Abstract
Purpose
Analyzing the clinical performance of restorative materials is important, as there is an expectation that these materials and procedures will restore teeth and do no harm. The objective of this research study was to characterize the clinical performance of metal-ceramic crowns, core ceramic crowns, and core ceramic/veneer ceramic crowns based on 11 clinical criteria.
Materials and Methods
An IRB-approved, randomized, controlled clinical trial was conducted as a single-blind pilot study. The following three types of full crowns were fabricated: (1) metal-ceramic crown (MC) made from a Pd-Au-Ag-Sn-In alloy (Argedent 62) and a glass-ceramic veneer (IPS d.SIGN veneer); (2) non-veneered (glazed) lithium disilicate glass-ceramic crown (LDC) (IPS e.max Press core and e.max Ceram Glaze); and (3) veneered lithia disilicate glass-ceramic crown (LDC/V) with glass-ceramic veneer (IPS Empress 2 core and IPS Eris). Single-unit crowns were randomly assigned. Patients were recalled for each of 3 years and were evaluated by two calibrated clinicians. Thirty-six crowns were placed in 31 patients. A total of 12 crowns of each of the three crown types were studied. Eleven criteria were evaluated: tissue health, marginal integrity, secondary caries, proximal contact, anatomic contour, occlusion, surface texture, cracks/chips (fractures), color match, tooth sensitivity, and wear (of crowns and opposing enamel). Numerical rankings ranged from 1 to 4, with 4 being excellent, and 1 indicating a need for immediate replacement. Statistical analysis of the numerical rankings was performed using a Fisher’s exact test.
Results
There was no statistically significant difference between performance of the core ceramic crowns and the two veneered crowns at year 1 and year 2 (p > 0.05). All crowns were rated either as excellent or good for each of the clinical criteria; however, between years 2 and 3, gradual roughening of the occlusal surface occurred in some of the ceramic-ceramic crowns, possibly caused by dissolution and wear of the glaze. Statistically significant differences in surface texture (p = 0.0013) and crown wear (p = 0.0078) were found at year 3 between the metal-ceramic crowns and the lithium-disilicate-based crowns.
Conclusion
Based on the 11 criteria, the clinical performance of ceramic-ceramic crowns was comparable to that of the metal-ceramic crowns after 2 years; however, gradual roughening occurred between years 2 and 3, which resulted in differences in surface texture and wear.
Keywords: Bilayer ceramics, clinical research, clinical performance, metal-ceramic, prosthodontics
Esthetics has become a major driving force for the development of restorative materials. Many materials are being introduced clinically without evidence from long-term clinical studies and without understanding the limitations of these materials. Ceramic-ceramic prostheses are becoming the restoration of choice for the replacement of coronal structures and missing teeth. Few publications of randomized and controlled clinical studies document the performance and survivability of ceramic-ceramic prostheses, particularly those used for posterior restorations. These types of prostheses have advantages over monolithic metal or metal-ceramic fixed dental prostheses (FDPs). Primarily, the esthetics of ceramic restorations is improved because of their increased translucency and light transmission.1,2 Other advantages include minimal tooth reduction3 compared with metal-ceramics, minimal thermal conductivity,3 less periodontal damage because of the supragingival placement of margins,4–6 and less susceptibility to metal allergies.
Meta analyses of clinical data have shown survival levels of 95.6% for metal-ceramic crowns and 93.3% for ceramic-ceramic crowns at 5 years.7 These survival levels for bi-layer ceramic crowns were affected by the type of material used and the position of the crowns in the mouth. Densely sintered alumina crowns had the highest survival rate at 5 years (96.7% anterior, 94.9% posterior), followed by reinforced glass-ceramics (95.9% anterior, 93.7% posterior), and In-Ceram Alumina crowns (96.7% anterior, 90.4% posterior).7 The glass-ceramic crowns had the lowest survival rate of all materials studied (91.4% anterior, 84.4% posterior).7 Interestingly, metal-ceramic crowns showed no significant difference in survival rates based on location in the mouth,8,9 although there were higher replacement rates for the anterior crowns, mostly as a result of fracture. In contrast, bilayer ceramic crowns had a higher survival rate and were more predictable if they were placed anteriorly and as single units.10,11 Since the bi-layer ceramic systems are relatively new, there have been few randomized, controlled clinical studies to evaluate their success. Validated design parameters based on clinical evidence are too limited for clinicians to apply these design principles to all new ceramics. Guidelines for the selection and design of ceramic-ceramic FDPs are based on recommendations from manufacturers, which are not generally based on peer-reviewed clinical research publications. Thus, there is limited guidance on how to minimize catastrophic failures of these restorations.
