Abstract
Neuronal voltage-gated calcium channels generate rapid, transient intracellular calcium signals in response to membrane depolarization. Neuronal CaV channels regulate a range of cellular functions and are implicated in a variety of neurological and psychiatric diseases including epilepsy, Parkinson’s disease, chronic pain, schizophrenia, and bipolar disorder. Each mammalian Cacna1 gene has the potential to generate tens to thousands of CaV channels by alternative pre-mRNA splicing, a process that adds fine granulation to the pool of CaV channel structures and functions. The precise composition of CaV channel splice isoform mRNAs expressed in each cell are controlled by cell-specific splicing factors. The activity of splicing factors are in turn regulated by molecules that encode various cellular features, including cell-type, activity, metabolic states, developmental state, and other factors. The cellular and behavioral consequences of individual sites of CaV splice isoforms are being elucidated, as are the cell-specific splicing factors that control splice isoform selection. Altered patterns of alternative splicing of CaV pre-mRNAs can alter behavior in subtle but measurable ways, with the potential to influence drug efficacy and disease severity. This article is part of a Special Issue entitled: Calcium channels.
Keywords: Splicing factor, Disease, Chronic pain, Morphine, Synaptic transmission, G protein coupled receptor, Mu-opioid receptor
1. Introduction
Voltage-gated calcium channel (CaV) currents originate from the activity of multiple classes of channels with different pharmacological sensitivities and unique functional properties. Neuronal CaV channels are implicated in a variety of neurological and psychiatric diseases including epilepsy, Parkinson’s disease, chronic pain, schizophrenia and bipolar disorder [1–9]. Relatively large differences in the voltage-dependence of channel activation between CaV3 (T-type) channels on the one hand, and CaV1 (L-type) and CaV2 (N, P/Q, R-types) channels on the other offered the first clues that many cells expressed more than one type of voltage-gated calcium channel [10–12]. Genome and transcriptome sequencing showed that mammalian neurons can express up to 9 of 10 different CaV α1 subunit genes [13,14]. Low threshold activation and slow deactivation characteristics are features of all CaV3 channels that set them apart from CaV1 and CaV2 family members [15].
With the exception of the skeletal muscle CaV1.1 that functions primarily as a voltage sensor, the unifying function of CaV channels is rapid control of voltage-dependent calcium entry [16,17]. Calcium entering through different CaV channels activates distinct signaling pathways depending on the unique sub-cellular localization, protein associations, and functional properties of CaV channels [13]. Thus, certain CaV channels are commonly associated with certain cellular functions, but new roles for CaV channels continue to be discovered. CaV1 channels expressed in neurons (encoded by Cacna1c, Cacna1d, and Cacna1f genes) support a range of calcium-dependent processes including modulation of gene expression, long and short-term changes in synaptic plasticity (CaV1.2, CaV1.3) [18–20], transmitter release at sensory nerve terminals (CaV1.3, CaV1.4) [21,22], and intrinsic spiking (CaV1.3) [23]. CaV2 channels (encoded by Cacna1a, Cacna1b, and Cacna1e genes) are primarily located at presynaptic nerve terminals where they control voltage-dependent calcium entry that triggers transmitter release [24,25]. CaV3 channels (Cacna1g, Cacna1h, Cacna1i genes) underlie pacemaking in many neurons including thalamic relay neurons [4,26].
This review is focused on the even finer granulation of structural and functional diversity among CaV channels that originates from each major CaV channel gene. Distinct CaV channels expressed in a given cell, distinguished by relatively small discrete differences in amino acid sequence, may number in the tens to hundreds depending on the extent of alternative pre-mRNA splicing [15,27]. Several sites of alternative splicing are present in Cacna1 genes predicting variations in amino acid sequence (e.g. see Fig. 1 for Cacna1b). Assuming each exon is regulated independent of the others, the number of discrete mRNA isoforms possible from each Cacna1 gene is potentially staggering (2N for N sites of alternative splicing). Analyses of different brain regions at different stages of development show that the composition of the pool of CaV mRNA splice isoforms varies with cell-type, state of development, and possibly neuronal activity [15,28]. This suggests that anticipated subtle functional differences among splice isoforms within a given CaV family are either individually or collectively contributing in important ways to neuronal processes. Pre-mRNA splicing is also implicated in many neurological diseases. Disease-causing mutations can disrupt splicing such as inherited frontotemporal dementia and Parkinsonism linked to chromosome 17, amyotrophic lateral sclerosis, spinocerebellar ataxia 8, and myotonic dystrophy [29]. Alternative splicing can play a role in modifying disease such as in the case of Timothy syndrome [30]. The cellular and behavioral consequences of only a few CaV splice isoforms are known (discussed below), but this should change as new methods are developed.
