Abstract
Aims
To test the hypotheses that some epithelial cells in the rabbit lacrimal gland (LG) are mucin-secreting cells that are also particularly rich in aquaporin 5 (AQP5) and sodium potassium ATPase β1 subunit (NKAβ1), LG-secreted mucins contribute to the total mucin pool in tear film, and that the rabbit LG is a heterogenic gland where proteins secreted in response to different agonists are varied.
Materials and methods
LGs were obtained from adult female rabbits and processed for paraffin sections for hematoxylin and eosin (HE) staining, periodic acid-Schiff (PAS), mucicarmine, and Alcian blue (pH 0.4, 1.0, and 2.5) for the detection of mucins. Serial sections were used for immunohistochemistry (IHC) and PAS. LG lysates and fluids were assayed by dot blot for detection of mucins, and by SDS-PAGE to detect differences in protein profiles of LG fluids stimulated by different agonists.
Results
HE staining demonstrated that the LG is a heterogeneous gland where most epithelial cells are serous, while all duct cells are mucous cells. Some acini and individual acinar cells within serous acini are also mucous or seromucous cells and these cells are particularly rich in AQP5 and NKAβ1. Dot blot assay showed the presence of mucins in the LG fluids. The protein profiles of LG fluids from pilocarpine, phenylephrine, and isoproterenol varied significantly, particularly in the mid range.
Conclusions
Our data indicated that the rabbit LG is a heterogeneous gland that is composed of both serous and mucin-secreting cells, and mucins produced by the LG contribute to the mucin pool in the tear film. The heterogeneity of the rabbit LG supports the notion of differential secretion, i.e. the volume and composition of the LG fluids vary depending on various circumstances in the ocular surface and the body’s needs.
Keywords: Lacrimal gland, Heterogeneity, Mucin, Dry eye, Differential secretion
INTRODUCTION
Lacrimal gland (LG) is the major source of tears that are essential for the health and function of the ocular surface. Dysfunction of the LG can result in decreased tear production, and subsequent dry eye symptoms.
Despite its importance, many of the questions remain unanswered regarding the structure and function of LG. LG has traditionally been thought as a serous gland, i.e. all the epithelial cells within the gland are serous cells that are mainly responsible for water, electrolytes, and protein secretion. Although the tear film contains a significant amount of mucins, it has been thought these mucins are mainly originated from goblet and epithelial cells in the conjunctiva,1 corneal epithelial cells,2 with minor contributions from some accessory glands.3,4
Although many earlier investigations, albeit often inconsistent, reported the presence of mucous cells in the LGs of human,5-9 rat,10,11 and suggested the LG also contains mucous cells that are capable of secreting mucins, other reports still described the LG as a homogenous gland and comprised of serous cells only.12
The rabbit has been a popular animal used for LG and dry eye studies. However, some controversies exist regarding whether and how many mucous cells are present in LG. Kühnel et al first reported very little PAS positive material (ductal cells) in the rabbit LG,13 while others reported that PAS positive acinar cells were found in most lobules,14,15 and the majority of acinar cells in rabbit LG were mucous producing.16
Millar et al. reported that some epithelial cells in the rabbit LG were mucous cells, forming demilune in some acini, along with some individual acinar cells in serous acini.17 Although the authors indicated that intercalated ducts were weakly PAS positive and interlobular ducts contained a mixture of PAS positive and Alcian blue granules in the apical regions, only one image of intercalated duct was presented.
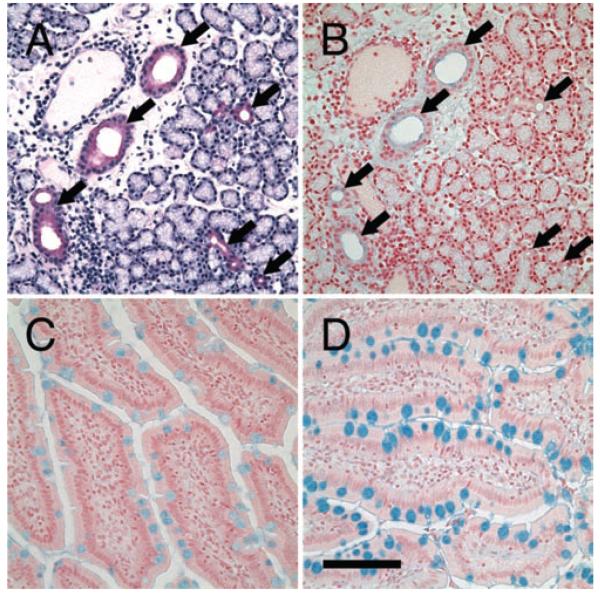
Due to the lack of knowledge of the LG duct system and confusions in interpreting data from different reports, we recently characterized the specific segments of the duct system in rabbit, based upon the morphological and functional characteristics, and established a standardized nomenclature of the duct system.18 The LG duct system can be divided into intralobular, interlobular, intralobar, interlobar, and main excretory ducts. During the course of our studies, we noticed in HE stained tissue sections that the rabbit LG was not a homogeneous tissue, i.e. some lobes/lobules/acini/acinar cells are more pinkish (eosinophilic, Figure 1) than others, and that duct cells looked bright red, suggesting that the rabbit LG may be a mixed gland, like the submandibular and sublingual salivary glands, composed of both serous and mucous cells.