Replacement of missing tooth structure with well-established metal-ceramic designs is very costly, yet new, unproven ceramics are commonly selected as viable treatment options. The estimated annual cost of $60 billion7 for dental services in the United States is primarily associated with the placement and replacement of restorations and prostheses. Reasons for failure and replacement include, but are not limited to, chipping and fracture of prostheses, secondary caries,9 periodontal disease, core/root fracture, and pulpal pathology.
The objective of this research was to compare the clinical performance of crowns made with a ceramic core with no veneer, a metal-ceramic system, and a bilayer ceramic-ceramic system. The following hypotheses were tested:
Bilayer ceramic crowns of a high strength core ceramic will exhibit good to excellent clinical performance based on 11 evaluative criteria.
Bilayer ceramic crowns have a clinical performance equivalent to or better than the metal-ceramic control crowns based on 11 evaluative criteria.
Materials and methods
Study design
A randomized, controlled clinical trial was performed to analyze the clinical performance of three ceramic materials based on 11 evaluative criteria. This single-blind pilot study involved a total of 32 patients with 37 teeth that needed full-coverage crowns opposing natural antagonist teeth. Patients’ teeth were randomly assigned to receive one of the three types of crowns.
Materials used in clinical study
Metal-ceramic crowns (MC) were made from Pd-Au-Ag-Sn-In alloy (Argedent 62, Argen Corporation, San Diego, CA) and glass-ceramic veneer (IPS d.SIGN veneer, Ivoclar Vivadent, Schaan, Liechtenstein).
Non-veneered (glazed) ceramic-ceramic crowns (LDC) were made from a lithium disilicate glass-ceramic (IPS e.max Press core and e.max Ceram glaze, Ivoclar Vivadent).
Core ceramic/veneer ceramic crowns (LDC/V) were made from lithium disilicate glass-ceramic and a glass-ceramic veneer (IPS Empress 2 core and IPS Eris, Ivoclar Vivadent).
Study population
Participants were recruited through broadcast e-mail and flyer advertisements. Participants were selected based on the following criteria:
Minimum age of 18 years, in good overall general health, and no contraindications to dental treatment.
Good overall dental health, no active tooth decay (caries), no periodontal disease, and periodontal pocket depths not greater than 4 mm.
No existing temporomandibular disorders, (e.g., clicking, popping, or pain on opening) or parafunctional habits (e.g., bruxism or clenching).
Crown required for second premolars, first molars, or second molars in any arch. Restorable teeth with a crown-to-root ratio of at least 1:1, and a full complement of opposing nonrestored or minimally restored natural teeth, (i.e., not larger than a Class II amalgam restoration).
No full-coverage restoration or partial denture in the opposing arch. Contralateral teeth must be present.
Good oral hygiene and compliance with oral hygiene instructions as determined by the amount of plaque present on tooth surfaces.
Normal flow of saliva. Participants with a reduced salivary volume or flow rate, who require medications that minimize flow of saliva, were excluded from the study.
Ability to pay $200 for the laboratory cost of a crown and are compliant with yearly appointments.
Study intervention
Thirty seven (37) teeth, in 32 enrolled participant (no patient having more than two study crowns) who needed full-coverage crowns were randomly assigned to receive either a metal-ceramic crown or a ceramic crown. For participant allocation, a computer-generated random number table was formulated to facilitate assignment of teeth to a material group. Participants were treated at the dental school of the University of Texas Health Science Center at San Antonio (UTHSCSA) between 2002 and 2007. The UTHSCSA Institutional Review Board approved the research protocol for treating human subjects. All participants were required to sign an informed consent form prior to initiating the study. The following baseline information was collected:
General medical history and physical examination.
Maximum bite force using a gnathodynamometer.
Periodontal pocket depths of abutment teeth.
In addition, periapical radiographs of abutment teeth and diagnostic casts of the maxillary and mandibular arches were made with vinylpolysiloxane (VPS) impression material.
