Abstract
The diagnosis and management of adrenal diseases hinge upon accurate determination of hormone concentrations in blood and other body fluids. The advent of immunoassays for various steroid hormones has enabled the remarkable progress in adrenal disease over the last several decades, with some limitation. Sequential immunoassay of single analytes is a tedious process, which requires aliquots for each assay. In many complex adrenal diseases, including adrenal cancer and congenital adrenal hyperplasia, the patterns or ratios of multiple steroids rather than the value of any one steroid is more relevant. Although gas chromatography/mass spectrometry of urinary steroid metabolites has been employed to profile steroid production, throughput is slow, and availability is sparse. Recent generations of liquid chromatography-tandem mass spectrometry instruments (LC-MS/MS) provide the throughput and sensitivity required to measure many steroids simultaneously using small samples for commercial and research uses. Even in the best hands, however, LC-MS/MS suffers from limitations and requires diligent attention to detail during method development and implementation. This article reviews the theory, instrumentation principles and terminology, and practical application of mass spectrometry to clinical adrenal disorders.
Keywords: mass spectrometry, adrenal glands, multiple reaction monitoring, steroid, pathways, congenital adrenal hyperplasia, adrenal adenoma, ion suppression, adrenal tumor, multiplex assay
1. Introduction
Steroid hormones derive from the coordinated regulation of multiple enzymes arranged in pathways to maximize final product formation (Miller and Auchus, 2011). Adrenal diseases occur when enzyme deficiencies disrupt this normal flow of precursors to product and lead to imbalances, with excess and deficiencies of particular steroids. Adrenal tumors often express steroidogenic enzymes via autonomous mechanisms and in aberrant proportions, leading to accumulation of precursors and mixed steroid products. Multiplexed steroid profiling with mass spectrometry is now available to guide diagnosis and treatment of these adrenal diseases, supplanting traditional immunoassays. The adrenal investigator, however, must understand the theory and limitations of mass spectrometry in order to use these techniques appropriately.
2. Mass spectrometer design
2.1 The triple quadrupole instrument
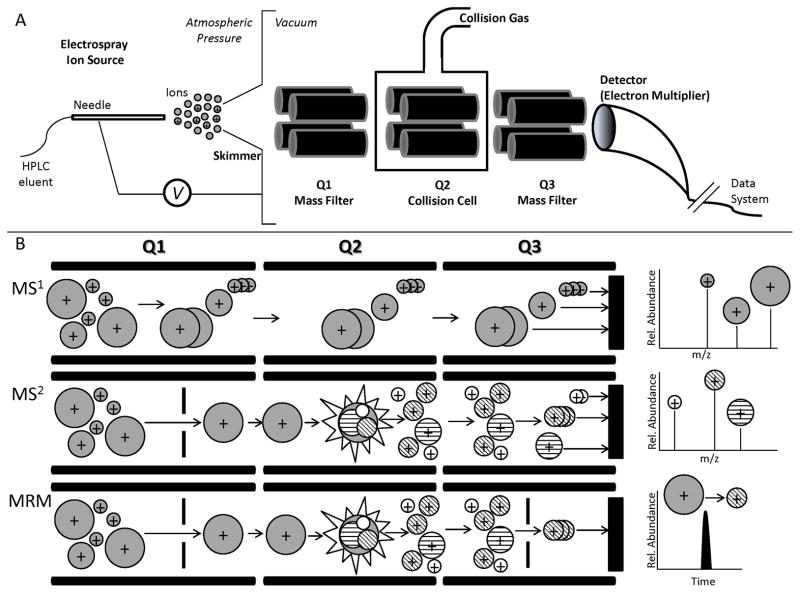
The most common tandem mass spectrometer used for quantitating steroids is the triple quadrupole because of its sensitivity and versatility. Triple quadrupole tandem mass spectrometers (the “back end”) coupled with high performance liquid chromatography (HPLC1, the “front end”, making a LC-MS/MS system, Figure 1A) can detect <1pg per injection (“on column”) and provide qualitative and quantitative data in the same analyte run; experiments can be multiplexed to detect and quantitate numerous compounds in the same sample. Instead of running numerous ELISA (enzyme-linked immunosorbent assay) plates to detect each steroid of interest, one sample is extracted, and all steroids are analyzed in the same experiment. Mass spectrometry yields primary data to unequivocally detect and confirm the presence of a steroid, whereas ELISA yields an indirect measurement, which can be confounded by cross-reactivity of similar compounds (Haisenleder et al., 2011).
Figure 1.
