3 for the first 14 days of culture. The culture media was changed three times a week.
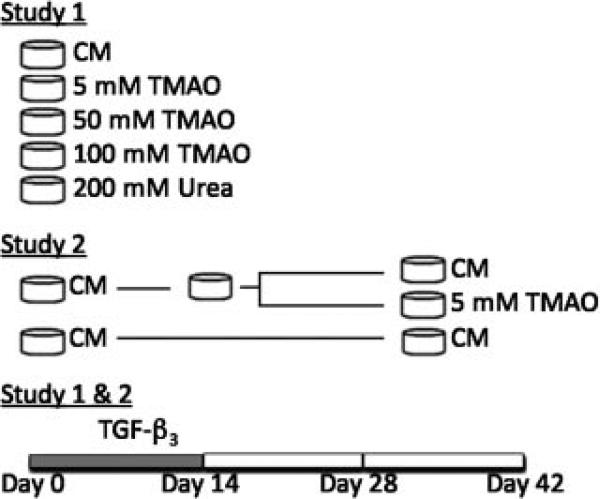
Two studies were performed to evaluate the effect of TMAO on the mechanical and biochemical properties of engineered cartilage (Fig. 1). Both studies were performed in duplicate to ensure robustness of the results. In Study 1, a wide range of TMAO concentrations was evaluated to optimize the ideal concentration for the culture media, including concentrations of 5, 50, 100, and 200 mM (Fig. 1). TMAO was supplemented fresh into the culture media during the first 14 days of culture. Constructs cultured without TMAO served as the control. Study 2 evaluated the effect of supplementing the culture media with TMAO following digestion of mature engineered cartilage constructs with chABC. Digestion of mature tissue engineered constructs with chABC temporarily suppresses the GAG content, increases the collagen content and improves the mechanical properties.6 At day 14, constructs cultured in CM were digested with 0.15 U/ml chABC (Sigma-Aldrich) for 48 h. Following the digestion, constructs were cultured in either CM or CM supplemented 5 mM of TMAO (Fig. 1).
Figure 1.
Schematic of the two experiments. Note that culture media used in both studies was supplemented with 10 ng/ml of TGF-β3 for the first 14 days in culture. In Study 1, the culture media was supplemented with different concentrations of TMAO for the first 2 weeks of culture. In Study 2, engineered cartilage cultured in CM was digested at day 14 then cultured either in CM or CM supplemented with 5 mM of TMAO.
Mechanical, biochemical and histological analysis were performed every 2 weeks (days 0, 14, 28, and 42). The equilibrium Young's modulus was determined using unconfined compression at 10% strain. A sinusoidal compressive strain (±1%, 0.5 Hz) was then applied to determine the dynamic modulus at that frequency. DNA content was determined using the PicoGreen Kit (Invitrogen, Inc., Grand Island, NY).26 The glycosaminoglycan (GAG) and collagen content were determined using the 1,9-dimethylmethylene blue (DMMB) and hydroxyproline assay, respectively. A two-way ANOVA with a Bonferroni post hoc test was performed, with factors set as time and culture media treatment, for mechanical and biochemical properties. A one-way ANOVA was performed to evaluate the effect of TMAO on the final dimensions and the wet weight of the engineered construct. Histological samples were imbedded in paraffin wax and slides were prepared (6–8 mm slices). Slides were stained with Alcian Blue and Picrosirius Red for GAG and collagen distribution, respectively. Immunohistochemistry was performed to determine the distribution of collagen type I, II, and VI (all primary and secondary antibodies from Abcam, Cambridge, MA).27
RESULTS
TMAO Effect on pH and Osmolarity
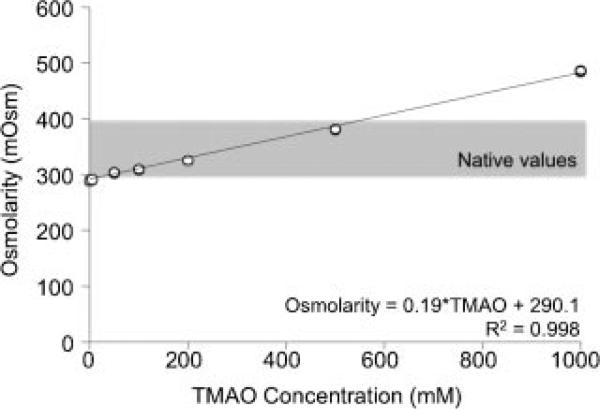
The osmolarity of the saline bath increased linearly with an increase in the TMAO concentration (Fig. 2). The saline bath osmolarity increased for all culture groups during the 10 days in culture (data not shown). The osmolarity of solutions with TMAO concentrations less than 200 mM consistently remained under the physiological limit of 400 mOsm.