Two investigators prepared all of the teeth for full crowns. Integrity (Dentsply, York, PA), a provisional bis-acryl resin material, was used to fabricate interim prostheses. Teeth were reduced from 1.0 mm for ceramic-ceramic crowns to 1.5 mm for metal-ceramic crowns. VPS impression material (Affinis, Coltene Whaledent, Alstatten, Switzerland) was used for final impressions. Master casts were mounted in centric relation.
Single-unit crowns were fabricated from each of the two ceramic material systems or the metal-ceramic system. The occlusal surface thickness of each finished crown was measured at baseline. Adjustments to the crown were made with a high-speed handpiece and a fine diamond bur (Brasseler, Savannah, GA, fine diamond bur, 15 μm grit) as needed. Prior to cementation, all adjusted surfaces were polished (Shofu porcelain polishing kit, Shofu Dental Corporation, Kyoto, Japan) or glazed depending on the size of the areas involved in the adjustment. Larger areas (involving more than 2 cusps) were typically glazed, while smaller areas were polished until they were deemed to be clinically as smooth as the nonadjusted surfaces. All crowns were cemented with a dual-cure resin luting agent (Variolink II, Ivoclar Vivadent).
The participants returned yearly after crown cementation for each of the following 3 years. Crowns were evaluated for the following criteria: (1) tissue health; (2) secondary caries; (3) occlusion; (4) proximal contact; (5) marginal integrity; (6) absence of sensitivity to percussion, heat, cold, and air; (7) color match; (8) surface texture; (9) absence of opposing tooth wear; (10) anatomic contour; and (11) presence or absence of cracks/chips or fracture. This evaluative system was derived from the California Dental Association quality assessment evaluation system.13 Crowns were examined by two independent clinicians who did not prepare the teeth or cement the prosthesis, and rankings of each criterion were made on a scale from 1 to 4: 4 as excellent, 3 as good, 2 as unacceptable (and needing repair or replacement in the near future), and 1 as unacceptable condition (but needing immediate replacement). Quantitative analysis of wear was performed by making VPS impressions at baseline and at all recall appointments. A Laser Scanner 3D (es 1, etkon, Willytec, Feldkirchen-Westerham, Germany) was used to superimpose images and to quantify wear. Wear was measured for the crowns, the opposing enamel, the teeth contralateral to the crowns, and the opposing enamel.
Statistical analysis
Fisher’s-exact test was used to determine statistical significance for the clinical performance variables for the three ceramics. The three groups of ceramics were compared for each evaluative criterion for years 1, 2, and 3.
Results
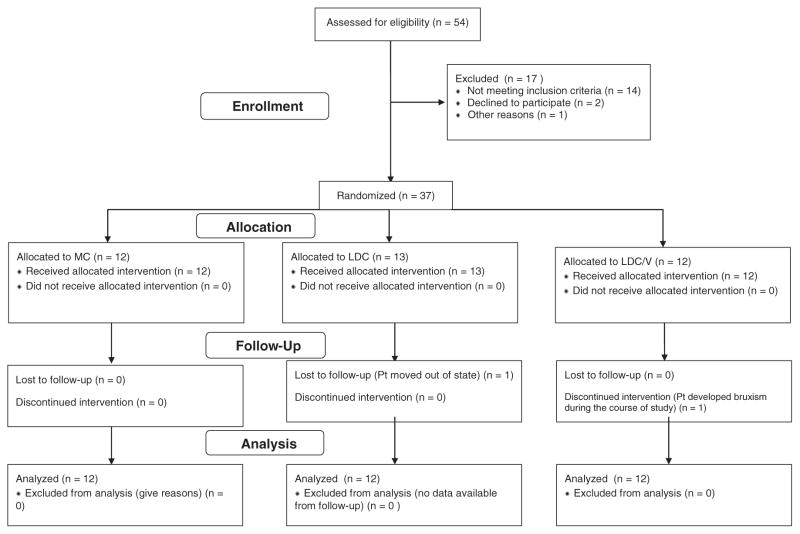
Participant recruitment, retention and allocation are detailed in Figure 1. One subject with one crown moved out of state prior to any of the follow-up exams being performed. This person was excluded from the data analysis, thereby resulting in a total of 36 crowns, with 12 crowns per ceramic group. There was no statistically significant difference among each of the criteria for the metal-ceramic, glazed core ceramic, and veneered ceramic crowns in year 1 or in year 2 based on the evaluative criteria. All crowns were rated as excellent or good for all 11 clinical criteria (Table 1); however, there was a significant difference in surface texture (p = 0.0013) and crown wear (p = 0.0078) at year 3 among the three ceramics. MC performed significantly better in the surface texture category than the ceramic-ceramic crowns LDC/V and LDC, which exhibited noticeable surface texture changes in several cases. Consistency between the evaluators ranged from 87% to 100%.
