Abstract
Purpose
We investigated the role that the cystic fibrosis transmembrane conductance regulator (CFTR) may play in Cl− transport in the acinar and ductal epithelial cells of rabbit lacrimal gland (LG).
Methods
Primary cultured LG acinar cells were processed for whole-cell patch-clamp electrophysiological recording of Cl− currents by using perfusion media with high and low [Cl−], 10 μM forskolin and 100 μM 3-isobutyl-1-methylxanthine (IBMX), the non-specific Cl− channel blocker 4,4′-disothiocyanostilbene-2, 2′ sulphonic acid (DIDS; 100 μM) and CFTRinh-172 (10 μM), a specific blocker for CFTR. Ex vivo live cell imaging of [Cl−] changes in duct cells was performed on freshly dissected LG duct with a multiphoton confocal laser scanning microscope using a Cl− sensitive fluorescence dye, N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide.
Results
Whole-cell patch-clamp studies demonstrated the presence of Cl− current in isolated acinar cells and revealed that this Cl− current was mediated by CFTR channel. Live cell imaging also showed the presence of CFTR-mediated Cl− transport across the plasma membrane of duct cells.
Conclusions
Our previous data showed the presence of CFTR in all acinar and duct cells within the rabbit LG, with expression most prominent in the apical membranes of duct cells. The present study demonstrates that CFTR is actively involved in Cl− transport in both acinar cells and epithelial cells from duct segments, suggesting that CFTR may play a significant role in LG secretion.
Keywords: CFTR, Chloride channel, Lacrimal gland, Dry eye
INTRODUCTION
Lacrimal gland (LG) secretion is mediated by an array of ion transporters and channels, which generate the electrogenic gradient that drives water into the lumen. Apical Cl− secretion across epithelia provides the primary driving force for fluid production and activation of apical Cl− channels is the rate-limiting step for fluid secretion by epithelial cells of most exocrine glands, e.g., salivary glands.1-3
Three ion transporters involved in Cl− transport have been identified in the LG: Na+-K+-2Cl− cotransporter (NKCC), chloride channel (ClC) and cystic fibrosis transmembrane conductance regulator (CFTR). NKCC, which mediates the transport of the three ions through plasma membranes, has been identified in the LG of mouse,4 rat5 and rabbit.6 ClC is a superfamily of poorly understood ion channels consisting of approximately 13 members and has also been found in the LG of rat5 and rabbit.6
CFTR is an ion channel that transports Cl− and thiocyanate across epithelial cell membranes and has been shown to play a critical role in Cl− transport within many epithelia, e.g., salivary glands,1-3,7-9 pancreas10-12 and sweat gland.13-15
CFTR has been detected in the LG of mouse16 and rat.5 Our recent studies also demonstrated the presence of CFTR mRNA and protein in the LG of rabbit,6 as well as its expression changes in a rabbit model of Sjögren’s syndrome.17 This suggests that CFTR may play a significant role in normal function and dysfunction of LG. However, little is known about the role that CFTR may function in LG secretion, and in fact, the above four reports were the only literature regarding CFTR and LG that we could find.
Therefore, the aim of the present study is to investigate whether CFTR is functionally involved in Cl− transport in acinar and duct epithelial cells from rabbit LG using whole-cell patch-clamp electrophysiological recording of isolated acinar cells and live cell imaging of dissected LG duct segments. Our data indicated that CFTR is actively involved in Cl− transport in both acinar and duct cells, which suggests that CFTR plays a significant role in LG secretion.
METHODS
Animals
Twenty-four New Zealand White female rabbits (Irish Farms, Norco, CA), weighing about 2 kg, were used throughout the study. Rabbits were narcotized with a mixture of ketamine (40 mg/ml) and xylazine (10 mg/ml), and given an overdose of Nembutal (80 mg/kg) for euthanasia. This study conformed to the standards and procedures for the proper care and use of animals as described in the ARVO Statement for the Use of Animals in Ophthalmic Research.
Chemicals
N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide (MQAE; Molecular Probes, Eugene, OR); 4,4′-disothiocyanostilbene-2, 2′ sulphonic acid (DIDS) (Sigma, St. Louis, MO); CFTRinh-172 (Sigma). Other chemicals were purchased from Sigma.
DIDS was dissolved in dimethyl sulfoxide (DMSO) to make a stock solution of 10 mM, and added to the perfusate to make the final concentration of 100 μM. CFTRinh-172 was dissolved in DMSO to make a stock solution of 1 mM, and added to the perfusate to make the final concentration of 10 μM. Stock solution of MQAE was made in water at 1 mg/ml, and added to the perfusate to make the final concentration of 100 μM.