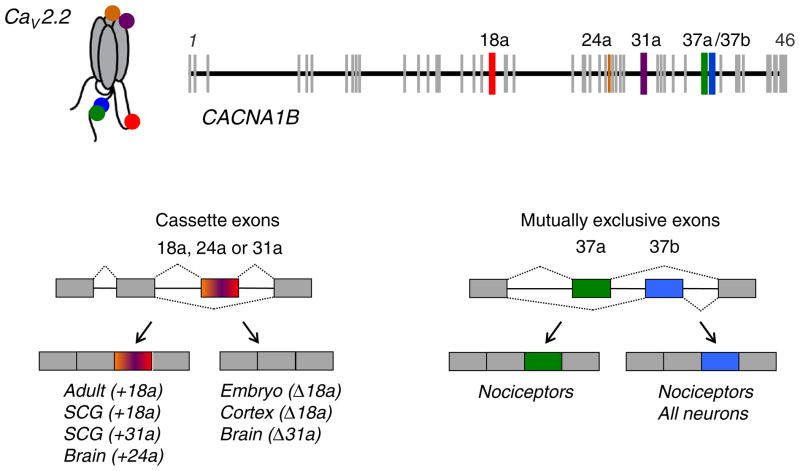
Fig. 1.
Alternatively spliced exons in Cacna1b and patterns of expression. Cacna1b pre-mRNA undergoes extensive alternative splicing generating 10 s to 100 s of unique CaV2.2 proteins in neurons. Alternatively spliced exons e18a, e24a, and e31a are cassette exons. E37a and e37b are mutually exclusive exons. Alternatively spliced exons are expressed in different regions of the nervous system, in different types of cells, and at different stages of development. Examples of different expression patterns of splice isoforms are shown for embryonic and adult brain, superior cervical ganglia (SCG), cortex, and nociceptors. The tissue distribution and functional consequences of these exons on CaV2.2 channel properties have been described in a series of publications [15,28,85,86,88,119,120].
1.1. Alternative pre-mRNA splicing
Alternative pre-mRNA splicing is particularly prevalent in the mammalian brain [31,32], consistent with the theory that alternative splicing evolved in parallel with biological complexity [33]. Alternative splicing is essential for normal neuronal development, axon targeting, neuronal excitability, and neural circuit formation [27,34–38]. Several excellent reviews outline the molecular interactions involved in alternative pre-mRNA splicing [39–42]. In brief, this form of pre-mRNA processing occurs in the cell’s nucleus and it is controlled by the concerted actions of cell-specific splicing factors (SFs). These cell-specific SFs bind to consensus motifs on pre-mRNAs and influence the action of the spliceosome by promoting or repressing inclusion of alternatively spliced exons, and promoting or repressing the use of alternative splice acceptor or donor sites at intron/exon boundaries. The cell-specific features of alternative pre-mRNA splicing are controlled by the collective action of cell-specific SFs that bind to elements encoded in each gene [40,43–49].
SFs known to control alternative pre-mRNA splicing in neurons include Nova1/2 [45], nPTB [50–52], rbFox1/2/3 [53–55] and SF2/AF [56]. Networks of genes targeted by specific SFs have been generated from genome-wide analyses of pre-mRNAs that bind them. Based on data from these types of studies, certain SFs are shown to associate with genes that control particular aspects of neuronal function. For example, Nova appears to preferentially regulate alternative splicing of pre-mRNAs encoding proteins found at inhibitory synapses [45,57,58]. Current knowledge of SFs that regulate alternative splicing of CaV pre-mRNAs is summarized below.
1.2. Alternative splicing and voltage-gated calcium channels
The large number of CaV channel splice isoforms expressed in the nervous system, combined with their distinct expression patterns according to region and cell type, is consistent with the remarkable range of neuronal functions and behaviors regulated by CaV channels. Experiments aimed at establishing the contributions of CaV channel splice isoforms to neuronal function have been hindered by the lack of isoform-specific tools to tease their contributions apart at the protein level. Nonetheless, experimental approaches that selectively target mRNA splice isoforms and that directly modify alternatively spliced exons at the gene level have proven valuable. Similarly, knowledge of cell-specific expression patterns of splice isoform mRNAs gives important clues about function (see below).
Sites of alternative splicing in Cacna1 genes are typically, although not exclusively, located in regions encoding hyper-variable domains of CaV proteins that presumably can accommodate changes in protein structures. These include the intracellular C-termini and the II–III intracellular linker (see Fig. 1 and see [59]). The location of certain alternatively spliced exons is conserved among Cacna1 genes. For example, all but one Cacna1 gene contain an alternatively spliced exon that encodes a peptide in the putative extracellular linker between transmembrane spanning helices S3 and S4 in domain IV of CaV channels. In some genes such as Cacna1a and Cacna1b, a homologous alternatively spliced exon also encodes a short peptide sequence in the S3–S4 linker of domain III [58,60,61]. The composition and/or length of the S3–S4 extracellular linker exons influences voltage-dependent gating of CaV channels, perhaps unsurprisingly given their proximity to putative S4 voltage-sensors [62,63] (Fig. 2). The modulation of CaV channel activation induced by these exons can be relatively small (5–7 mV) but such changes can significantly impact the total movement of calcium during action potential-like stimuli [62]. Thus, different CaV splice isoforms might be selected to fine-tune the coupling efficacy between membrane depolarization and calcium entry according to cell needs (Fig. 2). CaV1.1 channel splice isoforms with different IVS3–IVS4 linkers are also expressed in the skeletal muscle and these originate from different mRNAs that either contain or lack exon 29 of Cacna1s. The major form of CaV1.1 early in muscle development lacks e29, but it is included with greater frequency during pre-mRNA splicing with maturation [64]. CaV1.1 splice isoforms lacking e29a generate larger currents that activate at more negative voltages compared to clones that contain e29a [64].