Figure 1.
Research participant recruitment, allocation and retention chart.
Table 1.
Proportion of good to excellent ratings for the three ceramics
| YEAR ONE | MC | LDC | LDC/V | p-value |
|---|---|---|---|---|
| Tissue health | 92 | 100 | 100 | 0.99 |
| Marginal integrity | 92 | 100 | 100 | 0.99 |
| Secondary caries | 92 | 100 | 100 | 0.99 |
| Proximal contact | 85 | 100 | 92 | 0.76 |
| Anatomic contour | 92 | 92 | 100 | 0.99 |
| Occlusion | 85 | 100 | 92 | 0.76 |
| Surface texture | 85 | 100 | 100 | 0.32 |
| Crack/chip/fracture | 77 | 100 | 100 | 0.09 |
| Color/color match | 77 | 100 | 100 | 0.09 |
| Tooth sensitivity | 85 | 92 | 92 | 0.99 |
| Wear of antagonist | 92 | 100 | 100 | 0.99 |
| Wear of crown | 92 | 100 | 100 | 0.99 |
|
| ||||
| YEAR TWO | MC | LDC | LDC/V | p-value |
|
| ||||
| Tissue health | 92 | 100 | 100 | 0.99 |
| Marginal integrity | 92 | 100 | 100 | 0.99 |
| Secondary caries | 92 | 100 | 100 | 0.99 |
| Proximal contact | 84 | 100 | 100 | 0.32 |
| Anatomic contour | 92 | 100 | 100 | 0.99 |
| Occlusion | 92 | 100 | 100 | 0.99 |
| Surface texture | 92 | 100 | 92 | 0.76 |
| Crack/chip/fracture | 92 | 91 | 100 | 0.74 |
| Color/color match | 92 | 100 | 100 | 0.99 |
| Tooth sensitivity | 92 | 100 | 92 | 0.99 |
| Wear of antagonist | 92 | 100 | 100 | 0.99 |
| Wear of crown | 92 | 100 | 85 | 0.76 |
|
| ||||
| YEAR THREE | MC | LDC | LDC/V | p-value |
|
| ||||
| Tissue health | 91 | 100 | 100 | 0.99 |
| Marginal integrity | 91 | 100 | 100 | 0.99 |
| Secondary caries | 82 | 100 | 100 | 0.31 |
| Proximal contact | 91 | 91 | 100 | 0.99 |
| Anatomic contour | 91 | 91 | 100 | 0.99 |
| Occlusion | 91 | 100 | 100 | 0.99 |
| Surface texture | 91 | 18 | 27 | 0.0013 |
| Crack/chip/fracture | 73 | 91 | 91 | 0.58 |
| Color/color match | 82 | 82 | 73 | 0.99 |
| Tooth sensitivity | 82 | 100 | 100 | 0.31 |
| Wear of antagonist | 90 | 100 | 82 | 0.76 |
| Wear of crown | 90 | 27 | 45 | 0.0078 |
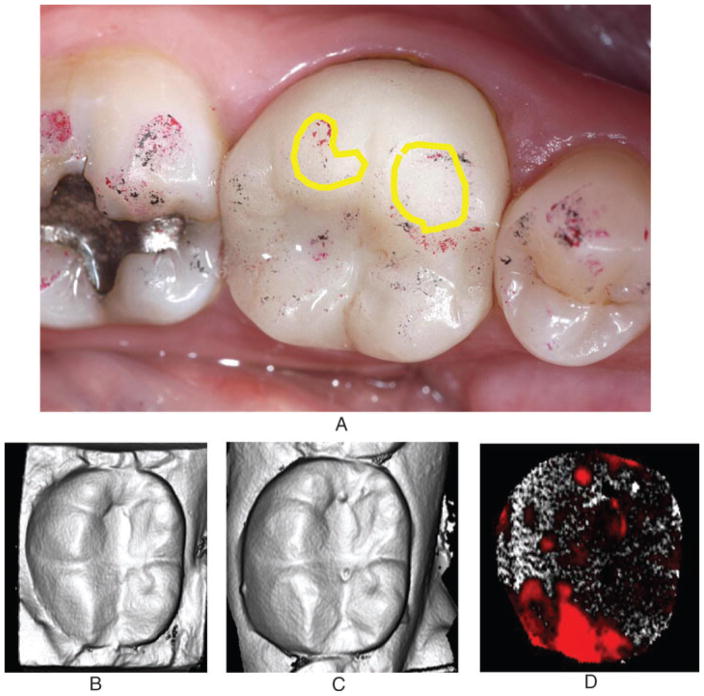
Roughness was noted mainly on the contact areas either along the buccal (for mandibular teeth) or lingual (for maxillary teeth) surface and the occlusal surfaces. An LDC/V crown is shown with roughness on the buccal and occlusal surfaces (Fig 2).14 Scanning electron microscopy was performed on the crown to characterize surface topography (Fig 3).