Figure 2.
Strong linear correlation between the TMAO concentration and the media osmolarity.
The pH decreased for increasing concentrations of TMAO in the saline bath solution (i.e., more basic), where the pH for the control group was 7.04 and increased to 7.08 for the 5 mM TMAO group. The 1,000 mM TMAO group increased the pH of the saline bath to 7.31, which is a reasonable pH for cell culture. After 1 day in culture (at 37°C, 5% CO2), the pH of the control group (i.e., saline bath) decreased from 7.04 to 6.53. This decrease was maintained over the following 10 days, such that the control group maintained a pH level between 6.45 and 6.65.
Transient Application of TMAO
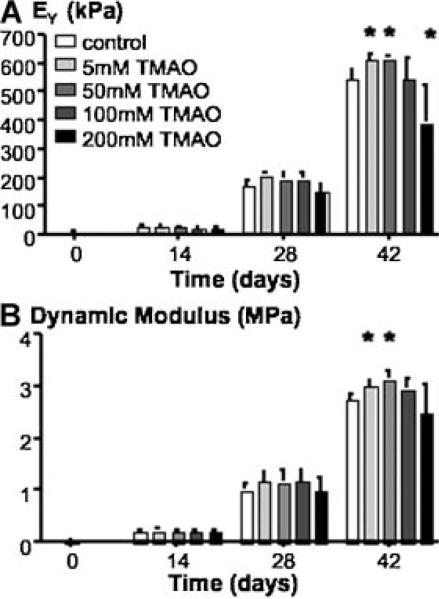
At day 42, the Young's modulus of constructs cultured in CM was 545.4 ± 28.8 kPa. The effect of TMAO on the mechanical properties was dose-dependent and had a peak near 50 mM TMAO (615.1 ± 7.8 kPa, p < 0.05; Fig. 3A). The Young's modulus of constructs cultured in CM supplemented with 200 mM TMAO was 30% lower than the control (p < 0.05). The dynamic modulus followed a similar trend as the Young's modulus (ANOVA p = 0.02; Fig. 3B). At day 42, engineered cartilage cultured in CM had a dynamic modulus of 2.74 ± 0.10 MPa, and the dynamic modulus of constructs cultured in CM supplemented with 50 mM TMAO was 3.13 ± 0.16 MPa (Fig. 3B).
Figure 3.
(A) The equilibrium Young's modulus and (B) dynamic modulus for engineered constructs from Study 1. *p < 0.05 compared to the control group (0 mM TMAO).
The dimension of the engineered constructs increased throughout the 6-week culture, where the final diameter for control constructs was 4.24 ± 0.07 mm, with a thickness of 2.73 ± 0.04 mm, and the variation in final construct dimensions was comparable to the TMAO dose-dependent response observed in the mechanical properties (data not shown). The final dimensions of the engineered constructs cultured with 5, 50, and 100 mM TMAO were not significantly different than the control group. The final thickness and diameter of constructs cultured in 200 mM TMAO media was significantly smaller than the control group (p < 0.05); however, the difference was less than 3% (diameter = 4.14 ± 0.07 mm, thickness = 2.65 ± 0.05 mm). In contrast, there was a strong negative correlation of the engineered cartilage final wet weight with the TMAO concentration (wet weight (g) = –0.023 g/mM × TMAO concentration (mM) + 41.2 g; p = 0.03, R = –0.92).