Since DMSO may potentially yield unwanted effects in cells, we performed vehicle control studies by adding 1% DMSO by itself in the media. Our results indicated that neither high [Cl−] nor low [Cl−] media could cause any holding current changes for up to 30 min. Nonetheless, to avoid any potential side effects of DMSO, we washed the cells in the DMSO-containing media for 2 min and then executed the voltage step protocols immediately, which typically lasted less than 10 min.
Cell Culture of LG Acinar Cells
Inferior LG were removed from rabbits and acinar cells were isolated and cultured for 2-3 days as described in detail previously.18
Whole-Cell Patch-Clamp Recording of Acinar Cells
For electrophysiological recordings of acinar cells, three different solutions were used for studying CFTR-mediated Cl− currents: 1) high Cl− external solution (in mM): 150 NaCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 20 sucrose, pH 7.4; 2) low Cl− external solution (in mM): 10 NaCl, 140 sodium gluconate, 1 CaCl2, 1 MgCl2, 10 HEPES, 20 sucrose, pH 7.4; and 3) internal pipette solution (in mM): 120 CsCl, 10 TEA-Cl, 1 MgCl2, 0.5 EGTA, 1 ATP, 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH 7.4.
An Axon Multiclamp 700B amplifier and Digidata 1440A interface (Molecular Devices, Sunnyvale, CA) were used with pClamp 10.2 software for data acquisition (Molecular Devices). Digital signal (high passed at 10 Hz, low passed at 5 kHz) was sampled at 10 kHz. Liquid junction potential was offset automatically for each recording or change of bath solution. Voltage step protocols were compiled in pClamp and the cells were held at 0 mV after whole-cell configuration was obtained. All current traces were leak-subtracted using p/n subtraction provided in pClamp. We monitored both access resistance and whole-cell input resistance during the entire recording period and only stable recordings (<10% change) with initial gigaseals were selected for analysis.
The resistance of the patch electrode was 5–7 MOhm and the series resistance was between 6–9 MOhm. Therefore, the access resistance did not contribute significantly to the series resistance nor affect the quality of recordings. Series resistance errors, although very small compared with cell input resistance, were routinely compensated at 70% level to increase the signal bandwidth of detection. We recorded a membrane capacity (Cm) of 18.6±/−4.2 pF, which is consistent with the uniformity of cell size. Statistics and graphing was done using GraphPad Prism software (GraphPad Software, La Jolla, CA).
To elicit CFTR-mediated Cl− currents, a series of voltage commands were applied to the cells (from −100 mV to +100 mV, with 20 mV steps; Figure 1). In some experiments, a cAMP-increasing cocktail (10 μM forskolin + 100 μM 3-isobutyl-1-methylxanthine [IBMX]) was added into the perfusate. To prevent ClC-2-mediated Cl− currents and ENaC-mediated Na+ currents, CdCl2 (300 μM) and amiloride (10 μM) were added to external solutions, respectively.2,3,19 At least four cells for each experimental parameter were recorded.
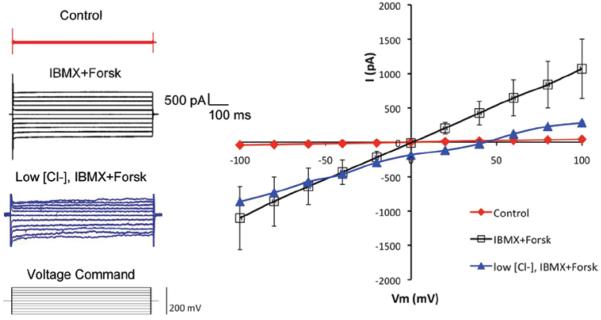
FIGURE 1.
Whole-cell patch clamp recordings of Cl− current in isolated rabbit LG acinar cells. Cells were initially perfused with external solution with high [Cl−] = 154 mM (control, n = 5). Addition of a cAMP-increasing cocktail of 100 μM IBMX and 10 μM forskolin (IBMX+Forsk, n = 5) elicited a strong Cl− current, suggesting the elicited current was mediated by CFTR, which is a cAMP-activated ATP-gated anion channel. Replacement of the external solution with low [Cl−] = 14 mM shifted the reversal potential from 0 mV to 41.95 ± 3.37 mV (n = 4). Data are presented as mean ± standard error of the mean (SEM). Student’s t-tests and/or ANOVA were performed by comparing data elicited from agent and control, and significant differences (p < 0.05) were observed at each holding potential.