Electrospray Ionization (ESI) Triple Quadrupole Mass Spectrometer. A. Schematic of an ESI source interfaced with a triple quadrupole mass spectrometer. After liquid chromatography separation, compounds elute into a high voltage needle, where a fraction exits as gas phase ions. The first and third quadrupoles (Q1 and Q3) function as mass analyzers whereas the second quadrupole (Q2) serves as a collision cell and ion guide. After ions pass through Q3, the ions collide with the detector, which measures ion abundance. The quadrupoles and detector function in conjunction to determine the mass of the ions as well as their abundance as described in B. B. MS1 experiments (top) are used to determine the mass spectrum of the ions in a sample. Q1 or Q3 scan the ions in a designated mass range and guide them to the detector; Q2 allows all ions in the designated mass range to pass through. MS2 experiments (center) determine the collision-induced product ions of a designated precursor ion. Q1 selects and guides the precursor ion of interest to Q2, where it undergoes collision-induced dissociation with an inert gas to yield unique product ions. Q2 transmits all product ions to mass filter Q3, which sorts the ions and transmits them to the detector. MRM (Multiple Reaction Monitoring, bottom) experiments are used to specifically quantitate multiple precursor ions using their characteristic product ions. Q1 selects the precursor ion, Q2 fragments it as in the MS2 experiment, and Q3 transmits only a specific product ion to the detector. Multiple analytes can be quantitated in a single experiment using MRM.
2.2. Quadrupole Mass Analyzer and MS Experiments
At the center of the triple quadrupole mass spectrometer is the quadrupole mass analyzer, which scans ions from the ion source to the detector. A quadrupole consists of four parallel cylindrical rods arranged at the corners of a square such that ions traverse through the area of the square between these rods. A superposition of direct current (DC) and radiofrequency (RF) voltage creates an electric field between the rods with opposite rods electrically paired. The rod-pair’s polarity oscillates at a specific frequency, which permits only ions in a selected mass range to reach the detector. In addition to mass ranges, individual ions can be selected for quantitative experiments. The first and third quadrupoles are RF and DC mass-resolving quadrupoles, while the second quadrupole is a RF-only collision cell or ion guide. The first and third quadrupoles can operate independent of each other or together in tandem.
In a triple quadrupole instrument, the three consecutive quadrupoles are aligned, so that ions selected in one quadrupole can be transferred to the next (Figure 1B). The triple quadrupole can be used for a number of experiments, including determining a traditional mass spectrum, MS1, of the precursor ions present to give an overall picture of the ions present in a sample (Figure 2A). In a product ion or MS2 experiment, the intact molecule is fragmented, and structural information derives from examining the pieces (Figure 2B). The first quadrupole (Q1) selects only the ion of interest (“precursor ion”) by varying the RF and DC so that only the ion of interest can avoid expulsion and pass completely through Q1 to the second quadrupole (Q2). The precursor ion collides with an inert gas such as nitrogen in a process referred to as collision-induced dissociation (CID) to yield smaller fragments or “product ions.” The second quadrupole acts as a collision cell and is designed so that all product ions formed will be sent to the third quadrupole (Q3). The third quadrupole serves as a mass analyzer to sort and inventory the product ions. The product ions collide into the detector triggering an electron cascade which is converted into an electric current and detected by a sensitive voltmeter. The combination of mass analyzers and detector yields information on the ion’s mass and intensity to yield a mass spectrum. The real advantage of the triple quadrupole, however, is its ability to perform multiple tandem mass spectrometry experiments in the same analytical run to gain both quantitative and qualitative information on multiple analytes simultaneously (Figure 2C,D).
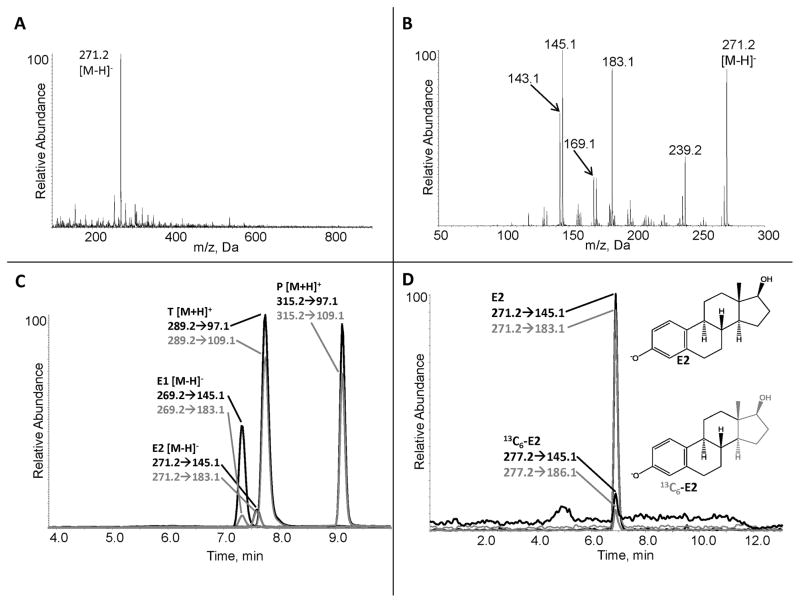
Figure 2.