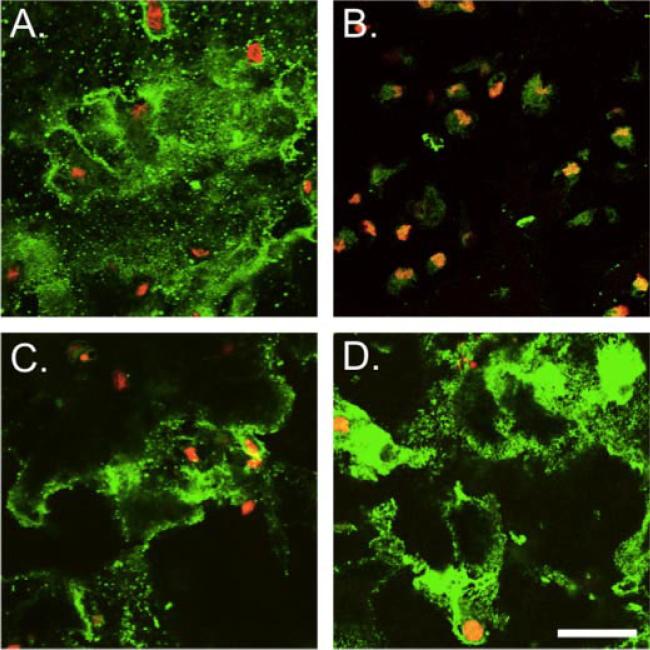
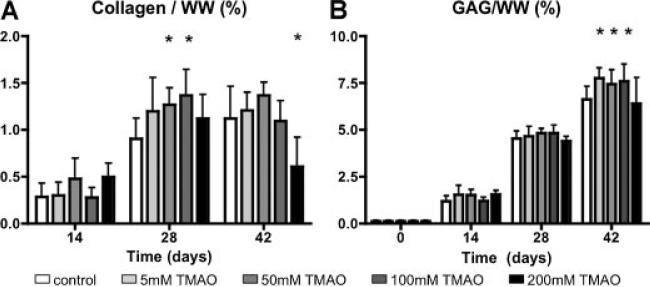
The number of chondrocytes in each construct doubled 2.5–3× within the 6-week culture. Generally, the TMAO concentration did not alter the DNA content, except in constructs cultured with 200 mM TMAO at day 28, which proliferated more slowly than the control group. The collagen content plateaued between day 28 and day 42, and was 1.12 ± 0.35%/wet weight (ww) for the control group (Fig. 5A). At day 28, the collagen content of the 50 and the 100 mM TMAO groups was approximately 45% greater than the control group (p < 0.05; Fig. 4A). Immunohistochemical staining for collagen type II in the control group was more dispersed around the cells, while staining was stronger around the pericellular matrix for the 50 mM TMAO groups (Fig. 5A,D). Staining for collagen type VI was more prominent in the 50 mM TMAO group than the control (Fig. 5). There was very little staining for collagen type I in the control and TMAO groups (data not shown).
Figure 5.
Immunohistochemistry of collagen type II (A,C) and type VI (B,D) for the control (top row) and the 50 mM TMAO group (bottom row). Collagen is shown in green and the cell nuclei are red. Bar = 100 μm. Type VI collagen staining was more prominent throughout engineered cartilage for constructs cultured in TMAO supplemented media (D). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jor]
Figure 4.
(A) Collagen and (B) GAG content normalized to wet weight (WW) for engineered cartilage in Study 1. *p < 0.05 compared to the control group (0 mM TMAO).