Microdissection of LG Ducts and Live Cell Imaging
Lacrimal ducts were micro-dissected from LG in dissection media at 4°C under a Leica dissecting microscope. The dissection medium was prepared from DMEM (DME mixture F-12; Sigma) with the addition of 1.2 g/l NaHCO3 and 3% fetal bovine serum (Hyclone). Before use, this solution was aerated with 95% O2 + 5% CO2 for 45 min, and pH adjusted to 7.4.
Dissected ducts were then transferred to a thermoregulated Lucite chamber mounted on a Leica DM IRE2 inverted microscope. The isolated duct was cannulated by a holding pipette and collection pipette (Figure 3), and perfused at 20 nl/min. The ductal perfusate was a Krebs-Ringer-HCO3 buffer (KRB) that contained (in mM) 115 NaCl, 5 KCl, 25 NaHCO3, 1.6 NaH2PO4, 0.4 Na2HPO4, 1 MgSO4, 1.5 CaCl2 and 5 D-glucose. The bath media was identical to the ductal perfusate and was continuously aerated with 95% O2 + 5% CO2 and exchanged at a rate of 1 ml/min. The preparation was kept in the dissection solution and the temperature was kept at 4°C until cannulation of the duct was completed, and then gradually raised to 37°C for the remainder of the experiment.20
FIGURE 3.
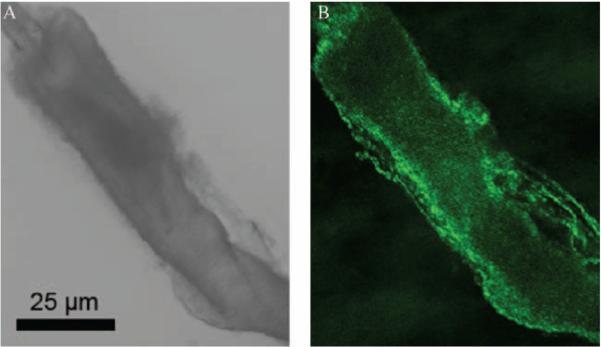
Live cell imaging of duct cells from dissected LG intralobular duct for MQAE fluorescence changes. Duct cells were loaded with fMQAE (100 = M) via the perfusate through a glass pipette, which can be seen in the top-left (A, B).
Multiphoton laser scanning microscopy was performed using a Leica TCS SP2 AOBS MP confocal microscope system (Leica Microsystems, Heidelberg, Germany). A Leica DM IRE2 inverted microscope is powered by a wideband, fully automated, infrared (710–920 nm) combined photo-diode pump laser and mode-locked titanium:sapphire laser (Mai-Tai, Spectra-Physics, Mountain View, CA) for multiphoton excitation, and/or by red (HeNe 633 nm/10 mW), orange (HeNe 594 nm/2 mW), green (HeNe 543 nm/1.2 mW) and blue (Ar 458 nm/5 mW; 476 nm/5 mW; 488 nm/20 mW; 514 nm/20 mW) lasers for conventional, one photon-excitation confocal microscopy. Images were collected in time (xyt) or z-series (xyz), depending on the purpose of study, with the Leica LCS imaging software.
Statistics
Data are presented as mean ± SEM (standard error of mean). Each experiment was performed on at least three samples from at least three animals (see text). Student’s t-test and analysis of variance (ANOVA) were performed to determine statistical significance, and p < 0.05 was considered significant.
RESULTS
Patch-Clamp of LG Acinar Cells
By using whole-cell patch-clamp, we were able to record strong Cl− current in isolated acinar cells. When a cAMP-increasing cocktail of 100 μM 3-isobutyl-1-methylxanthine (IBMX) and 10 μM forskolin was added into the perfusate that has a [Cl−] of 154 mM, a strong Cl− current was elicited (Figure 1), indicating that the elicited current was mediated by CFTR, a Cl− channel that is activated by cAMP.3,21,22 Moreover, replacing the external solution with low [Cl−]=14 mM shifted the reversal potential from 0 to 41.95 ± 3.37 mV. The discrepancy between this reversal potential and the calculated reversal potential based on the Nernst equation for chloride (~57 mV) may be due to other transporters and channels involved in Cl− transport, i.e., NKCC1, KCC1 and ClC2γ in the rabbit LG acinar cells.6 Chloride channels may also have certain conductance for other ions, albeit these conductance tend to be insignificant as compared to Cl−. However, no data exists regarding this phenomenon in the LG, as far as we know. We set out to investigate whether the elicited Cl− current was mediated by CFTR, rather than other Cl− transporters by using various Cl− channel blockers.