Figure 2.
LDC/V mandibular crown exhibiting roughness on buccal cusp areas circumscribed by yellow (A). Laserscanner images at baseline (B) and at 2 years (C) showing areas of wear along the distal and buccal cusps (D).
Reprint from: Esquivel-Upshaw JF, Rose WF, Jr., Barrett AA, Oliveira ER, Yang MC, Clark AE, Anusavice KJ. Three years in vivo wear: Core-ceramic, veneers, and enamel antagonists. Dent Mater 2012;28:615–621.
Figure 3.
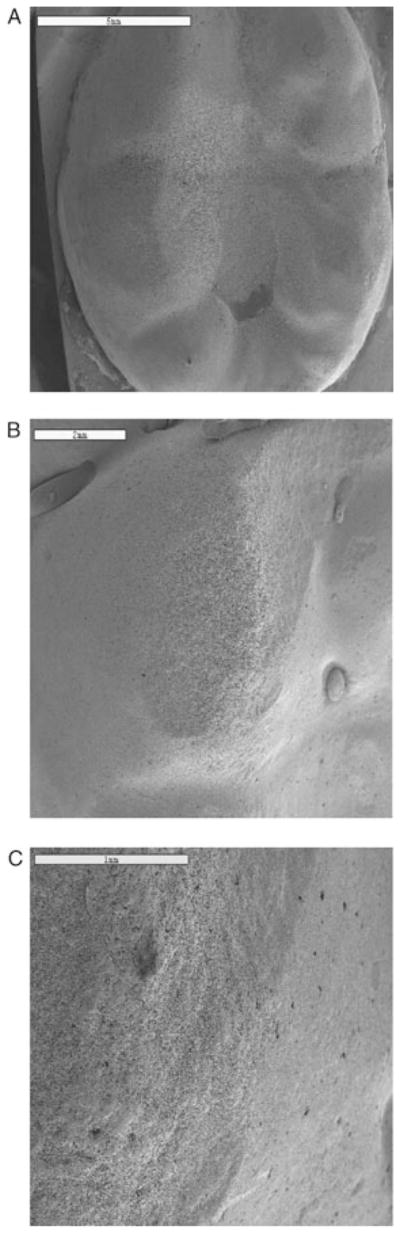
SEM images of buccal cusps in Figure 2 showing roughening of buccal cusps (A), and at higher magnifications (B,C).
Bite force measured on the patients ranged from 125 to 1272 N.12 There was no correlation between bite force and wear through years 1 and 2; however, there was a significant effect of bite force on wear at year 3 when the enamel contralateral antagonist (ECCA) was analyzed alone (p < 0.0001).