By day 42, the GAG content reached near native values and was 6.6 ± 0.7%/ww for the control group (Fig. 4B). The GAG content of engineered cartilage cultured in media supplemented with less than 100 mM of TMAO was 15% higher than the control group (p < 0.05; Fig. 4B).
Application of TMAO Following chABC Digestion
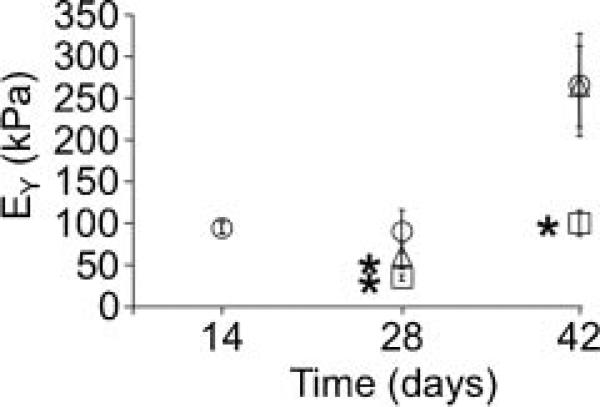
In Study 2, constructs were cultured in CM for 2 weeks then GAGs were digested with chABC at day 14. Following digestion, constructs were cultured either in CM or CM supplemented with 5 mM TMAO. Digestion of constructs with chABC decreased the Young's modulus from 94.3 ± 7.1 kPa at day 14 to 15 ± 1.3 kPa at day 16. By day 28, the Young's modulus of the digested engineered constructs cultured in TMAO media had recovered approximately 65% of the pre-digestion modulus (i.e., day 14) and was 1.8× greater than the digested control group (Fig. 6). At day 42, digested constructs cultured in TMAO media continued to grow at a faster rate than the digested control group. The Young's modulus of the digested engineered constructs cultured in TMAO media was and 235.6 ± 75.8 kPa and was not significantly different than the undigested control (Fig. 6). In contrast, the Young's modulus of digested constructs cultured in CM following digestion was 115.6 ± 38.1 kPa, which was 60% lower than the undigested control group (Fig. 6). Similarly, the dynamic modulus of the digested engineered constructs cultured with TMAO media recovered towards the undigested control group at a quicker rate than the digested constructs cultured in CM (data not shown). However, the dynamic modulus of the digested constructs cultured with TMAO media was 1.93 ± 0.26 MPa and did not reach the undigested control values by day 42 (2.78 ± 0.87 MPa, p < 0.001).
Figure 6.
Young's modulus of samples digested with chABC at day 14. Following the digestion, samples were cultured in CM (square) or CM supplemented with 5 mM of TMAO (triangle). Undigested samples served as the control (circle). *Significant difference between the undigested control and the chABC-digested constructs (p ≤ 0.05).
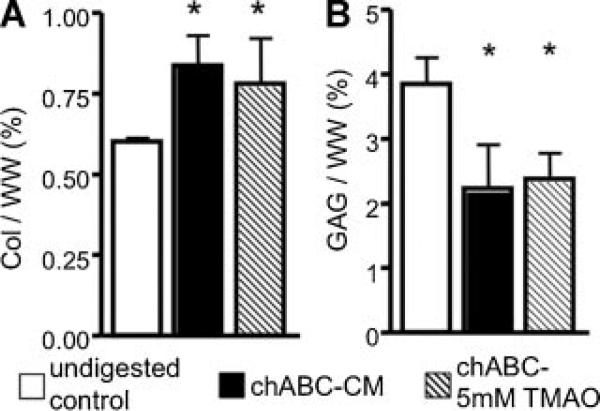
The DNA content of the undigested control group increased throughout the 6-week culture period (p < 0.0001). There were no differences between the DNA content of the undigested control group and the chABC-digested constructs (p = 0.5). By day 42, the collagen content of the undigested control group was 0.60 ± 0.11%/ww (Fig. 7A). Although the compressive mechanical properties of the digested engineered cartilage cultured with TMAO media were similar to the undigested control group at day 42, the collagen content of the digested constructs was approximately 30% higher than the undigested control (p < 0.05; Fig. 7A). The GAG content of the undigested control group was 3.85 ± 0.41%/ww at day 42 and was 30% lower than the undigested control group (p < 0.01; Fig. 7B). The GAG and collagen content was not significantly different between digested constructs cultured with CM and TMAO media (p > 0.05; Fig. 7).