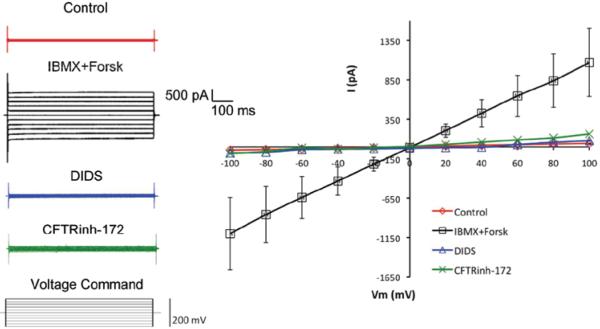
Addition of 100 μM DIDS, a non-specific Cl− channel blocker, into the external solution completely abolished the IBMX and forskolin elicited Cl− current (Figure 2), suggesting that the elicited current was indeed mediated by Cl− channels. Furthermore, addition of 10 μM of CFTRinh-172, a selective CFTR channel inhibitor,23 also completely abolished the Cl− current, confirming that the Cl− current was mediated by CFTR channel. At least four cells for each experimental parameter were analyzed. Recording data elicited from each agent and control was analyzed by Student’s t-test and/or ANOVA, and significant differences (p ≤ 0.05) were observed at each holding potential (Figures 1 and 2).
FIGURE 2.
Similar to Figure 1, in acinar cells perfused with external solution with [Cl−] = 154 mM, IBMX and forskolin elicited Cl− current was completely abolished by adding 100 μM DIDS, a non-specific Cl− channel blocker, into the external solution (n = 4). This suggests that the elicited current was mediated by Cl− channel. Furthermore, addition of 10 μM of CFTRinh-172, a selective CFTR channel inhibitor, also completely abolished the Cl− current (n = 5), confirming that the Cl− current was mediated by CFTR channel. Data are presented as mean ± SEM. Student’s t-tests and/or ANOVA were performed by comparing data elicited from agent and control, and significant differences (p < 0.05) were observed at each holding potential.
Live Cell Imaging of Duct Cells from Dissected LG Duct
In addition to acinar cells, the LG duct epithelial cells are also actively involved in LG fluid production. As shown in our previous report,6 CFTR was particularly rich in the apical membranes of LG duct. Therefore, we used the Cl− sensitive probe MQAE to study [Cl−]-sensitive fluorescence intensity changes in duct cells and its changes in response to DIDS and CFTRinh-172.
Duct cells were initially loaded with MQAE (100 μM) via the perfusate, a Ringer solution that contains 135 mM NaCl which is identical to the bath medium, through a glass pipette (Figure 3). Replacement of the luminal perfusate with a modified Ringer solution containing 10 mM NaCl and 125 mM Na-gluconate caused the cytosolic MQAE intensity to increase dramatically (Figure 4) due to the decreased quenching resulting from increased Cl− efflux into luminal space. This suggests that the duct cells were actively involved in Cl− transport.
FIGURE 4.
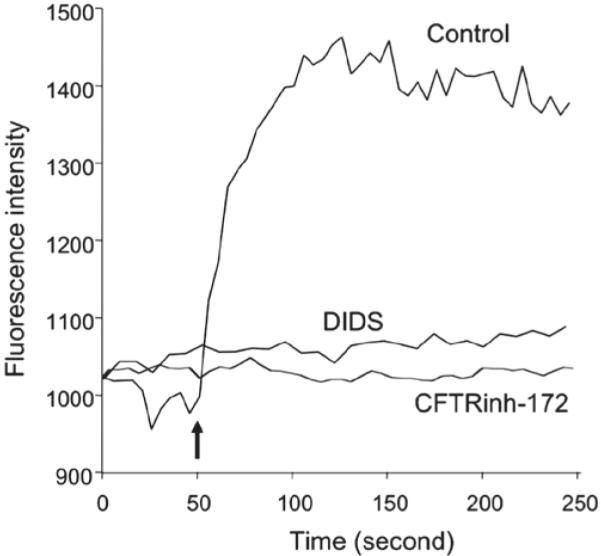
Representative recording of the fluorescence intensity changes of the chloride-sensitive probe MQAE in duct cells by using isolated duct segment. Imaging was performed using a Leica TCS SP2 confocal microscope system and two-photon excitation at 750 nm. Emitted photons at 440 nm were detected by a photomultiplier. Control: Duct lumen was originally perfused with Ringer solution containing 135 mM NaCl, identical to the bath medium. At 50 s (arrow), luminal perfusate was switched to a modified Ringer solution containing 10 mM NaCl and 125 mM Na-gluconate. Increased cytosolic MQAE intensity was caused by decreased quenching effect by Cl− due to its increased efflux into lumen (n = 5). DIDS: When the same maneuver was performed with 100 = M DIDS present in the perfusate, the change in fluorescence intensity was almost completely prevented (n = 4), indicating that the increased fluorescence intensity was mediated by Cl− channel. CFTRinh-172: adding 10 μM CFTRinh-172 into the perfusate caused a complete abolishment of fluorescence intensity increase (n = 4), confirming the increased fluorescence intensity was mediated by CFTR.