Discussion
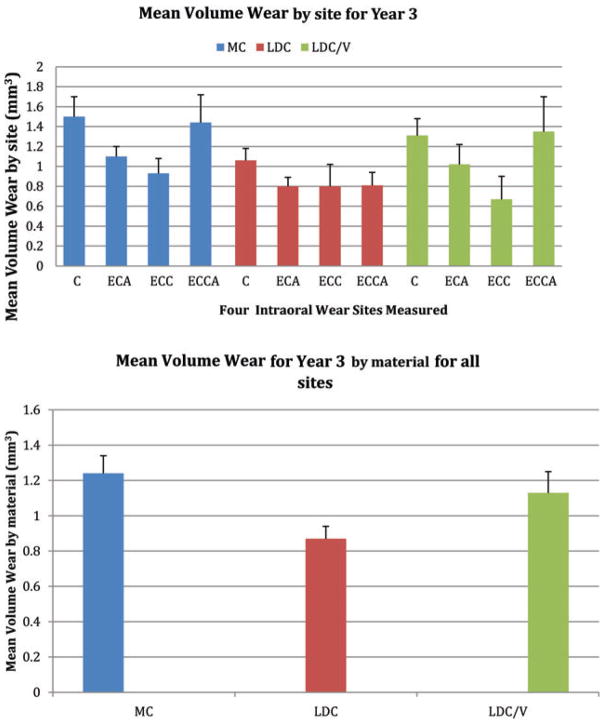
This study characterized the clinical performance of three crown materials according to 11 evaluative criteria. For the first 2 years, all crowns made from the three ceramics performed very well and were rated either as excellent or good based on the clinical criteria; however, roughening of some surfaces was observed in year 3. Based on the visual evaluative rankings, LDC and LDC/V exhibited visible changes in surface texture and increased wear at year 3. Therefore, we rejected both hypotheses because of the clinical roughness demonstrated by these crowns at year 3; however, quantitative data from the same study measured with a laser scanner revealed no significant difference in mean volume wear between LDC and LDC/V compared with natural enamel (Fig 4).15 In fact, both ceramics exhibited wear comparable to that of enamel wear against enamel.16
Figure 4.
(Top) Mean volume wear at year 3 for different sites C (crown); ECA (enamel crown antagonist); ECC (enamel crown contralateral); ECCA (enamel crown contralateral antagonist): (Bottom) Mean volume wear by material
Group MC exhibited the highest quantitative volume loss, but the surfaces remained smoother than those of the other two ceramics as determined by the clinical examiners. The ratio of ceramic wear to antagonist enamel wear was 1.3 for MC, 1.28 for LDC/V, and 1.3 for LDC, which shows that the wear of the three systems compared to their opposing enamel was essentially the same. Since MC remained clinically smooth as judged by examiners, there is a possibility that the composition and distribution of glass matrix and the residual crystals in the MC glass-ceramic resulted in a more uniform dissolution, while the other two ceramics exposed more of the crystalline phase, thereby increasing surface roughness. If this hypothesis is correct, the surface roughness should continue to increase over time. Although LDC and LDC/V exhibited rougher surfaces, this roughness did not cause greater wear of the opposing enamel during the three-year period. There is a possibility that these rougher surfaces will produce greater wear in the future.
Dental ceramics are required to be stable and chemically durable according to international standard, ISO 6872. This property is important because surface degradation of ceramics can lead to increased abrasiveness of the ceramic material and increased wear of the opposing enamel. Surface degradation of ceramic can also lead to increased surface roughness, which can lead to increased plaque accumulation and periodontal disease.17,18 Surface dissolution produces critical flaws in the surface of the ceramic, which can lead to crack formation and eventual bulk fracture of the restoration; however, these clinical data reveal that roughening of the surface occurred with very minimal loss of ceramic structure, comparable to normal enamel wear. This may indicate a possible erosion mechanism for the glaze ceramic, which ranged between 25 and 50 μm in thickness. Previous studies revealed that glass-matrix ceramics exhibit ion leaching in the presence of an aqueous medium.19,20 Sodium and potassium were leached from the ceramic surface as a result of the interaction of water with the silica bonds; however, these studies revealed no difference in ion leaching rates between glass-ceramic veneers and metal-ceramic crown veneers or between high- and low-fusing ceramics. Another study that focused on the surface roughness of ceramic after exposure to acidulated phosphate fluoride21 revealed that different ceramics exhibit variations in surface roughness and loss of structure. The surface roughness had a direct correlation with the mass of the ceramic. This finding is in contrast to what we found in this clinical study. Although surface roughness was evident upon clinical examination, the crowns did not exhibit excessive loss of material as measured quantitatively by differences between surfaces determined from in 3D laser-scanning images.