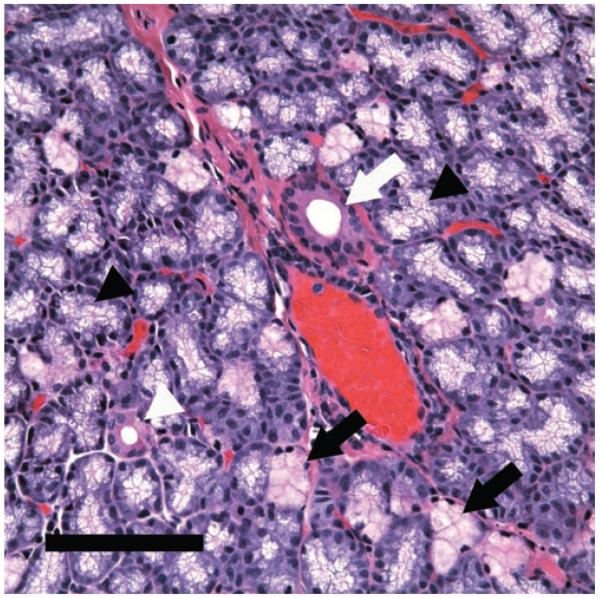
FIGURE 1.
HE staining demonstrated the heterogeneity of rabbit LG. Acinar cells showed a foamy appearance because of the numerous secretory granules in their cytoplasm but their shade did vary. Most of the acinar cells show a pale appearance (black arrowheads), indicating they are serous cells, while some acini (black arrows) showed a pinkish red appearance, suggesting that the contents in these cells are more eosinophilic. Ducts, in contrast, were stained bright red (eosinophilic) in a smooth pattern. White arrowhead: intralobular duct. White arrow: interlobular duct. Scale bar, 100 μm.
Our recent studies revealed that aquaporin 5 (AQP5) and sodium potassium ATPase β1 subunit (NKAβ1) were distributed unevenly within the rabbit LG.18 AQP5 immunoreactivity (IR) was distributed among acini in a mosaic pattern, with some acini and acinar cells demonstrating much stronger AQP5-IR than the rest of the acini/acinar cells, although minimal AQP5-IR was detected in duct cells. NKAβ1-IR was present in all acinar cells, however, the levels also differed in a mosaic pattern: higher in some acinar cells and acini than in others.
These immunological findings, along with morphological observations that the LG epithelial cells showed a heterogenic appearance, prompted us to hypothesize that the acinar cells which demonstrated higher AQP5-IR and NKAβ1-IR were mucin-secreting cells, and that the rabbit LG is capable of secreting mucins that contribute to the mucin pool in the ocular surface. The heterogeneity of the rabbit LG suggests that each lobe/lobule/acini/acinar cells may play a unique role and the compositions of tears produced by the rabbit LG may differ depending on the needs of the body and environment that resulting in differential secretion.
METHODS
Chemicals
Pilocarpine, isoproterenol bitartrate, l-phenylephrine hydrochloride, were purchased from Sigma-Aldrich (St. Louis, MO). All reagents were of the highest purity available.
Animals and LG Collection
Twelve adult female New Zealand White rabbits (Irish Farms, Norco, CA) weighing approximately 4.0 kg were used. Rabbits were narcotized with a mixture of ketamine (40 mg/mL) and xylazine (10 mg/mL) and given an overdose of pentobarbital (80 mg/kg) for euthanasia. Inferior LGs were removed and fixed with 10% neutral buffered formalin, pH 6.9 to 7.1 at 25°C (Electron Microscopy Sciences, Ready-to-use, Fort Washington, PA) for paraffin embedding. This study conformed to the standards and procedures for the proper care and use of animals as described in the ARVO Statement for the Use of Animals in Ophthalmic Research.
Histochemical Staining
Paraffin sections, at 4 μm, were stained with hematoxylin and eosin (HE) for general morphology. For the detection of mucins, serial paraffin sections were stained with the following staining methods: 1) periodic acid-Schiff (PAS), which stains glycogen, mucin, and some basement membrane as red to purple; 2) diastase/PAS: diastase treatment before PAS staining breaks down glycogen, and therefore the staining is due to mucin only; 3) mucicarmine, which stains mucin as dark rose; 4) Alcian blue at pH 2.5, which stains weakly acidic sulfated mucosubstances, hyaluronic acid, and sialomucins dark blue; 5) Alcian blue at pH 1.0, which stains sulfated mucosubstances dark blue; 6) Alcian blue at pH 0.4, which stains strongly acidic sulfated mucosubstances blue. For Alcian blue, the slides were counterstained with nuclear fast red. Staining techniques follow standard protocols.19
Slides were evaluated with a compound microscope (Eclipse 80i; Nikon, Tokyo, Japan), and images were captured with a digital camera (Optronics, Goleta, CA) and analyzed by the manufacturer’s software (PictureFrame).