When the same maneuver was performed with 100 μM DIDS present in the perfusate, the change in cytosolic [Cl−] was almost completely prevented, indicating that the increased fluorescence intensity was mediated by Cl− channel. Furthermore, addition of 10 μM CFTRinh-172 into the perfusate caused a complete abolishment of fluorescence intensity change, confirming that the increased fluorescence intensity was mediated by CFTR.
DISCUSSION
LG fluid secretion is an osmotic process driven by the transepithelial secretion of electrolytes that is mediated by many ion transporters/channels and following water transport.4-6,24-28 As in other exocrine gland secretions, LG fluid is believed to be produced in two stages: (i) secretion of primary LG fluid in the acini and (ii) modification into the final LG fluid during transit through the duct system.
It has been demonstrated that the primary LG fluid resembles an isotonic ultrafiltrate of plasma, while the final LG fluid has a much higher [K+] and [Cl−].29,30 Non-mutually exclusive explanations for the elevated ions in final LG fluid are: (i) duct cells secrete increased amounts of K+ and Cl− and (ii) duct cells reabsorb Na+ and water to make the [K+] and [Cl−] in final LG fluid relatively higher.
Although CFTR has been detected in the LG of mouse,16 rat5 and rabbit,6,17 little is known about its functional role in LG secretion. Our previous data indicated that CFTR is present in all acinar cells and epithelial cells from all duct segments, suggesting that it may play a significant role in the elevated [Cl−] in final LG fluid.
In this report, our electrophysiological studies of acinar cells using various perfusion media with different [Cl−] and channel blockers confirmed both the existence of Cl− current in these cells and that CFTR is the major transporter responsible for this current. With ex vivo live cell imaging studies of dissected LG duct segment using a specific Cl− sensitive fluorescence dye, MQAE, we also demonstrated that CFTR clearly played a role in Cl− transport in duct cells.
These data taken together, for the first time to the extent of our knowledge, suggest that CFTR is functionally involved in Cl− transport in both acinar and duct LG epithelial cells.
It has been shown that CFTR plays a critical role in Cl− transport in many epithelia. Mutations of CFTR can cause cystic fibrosis (CF), the most common and fatal genetic disease in Caucasians affecting one in every 3200 live births.31 Disruption of CFTR-mediated Cl− transport results in defective fluid and electrolyte movement in epithelial cells of many tissues/organs, such as the pancreas,10-12 salivary glands1-3,7-9 and sweat glands.13-15 A recent report also verified that gating of CFTR is determined by membrane stretch that points to the sensitivity of CFTR-mediated Cl− current to changes in cell volume.32
While we were unable to find any literature regarding CFTR’s functional role in LG secretion, data from salivary glands, another exocrine gland that closely resembles to LG anatomically and functionally, may help us to elucidate the role CFTR may play in LG function. A recent report by Catalan et al.3 using CFTR knockout mice elegantly demonstrated that CFTR plays a critical role in the function of duct cells from mouse submandibular glands. Whole-cell patch-clamp failed to record CFTR-mediated Cl− currents from isolated duct cells from submandibular glands of CFTR knockout mice and significant changes in flow rate and total volume of saliva were detected in CFTR knockout mice. However, unlike the rabbit LG, no CFTR was detected in the acinar cells of mouse submandibular glands.