The clinical performance of all three ceramics was considered clinically acceptable. Survival of all crowns was 100% after a 3-year observation period; however, surface roughening appears to have occurred gradually between years 2 and 3 for LDC and LDC/V with 73% to 82% of the population rated at 2 or below in the surface texture category (Fig 2). A similar study on clinical performance of ceramic crowns22 reported that alumina-based core ceramic crowns (Procera All Ceram) showed not only wear, but also deformation of occlusal surfaces after 3 years compared with lithium-disilicate-based glass-ceramic crowns. The authors concluded that lithium-disilicate-based crowns and metal-ceramic crowns were more wear resistant than alumina-based crowns.
This finding, along with our quantitative analysis of crown wear, substantiates our theory that surface roughening was not a result of ceramic degradation of the veneer ceramic or core ceramic surfaces, but a degradation of the overglaze, which is only several micrometers thick. These findings also support anecdotal reports of glaze degradation leading to surface roughness and not necessarily wear of the veneering ceramic. We have learned that the manufacturer has discontinued marketing both the overglaze and the veneering ceramic (personal communication, Patrik Oehri, October 3, 2011). These materials were reformulated to ensure greater resistance to oral degradation.
Bias of the examiners may have impacted negatively on the results. There was a tendency for the examiners to assume that a change in surface texture of the crowns produced significant surface wear of the veneering ceramic. In reality, wear is difficult to visualize intraorally unless there are visible wear facets on the surfaces. A quantitative analysis has shown that no significant wear occurred on any of the three materials compared with enamel.
The effect of bite force was previously examined, and there was no correlation between the magnitude of bite force and the amount of wear.14 For this study, bite force also had no effect in years 1 and 2 but was significant in year 3 for the ECCA tooth surface. We can theorize that if the crown and its antagonist enamel (C and ECA) do not exhibit much wear for these initial years, then the contralateral or opposite side sustains the damage, up to a point where the ceramic side prevents excessive wear damage on the contralateral side (i.e., natural equilibration occurs). If so, the wear on CCA could be expected to plateau in year 4 or year 5.
Conclusion
This study indicates that the clinical performance for the first 2 years of the two types of ceramic-ceramic crowns were comparable to that of metal-ceramic crowns based on 11 evaluative criteria. Gradual surface roughening was observed between years 2 and 3 for the veneered lithium-disilicate-based ceramics with no apparent volume wear loss. The loss or surface degradation of the glaze can account for increased surface roughness, although these ceramics have been proven to be wear-resistant and comparable in wear to natural enamel. The glass-ceramic veneer for metal-ceramic crowns performed better clinically with regard to surface texture. Further clinical studies are needed to determine the mechanism responsible for surface degradation of glazes and ceramics and their potential effects on oral health.
Acknowledgments
The authors thank Robert B. Lee of the University of Florida, Dr. Chuchai Anunmana of Mahidol University, and Javier Luna of Creative Smiles Labs, San Antonio, TX, for their research contributions. This study was supported in part by Ivoclar Vivadent and NIH grants K23-DE01841 and R01-DE06672.
Footnotes
Previously presented at IADR, San Diego, March 2011.
The authors deny any conflicts of interest.