Fig. 2.
The potential impact of alternative splicing of CaV channels on the voltage range over which each channel subtype can operate. Boltzmann activation curves are plotted using V1/2 and k values from the literature from recordings using 1–2 mM calcium as charge carrier, except for data for CaV1.1 obtained from recordings using 10 mM calcium. A, CaV1.1 (V1/2 =6.2 mV, k=5.3 mV; [121]), CaV1.2 (V1/2 = −17 mV, k=8 mV; [122]), CaV1.3 (V1/2 = −36 mV, k=8 mV; [123,124]), CaV2.2 (V1/2 = −0.1 mV, k=7.5 mV; [125]), and CaV3.1 (CaV3.1, V1/2 = −46 mV, k=4.11; [126]). B, Alternative splicing of exons can modify voltage-dependence of activation and this will increase the operating voltage range for each CaV channel family. Approximate locations of regions encoded by alternatively spliced exons that influence the voltage-dependence of channel activation are shown. CaV2.2 data are from [125]; CaV1.1 data are from [64]; and CaV3.1 data are from [127]. CaV1.2 [128] and CaV1.3 [67,129] isoforms that have different activation properties were compared using 11 mM barium as charge carrier, this will shift the voltage-dependence of activation relative to recordings with 2 mM Ca. For comparison with other data, we illustrate an approximate operating range for CaV1.2 and CaV1.3 (dotted lines) based on the different V1/2 values from [67,128,129] but with reference to the activation curves shown in A obtained with 2 mM Ca.
Regardless of their location relative to the voltage sensors and putative gating domains, many alternatively spliced Cacna1 exons impact CaV channel gating. For example, alternatively spliced cassette exons encoding peptide sequences and the use of alternative splice junctions in intracellular regions of CaV2.2, CaV1.2, and CaV1.3 channels influence the voltage dependence of channel activation and inactivation, channel gating kinetics, and calcium-dependent inactivation [65–69]. The different C-termini splice isoforms of CaV1.3 channels have distinct tissue distributions with a short C-terminus isoform devoid of tonic inhibitory control exerted by full length C-termini [68]. This short C-terminus isoform is expressed in the brain but not in the heart and passes substantially more current during short burst action potential-like stimuli [68].
1.3. Cell-specific factors that control alternative splicing of exons of Cacna1 genes
Comparative analyses of CaV mRNAs expressed in different regions of the nervous system provide strong evidence of cell-specific and development-specific splicing events [15,27,28,70–72]. Splicing factors bind their unique motifs which are often located in introns that flank target AS exons, but these motifs can also exist within the target exon itself [73]. SF binding location relative to the target exon is often predictive of the overall impact of the SF. For example, Nova and rbFox proteins typically repress exon inclusion when they bind their respective consensus sequences upstream of the target exon, and typically promote exon inclusion when they bind downstream of the target exon [74,75]. The coordinated expression and/or activity of available SFs determines the composition of the pool of CaV channel isoforms in a given cell type [27]. Splicing factors are in turn regulated by cell-specific molecules including miRNAs [76], kinases, and phosphatases [77,78]. The potential exists to approximate which CaV mRNA isoforms are expressed when, and in which cell-type, based on SF binding sites. Cacna1-derived sequences are present in recent genome-wide, high throughout sequence analyses of the sites of splicing factor binding, and certain SF binding sites have been functionally validated. For example Nova-2, a brain-specific splicing factor, binds Cacna1b and Cacna1a pre-mRNAs to promote inclusion of e24a and repression of e31a thereby influencing the composition of both CaV2.1 and CaV2.2 mRNAs [58,60]. E24a and e31a encode short peptide sequences in the IIIS3–IIIS4 and IVS3–IVS4 linkers, respectively, and as discussed above, they modulate the voltage-dependence of CaV2 channel activation (Fig. 2). RbFox proteins, a family of splicing factors implicated in the control of alternative splicing during development, were recently shown to repress e9* and promote inclusion of e33 of Cacna1c, influencing the composition of CaV1.2 mRNAs expressed in the cortex [79,80]. At a different splice site, polypyrimidine tract-binding protein (PTB) and its neuronal homolog (nPTB) control the developmental switch from exon 8 to exon 8a-containing CaV1.2 mRNAs in neurons [81]. Many more cell-specific splicing events involving CaV pre-mRNAs are described for which the corresponding SFs have not yet been identified. Identifying these SFs is necessary to understand how cells control CaV calcium signaling.
1.4. Mutually exclusive splicing
The alternatively spliced exons that encode short peptide sequences in the S3–S4 linkers of CaV channels are cassette exons, they are either included or excluded during pre-mRNA processing. In either form, exon included or skipped, the reading frames of the resultant mRNAs are typically preserved and translated into functional CaV isoforms. An exception to this involves cassette exons that shift the reading frame, when included or when excluded, leading to non-functional proteins. This form of alternative splicing regulates expression levels of critical proteins, including SFs, and is known to be critical for early cellular differentiation [82]. By contrast, mutually exclusive splicing involves the selection of one of a pair of exons that encode slightly different sequences. In mutually exclusive splicing, the mechanism of exon selection must incorporate a form of steric hindrance to ensure exon selection is strictly mutually exclusive. If neither one of the pair of mutually exclusive exons e37a and e37b of Cacna1b is included during pre-mRNA splicing, this results in a frame shift which creates an early stop and a non-functional truncated CaV protein [66]. The same is true for the homologous exons e37a and e37b of Cacna1a [83,84] and e8 and e8a of Cacna1c [81]. Thus, aberrant splicing involving mutually exclusive exons is expected to be deleterious resulting in non-functional protein.