Immunohistochemistry (IHC)
Paraffin sections were processed for IHC detection of goat polyclonal anti-aquaporin 5 (AQP5, C-19, 1:400, Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-NKAβ1, 1:100, (Santa Cruz Biotechnology). Secondary antibodies used were donkey anti-goat and mouse IgG, all at a dilution of 1:750 and then streptavidin peroxidase at 1:500 (Jackson ImmunoResearch Laboratories, West Grove, PA). Tissue sections were stained with DAB (Sigma).
Protein Assay
The samples were analyzed for their total protein by using QuickStart™ Bradford protein assay (Bio-Rad, Hercules, CA). Bovine serum albumin (BSA) was used as a standard protein, and standards were run with each assay. Estimates of protein concentrations were determined from the standard curves measured with each assay. The assays were performed on a microplate reader (EL 808; Bio-Tek Instruments, Winooski, VT), read at 595 nm. Both samples and standards were read in duplicate in 96-well flat-bottomed microplates (Greiner bio-one GmbH; Frickerhausen, Germany). Total protein concentration was determined using the software provided by the manufacturer (KC4; Bio-Tek). Proteins secreted in response to various agonists (stimulated secretion) were measured by the difference between total and basal secretions. The readings were then converted to micrograms per milliliter per gram tissue per minute (μg/mL/g/min).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Samples were denatured in SDS-PAGE sample buffer for 20 minutes at 60 °C, resolved on a 4–20% gradient gel (Bio-Rad) at 80 V for 20 min and 100 V for 60 min, and then stained with GelCode Blue Stain Reagent (Thermo Scientific, Waltham, MA) and scanned with an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE).
LG Fluids Collection
LG fluid was collected using the local technique we developed recently.20 In brief, after the lacrimal duct opening was cannulated by a small-bore polyethylene tubing in an anesthetized rabbit, the inferior LG was locally bathed in agonists (pilocarpine, phenylephrine, and isoproterenol, all at 1.2 mg/mL), and stimulated LG fluids were collected in centrifuge tubes which were stored at −80°C until use.
Dot Blot Assay
For dot blot of LG lysates and pilocarpine stimulated LG fluids, nitrocellulose membranes were placed on a 96-well Hybri-dot Manifold apparatus (Bethesda Research Laboratories, MD) and samples were immobilized by water suction. After filtration, water was added under vacuum to each well to wash out the salt attached to the membrane, which were then subject to subsequent staining with PAS and Alcian blue at pH 2.5.21,22
RESULTS
Histochemical Staining
HE staining showed that the rabbit LG is a heterogeneous gland, where most of the acinar cells are serous cells, with a pale and foamy appearance due to the numerous secretory granules within their cytoplasm. However, some acini and acinar cells within the serous acini were stained pinkish red, i.e. more eosinophilic. Ducts were stained as bright red, i.e. even more eosinophilic, and with a smooth pattern, in contrast to the foamy appearance of acinar cells (Figure 1).
The heterogeneous staining pattern suggested that these pinkish and red colored acinar and duct cells may be mucous cells, as seen in other mixed glands such as submandibular and sublingual salivary glands. Therefore we decided to use a number of histochemical stains that specifically stains mucin to test our hypothesis. Indeed, PAS staining indicated that all duct cells were stained as magenta, suggesting that they are mucin-secreting mucous cells, along with some acini and individual acinar cells within serous acini that were also stained bright magenta (Figure 2A). Like HE staining, some acini were also lightly stained pinkish red, suggesting that they were seromucous cells capable of producing both serous and mucous products. However, most of the acini were serous cells. The heterogeneous characteristics of the rabbit LG was also reflected by the fact that some areas of the gland were particularly rich in mucin-secreting cells, while other parts of the gland had considerably less of these cells (Figure 2B).
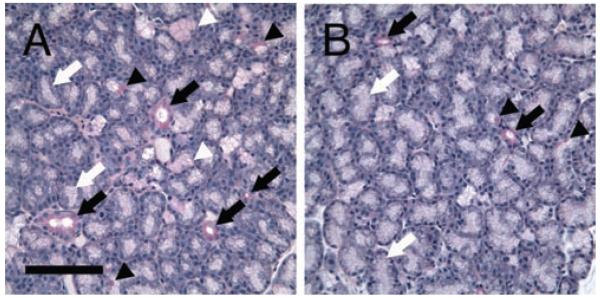
FIGURE 2.
PAS staining demonstrated the heterogeneity of serous, seromucous, and mucous cells in LG. A) In this area of the LG, there are many mucous acinar cells stained as intense magenta (black arrowheads), and seromucous acinar cells stained as pinkish red (white arrowheads). However, most acini are composed of serous cells that showed pale appearance (white arrows). Ducts are stained as intense magenta by PAS (black arrows). B) In other areas of the LG, much less mucous and seromucous acinar cells are found. Scale bar, 100 μm.