Figure 7.
(A) Collagen and (B) GAG normalized to wet weight for the control group (white bar) and digested samples cultured in CM (black) or TMAO media (diagonal) at day 42. *Significant difference to the control group (p ≤ 0.05).
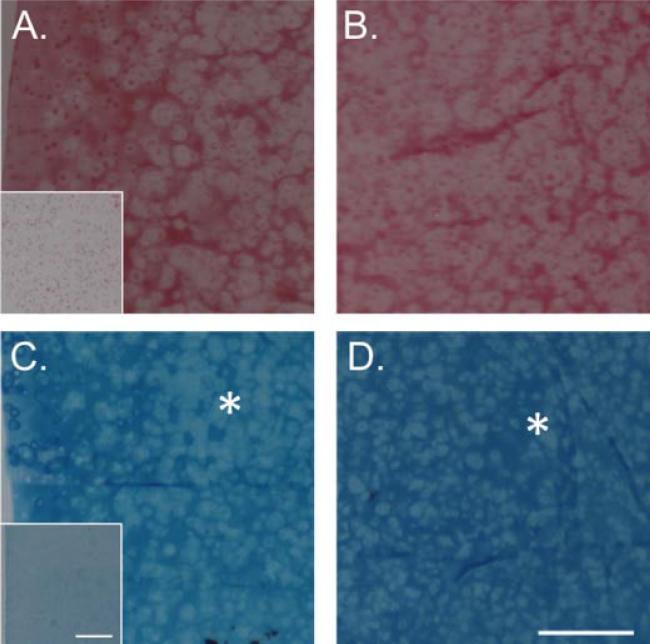
Picrosirius Red staining for collagen showed an uneven distribution of collagen through the depth of the chABC-digested constructs, where the staining was stronger on the edges (Fig. 8A,B). In contrast, Alcian Blue staining for proteoglycans was stronger in the center of the construct, which may be due to limited diffusion of chABC into the center of the constructs (Fig. 8C,D). There were no differences in the collagen or proteoglycan distribution between the groups (data not shown). The immunohistochemical staining for collagen I, II, and VI were comparable to the observations in Study 1. That is, the digested constructs cultured in CM media had very limited collagen type VI staining compared to the TMAO group (Fig. 5).
Figure 8.
Picrosirius Red staining for collagen in the (A) edge and (B) center of chABC-digested constructs cultured in CM supplemented with 5 mM TMAO at day 42. Alcian Blue staining for proteoglycans in the (C) edge and (D) center of chABC-digested constructs cultured in CM supplemented with 5 mM TMAO. Inset represents staining of constructs at day 0. The asterisk represents regions of lighter Alcian Blue staining at the edge and darker staining at the center. Bar = 500 μm. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jor]
DISCUSSION
The culture media for tissue-engineered cartilage is defined with many components that are crucial to the growth of chondrocytes in a 3D scaffold system. For example, dexamethasone and transient supplementation of TGF-β3 have been shown to be crucial components in the growth and maintenance of engineered cartilage and cartilage explants.8,9 The findings of our study lead us to accept the hypothesis that TMAO supplementation of the culture media improves collagen content and recovery of mechanical properties following enzymatic digestion of engineered cartilage under the conditions in the current investigation.