References
- 1.Raigrodski AJ, Chiche GJ. The safety and efficacy of anterior ceramic fixed partial dentures: a review of the literature. J Prosthet Dent. 2001;86:520–525. doi: 10.1067/mpr.2001.120111. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen JA. The IPS Empress 2 system: defining the possibilities. Quintessence Dent Technol. 1999;22:153–163. [Google Scholar]
- 3.Rosenstiel SL, Land MF, Fujimoto J. Contemporary Fixed Prosthodontics. 4. St. Louis, Missouri: Mosby Elsevier; 2006. [Google Scholar]
- 4.Sorensen JA, Cruz M, Mito WT, Raffeiner O, Meredith HR, Foser HP. A clinical investigation on three-unit fixed partial dentures fabricated with a lithium disilicate glass–ceramic. Pract Periodontics Aesthet Dent. 1999;11:95–106. quiz 08. [PubMed] [Google Scholar]
- 5.Gargiulo AW, Wentz FM, Orban B. Dimensions and relationships of the dentogingival junction in humans. J Periodontol. 1961;32:261–267. [Google Scholar]
- 6.Silness J. Periodontal conditions in patients treated with dental bridges. 3. The relationship between the location of the crown margin and the periodontal condition. J Periodontal Res. 1970;5:225–229. doi: 10.1111/j.1600-0765.1970.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 7.Newcomb GM. The relationship between the location of subgingival crown margins and gingival inflammation. J Periodontol. 1974;45:151–154. doi: 10.1902/jop.1974.45.3.151. [DOI] [PubMed] [Google Scholar]
- 8.Pjetursson BE, Sailer I, Zwahlen M, Hammerle CH. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part I. Single crowns. Clin Oral Implants Res. 2007;18(Suppl 3):73–85. doi: 10.1111/j.1600-0501.2007.01467.x. [DOI] [PubMed] [Google Scholar]
- 9.Walton TR. A 10-year longitudinal study of fixed prosthodontics: clinical characteristics and outcome of single-unit metal-ceramic crowns. Int J Prosthodont. 1999;12:519–526. [PubMed] [Google Scholar]
- 10.De Backer H, Van Maele G, De Moor N, Van den Berghe L, De Boever J. An 18-year retrospective survival study of full crowns with or without posts. Int J Prosthodont. 2006;19:136–142. [PubMed] [Google Scholar]
- 11.Heintze SD, Rousson V. Fracture rates of IPS Empress all-ceramic crowns—a systematic review. Int J Prosthodont. 2010;23:129–133. [PubMed] [Google Scholar]
- 12.Della Bona A, Kelly JR. The clinical success of all-ceramic restorations. J Am Dent Assoc. 2008;139(Suppl):8S–13S. doi: 10.14219/jada.archive.2008.0361. [DOI] [PubMed] [Google Scholar]
- 13.Quality Evaluation for Dental Care. Guidelines for the Assessment of Clinical Quality and Professional Performance. Los Angeles: California Dental Association; 1977. [Google Scholar]
- 14.Esquivel-Upshaw JF, Rose WF, Jr, Barrett AA, Oliveira ER, Yang MC, Clark AE, Anusavice KJ. Three years in vivo wear: core-ceramic, veneers, and enamel antagonists. Dent Mater. 2012;28:615–621. doi: 10.1016/j.dental.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva NR, Thompson VP, Valverde GB, Coelho PG, Powers JM, Farah JW, Esquivel-Upshaw JF. Comparative reliability analyses of zirconium oxide and lithium disilicate restorations in vitro and in vivo. J Am Dent Assoc. 2011;142(Suppl 2):4S–9S. doi: 10.14219/jada.archive.2011.0336. [DOI] [PubMed] [Google Scholar]
- 16.Lambrechts P, Braem M, Vuylsteke-Wauters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Res. 1989;68:1752–1754. doi: 10.1177/00220345890680120601. [DOI] [PubMed] [Google Scholar]
- 17.Anusavice KJ. Degradability of dental ceramics. Adv Dent Res. 1992;6:82–89. doi: 10.1177/08959374920060012201. [DOI] [PubMed] [Google Scholar]
- 18.Anusavice KJ, Zhang NZ. Chemical durability of Dicor and fluorocanasite-based glass–ceramics. J Dent Res. 1998;77:1553–1559. doi: 10.1177/00220345980770071101. [DOI] [PubMed] [Google Scholar]
- 19.Milleding P, Haraldsson C, Karlsson S. Ion leaching from dental ceramics during static in vitro corrosion testing. J Biomed Mater Res. 2002;61:541–550. doi: 10.1002/jbm.10109. [DOI] [PubMed] [Google Scholar]
- 20.Milleding P, Karlsson S, Nyborg L. On the surface elemental composition of non-corroded and corroded dental ceramic materials in vitro. J Mater Sci Mater Med. 2003;14:557–566. doi: 10.1023/a:1023416232222. [DOI] [PubMed] [Google Scholar]
- 21.Ccahuana VZ, Ozcan M, Mesquita AM, Nishioka RS, Kimpara ET, Bottino MA. Surface degradation of glass ceramics after exposure to acidulated phosphate fluoride. J Appl Oral Sci. 2010;18:155–165. doi: 10.1590/S1678-77572010000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etman MK, Woolford MJ. Three-year clinical evaluation of two ceramic crown systems: a preliminary study. J Prosthet Dent. 2010;103:80–90. doi: 10.1016/S0022-3913(10)60010-8. [DOI] [PubMed] [Google Scholar]