Our recent data17 indicated that in a rabbit model of Sjögren’s syndrome, induced autoimmune dacryoadenitis (IAD), there were significant changes of mRNA for CFTR from whole LG as well as acinar cells and epithelial cells from various duct segments from rabbits with IAD. This may translate into altered transport of Cl−, strongly suggesting that these changes may contribute to the reduced LG secretion in rabbits with IAD, as demonstrated before.33-35 These data were consistent with the recent report of Catalan et al.3, which showed that submandibular secretion was dependent on CFTR-mediated Cl− transport. Patients with CF have also been reported to exhibit ocular surface changes characteristic of dry eye, e.g., decreased tear production, increased corneal fluorescein staining, as well as corneal and conjunctival squamous metaplasia that have been implicated as primary and direct manifestations of CF.36-39
In most exocrine glands, apical Cl− secretion provides the primary driving force for fluid secretion. Activation of apical Cl− channels, including CFTR along with Cl− and Na+ coupled entry mechanisms in apical and basolateral membranes, is the rate-limiting step for fluid secretion.2,3,40 The fact that immunofluorescence data6 revealed CFTR localization in the apical cytoplasm of acinar cells instead of the apical membranes implies that CFTR in acinar cells are quiescent in the non-stimulated state. Upon activation, CFTR may translocate to the apical membranes as has been demonstrated in the mouse mandibular duct cells.41
CFTR is predominantly present in the LG ducts where they are primarily located on the apical membrane. This strongly implies the ducts’ active involvement in Cl− transport. These results are also consistent with a recent report that showed the presence of CFTR in the apical membranes of ductal cells in rat LG.5 An earlier report, using mouse mutants with defective CFTR gene, demonstrated dilated acini that were presumably caused by back-pressure from blocked ducts. These mutant mice also developed eye infections and were prone to persistent eye closure.16
The presence of significant amounts of CFTR in the ducts along with the detection of other channels and transporters in the LG ducts5,6,24,25,42 strongly supports the notion that LG ducts play a critical role in lacrimal function. A recent functional study of the LG ducts by Haarsma et al.43 using perforated patch-clamp on dissociated rat LG duct cells elegantly recorded the presence of voltage-activated K+ channels and confirmed these duct cells’ functional involvement in LG secretion.
In addition to mediating anion transport, CFTR has also been shown directly involved in the secretion of mucins and serous proteins.44,45 In fact, the LG duct cells of rabbit46,47 are all mucous cells capable of secreting mucin. This appears to support the report from Ratcliff et al.16 that suggested that dilated acini in mutant CFTR mouse may result from the increased back-pressure caused by blocked ducts and clinical findings that CF patients demonstrated dry eye symptoms.36-39
In summary, the data presented here demonstrated that CFTR is functionally involved in Cl− transport in both acinar and duct cells in the rabbit LG and suggests that CFTR plays a significant role in LG function and dysfunction. However, the exact mechanisms of how these transporters mediate LG secretion in physiological and pathological conditions are unknown and certainly warrant further investigations. Direct secretion studies, e.g., in vivo secretion studies by using CFTR knockout mice, may help to elucidate its role in LG secretion.
ACKNOWLEDGMENTS
The authors greatly thank Dr. Shenfeng Qiu for his expert advice in electrophysiological recordings of acinar cells and data analysis; Dr. Sarah Hamm-Alvarez for help in acinar cell culture; Dr. Janos Peti-Peterdi for help in duct cells imaging; Drs. Austin Mircheff and Joel Schechter for helpful discussions; Hua Pei and Francie Yarber for excellent technical support.
This work was supported by NIH grants EY017731, EY016985, EY10550, EY005801, RR024754.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- [1].Noël S, Strale PO, Dannhoffer L, et al. Stimulation of salivary secretion in vivo by CFTR potentiators in Cftr+/+ and Cftr−/− mice. J Cyst Fibros. 2008;7:128–133. doi: 10.1016/j.jcf.2007.06.005. [DOI] [PubMed] [Google Scholar]