Because PAS also stains glycogen as intense magenta, in addition to mucins, we used serial sections and diastase, which breaks down glycogen, to confirm that there were no glycogen present in the LG and that all the PAS positive staining was indicative of mucins (Figure 3).
FIGURE 3.
Serial sections of LG demonstrated that those stained with PAS was mucin, not glycogen. A) PAS only. All ducts were stained as intense magenta. B) PAS with diastase. Same staining pattern as A, indicating that only mucins were stained, not glycogen. Scale bar, 100 μm.
By using serial sections, the PAS positive staining was confirmed by mucicarmine, another histochemical stain (Figure 4). Alcian blue, which selectively stains acidic mucins, indicated that some mucins in ducts, particularly those in the apical regions, were acidic, and that these mucins were more prominent in larger ducts (Figure 5), results consistent with previous report in rabbit, although no image was presented.17
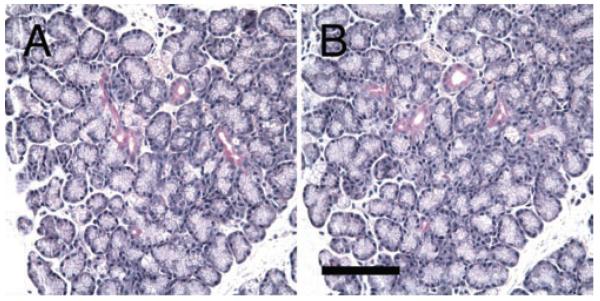
FIGURE 4.
Serial sections of LG that have been stained with PAS and mucicarmine. A) All ducts are stained with PAS as magenta (black arrows), which are confirmed by mucicarmine staining (B). As a positive control, goblet cells in intestine were also stained with mucicarmine (C). Scale bar, 100 μm.
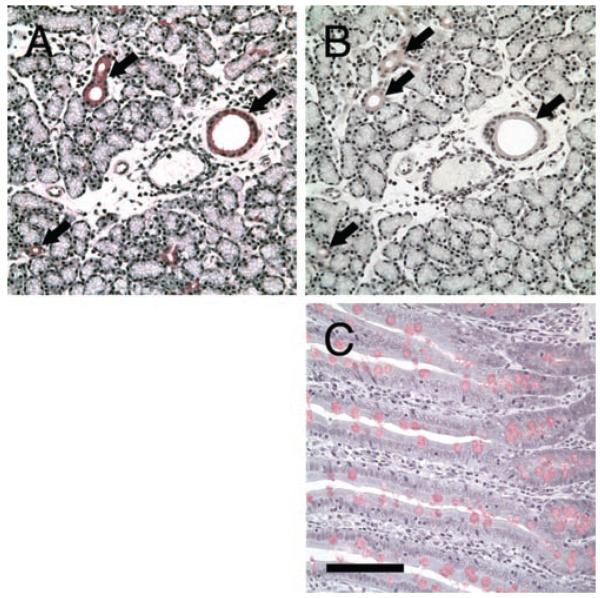
FIGURE 5.
Serial sections of LG showed the presence of acidic mucins in all duct cells. A) All ducts are stained as intense magenta by PAS (arrows). B) All ducts are also stained with Alcian blue at pH 0.4, although the staining appeared to be concentrated in the apical regions and more prominent in larger ducts, which is in consistent with previous report from Millar et al, 1996, although no images were presented. As positive controls, goblet cells in intestines were also stained with Alcian blue, at pH 0.4 (C) and pH 2.5 (D), respectively. Scale bar, 100 μm.
It has been suggested that mucicarmine staining is non-specific for the demonstration of acid mucosubstances.19 The weak staining we observed with mucicarmine, along with the results from Alcian blue in duct cells, support this notion.
Because our previous report demonstrated that the rabbit duct system can be separated into 4 distinct segments, and each segment has its anatomical and morphological characteristics,18 we also characterized the PAS staining pattern of each duct segment. Our results indicated that all duct segments were stained with PAS (Figures 2-6). It should be noted that the staining intensity differences, as we discussed here, were subjective and personal interpretation, and no quantitative analysis was performed.
FIGURE 6.