Representative Data for Steroid Analysis with Mass Spectrometry A. Negative-ion MS1 spectrum of estradiol (E2) standard used to determine the ions in a sample as well as the precursor ion [M–H]−. B. Negative-ion tandem mass spectrometry (MS2) experiment used to determine the collision-induced product ions of E2, which in this case is equivalent to a full mass spectrum, since the precursor ion is the molecular ion. C. MRM ion chromatogram of estrone (E1), E2, testosterone (T) and progesterone (P). Negative (E1, E2) and positive (T, P) mass transitions were acquired during the same mass spectrometry experiment and overlaid for chromatographic comparison. To be certain a mass transition is from the steroid of interest and not contaminants, a minimum of two mass transitions (quantifier and qualifier product ions) are used; the peaks must co-elute to be validated for quantitation. The ratios of the two transitions are monitored to detect the presence of interferences. Contaminants can have an identical mass transition as the analyte, but it is very unlikely that a different compound will have two or more transitions with the same ratios as the analyte. The ratio of the two mass transitions for the endogenous analyte should be the same as the stable isotope internal standard mass transitions. D. Negative-ion MRM chromatogram of unlabeled E2 and [13C6]-E2 internal standard, which elute simultaneously. To unequivocally ensure that the mass transitions being quantitated derive from the steroid of interest and not a contaminant, it is required that the target steroid’s mass transitions co-elute with the internal standard. In order for an endogenous steroid to be quantitated, it must have two mass transitions co-eluting with the corresponding mass transitions of the isotopically labeled internal standard steroid.
The triple quadrupole arrangement can be changed from the product ion configuration and be used in other useful tandem mass spectrometry experiments including multiple reaction monitoring (MRM) and MS3. The most sensitive and specific quantitative mass spectrometry experiment to quantitate multiple precursor ions is the MRM experiment. Briefly, Q1 is tuned so that only one ion can pass through to the collision cell (Q2). The ion is fragmented and passed along to Q3, which is also tuned to allow only one fragment ion to pass to the detector. The instrument is monitoring the mass transition from a specific precursor ion to a specific product ion in these experiments. Often, two mass transitions, from a single precursor ion to both a quantifier ion and a qualifier ion, are used to quantitate and to confirm the identity of a specific compound, respectively (Kushnir et al., 2005).
A reference standard, typically the desired analyte bearing a stable isotope label (three or more 2H or 13C atoms) is added during sample preparation for comparison in MRM experiments, to translate a chemical concentration to the ion intensity. The specificity and robustness of the MRM experiment is enhanced by the requirement that the endogenous elution peak must coincide with the reference standard, and the labeled and unlabeled compounds are distinguished by parent ion masses (Figure 2C,D). In this manner, if the elution time is altered, the endogenous compound can still be quantitated. The ratio of the two mass transition signal intensities should reflect those of the stable isotope reference standard; a significant difference indicates a contamination. Despite several precautions taken to ensure that only the analyte of interest is quantitated, the numerous molecules present in a biological specimen can contaminate a measurement and significantly affecting the accuracy and precision of an assay. Interfering substances can also derive from solvents, tubes, and trays, emphasizing the need to always perform blanks, calibrators, and control samples in each run.
MS3 experiments are used to further fragment product ions and yield more structural information. In some modern mass spectrometers, Q3 can also function as a linear trapping quadrupole (LTQ). Product ions are trapped in Q3, further fragmented and then passed along to the detector. MS2 experiments yield information on the overall precursor ion while MS3 experiments yield structural information on the product ions. For example, the MS2 experiment can indicate whether a precursor ion is testosterone or an isobaric contaminant in the sample by the ratio of product ion intensities, which assures accurate measurement of a known compound in a mixture. In contrast, MS3 experiments can identify the functional groups present at different locations on the precursor ion, which enhances structure determination of an unknown compound.
2.3. HPLC and ESI Ionization Source
The attention often focuses on the expensive and sophisticated mass spectrometer at the “back end,” but optimal use of a triple quadrupole instrument requires expert HPLC use in the “front end.” Steroid HPLC separation can be either “normal phase,” a very polar silica column with progressively more polar organic solvents, or “reverse phase,” a hydrophobic carbon chain column with progressively less polar aqueous-organic solvent mixtures. In normal phase chromatography, the more polar compounds elute last as the polarity of the solvent (“mobile phase”) increases, whereas in reverse phase HPLC the more polar compounds bind poorly to the hydrophobic column material (“stationary phase”) and elute first.