- [2].Romanenko VG, Nakamoto T, Catalán MA, et al. Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1058–G1067. doi: 10.1152/ajpgi.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Catalán MA, Nakamoto T, Gonzalez-Begne M, et al. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol (Lond) 2010;588:713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walcott B, Birzgalis A, Moore LC, Brink PR. Fluid secretion and the Na+-K+-2Cl-cotransporter in mouse exorbital lacrimal gland. Am J Physiol, Cell Physiol. 2005;289:C860–C867. doi: 10.1152/ajpcell.00526.2004. [DOI] [PubMed] [Google Scholar]
- [5].Ubels JL, Hoffman HM, Srikanth S, Resau JH, Webb CP. Gene expression in rat lacrimal gland duct cells collected using laser capture microdissection: evidence for K+ secretion by duct cells. Invest Ophthalmol Vis Sci. 2006;47:1876–1885. doi: 10.1167/iovs.05-0363. [DOI] [PubMed] [Google Scholar]
- [6].Ding C, Parsa L, Nandoskar P, Zhao P, Wu K, Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 2010;51:2960–2967. doi: 10.1167/iovs.09-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ishibashi K, Yamazaki J, Okamura K, Teng Y, Kitamura K, Abe K. Roles of CLCA and CFTR in electrolyte re-absorption from rat saliva. J Dent Res. 2006;85:1101–1105. doi: 10.1177/154405910608501207. [DOI] [PubMed] [Google Scholar]
- [9].Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(−) reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1729–R1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]
- [10].Park HW, Nam JH, Kim JY, et al. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- [11].Gouyer V, Leir SH, Tetaert D, et al. The characterization of the first anti-mouse Muc6 antibody shows an increased expression of the mucin in pancreatic tissue of Cftr-knockout mice. Histochem Cell Biol. 2010;133:517–525. doi: 10.1007/s00418-010-0688-8. [DOI] [PubMed] [Google Scholar]
- [12].Ko SB, Mizuno N, Yatabe Y, et al. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology. 2010;138:1988–1996. doi: 10.1053/j.gastro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reddy MM, Quinton PM. PKA mediates constitutive activation of CFTR in human sweat duct. J Membr Biol. 2009;231:65–78. doi: 10.1007/s00232-009-9205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brown MB, Haack KK, Pollack BP, Millard-Stafford M, McCarty NA. Low abundance of sweat duct Cl− channel CFTR in both healthy and cystic fibrosis athletes with exceptionally salty sweat during exercise. Am J Physiol Regul Integr Comp Physiol. 2011;300:R605–R615. doi: 10.1152/ajpregu.00660.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muhammad E, Leventhal N, Parvari G, et al. Autosomal recessive hyponatremia due to isolated salt wasting in sweat associated with a mutation in the active site of Carbonic Anhydrase 12. Hum Genet. 2011;129:397–405. doi: 10.1007/s00439-010-0930-4. [DOI] [PubMed] [Google Scholar]
- [16].Ratcliff R, Evans MJ, Cuthbert AW, et al. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- [17].Nandoskar P, Wang Y, Wei R, et al. Changes of chloride channels in the lacrimal glands of a rabbit model of Sjögren syndrome. Cornea. 2012;31:273–279. doi: 10.1097/ICO.0b013e3182254b42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jerdeva GV, Wu K, Yarber FA, et al. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005;118:4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dinudom A, Young JA, Cook DI. Amiloride-sensitive Na+ current in the granular duct cells of mouse mandibular glands. Pflugers Arch. 1993;423:164–166. doi: 10.1007/BF00374977. [DOI] [PubMed] [Google Scholar]
- [20].Peti-Peterdi J, Chambrey R, Bebok Z, et al. Macula densa Na(+)/H(+) exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am J Physiol Renal Physiol. 2000;278:F452–F463. doi: 10.1152/ajprenal.2000.278.3.F452. [DOI] [PubMed] [Google Scholar]
- [21].Nehrke K, Arreola J, Nguyen HV, et al. Loss of hyperpolarization-activated Cl(−) current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem. 2002;277:23604–23611. doi: 10.1074/jbc.M202900200. [DOI] [PubMed] [Google Scholar]
- [22].Garcia-Olivares J, Alekov A, Boroumand MR, Begemann B, Hidalgo P, Fahlke C. Gating of human ClC-2 chloride channels and regulation by carboxy-terminal domains. J Physiol (Lond) 2008;586:5325–5336. doi: 10.1113/jphysiol.2008.158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma T, Thiagarajah JR, Yang H, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dartt DA, Møller M, Poulsen JH. Lacrimal gland electrolyte and water secretion in the rabbit: localization and role of (Na+ + K+)-activated ATPase. J Physiol (Lond) 1981;321:557–569. doi: 10.1113/jphysiol.1981.sp014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mircheff AK. Lacrimal fluid and electrolyte secretion: a review. Curr Eye Res. 1989;8:607–617. doi: 10.3109/02713688908995761. [DOI] [PubMed] [Google Scholar]