The splicing factors that regulate mutually exclusive splicing of e37a and e37b of Cacna1b and Cacna1a are not known. E37a of Cacna1b is expressed in a limited number of cell types including nociceptors, whereas 37b is abundant throughout the nervous system [28,85]. Several mechanisms could explain the observed cell-specific expression pattern but all involve cell-specific splicing factors. Based on our analyses of CaV2.2 mRNAs expressed in dorsal root ganglia and brain of mice that contain either tandem e37a or tandem e37b exons, we favor a mechanism involving a splicing repressor that binds to e37a [86]. We predict this putative splicing repressor is expressed in most neurons but is absent or at low levels in nociceptors and other neurons that express e37a-contaiing CaV2.2 mRNAs (Fig. 3).
Fig. 3.
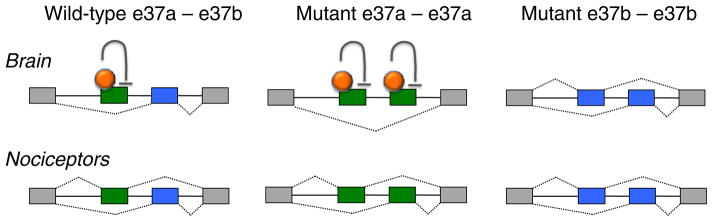
Theoretical model showing how the action of splicing factors might regulate the expression of e37a in different neurons. A putative splicing repressor (orange) binds to e37a sequence in CaV2.2 pre-mRNA preventing its inclusion during alternative pre-mRNA splicing. The putative splicing repressor is expressed in most neurons except in nociceptors and other neurons that express e37a. In the absence of the splicing repressor, e37a and e37b are equally likely to be included during pre-mRNA splicing. The model is based on data published in [86]. When e37a is moved to the e37b position, e37a is repressed consistent with the presence of an exonic repressor element [86]. When e37b is moved to the e37a position, it is expressed at wild-type levels inconsistent with the presence of a repressor element that resides exclusively in the intron [86].
1.5. Functional consequences of alternative splicing
Alternatively spliced exons are present in >95% of multi-exon genes [87] arguing that cellular control over exon selection must play a critical role in normal development and cell function [43]. By definition, cell-specific splicing events occur in a limited population of cells and tissues making it difficult to locate the products and, most of the time, tools such as antibodies and modulators to selectively identify and specifically control the activity of individual splice isoforms do not exist. The enrichment of the mutually exclusive e37a of Cacna1b in capsaicin-responsive nociceptors of dorsal root ganglia therefore offered a unique opportunity to assess its functional importance. Several other features of this system, as well as knowledge of the functional differences between e37a and e37b CaV2.2 splice isoforms, were advantageous. Among these features are: 1) A measurable behavior that is relatively easy to isolate and characterize; nociceptors have a well-defined role in detection of thermal stimuli and standard methods exist to monitor thermal responsiveness in vivo. 2) CaV2.2 channels dominate in controlling calcium entry to trigger glutamate release from presynaptic terminals of nociceptors in the spinal dorsal horn. 3) Several neurotransmitters and drugs down regulate synaptic transmission by inhibiting the gating of presynaptic CaV2.2 channels at nociceptor terminals through G protein coupled receptor activation. 4) E37a promotes Gi/o protein coupled receptor inhibition of CaV2.2 by a mechanism that persists independent of the stimulus. This pathway contrasts with Gi/o protein coupled receptor inhibition of e37b CaV2.2 channel isoforms that is reversed by membrane depolarization. Two complementary approaches were used to assess the functional importance of e37a in vivo [88].
1.6. Behavioral significance of e37a of Cacna1b
Without tools to selectively inhibit e37a-CaV2.2 and to leave e37b-CaV2.2 channels unaffected, isoform-specific siRNAs were used to reduce mRNA levels and protein levels in vivo [88]. Behavior analyses of thermal and mechanical thresholds in mice, following intrathecal application of isoform-selective siRNAs, suggested that e37a-CaV2.2 channels preferentially participate in the transmission of basal thermal nociception. E37a-CaV2.2 channels also mediated thermal hyperalgesia that accompanied peripheral nerve injury based on the complete reversal of thermal withdrawal thresholds in mice injected with siRNAs targeting e37a-CaV2.2 mRNAs [88]. This study showed that e37a-CaV2.2 splice isoforms are targeted to nociceptors nerve terminals and that they have a preferred role in mediating synaptic transmission in thermosensing.
An alternative experimental strategy was needed to establish if functional differences in Gi/o protein inhibition between e37 CaV2.2 splice isoforms impact behavior. In order to do this, e37a in the mouse Cacna1b gene was replaced with a second copy of e37b pre-serving wild-type CaV2.2 levels [86]. No obvious behavioral deficits were observed in e37a-null mice and withdrawal reaction times to thermal stimuli were not different from wild-type mice. However, in mice lacking e37a spinal morphine was a less effective analgesic to thermal stimuli compared to wild-type mice. This report offers evidence that disrupting an individual splicing event can impact animal behavior in subtle but measurable ways, in this case by altering the pharmacological efficacy of morphine as an analgesic [86].