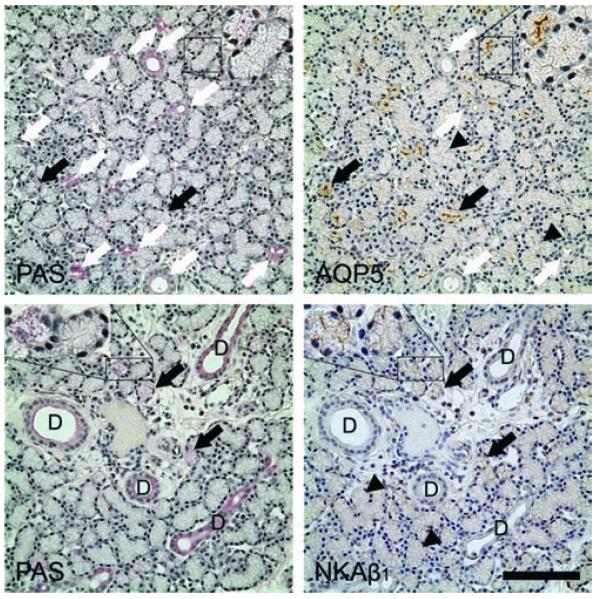
Mucous acinar cells are particularly rich in AQP5-IR and NKAβ1-IR. Top Row: Serial sections indicated that the acinar cells that have been stained with PAS, with numerous granules in the cytoplasm stained as light magenta (black arrows), were also particularly rich with AQP5-IR, while all ducts that have been stained intensely by PAS were minimally immunoreactive to AQP5 (white arrows). Most acinar cells demonstrated AQP5-IR (black arrowheads) although the intensity was much lower than those acinar cells which were particularly rich in AQP5-IR, giving the gland a “mosaic” pattern. AQP5-IR was mostly in the apical membranes. Insets: higher magnification of 1 acinus (top left) with PAS positivity is also particularly rich in AQP5-IR, while another acinus showing no PAS positivity and minimal AQP5-IR. Bottom Row: Serial sections indicated that acinar cells that have been stained with PAS (black arrows), were also particularly rich with NKAβ1-IR. Most acinar cells, although demonstrating NKAβ1-IR (black arrowheads), had a much lower intensity than those acinar cells which were particularly rich in NKAβ1-IR, giving the gland a “mosaic” pattern. NKAβ1-IR was most prominent in the basolateral membranes, particularly the lateral membrane. Insets: higher magnification of 1 acinus (left) with PAS positivity is also particularly rich in NKAβ1-IR, while another acinus showing no PAS positivity and much less NKAβ1-IR. Scale bar = 100 μm.
Immunohistochemistry
Our previous data indicated that AQP5 and NKAβ1 were distributed in the rabbit LG in a “mosaic” pattern, with some acini and acinar cells particularly rich in these two molecules,18 this prompted us to hypothesize that these acini and acinar cells may be the mucin-secreting cells as we demonstrated by PAS. Therefore, we used serial sections of the LG, and our results indeed showed that these AQP5- and NKAβ1-rich acinar cells were mucinsecreting cells, while duct cells, although also capable of secreting mucins, were minimally immunoreactive to AQP5 and NKAβ1 (Figure 6).
Dot Blot Assay
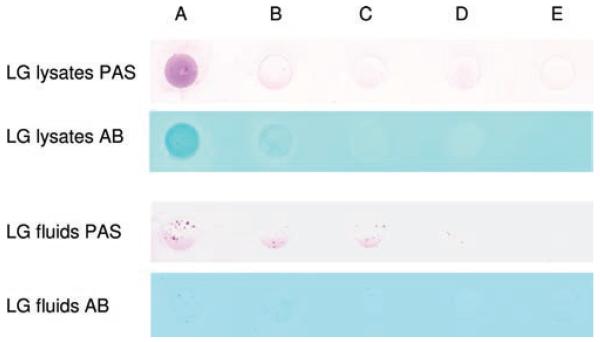
Because there were two types of mucins in the epithelium, membrane tethered and secreted, we hypothesized that the mucin-secreting cells in the LG also contribute to the mucin pool in the tear film. Our dot blot assays indicated the presence of mucins in the LG lysates and LG fluids (Figure 7), confirming that the mucins in the tear film are also secreted by mucin-secreting cells from the LG, in addition to goblet cells in conjunctiva. However, it should be noted that although mucins were present in both LG lysates and fluids, their amount in the LG fluids was considerably less than those in the lysates.
FIGURE 7.
Dot blot assays of LG lysates and fluids, as detected by PAS and Alcian blue (AB, pH = 2.5), demonstrated the presence of mucins. Protein concentration of LG lysates was 13.33 μg/μL, and 13.68 μg/μL for LG fluids. Each picture was a representative of at least 3 experiments. Note that although the amount of total proteins in LG fluids was similar to that in LG lysates, the amount of mucins was considerably less, and these mucins tend to be in clusters. Sample volume in each dot: A = 20 μL; B = 10 μL; C = 5 μL; D = 2.5 μL; E = 0 μL.
SDS-PAGE
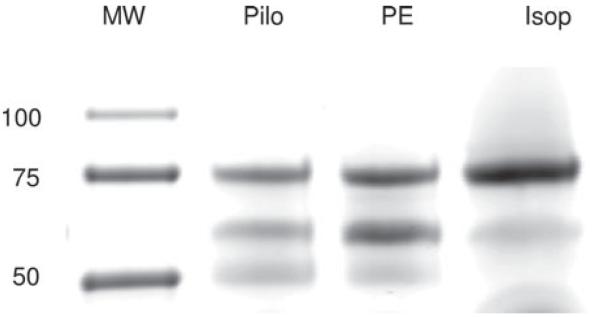
It has been suggested that the LG fluids produced in response to different agonists and at various situations may differ in their compositions, i.e. differential secretion,23,24 a notion that appears to be consistent with our data presented here (Figure 8). SDS-PAGE of LG fluids in response to various agonists indicated that the protein profiles were significantly different, most prominent in the mid range.