A typical normal phase system utilizes a silica analytical column and organic solvents such as hexanes or chloroform of low polarity and high polarity solvents such as methyl tert-butyl ether (MTBE), methylene chloride, ethyl acetate, isopropanol, acetonitrile, or methanol. The mobile phase starts as predominantly low polarity solvent (pure hexanes) and becomes increasingly more polar as the higher polarity solvent (ethyl acetate) is added, often in a defined gradient. In this manner, less polar compounds elute first while more polar compounds elute later with the high polarity solvent. A normal phase approach is generally used for separating rather polar compounds, which require a moderately polar organic solvent for elution.
Reverse phase chromatography utilizes hydrophobic hydrocarbon chains, usually C8 or C18 aliphatic groups, attached to polymer beads for a stationary phase. Reverse phase HPLC uses a weak solvent, usually water, to load the steroids on the column and a strong solvent, usually methanol or acetonitrile, for elution. The more polar steroids elute first in reverse phase chromatography instead of last as in normal phase chromatography. A reverse phase approach is used when the compounds to be separated are all rather hydrophobic and would not resolve well on a normal phase system, and reverse phase is ideal for mixtures with different functional groups. Reverse phase is generally the more effective system for steroid separation because of the hydrophobic nature of the unconjugated steroids. Although reverse phase columns come with a variety of chemical structures including phenyl and cyano groups, steroid methods usually employ simple C8 or C18 aliphatic chains; the longer the chain, the stronger the hydrophobic interaction.
After liquid chromatographic separation, the steroid-solvent mixture flows into an interface between the HPLC and the mass spectrometer called a “source,” in which the solute ions are formed and routed to the mass spectrometer. Among the various types of sources, the most commonly used for steroid work is the electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) sources, in which solvated neutral steroids are converted to gaseous ions at atmospheric pressure. The HPLC effluent flows into a high voltage needle and exits as a fine spray of highly charged droplets, which are directed towards the mass spectrometer via an electric field between the needle and mass spectrometer orifice. The solvent-analyte droplets are desolvated by a heated gas, usually nitrogen, which evaporates solvent until the charge density on the droplet surface rises so high that the electrostatic repulsion force exceeds the surface tension of the solvent. At that point, a coulombic explosion occurs, which generates much smaller droplets and deposits the charge onto the steroid molecules, forming parent ions, which enter the mass analyzer as completely desolvated ions.
Nanospray ESI (nanoESI) uses smaller capillaries and lower flow rates, which increases the efficiency of ion formation because less solvent is required. Consequently, nanoESI is a more sensitive technique than conventional ESI; however, the smaller capillaries predispose nanoESI to clogging with dirtier samples, rendering this method inappropriate for high-throughput assays. It is important to realize that only those steroid molecules that are ionized are measured, and a very small fraction of the steroid molecules are ionized by ESI, ~1–3% of the total mass exiting the source.
Atmospheric pressure chemical ionization (APCI) is similar to ESI, and some ionization sources are able to perform both methods. APCI utilizes a similar desolvation process with heated gas (usually nitrogen) but unlike ESI, uses corona discharge from an adjacent electrode to generate ions instead of applying a voltage to the eluent needle. The desolvated molecules and solvent gas enter the corona discharge area, where the abundant individual solvent molecules are ionized; these solvent ions collide with analyte molecules to form the molecular ions. APCI ionization occurs in the gas phase, whereas ESI occurs in the liquid phase; this distinction allows for the use of nonpolar solvents with APCI. The ESI process tends to generate multiply charged species whereas APCI generates singly charged species, making APCI only useful for small molecule work. ESI ionizes molecules through a “soft” ionization process where the analyte remains solvated through the ionization process; the APCI process ionizes gas-phase molecules in a higher energy process, which can form multiple fragment ions (Huang et al., 2010, Zaikin and Halket, 2006). Atmospheric pressure photochemical ionization (APPI) is yet another ionization method for LC-MS/MS. Although APPI is a robust ionization method, particularly for steroid analysis (Guo et al., 2006), the instrumentation setup is more costly and cumbersome than ESI and APCI sources, limiting its use.
2.4. Stable Isotope Dilution Mass Spectrometry
To quantitate endogenous steroid hormones, a stable isotope dilution mass spectrometry strategy is employed because of its sensitivity, reliability and scalability for high-throughput assays. Briefly, a known amount of a stable isotope reference standard (same steroid with three or more 2H or 13C atoms) is added to the biological sample before the steroids are extracted and subjected to mass spectrometry analysis. The ion intensities of the endogenous and reference standards are compared to a standard curve for quantitation. The stable isotope standard accounts for sample loss during extraction and analysis steps and also serves as an elution indicator, so that only the endogenous steroid eluting with the stable isotope standard is quantitated. The use of the labeled internal standard compensates for chromatogram drift during a run and prevents measurement errors from contaminants having the same precursor and product ion mass transition yet slightly different retention times.