1.7. Alternative pre-mRNA splicing, calcium channels, disease, and therapeutics
The most widely cited example of alternative splicing involves a gene critical in sex determination pathways in the fruit fly Drosophila melanogaster [89,90]. The splicing factor transformer (tra) controls alternative splicing of doublesex (dsx), the master controller of somatic sexual dimorphism in flies [91,92]. The consequences of aberrant splicing in the sex determination pathway are dramatic and global [93]; however, the majority of alternative pre-mRNA splicing events likely participate in delimited cellular processes.
Assessing the impact of such events on animal behavior, while more challenging, is potentially very relevant to processes that could contribute to neurological and psychiatric diseases [35,94–98]. In some cases altered splicing patterns for a particular gene or a subset of genes explain the pathophysiological manifestations of a disease such as cystic fibrosis [99,100]. In these cases, the presence of certain alternatively spliced mRNAs and proteins serves as disease biomarkers and, in the case of certain cancers, the presence of specific isoforms of MDM2, survivin-2B and CD44 defines cancer type [101]. In a very recent study, the loss of the splicing factor muscleblind-like protein 2 (Mbnl2) disrupts normal splicing of hundreds of exons and produces neurological symptoms similar to those that characterize human myotonic dystrophy [102]. Exon 12a of Cacna1d is among the list of affected splicing events in mice lacking Mbnl2 and, altered levels of e12a-containing CaV1.2 mRNAs are also observed in brains of human patients with myotonic dystrophy [102]. In humans, myotonic dystrophy is associated with abnormal CUG expansions in RNAs. A hypothesis presented in this study posits that CUG repeats sequester Mbln2, interfering with target exon splicing, and generating abnormal patterns of splice isoforms, including CaV1.2 mRNAs, in the nervous system.
In other cases, alternative splicing acts as a disease modifier. For example, mutually exclusive alternatively spliced exons e8 and e8a of human CACNA1C are mutated in two different types of Timothy’s syndrome respectively [30]. Because these exons are mutually exclusive, the effect of a gain-of-function mutation in one exon might be mitigated to some degree by the activity of the alternative wild-type exon in some tissues [30]. One of a pair of mutually exclusive exons, e8B, in CACNA1D is mutated in the human disorder SANDD syndrome and is associated with deafness and bradycardia [103]. Interestingly, the exons affected in both Timothy syndrome and SANDD map to homologous regions of CACNA1C and CACNA1D genes and encode homologous regions of CaV1.2 and CaV1.3 channels, respectively. Changes in the relative abundance of CaV1.2 channel splice isoforms have also been documented during cardiac disease [104]. Finally, spinocerebellar ataxia type 6 (SCA6) is associated with poly-glutamine repeats in the C-terminus of CaV2.1. The choice of an alternative 3′ acceptor during pre-mRNA splicing determines whether e47 of CACNA1A is in frame or out of frame resulting in an early stop and a truncated C-terminus. In patients with SCA6, inclusion of the complete e47 during splicing leads to CaV2.1 mRNAs containing expanded CAG repeats; the translation of these mRNAs generates CaV2.1 channels with poly-glutamine repeats that are responsible for the severity of SCA6 [65,105,106].
The role of alternative pre-mRNA splicing of CaV channels in disease, particularly as a disease modifier, adds credence to therapeutic strategies designed to target splicing factors [107–109] and specific splice isoforms [110,111]. For example, in Timothy’s syndrome, it might be possible to shift the balance of splicing toward the non-mutated exon by targeting the splicing factors that control exon selection or by knocking down the mutated splice isoforms that underlie the gain of function phenotype [30]. Isoform-specific antisense oligonucleotides and interference RNAs have been developed to knockdown mutated mRNA isoforms as a strategy to treat certain types of thalassemias and dystrophies, cystic fibrosis, cancer, and pain [88,112–115]. Other approaches to modify splicing events involve the use of bifunctional oligonucleotides. These hybrid molecules are designed to bind to specific regions of pre-mRNAs and to modify splicing via an antisense-targeting domain. For example, bifunctional oligonucleotides have been used to enhance e7 inclusion in SMN2 to reduce the severity of spinal muscular atrophy [116–118].
2. Concluding remarks
Cell-specific control of alternative pre-mRNA splicing is used to optimize depolarization-dependent calcium signaling in different parts of the nervous system, at different stages of development, and potentially depending on neuronal activity. Comparing unique functional characteristics among splice isoforms points to domains on CaV channels that regulate function. Identification of the cell-specific splicing factors that determine the composition of CaV splice isoforms is now needed. This information will be valuable not only to understand the coordinated expression of CaV splice isoforms and their relationship to other functionally related genes, but also to develop strategies to manipulate splicing patterns. The role that alternative splicing plays in disease particularly as a disease modifier suggests novel therapeutic approaches based on selectively down regulating disease causing isoforms and up-regulating isoforms that have therapeutic benefits. Continued technical advances in transcriptome analyses according to cell-type will greatly assist in identifying functionally relevant CaV channel splice isoforms, and ultimately their behavioral significance.