FIGURE 8.
SDS-PAGE of proteins secreted from LG in response to various agonists. Samples were run on 4–20% gradient gel. There was a significant difference of the protein profile that was most evident in the mid range. Pilo = pilocarpine. PE = phenylephrine. Isop = isoproterenol. The picture was a representative of at least three experiments from three animals in each group.
DISCUSSION
The LG is a tubuloacinar gland that is made up of lobes and lobules, and each lobule contains many acini–the secretory unit of LG. Each acinus consists of acinar cells with their apices pointed to a central lumen, and lumens from many acini unite to form ducts that eventually exit onto the ocular surface.
It has been thought that mucins in the tear film originate mainly from goblet cells in the conjunctiva and that the LG contains only serous cells that are responsible for producing a watery secretion composed of water, electrolytes, and proteins,12 despite some previous reports, albeit often inconsistent, which suggested the LG also contains mucous cells that are capable of secreting mucins. Kühnel et al first reported very little PAS positive material (ductal cells) in the rabbit LG and did not seem to recognize the presence of mucous acini,13 while others reported that PAS positive acinar cells were found in most lobules and these formed either true acini, demilunes similar to those seen for serous cells of the submandibular gland, or were individual cells in serous acini.14,15 However, other studies have also indicated that the majority of acinar cells in rabbit LG were mucous producing but were based on structural characteristics rather than PAS staining.16 Mucin-secreting epithelial cells were also found in LG from human,5-9 and rat.10,11
Millar et al. reported that the majority of epithelial cells in the rabbit LG were serous, while some acini contained a small percentage of PAS positive cells, which sometimes formed part of the acinus and on other occasions appeared to form demilune.17 They also reported that PAS positive cells varied between lobules from almost none to about 10%, and that the inferior LG had more PAS positive acinar cells than the superior LG. The authors also reported that PAS positive staining was seen in intercalated duct cells, which were stained much weaker than acinar cells. Interlobular ducts contained a mixture of PAS positive and Alcian blue granules in the apical regions, although no image was presented.
Our data presented here clearly showed that all the ductal cells in the rabbit LG were mucous cells, in addition to some acini and some individual acinar cells within serous acini, which are either mucinsecreting mucous or seromucous cells. Some acini and acinar cells appeared to be mixed cells, i.e. they can secrete both serous and mucous contents, as demonstrated by the light pinkish staining by PAS. Some serous acini also showed a mucous demilune. These data were generally in agreement with findings from Millar et al.17 However, the duct cells in our studies appeared to be stained much stronger than theirs. What’s more, duct cells from all duct segments were stained similarly and Alcian blue positive staining was found in the apical regions.
These data suggest that the rabbit LG is a mixed gland where most of the acinar cells are serous cells, while some acinar cells and all ductal cells are mucin-secreting mucous or seromucous cells, more like the submandibular and sublingual glands rather than the parotid gland. Our dot blot assay also clearly demonstrated the presence of mucins in the LG fluids, which provides direct evidence that the rabbit LG synthesizes mucins that contribute to the mucin pool in the tear film.
These data also indicate that the rabbit LG is a heterogenic gland in which the lobes and lobules, in addition to containing both serous and mucous cells, are composed of different populations of secretory cells, suggesting that acini and ducts, even acinar cells within the same acinus, may perform different roles. The notion of differential secretion, i.e. the variance in rate and composition of LG fluid secretion, which depends on the various circumstances in the ocular surface and the body’s needs,23-25 is also supported by our morphological and SDS-PAGE studies.
Mucins, as either membrane-associated or secreted forms, are a family of high molecular weight, heavily glycosylated proteins produced by epithelial tissues that are vital for the maintenance of a healthy moist ocular surface.26,27 Mucins have been shown to be present on all wet-surfaced epithelia and their major functions include lubrication, water retention, and pathogen blockage.13 Glandular mucins have also been proposed to be required for maintenance of a patent acinar and ductal lumen, because they prevent apical to apical adherence.28 Recent data indicated that mucins in LG may also play a role in signaling events,10,11 and may be involved in the pathological events that occur at the ocular surface such as dry eye.9
The presence of mucins in the LG, particularly in the apical membranes of duct cells, suggests that they may play a role in LG fluids rheology by reducing the shearing force while being transported in the ducts, while at the same time also serve as a protective barrier for potential pathogens that may travel upstream from the ocular surface.9,29 Our Alcian blue staining, which preferentially stains acidic mucins, demonstrated the presence of acidic mucins in the apical membranes of all duct cells, most prominent in bigger ducts. The mucinsecreting capacity within the LG, in addition to goblet cells in conjunctiva and accessory glands in eyelids, may serve as a necessary redundancy given the critical role mucins play in maintaining the health and function of the eye.