It should be noted that heavily deuterated compounds will elute slightly earlier than unlabeled compounds in reverse-phase chromatography, because of a chromatographic deuterium isotope effect. This phenomenon can interfere with co-elution of internal standards and trace endogenous analytes in complex mixtures, particularly for long runs using ultra performance HPLC systems (UPLC) (Berg and Strand, 2011). For this reason 13C-labeled standards are preferable to deuterated standards; however, these 13C-labeled steroids are usually considerably more expensive and less available than deuterated standards.
3. Sample Preparation and Clinical Uses, and Limitations
3.1 Steroid Extraction From Serum
To analyze steroids from a biological matrix, usually serum or plasma but also urine and saliva and more recently hair for cortisone and cortisol (D’Anna-Hernandez et al., 2011, Gao et al., 2010, Vanaelst et al., 2012), the first step is usually some type of extraction, either liquid-liquid or solid-phase. This step removes interfering compounds from a sample, improves assay sensitivity, and extends the life of the chromatography columns. A liquid-liquid extraction method for steroids usually begins with the addition of stable isotope standards to serum and plasma samples followed in many but not all methods by a protein precipitation step using methanol or acetonitrile to free the steroids from their carrier proteins. After centrifugation, steroids are typically extracted from biological matrix with organic solvents, evaporated and resuspended in a sample solvent for injection into the mass spectrometer.
Alternatively, a solid phase approach extracts steroids utilizing an immobile hydrophobic adsorbent material followed by washing with water and selected elution with compound-specific solvents such as methanol. The solid phase adsorbent can be packed into a cartridge for a few samples, assembled into 96-well plates or manifold assemblies for many samples, or plumbed into the HPLC system using additional pumps and switching valves for on-line extraction in high-throughput assays, basically a second extraction column with lower affinity for steroids than the analytical column.
All assays are less sensitive when measuring analytes in a biological specimen or fluid matrix than when constructing a standard curve with pure compounds in pure solvent, the so-called “matrix effect.” The major matrix effect for mass spectrometry assays occurs when contaminant compounds co-elute with the analyte of interest. Even though the contaminants might not have the same mass transitions as the analyte and thus not directly impair the accuracy of the measurement, the contaminants compete with the analyte for the limited amount of electric charge applied to the microdroplets exiting the ESI source. This competition reduces the number of analyte ions formed, a process called “ion suppression.” Although the equivalently suppressed ionization of the internal standards automatically corrects the calculation of steroid mass, the sensitivity of the measurement suffers, since fewer ions are delivered to the mass spectrometer. In addition, contaminants can irreversibly attach to the column, increasing the “back pressure,” the pump pressure required to achieve a certain flow rate, and also degrade chromatographic resolution for subsequent samples. For this reason, a guard column and/or filter are frequently used to protect the analytical column from contaminating biomolecules and particles, respectively. The guard column is a thin disk made of the same or similar material as the analytical column attached directly before the analytical column, too small to resolve individual steroid components yet large enough to adsorb most hydrophobic contaminants before reaching the analytical column. The guard columns are disposed of when the HPLC back pressure increases or peak broadening occurs.
3.2. Applications for Adrenal Steroid Analyses
The clinical diagnosis of adrenal diseases mainly employs basal and dynamic measurements of serum steroids, for which LC-MS/MS is an attractive alternative to immunoassays (Kushnir et al., 2011, Penning et al., 2010, Rauh, 2010). LC-MS/MS assays have the advantage of moderately high throughput, exquisite accuracy, wide dynamic range of up to 5 orders of magnitude, relative freedom from interferences and multiplexing of many analyte measurements in a single assay with a single small sample. An LC-MS/MS assay, which measures a panel of steroids, including 17-hydroxyprogesterone, androstenedione, 11-deoxycortisol, cortisol, and the highly specific marker 21-deoxycortisol, is employed as second-tier screening for 21-hydroxylase deficiency (Janzen et al., 2007, Minutti et al., 2004). The power of the LC-MS/MS method is the capacity to quantitate steroids above and below the enzymatic block simultaneously, which provides ratios and distinguishes the enzymatic block from other causes of elevated 17-hydroxyprogesterone. Furthermore, the high sensitivity and assay multiplexing are essential for analyses with small samples such as blood spots for newborn screening. In addition, the multiplexed assays can distinguish amongst the various causes of congential adrenal hyperplasia, such as 11-hydroxylase deficiency (high 11-deoxycortisol) versus 21-hydroxylase deficiency (high 21-deoxycortisol) (Janzen et al., 2012).
Another example of a scientific problem of steroid biochemistry not approachable with immunoassay but amenable to LC-MS/MS analysis is androgen measurements in tumor tissues, for the study of castration-resistant prostate cancer (CRPC). Using multiplexed assays, often with derivatization such as oximes and/or picolinic acid esters, progress has been made to defining the pathways and key enzymes responsible for CRPC progression (Montgomery et al., 2008, van der Sluis et al., 2012, van der Sluis et al., 2012). These studies use tiny samples of rare tumor specimens, and numerous controls are necessary to exclude artifacts when the assays are pushed to their limits.