In this study, we evaluated the effect of adding TMAO into the culture media of engineered cartilage using two experimental designs. First, the culture media of immature constructs was supplemented with TMAO during the first 2 weeks of culture. We chose to study this short-term application of TMAO, as our laboratory and others have established that transient application of growth factors has a greater beneficial impact on development of engineered cartilage derived from juvenile chondrocytes, than continuous application of growth factors (i.e., the entire culture period).28–30 We observed improved mechanical and biochemical properties in engineered cartilage cultured in media supplemented with TMAO at concentrations less than 100 mM. Although the TMAO was only added to the culture media for the first 2 weeks, differences persisted throughout the 6-week culture period.
The second experiment evaluated the effect of supplementing the culture media with TMAO after chABC digestion. Previous studies have demonstrated that chABC digestion mediates an increase in collagen content of engineered constructs by temporarily suppressing the GAG content.6,22 In this study, we observed a 30% increase in the collagen content of engineered constructs digested with chABC. Furthermore, the functional properties of digested constructs cultured in TMAO media improved at a significantly faster rate than constructs cultured in the control medium (p < 0.05; Fig. 6). Although the functional properties were improved, the collagen and proteoglycan content of digested constructs cultured in TMAO were not significantly greater than digested constructs cultured in CM. It is likely that the improved functional properties are due to other extracellular matrix components that were not measured in this study (e.g., cartilage oligomeric matrix protein, COMP). Together, these findings suggest that an optimized concentration of TMAO can be supplemented to the culture medium to increase the rate of tissue growth and the overall collagen content.
Sharks store a large concentration of urea to stabilize their internal osmolarity with their salty environment. TMAO is thought to counteract the negative effect of urea by stabilizing protein folding.15–18 Preliminary studies suggest that low concentrations of urea (90 mM) did not have an adverse effect on the ability of juvenile chondrocytes to produce engineered cartilage. However, concentrations of urea (360 mM), which was within the range of physiological concentrations for sharks (400 mM), caused cell death as expected and, therefore, a lack of tissue growth. Combining TMAO and urea using physiological ratios (1:2, TMAO:urea) moderately lessened the effects of urea on bovine chondrocytes. These findings do support the notion that TMAO may help cells counteract the negative effects of urea. Since the addition of only TMAO in these preliminary studies showed improvements in functional properties of engineered cartilage, we furthered our investigation with the presented studies to optimize the TMAO concentration for bovine chondrocytes.
There are various potential mechanisms by which TMAO may exert its beneficial effects, including its role as a chemical chaperone with effects on protein folding/stabilization and osmolyte.18 We do not believe the results are due directly to osmotic-loading induced stimulation of chondrocytes. The native osmotic environment of juvenile bovine chondrocytes ranges from 340 mOsm at the superficial zone to 400 mOsm in the deep zone.23 TMAO is a natural osmolyte that increases the osmolarity of the culture media from 307 mOsm for the control group to 345 mOsm for the 200 mM TMAO group (Fig. 2). Previous studies have shown that increasing the culture media osmolarity above 350 mOsm decreases cell proliferation, mechanical properties, GAG and collagen production.26,31,32 Similarly, we observed a significant decrease in the cell proliferation and mechanical properties of engineered cartilage culture in the TMAO media with the highest osmolarity (i.e., 200 mM TMAO). It should be noted that the absolute difference in the osmolarity of the 5 mM TMAO group and the control group was less than 2 mOsm; however, the mechanical properties and the GAG content was significantly higher in the 5 mM TMAO group. Together, these findings suggest that although our initial aim was to use TMAO as a natural osmolyte, these findings suggest that improved functional properties of engineered cartilage may be due more to the protein stabilization properties TMAO, rather than the osmotic effects. Future studies will be performed to better understand the mechanism underlying TMAO's beneficial effects.