Mucins undergo massive swelling by hydration during and after exocytosis from epithelial cells.30-32 Because of the presence of mucins, the LG fluids will be more viscous and could potentially block the luminal space in acini; therefore, it is essential that sufficient water secreted by acinar cells during and after the exocytosis of mucins. The increased presence of AQP5 and NKAβ1 in mucin-secreting acinar cells appear to be the reasonable explanation, i.e. facilitate mucin secretion and transport.
AQP are a group of channels that are responsible for fast water movement across membranes,33 and NKA are responsible for providing a Na+ gradient that can be used by other carrier processes.34 Indeed, it has been proposed that salivary gland serous and mucous secretions may involve different mechanisms.35 AQP5 and mucin are both secreted from human salivary glands, and their concentrations in saliva correlate well at various collection times and conditions, suggesting these mucins are secreted by mucous cells from the LG epithelial cells that are also rich in AQP5.36
The minimal presence of AQP5 and NKAβ1 in duct cells, however, suggest the increased presence of these 2 molecules may not be needed due to the larger luminal space in ducts, as compared to the acinar lumen. Other explanations could be that there may be other isoforms of AQP present in ducts or the mucins secreted by duct cells are membrane-associated only, as evidenced by Alcian blue staining, and these mucins mainly facilitate the transport of LG fluids in the ducts.
In summary, data presented here indicates that the rabbit LG is a heterogeneous exocrine gland that is composed of both serous and mucin-secreting cells, like the submandibular and sublingual salivary glands. All the duct cells are mucous cells, while some acini and individual acinar cells in serous acini are also mucin-secreting cells. Mucins produced by the LG are secreted and transported onto the ocular surface. AQP5 and NKAβ1 were particularly rich in the mucin-secreting acinar cells, but not in the duct cells. LG fluids produced in response to different agonists have different protein compositions, supporting the notion of differential secretion.
ACKNOWLEDGEMENTS
This work was supported by NIH grants EY017731 (CD), DK048522 (Core Facilities of the USC Research Center for Liver Diseases), and EY03040 (Doheny Eye Institute Core). The authors thank Maria Edman, Ernesto Barron, and Qinggao Deng for helpful comments and discussions.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- [1].Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease. A better indicator than tear mucin. Arch Ophthalmol. 1983;101:1284–1287. doi: 10.1001/archopht.1983.01040020286025. [DOI] [PubMed] [Google Scholar]
- [2].Watanabe H, Fabricant M, Tisdale AS, Spurr-Michaud SJ, Lindberg K, Gipson IK. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36:337–344. [PubMed] [Google Scholar]
- [3].Krause WJ, Ivey KJ, Baskin WN, MacKercher P. Ultrastructure of the human pyloric glands with emphasis on the mucous cell component. Part I. Acta Anat (Basel) 1977;99:1–10. doi: 10.1159/000144828. [DOI] [PubMed] [Google Scholar]
- [4].Jäger K, Wu G, Sel S, Garreis F, Bräuer L, Paulsen FP. MUC16 in the lacrimal apparatus. Histochem Cell Biol. 2007;127:433–438. doi: 10.1007/s00418-006-0246-6. [DOI] [PubMed] [Google Scholar]
- [5].Paulsen FP, Hinz M, Schaudig U, Thale AB, Hoffmann W. TFF peptides in the human efferent tear ducts. Invest Ophthalmol Vis Sci. 2002;43:3359–3364. [PubMed] [Google Scholar]
- [6].Paulsen FP, Berry MS. Mucins and TFF peptides of the tear film and lacrimal apparatus. Prog Histochem Cytochem. 2006;41:1–53. doi: 10.1016/j.proghi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [7].Paulsen F. Functional anatomy and immunological interactions of ocular surface and adnexa. Dev Ophthalmol. 2008;41:21–35. doi: 10.1159/000131068. [DOI] [PubMed] [Google Scholar]
- [8].Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–45. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- [9].Paulsen F, Langer G, Hoffmann W, Berry M. Human lacrimal gland mucins. Cell Tissue Res. 2004;316:167–177. doi: 10.1007/s00441-004-0877-7. [DOI] [PubMed] [Google Scholar]
- [10].Arango ME, Li P, Komatsu M, Montes C, Carraway CA, Carraway KL. Production and localization of Muc4/sialomucin complex and its receptor tyrosine kinase ErbB2 in the rat lacrimal gland. Invest Ophthalmol Vis Sci. 2001;42:2749–2756. [PubMed] [Google Scholar]