In the diagnosis of primary aldosteronism, a critical step is to distinguish unilateral from bilateral aldosterone production. LC-MS/MS has been used to measure 18-hydroxycorticosterone in specimens drawn during adrenal vein sampling. Although the discriminatory power of the 18-hydroxycorticosterone/aldosterone and 18-hydroxycorticosterone/cortisol ratios was not superior to the aldosterone/cortisol ratio, this study found that the 18-hydroxycorticosterone/aldosterone ratio in adrenal vein blood on the side with an aldosterone-producing adenoma was consistently very close to 2, lower than that found in bilateral disease and much lower than found in the contralateral adrenal vein (Auchus et al., 2007). Similarly, 18-oxocortisol and 18-hydroxycortisol, measured by LC-MS/MS, are disproportionately higher in adrenal vein specimens draining an aldosterone-producing adenoma than in the contralateral side or in bilateral disease (Nakamura et al., 2011). These multiplexed assays are particularly useful in cases when the lateralization index (ratio of aldosterone/cortisol ratios) is equivocal (Auchus et al., 2010), in the 2–4 range with cosyntropin stimulation. Such secondary criteria might help to standardize the approach to interpretation of these studies, which vary considerably even amongst referral centers (Rossi et al., 2012).
3.3. Advantages and Disadvantages of Steroid Assays by LC-MS/MS
A modern triple quadrupole LC-MS/MS instrument, including nitrogen source, columns, and supplies to start analyses, costs at least $400,000 and requires an expert analytical chemist for method development and implementation. Pure authentic standards and stable-isotope labeled internal standards, about 10 mg for each analyte, can be expensive but will last for many years. In some cases, the internal standard for one or more analyte might not be commercially available, and the investigator must either have the compound prepared via custom synthesis, use a chemical analog as internal standard, or use a limited set of internal standards in a multi-steroid profile, where the standards are spread throughout the chromatogram with key analytes. The system requires frequent cleaning and maintenance, column replacement, and software upgrades. A service contract costs roughly 10% of the purchase price of the instrument per year, which is generally a good investment if the instrument is used daily. Facilities with 10 or more instruments often hire an in-house service engineer for maintenance, and on this scale such a hire is cost-effective and provides quicker service.
Despite the large setup costs and expertise required for LC-MS/MS measurements, the superior accuracy over platform immunoassays—particularly for low steroid concentrations, the wide dynamic range, and the ability to perform moderately high-throughput assays without reliance on radioactivity and proprietary or finite antibody sources make LC-MS/MS assays attractive for steroid assays in reference laboratories. On the other hand, LC-MS/MS offers little advantage over platform immunoassays for abundant steroids such as cortisol, unless part of a multiplex steroid profile; however, a second injection with dilution might be necessary to bring the cortisol quantity into the linear range of the assay. The Center for Disease Control (CDC) has developed the HoST Program for testosterone assay certification, designed to establish inter-laboratory assay harmonization similar to cholesterol assays. Given the poor performance of testosterone platform immunoasssays for samples from women and children (Rosner et al., 2007), LC-MS/MS testosterone assays are achieving certification through the HoST program. Although reference laboratories have transitioned many of their steroid assays to LC-MS/MS, these assays are generally designed for rapid measurement of single steroids, whereas profiling to measure a dozen or more steroids at one time on small amounts of serum has been mainly the providence of academic laboratories (Guo et al., 2006). Nevertheless, instrument manufactures have developed method “packages,” which are now marketed for steroid profiling applications. In the absence of a single protocol for all steroids and all instruments, it is unlikely that a universal approach will arise for some time.
It is important to recognize that the sensitivity of LC-MS/MS measurement is quite dissimilar for different types of steroids (McDonald et al., 2011). Most delta-4 steroids ionize well with ESI (positive ion mode) and fragment predictably to abundant and characteristic product ions, and hence assays for these steroids detect 100 fg on column or less. Steroid sulfates and glucuronides also ionize and fragment well (negative ion mode), but the mass spectrometry properties of delta-5 steroids, 5α-reduced androgens, and estrogens are not as favorable. These steroids require considerable method development, often involving derivatization. Steroids with free hydroxyl groups (dehydroepiandrosterone, dihydrotestosterone) are efficiently converted to picolinic acid esters, which guides [M+H]+ ion formation and fragmentation with markedly improved sensitivity (Yamashita et al., 2009). Ketosteroids are readily converted to oximes, which improves ionization of delta-5 steroids (Keski-Rahkonen et al., 2011) and thus affords much better sensitivity. Addition of ammonium fluoride improves sensitivity for estrone and estradiol assays in negative ion mode (Fiers et al., 2012). The quandary posed to the investigator is how to measure as many steroids of interest as possible with the fewest manipulations, losses, and sample splitting for different derivitization protocols.