In the current study, amphotericin B was a constituent of the culture media, which we have shown to yield mechanically functional engineered cartilage. It is possible that this anti-fungal agent known to induce pores in the cell membrane33 may play a role in the observed beneficial action of TMAO. Future studies are aimed at further optimization of TMAO as a media supplement for cartilage tissue engineering as well as for elucidating the underlying mechanisms that mediate its effects.
Temporary suppression of GAGs in engineered cartilage has been shown to increase collagen production and the tensile and compressive stiffness of engineered cartilage.6,22 Similarly, the findings in Study 2 showed an increase in the collagen content of engineered cartilage following chABC digestion at day 14. Importantly, recovery of the digested engineered cartilage mechanical properties was significantly faster for constructs cultured in CM media supplemented with a nominal amount of TMAO (i.e., 5 mM TMAO; Fig. 6). The findings of this study suggest that supplementing the culture media with TMAO, following chABC digestion, provides the added biochemical enhancement of collagen content offered by chABC digestion (Fig. 7), and decreases the time necessary for full recovery of mechanical properties towards native values.
We have been able to successfully grow engineered cartilage using agarose as a three-dimensional scaffold. The 3D agarose scaffold used here provides a biocompatible, clinically relevant scaffold system that can be molded to fit the contours of the knee joint.34,35 Within 6 weeks of in vitro culture, we have demonstrated that this culture system can produce engineered cartilage with compressive properties and GAG contents within the range of native tissue. However, growing engineered cartilage with collagen values close to native tissue remains a complex challenge within the field. The findings of this initial study, using TMAO as a culture media supplement, suggest that TMAO can be employed to increase collagen content moderately. The studies presented here evaluated the effects of TMAO for transient periods during the culture, that is, during the first 2 weeks of in vitro culture or after chABC digestion. Future work will focus on optimizing length of TMAO supplementation and the best time to introduce TMAO into the culture system (i.e., at day 0 or after chABC digestion). In conclusion, supplementing the culture media with TMAO shows promise for improving the compressive mechanical properties, increasing extracellular matrix production, and increasing the recovery time following chABC digestion.
Acknowledgments
Grant sponsor: NIAMS; Grant numbers: AR52871, AR46568.
REFERENCES
- 1.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mov VC, Hayes WC, editors. Basic orthopaedic biomechanics. Raven Press; New York: 1997. pp. 197–207. [Google Scholar]
- 2.Mow VC, Bachrach NM, Setton LA, et al. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In: Mov VC, Guilak F, Hayes WC, editors. Cell mechanics and cellular engineering. Springer; New York: 1994. pp. 345–379. [Google Scholar]
- 3.Guilak F, Estes BT, Diekman BO, et al. Nicolas Andry Award: multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530–2540. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo CK, Li WJ, Mauck RL, et al. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 5.Toh WS, Lee EH, Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. 2011;7:544–559. doi: 10.1007/s12015-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 6.Bian L, Crivello KM, Ng KW, et al. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A. 2010;15:2065–2072. doi: 10.1089/ten.tea.2008.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JJ, Nicodemus GD, Greenwald EC, et al. Degradation improves tissue formation in (un)loaded chondrocyte-laden hydrogels. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-1823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian L, Stoker AM, Marberry KM, et al. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78–85. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang AH, Stein A, Tuan RS, et al. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461–3472. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smetana K., Jr Cell biology of hydrogels. Biomaterials. 1993;14:1046–1050. doi: 10.1016/0142-9612(93)90203-e. [DOI] [PubMed] [Google Scholar]
- 11.Hatic SO, II, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3:361–364. doi: 10.1177/1938640010388602. [DOI] [PubMed] [Google Scholar]