Acknowledgments
We are grateful for the funding from NIH grants NS055251 (D.L.) and F31NS066691 (SEA).
Footnotes
This article is part of a Special Issue entitled: Calcium channels.
References
- 1.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 2.Belardetti F, Zamponi GW. Linking calcium-channel isoforms to potential therapies. Curr Opin Investig Drugs. 2008;9:707–715. [PubMed] [Google Scholar]
- 3.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 4.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 5.Santi CM, Cayabyab FS, Sutton KG, McRory JE, Mezeyova J, Hamming KS, Parker D, Stea A, Snutch TP. Differential inhibition of T-type calcium channels by neuroleptics. J Neurosci. 2002;22:396–403. doi: 10.1523/JNEUROSCI.22-02-00396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Wellcome Trust Case Control, Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bear B, Asgian J, Termin A, Zimmermann N. Small molecules targeting sodium and calcium channels for neuropathic pain. Curr Opin Drug Discov Dev. 2009;12:543–561. [PubMed] [Google Scholar]
- 8.Norton RS, McDonough SI. Peptides targeting voltage-gated calcium channels. Curr Pharm Des. 2008;14:2480–2491. doi: 10.2174/138161208785777478. [DOI] [PubMed] [Google Scholar]
- 9.Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagiwara S, Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- 11.Lipscombe D, Raingo J. Alternative splicing matters: N-type calcium channels in nociceptors. Channels (Austin) 2007;1:225–227. doi: 10.4161/chan.4809. [DOI] [PubMed] [Google Scholar]
- 12.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 13.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Gray AC, Raingo J, Lipscombe D. Neuronal calcium channels: splicing for optimal performance. Cell Calcium. 2007;42:409–417. doi: 10.1016/j.ceca.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- 17.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 18.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 19.Weick JP, Groth RD, Isaksen AL, Mermelstein PG. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. Ca1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci. 2006;23:2297–2310. doi: 10.1111/j.1460-9568.2006.04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- 22.Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surmeier DJ, Mercer JN, Chan CS. Autonomous pacemakers in the basal ganglia: who needs excitatory synapses anyway? Curr Opin Neurobiol. 2005;15:312–318. doi: 10.1016/j.conb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 27.Lipscombe D. Neuronal proteins custom designed by alternative splicing. Curr Opin Neurobiol. 2005;15:358–363. doi: 10.1016/j.conb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 29.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Sugnet CW, Srinivasan K, Clark TA, O’Brien G, Cline MS, Wang H, Williams A, Kulp D, Blume JE, Haussler D, Ares M., Jr Unusual intron conservation near tissue-regulated exons found by splicing microarrays. PLoS Comput Biol. 2006;2:e4. doi: 10.1371/journal.pcbi.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo G, Holste D, Kreiman G, Burge CB. Variation in alternative splicing across human tissues. Genome Biol. 2004;5:R74. doi: 10.1186/gb-2004-5-10-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nat Rev Genet. 2010;11:345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 34.Dredge BK, Polydorides AD, Darnell RB. The splice of life: alternative splicing and neurological disease. Nat Rev Neurosci. 2001;2:43–50. doi: 10.1038/35049061. [DOI] [PubMed] [Google Scholar]
- 35.Licatalosi DD, Darnell RB. Splicing regulation in neurologic disease. Neuron. 2006;52:93–101. doi: 10.1016/j.neuron.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, Black DL. Maps, codes, and sequence elements: can we predict the protein output from an alternatively spliced locus? Neuron. 2006;52:574–576. doi: 10.1016/j.neuron.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Ule J, Darnell RB. RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr Opin Neurobiol. 2006;16:102–110. doi: 10.1016/j.conb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Zipursky SL, Wojtowicz WM, Hattori D. Got diversity? Wiring the fly brain with Dscam. Trends Biochem Sci. 2006;31:581–588. doi: 10.1016/j.tibs.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Kalsotra A, Cooper TA. Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet. 2011;12:715–729. doi: 10.1038/nrg3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabowski P. Alternative splicing takes shape during neuronal development. Curr Opin Genet Dev. 2011;21:388–394. doi: 10.1016/j.gde.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Luco RF, Misteli T. More than a splicing code: integrating the role of RNA, chromatin and non-coding RNA in alternative splicing regulation. Curr Opin Genet Dev. 2011;21:366–372. doi: 10.1016/j.gde.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caceres JF, Kornblihtt AR. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 2002;18:186–193. doi: 10.1016/s0168-9525(01)02626-9. [DOI] [PubMed] [Google Scholar]