Lastly, LC-MS/MS assays must be approached with the same compulsive care as any other clinical assay. Despite all the cost and automation, assay validation and quality control cannot be dismissed. Every step from mobile phase and stock solution preparation to specimen aliquotting is a potential source for error. Successful laboratories will assiduously follow written standard operating procedures with frequent internal checks and validations.
3.4. Comparison with GC/MS
Gas chromatography/mass spectrometry (GC/MS) differs from LC-MS/MS primarily in that the analytes must be chromatographed in the gas phase. While GC/MS is ideal for volatile compounds, steroids are not inherently volatile and must be derivatized, usually converting free hydroxyl and ketone groups silyl ethers, silyl enol ethers, and silated oximes. The steroid derivatives are injected into an oven containing a long capillary column, and the steroids are separated with a carrier gas of helium or hydrogen. Upon exiting the column, the molecules are ionized with electron impact (EI) or chemical ionization (CI) before entering the mass spectrometer. Typically, a single ion fragment for each analyte is tracked using selected ion monitoring (SIM), and quantitation is accomplished by comparing the ion current against a standard curve. Although a very powerful technique, the sensitivity is lower than LC-MS/MS, and the derivatization and chromatography processes are slow and thus limit throughput. More detailed discussions of GC/MS and its application to steroid analysis have appeared recently (Krone et al., 2010, McDonald et al., 2011).
4. Conclusions
The advent of highly sensitive LC-MS/MS assays for steroid hormones makes previously unthinkable experiments possible and forces adrenal investigators to reconsider their approach to adrenal biology. Mass spectrometry assays can be applied to tissue culture medium, tumors, and body fluid from human beings and experimental animals. Instrumentation and methods are constantly evolving at an accelerating pace. The investigator is no longer limited to the list of steroids offered on commercial immunoassay menus but is now limited by their own imagination.
LC-MS/MS measures several steroids in one experiment on a single small sample.
Pre-analytical extraction prevents instrument contamination and ion suppression.
The sensitivity of LC-MS/MS assays varies for different structural classes of steroids.
Derivatization improves sensitivity, particularly for specific types of steroids.
Acknowledgments
This work was supported by a Clinician-Scientist Award in Translational Research from the Burroughs-Wellcome Fund (#1005954 to R.J.A.) and grants for the Michigan Nutrition Obesity Center (DK089503) and Michigan Regional Comprehensive Metabolomics Research Core (U24DK097153). We thank Drs. Robert M. Barkley and Miguel A. Gijón for their expert advice and guidance with the design of the figures.
Footnotes
Abbreviations: APCI, atmospheric pressure chemical ionization; APPI, atmospheric pressure photochemical ionization; CDC, Center for Disease Control; CI, chemical ionization; CID, collision-induced dissociation; CRPC, castration-resistant prostate cancer; ELISA, enzyme-linked immunosorbent assay; ESI, electrospray ionization; GC, gas chromatography; HPLC, high performance liquid chromatography; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LTQ, linear trapping quadrupole; MTBE, methyl tert-butyl ether; MRM, multiple reaction monitoring; MSn, mass spectrometry to the nth, meaning n cycles of iterative mass spectrometry; Qn, quadrupole number n; RF, radio frequency; UPLC, ultra performance liquid chromatography.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auchus RJ, Chandler DW, Singeetham S, Chokshi N, Nwariaku FE, Dolmatch BL, Holt SA, Wians FH, Jr, Josephs SC, Trimmer CK, Lopera J, Vongpatanasin W, Nesbitt SD, Leonard D, Victor RG. Measurement of 18-hydroxycorticosterone during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2007;92:2648–2651. doi: 10.1210/jc.2006-2631. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Wians FH, Jr, Anderson ME, Dolmatch BL, Trimmer CK, Josephs SC, Chan D, Toomay S, Nwariaku FE. What we still do not know about adrenal vein sampling for primary aldosteronism. Horm Metab Res. 2010;42:411–415. doi: 10.1055/s-0030-1252060. [DOI] [PubMed] [Google Scholar]
- Berg T, Strand DH. 13C labelled internal standards--a solution to minimize ion suppression effects in liquid chromatography-tandem mass spectrometry analyses of drugs in biological samples? J Chromatogr A. 2011;1218:9366–9374. doi: 10.1016/j.chroma.2011.10.081. [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011;104:348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman JM. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;893–894:57–62. doi: 10.1016/j.jchromb.2012.02.034. [DOI] [PubMed] [Google Scholar]