- 12.Kielpinski G, Prinzi S, Duguid J, et al. Roadmap to approval: use of an automated sterility test method as a lot release test for Carticel, autologous cultured chondrocytes. Cytotherapy. 2005;7:531–541. doi: 10.1080/14653240500361079. [DOI] [PubMed] [Google Scholar]
- 13.Mauck RL, Soltz MA, Wang CC, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 14.Ng KW, Kugler LE, Doty SB, et al. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthritis Cartilage. 2009;17:220–227. doi: 10.1016/j.joca.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 16.Lin TY, Timasheff SN. Why do some organisms use a urea-methylamine mixture as osmolyte? Thermodynamic compensation of urea and trimethylamine N-oxide interactions with protein. Biochemistry. 1994;33:12695–12701. doi: 10.1021/bi00208a021. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Haque I, Ahmad F. Counteracting osmolyte trimethylamine N-oxide destabilizes proteins at pH below its pKa. Measurements of thermodynamic parameters of proteins in the presence and absence of trimethylamine N-oxide. J Biol Chem. 2005;280:11035–11042. doi: 10.1074/jbc.M410716200. [DOI] [PubMed] [Google Scholar]
- 18.Meersman F, Bowron D, Soper AK, et al. Counteraction of urea by trimethylamine N-oxide is due to direct interaction. Biophys J. 2009;97:2559–2566. doi: 10.1016/j.bpj.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancey PH, Siebenaller JF. Trimethylamine oxide stabilizes teleost and mammalian lactate dehydrogenases against inactivation by hydrostatic pressure and trypsinolysis. J Exp Biol. 1999;202:3597–3603. doi: 10.1242/jeb.202.24.3597. [DOI] [PubMed] [Google Scholar]
- 20.Treberg JR, Speers-Roesch B, Piermarini PM, et al. The accumulation of methylamine counteracting solutes in elasmobranchs with differing levels of urea: a comparison of marine and freshwater species. J Exp Biol. 2006;209:860–870. doi: 10.1242/jeb.02055. [DOI] [PubMed] [Google Scholar]
- 21.Porter ME, Beltran JL, Koob TJ, et al. Material properties and biochemical composition of mineralized vertebral cartilage in seven elasmobranch species (Chondrichthyes). J Exp Biol. 2006;209:2920–2928. doi: 10.1242/jeb.02325. [DOI] [PubMed] [Google Scholar]
- 22.Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A. 2009;15:3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oswald ES, Chao PH, Bulinski JC, et al. Dependence of zonal chondrocyte water transport properties on osmotic environment. Cell Mol Bioeng. 2008;1:339–348. doi: 10.1007/s12195-008-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76:1044–1056. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampat SR, O'Connell GD, Fong JV, et al. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2011.0155. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oswald ES, Ahmed HS, Kramer SP, et al. Effects of hypertonic (NaCl) two-dimensional and three-dimensional culture conditions on the properties of cartilage tissue engineered from an expanded mature bovine chondrocyte source. Tissue Eng Part C Methods. 2011;17:1041–1049. doi: 10.1089/ten.tec.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly TA, Wang CC, Mauck RL, et al. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223–237. [PubMed] [Google Scholar]
- 28.Byers BA, Mauck RL, Chiang IE, et al. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima EG, Bian L, Ng KW, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng KW, O'Conor CJ, Kugler LE, et al. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39:2491–2500. doi: 10.1007/s10439-011-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo J, Kim KI, Min BH, et al. Controlling medium osmolality improves the expansion of human articular chondrocytes in serum-free media. Tissue Eng Part C Methods. 2010;16:957–963. doi: 10.1089/ten.TEC.2009.0525. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Urban JP, Tirlapur UK, et al. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthritis Cartilage. 2010;18:433–439. doi: 10.1016/j.joca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Marty A, Finkelstein A. Pores formed in lipid bilayer membranes by nystatin, Differences in its one-sided and two-sided action. J Gen Physiol. 1975;65:515–526. doi: 10.1085/jgp.65.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung CT, Mauck RL, Wang CC, et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 35.O'Connell GD, Lima EG, Bian L, et al. Toward engineering a biological joint replacement. J Knee Surg. 2012 doi: 10.1055/s-0032-1319783. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]