- [11].Carraway KL, Carvajal ME, Li P, Carraway CA. ErbB2 and its ligand Muc4 (sialomucin complex) in rat lacrimal gland. Adv Exp Med Biol. 2002;506:289–295. doi: 10.1007/978-1-4615-0717-8_40. [DOI] [PubMed] [Google Scholar]
- [12].Delporte C. Aquaporins in secretory glands and their role in Sjögren’s syndrome. Handb Exp Pharmacol. 2009;190:185–201. doi: 10.1007/978-3-540-79885-9_9. [DOI] [PubMed] [Google Scholar]
- [13].Kühnel W. [Comparative histological, histochemical, and electron microscopical studies on lacrimal glands. I. Rabbit and cat] Z Zellforsch Mikrosk Anat. 1968;85:408–410. [PubMed] [Google Scholar]
- [14].Burger G. [On the morphology and histochemistry of the glandula infraorbitalis of rabbits] Z Zellforsch Mikrosk Anat. 1968;87:451–462. [PubMed] [Google Scholar]
- [15].Oikawa M, Okano T. [The relations between the lacrimal gland, infraorbital gland and zygomatic gland of the rabbit (author’s transl)] Aichi Gakuin Daigaku Shigakkai Shi. 1978;16:132–138. [PubMed] [Google Scholar]
- [16].Ubels JL, Harkema JR. The rabbit lacrimal gland in vitamin A deficiency. Invest Ophthalmol Vis Sci. 1994;35:1249–1253. [PubMed] [Google Scholar]
- [17].Millar TJ, Herok G, Koutavas H, Martin DK, Anderton PJ. Immunohistochemical and histochemical characterisation of epithelial cells of rabbit lacrimal glands in tissue sections and cell cultures. Tissue Cell. 1996;28:301–312. doi: 10.1016/s0040-8166(96)80017-6. [DOI] [PubMed] [Google Scholar]
- [18].Ding C, Parsa L, Nandoskar P, Zhao P, Wu K, Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 2010;51:2960–2967. doi: 10.1167/iovs.09-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Prophet EB, Mills B, Arrington JB, Sobin LH. AFIP laboratory methods in histotechnology. American Registry of Pathology. Washington DC: 1994. [Google Scholar]
- [20].Ding C, Rife L, Nakamura T, Wang YW, Kopp K, Schechter JE. A novel, local technique for studying rabbit lacrimal gland secretion in situ. Ophthalmic Res. 2008;40:49–52. doi: 10.1159/000111159. [DOI] [PubMed] [Google Scholar]
- [21].Goso Y, Hotta K. Dot blot analysis of rat gastric mucin using histochemical staining methods. Anal Biochem. 1994;223:274–279. doi: 10.1006/abio.1994.1584. [DOI] [PubMed] [Google Scholar]
- [22].Thornton DJ, Holmes DF, Sheehan JK, Carlstedt I. Quantitation of mucus glycoproteins blotted onto nitrocellulose membranes. Anal Biochem. 1989;182:160–164. doi: 10.1016/0003-2697(89)90735-5. [DOI] [PubMed] [Google Scholar]
- [23].Walcott B, Cameron RH, Brink PR. The anatomy and innervation of lacrimal glands. Adv Exp Med Biol. 1994;350:11–18. doi: 10.1007/978-1-4615-2417-5_2. [DOI] [PubMed] [Google Scholar]
- [24].Walcott B. The Lacrimal Gland and Its Veil of Tears. News Physiol Sci. 1998;13:97–103. doi: 10.1152/physiologyonline.1998.13.2.97. [DOI] [PubMed] [Google Scholar]
- [25].Ding C, Walcott B, Keyser KT. Sympathetic neural control of the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2003;44:1513–1520. doi: 10.1167/iovs.02-0406. [DOI] [PubMed] [Google Scholar]
- [26].Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- [27].Gipson IK, Hori Y, Argüeso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- [28].Komatsu M, Carraway CA, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- [29].Bron AJ. Non-Sjögren dry eye: pathogenesis diagnosis and animal models. Adv Exp Med Biol. 1994;350:471–488. doi: 10.1007/978-1-4615-2417-5_81. [DOI] [PubMed] [Google Scholar]
- [30].Tam PY, Verdugo P. Control of mucus hydration as a Donnan equilibrium process. Nature. 1981;292:340–342. doi: 10.1038/292340a0. [DOI] [PubMed] [Google Scholar]
- [31].Verdugo P. Hydration kinetics of exocytosed mucins in cultured secretory cells of the rabbit trachea: a new model. Ciba Found Symp. 1984;109:212–225. doi: 10.1002/9780470720905.ch15. [DOI] [PubMed] [Google Scholar]
- [32].Verdugo P, Deyrup-Olsen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding. J Dent Res. 1987;66:506–508. doi: 10.1177/00220345870660022001. [DOI] [PubMed] [Google Scholar]
- [33].Delporte C, O’Connell BC, He X, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- [35].Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- [36].Pan Y, Iwata F, Wang D, et al. Identification of aquaporin-5 and lipid rafts in human resting saliva and their release into cevimeline-stimulated saliva. Biochim Biophys Acta. 2009;1790:49–56. doi: 10.1016/j.bbagen.2008.08.009. [DOI] [PubMed] [Google Scholar]