- 45.Jensen KB, Dredge BK, Stefani G, Zhong R, Buckanovich RJ, Okano HJ, Yang YY, Darnell RB. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron. 2000;25:359–371. doi: 10.1016/s0896-6273(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 46.Lin WH, Gunay C, Marley R, Prinz AA, Baines RA. Activity-dependent alternative splicing increases persistent sodium current and promotes seizure. J Neurosci. 2012;32:7267–7277. doi: 10.1523/JNEUROSCI.6042-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daoud R, Da Penha Berzaghi M, Siedler F, Hubener M, Stamm S. Activity-dependent regulation of alternative splicing patterns in the rat brain. Eur J Neurosci. 1999;11:788–802. doi: 10.1046/j.1460-9568.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 48.Iijima T, Wu K, Witte H, Hanno-Iijima Y, Glatter T, Richard S, Scheiffele P. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147:1601–1614. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An P, Grabowski PJ. Exon silencing by UAGG motifs in response to neuronal excitation. PLoS Biol. 2007;5:e36. doi: 10.1371/journal.pbio.0050036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gil A, Sharp PA, Jamison SF, Garcia-Blanco MA. Characterization of cDNAs encoding the polypyrimidine tract-binding protein. Genes Dev. 1991;5:1224–1236. doi: 10.1101/gad.5.7.1224. [DOI] [PubMed] [Google Scholar]
- 51.Patton JG, Mayer SA, Tempst P, Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- 52.Kikuchi T, Ichikawa M, Arai J, Tateiwa H, Fu L, Higuchi K, Yoshimura N. Molecular cloning and characterization of a new neuron-specific homologue of rat polypyrimidine tract binding protein. J Biochem. 2000;128:811–821. doi: 10.1093/oxfordjournals.jbchem.a022819. [DOI] [PubMed] [Google Scholar]
- 53.Jin Y, Suzuki H, Maegawa S, Endo H, Sugano S, Hashimoto K, Yasuda K, Inoue K. A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG. EMBO J. 2003;22:905–912. doi: 10.1093/emboj/cdg089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Zhang Z, Castle J, Sun S, Johnson J, Krainer AR, Zhang MQ. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev. 2008;22:2550–2563. doi: 10.1101/gad.1703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge H, Zuo P, Manley JL. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991;66:373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- 57.Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, Darnell JC, Darnell RB. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 59.Lipscombe D, Pan JQ, Gray AC. Functional diversity in neuronal voltage-gated calcium channels by alternative splicing of Ca(v)alpha1. Mol Neurobiol. 2002;26:21–44. doi: 10.1385/MN:26:1:021. [DOI] [PubMed] [Google Scholar]
- 60.Allen SE, Darnell RB, Lipscombe D. The neuronal splicing factor Nova controls alternative splicing in N-type and P-type CaV2 calcium channels. Channels (Austin) 2010;4:483–489. doi: 10.4161/chan.4.6.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ule J, Ule A, Spencer J, Williams A, Hu JS, Cline M, Wang H, Clark T, Fraser C, Ruggiu M, Zeeberg BR, Kane D, Weinstein JN, Blume J, Darnell RB. Nova regulates brain-specific splicing to shape the synapse. Nat Genet. 2005;37:844–852. doi: 10.1038/ng1610. [DOI] [PubMed] [Google Scholar]
- 62.Lin Z, Lin Y, Schorge S, Pan JQ, Beierlein M, Lipscombe D. Alternative splicing of a short cassette exon in alpha1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci. 1999;19:5322–5331. doi: 10.1523/JNEUROSCI.19-13-05322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakai J, Adams BA, Imoto K, Beam KG. Critical roles of the S3 segment and S3-S4 linker of repeat I in activation of L-type calcium channels. Proc Natl Acad Sci U S A. 1994;91:1014–1018. doi: 10.1073/pnas.91.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J. 2009;96:35–44. doi: 10.1016/j.bpj.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao P, Zhang HY, Soong TW. Alternative splicing of voltage-gated calcium channels: from molecular biology to disease. Pflugers Arch. 2009;458:481–487. doi: 10.1007/s00424-009-0635-5. [DOI] [PubMed] [Google Scholar]
- 66.Pan JQ, Lipscombe D. Alternative splicing in the cytoplasmic II–III loop of the N-type Ca channel alpha 1B subunit: functional differences are beta subunit-specific. J Neurosci. 2000;20:4769–4775. doi: 10.1523/JNEUROSCI.20-13-04769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y, Yu D, Hiel H, Liao P, Yue DT, Fuchs PA, Soong TW. Alternative splicing of the Ca(v)1.3 channel IQ domain, a molecular switch for Ca2+-dependent inactivation within auditory hair cells. J Neurosci. 2006;26:10690–10699. doi: 10.1523/JNEUROSCI.2093-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, Striessnig J, Koschak A. Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. J Biol Chem. 2011;286:42736–42748. doi: 10.1074/jbc.M111.269951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao P, Yu D, Lu S, Tang Z, Liang MC, Zeng S, Lin W, Soong TW. Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1.2 calcium channels. J Biol Chem. 2004;279:50329–50335. doi: 10.1074/jbc.M409436200. [DOI] [PubMed] [Google Scholar]
- 70.Vendel AC, Terry MD, Striegel AR, Iverson NM, Leuranguer V, Rithner CD, Lyons BA, Pickard GE, Tobet SA, Horne WA. Alternative splicing of the voltage-gated Ca2+ channel beta4 subunit creates a uniquely folded N-terminal protein binding domain with cell-specific expression in the cerebellar cortex. J Neurosci. 2006;26:2635–2644. doi: 10.1523/JNEUROSCI.0067-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diebold RJ, Koch WJ, Ellinor PT, Wang JJ, Muthuchamy M, Wieczorek DF, Schwartz A. Mutually exclusive exon splicing of the cardiac calcium channel alpha 1 subunit gene generates developmentally regulated isoforms in the rat heart. Proc Natl Acad Sci U S A. 1992;89:1497–1501. doi: 10.1073/pnas.89.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angelotti T, Hofmann F. Tissue-specific expression of splice variants of the mouse voltage-gated calcium channel alpha2/delta subunit. FEBS Lett. 1996;397:331–337. doi: 10.1016/s0014-5793(96)01205-7. [DOI] [PubMed] [Google Scholar]