- [26].Herok GH, Millar TJ, Anderton PJ, Martin DK. Characterization of an inwardly rectifying potassium channel in the rabbit superior lacrimal gland. Invest Ophthalmol Vis Sci. 1998;39:308–314. [PubMed] [Google Scholar]
- [27].Herok GH, Millar TJ, Anderton PJ, Martin DK. Role of chloride channels in regulating the volume of acinar cells of the rabbit superior lacrimal gland. Invest Ophthalmol Vis Sci. 2008;49:5517–5525. doi: 10.1167/iovs.07-0435. [DOI] [PubMed] [Google Scholar]
- [28].Selvam S, Thomas PB, Gukasyan HJ, et al. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol, Cell Physiol. 2007;293:C1412–C1419. doi: 10.1152/ajpcell.00200.2007. [DOI] [PubMed] [Google Scholar]
- [29].Alexander JH, van Lennep EW, Young JA. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflugers Arch. 1972;337:299–309. doi: 10.1007/BF00586647. [DOI] [PubMed] [Google Scholar]
- [30].Rismondo V, Osgood TB, Leering P, Hattenhauer MG, Ubels JL, Edelhauser HF. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J. 1989;15:222–228. [PubMed] [Google Scholar]
- [31].Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- [32].Zhang WK, Wang D, Duan Y, Loy MM, Chan HC, Huang P. Mechanosensitive gating of CFTR. Nat Cell Biol. 2010;12:507–512. doi: 10.1038/ncb2053. [DOI] [PubMed] [Google Scholar]
- [33].Guo Z, Song D, Azzarolo AM, et al. Autologous lacrimal-lymphoid mixed-cell reactions induce dacryoadenitis in rabbits. Exp Eye Res. 2000;71:23–31. doi: 10.1006/exer.2000.0855. [DOI] [PubMed] [Google Scholar]
- [34].Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Atkinson R, Trousdale MD. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32. doi: 10.1097/00003226-200301000-00007. [DOI] [PubMed] [Google Scholar]
- [35].Thomas PB, Zhu Z, Selvam S, et al. Autoimmune dacryoadenitis and keratoconjunctivitis induced in rabbits by subcutaneous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. J Autoimmun. 2008;31:116–122. doi: 10.1016/j.jaut.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sheppard JD, Orenstein DM, Chao CC, Butala S, Kowalski RP. The ocular surface in cystic fibrosis. Ophthalmology. 1989;96:1624–1630. doi: 10.1016/s0161-6420(89)32676-5. [DOI] [PubMed] [Google Scholar]
- [37].Morkeberg JC, Edmund C, Prause JU, Lanng S, Koch C, Michaelsen KF. Ocular findings in cystic fibrosis patients receiving vitamin A supplementation. Graefes Arch Clin Exp Ophthalmol. 1995;233:709–713. doi: 10.1007/BF00164674. [DOI] [PubMed] [Google Scholar]
- [38].Ansari EA, Sahni K, Etherington C, et al. Ocular signs and symptoms and vitamin A status in patients with cystic fibrosis treated with daily vitamin A supplements. Br J Ophthalmol. 1999;83:688–691. doi: 10.1136/bjo.83.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mrugacz M. CCL4/MIP-1beta levels in tear fluid and serum of patients with cystic fibrosis. J Interferon Cytokine Res. 2010;30:509–512. doi: 10.1089/jir.2009.0102. [DOI] [PubMed] [Google Scholar]
- [40].Turner JT, Redman RS, Camden JM, Landon LA, Quissell DO. A rat parotid gland cell line, Par-C10, exhibits neurotransmitter-regulated transepithelial anion secretion. Am J Physiol. 1998;275:C367–C374. doi: 10.1152/ajpcell.1998.275.2.C367. [DOI] [PubMed] [Google Scholar]
- [41].Dinudom A, Komwatana P, Young JA, Cook DI. A forskolin-activated Cl− current in mouse mandibular duct cells. Am J Physiol. 1995;268:G806–G812. [Google Scholar]
- [42].Tóth-Molnár E, Venglovecz V, Ozsvári B, et al. New experimental method to study acid/base transporters and their regulation in lacrimal gland ductal epithelia. Invest Ophthalmol Vis Sci. 2007;48:3746–3755. doi: 10.1167/iovs.06-1291. [DOI] [PubMed] [Google Scholar]
- [43].Haarsma LD, Bardolph SL, Ubels JL. Recording of K+ currents from lacrimal gland duct cells. 2010. ARVO E-Abstract 4175. [Google Scholar]
- [44].Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- [45].Kuver R, Ramesh N, Lau S, Savard C, Lee SP, Osborne WR. Constitutive mucin secretion linked to CFTR expression. Biochem Biophys Res Commun. 1994;203:1457–1462. doi: 10.1006/bbrc.1994.2348. [DOI] [PubMed] [Google Scholar]
- [46].Ding C, Huang J, MacVeigh-Aloni M, Lu M. Not all lacrimal epithelial cells are created equal—heterogeneity of the rabbit lacrimal gland and differential secretion. Curr Eye Res. 2011;11:971–978. doi: 10.3109/02713683.2011.602814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Millar TJ, Herok G, Koutavas H, Martin DK, Anderton PJ. Immunohistochemical and histochemical characterisation of epithelial cells of rabbit lacrimal glands in tissue sections and cell cultures. Tissue Cell. 1996;28:301–312. doi: 10.1016/s0040-8166(96)80017-6. [DOI] [PubMed] [Google Scholar]