- Gao W, Xie Q, Jin J, Qiao T, Wang H, Chen L, Deng H, Lu Z. HPLC-FLU detection of cortisol distribution in human hair. Clin Biochem. 2010;43:677–682. doi: 10.1016/j.clinbiochem.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MZ, Yuan CH, Cheng SC, Cho YT, Shiea J. Ambient ionization mass spectrometry. Annu Rev Anal Chem (Palo Alto Calif) 2010;3:43–65. doi: 10.1146/annurev.anchem.111808.073702. [DOI] [PubMed] [Google Scholar]
- Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, Sander J. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007;92:2581–2589. doi: 10.1210/jc.2006-2890. [DOI] [PubMed] [Google Scholar]
- Janzen N, Riepe FG, Peter M, Sander S, Steuerwald U, Korsch E, Krull F, Muller HL, Heger S, Brack C, Sander J. Neonatal screening: identification of children with 11β-hydroxylase deficiency by second-tier testing. Horm Res Paediatr. 2012;77:195–199. doi: 10.1159/000337974. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen P, Huhtinen K, Poutanen M, Auriola S. Fast and sensitive liquid chromatography-mass spectrometry assay for seven androgenic and progestagenic steroids in human serum. J Steroid Biochem Mol Biol. 2011;127:396–404. doi: 10.1016/j.jsbmb.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J Steroid Biochem Mol Biol. 2010;121:496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir MM, Rockwood AL, Nelson GJ, Yue B, Urry FM. Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clin Biochem. 2005;38:319–327. doi: 10.1016/j.clinbiochem.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kushnir MM, Rockwood AL, Roberts WL, Yue B, Bergquist J, Meikle AW. Liquid chromatography tandem mass spectrometry for analysis of steroids in clinical laboratories. Clin Biochem. 2011;44:77–88. doi: 10.1016/j.clinbiochem.2010.07.008. [DOI] [PubMed] [Google Scholar]
- McDonald JG, Matthew S, Auchus RJ. Steroid profiling by gas chromatography-mass spectrometry and high performance liquid chromatography-mass spectrometry for adrenal diseases. Horm Cancer. 2011;2:324–332. doi: 10.1007/s12672-011-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, Cheillan D, Dorche C, Chace DH, Lymp JF, Zimmerman D, Rinaldo P, Matern D. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–3693. doi: 10.1210/jc.2003-032235. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Satoh F, Morimoto R, Kudo M, Takase K, Gomez-Sanchez CE, Honma S, Okuyama M, Yamashita K, Rainey WE, Sasano H, Ito S. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab. 2011;96:E1272–E1278. doi: 10.1210/jc.2010-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA. Liquid chromatography-mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J Steroid Biochem Mol Biol. 2010;121:546–555. doi: 10.1016/j.jsbmb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh M. Steroid measurement with LC-MS/MS. Application examples in pediatrics. J Steroid Biochem Mol Biol. 2010;121:520–527. doi: 10.1016/j.jsbmb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position Statement: Utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, Degenhart C, Deinum J, Fischer E, Gordon R, Kickuth R, Kline G, Lacroix A, Magill S, Miotto D, Naruse M, Nishikawa T, Omura M, Pimenta E, Plouin PF, Quinkler M, Reincke M, Rossi E, Rump LC, Satoh F, Schultze Kool L, Seccia TM, Stowasser M, Tanabe A, Trerotola S, Vonend O, Widimsky J, Jr, Wu KD, Wu VC, Pessina AC. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97:1606–1614. doi: 10.1210/jc.2011-2830. [DOI] [PubMed] [Google Scholar]
- van der Sluis TM, Meuleman EJ, van Moorselaar RJ, Bui HN, Blankenstein MA, Heijboer AC, Vis AN. Intraprostatic testosterone and dihydrotestosterone. Part II: concentrations after androgen hormonal manipulation in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 2012;109:183–188. doi: 10.1111/j.1464-410X.2011.10652.x. [DOI] [PubMed] [Google Scholar]
- van der Sluis TM, Vis AN, van Moorselaar RJ, Bui HN, Blankenstein MA, Meuleman EJ, Heijboer AC. Intraprostatic testosterone and dihydrotestosterone. Part I: concentrations and methods of determination in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 2012;109:176–182. doi: 10.1111/j.1464-410X.2011.10651.x. [DOI] [PubMed] [Google Scholar]
- Vanaelst B, Huybrechts I, Bammann K, Michels N, de Vriendt T, Vyncke K, Sioen I, Iacoviello L, Gunther K, Molnar D, Lissner L, Rivet N, Raul JS, de Henauw S. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49:1072–1081. doi: 10.1111/j.1469-8986.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Miyashiro Y, Maekubo H, Okuyama M, Honma S, Takahashi M, Numazawa M. Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2009;74:920–926. doi: 10.1016/j.steroids.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Zaikin VG, Halket JM. Derivatization in mass spectrometry--8. Soft ionization mass spectrometry of small molecules. Eur J Mass Spectrom (Chichester, Eng) 2006;12:79–115. doi: 10.1255/ejms.798. [DOI] [PubMed] [Google Scholar]