- 73.Black DL, Grabowski PJ. Alternative pre-mRNA splicing and neuronal function. Prog Mol Subcell Biol. 2003;31:187–216. doi: 10.1007/978-3-662-09728-1_7. [DOI] [PubMed] [Google Scholar]
- 74.Dredge BK, Darnell RB. Nova regulates GABA(A) receptor gamma2 alternative splicing via a distal downstream UCAU-rich intronic splicing enhancer. Mol Cell Biol. 2003;23:4687–4700. doi: 10.1128/MCB.23.13.4687-4700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dredge BK, Stefani G, Engelhard CC, Darnell RB. Nova autoregulation reveals dual functions in neuronal splicing. EMBO J. 2005;24:1608–1620. doi: 10.1038/sj.emboj.7600630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin C, Manley JL. Cell signalling and the control of pre-mRNA splicing. Nat Rev Mol Cell Biol. 2004;5:727–738. doi: 10.1038/nrm1467. [DOI] [PubMed] [Google Scholar]
- 78.Heyd F, Lynch KW. Degrade, move, regroup: signaling control of splicing proteins. Trends Biochem Sci. 2011;36:397–404. doi: 10.1016/j.tibs.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Damianov A, Black DL. Autoregulation of Fox protein expression to produce dominant negative splicing factors. RNA. 2010;16:405–416. doi: 10.1261/rna.1838210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang ZZ, Zheng S, Nikolic J, Black DL. Developmental control of CaV1.2 L-type calcium channel splicing by Fox proteins. Mol Cell Biol. 2009;29:4757–4765. doi: 10.1128/MCB.00608-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang ZZ, Sharma S, Zheng S, Chawla G, Nikolic J, Black DL. Regulation of the mutually exclusive exons 8a and 8 in the CaV1.2 calcium channel transcript by polypyrimidine tract-binding protein. J Biol Chem. 2011;286:10007–10016. doi: 10.1074/jbc.M110.208116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fairbrother WG, Lipscombe D. Repressing the neuron within. Bioessays. 2008;30:1–4. doi: 10.1002/bies.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang SY, Yong TF, Yu CY, Liang MC, Pletnikova O, Troncoso J, Burgunder JM, Soong TW. Age and gender-dependent alternative splicing of P/Q-type calcium channel EF-hand. Neuroscience. 2007;145:1026–1036. doi: 10.1016/j.neuroscience.2006.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vigues S, Chabret C, Valentin S, Valmier J. Rat embryonic hippocampal neurons express a new class A calcium channel variant. Neurosci Lett. 1998;258:37–40. doi: 10.1016/s0304-3940(98)00842-8. [DOI] [PubMed] [Google Scholar]
- 85.Castiglioni AJ, Raingo J, Lipscombe D. Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels. J Physiol. 2006;576:119–134. doi: 10.1113/jphysiol.2006.115030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrade A, Denome S, Jiang YQ, Marangoudakis S, Lipscombe D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat Neurosci. 2010;13:1249–1256. doi: 10.1038/nn.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 88.Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagoshi RN, McKeown M, Burtis KC, Belote JM, Baker BS. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell. 1988;53:229–236. doi: 10.1016/0092-8674(88)90384-4. [DOI] [PubMed] [Google Scholar]
- 90.Bell LR, Horabin JI, Schedl P, Cline TW. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991;65:229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- 91.Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991;252:833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- 92.Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 93.Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987;50:739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X, Nicholls PJ, Laje G, Sotnikova TD, Gainetdinov RR, Albert PR, Rajkowska G, Stockmeier CA, Speer MC, Steffens DC, Austin MC, McMahon FJ, Krishnan KR, Garcia-Blanco MA, Caron MG. A functional alternative splicing mutation in human tryptophan hydroxylase-2. Mol Psychiatry. 2011;16:1169–1176. doi: 10.1038/mp.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, Law AJ. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proc Natl Acad Sci U S A. 2010;107:15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang GS, Kearney DL, De Biasi M, Taffet G, Cooper TA. Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy. J Clin Invest. 2007;117:2802–2811. doi: 10.1172/JCI32308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007;8:819–831. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 99.Chu CS, Trapnell BC, Curristin S, Cutting GR, Crystal RG. Genetic basis of variable exon 9 skipping in cystic fibrosis transmembrane conductance regulator mRNA. Nat Genet. 1993;3:151–156. doi: 10.1038/ng0293-151. [DOI] [PubMed] [Google Scholar]
- 100.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 101.Pajares MJ, Ezponda T, Catena R, Calvo A, Pio R, Montuenga LM. Alternative splicing: an emerging topic in molecular and clinical oncology. Lancet Oncol. 2007;8:349–357. doi: 10.1016/S1470-2045(07)70104-3. [DOI] [PubMed] [Google Scholar]
- 102.Charizanis K, Lee KY, Batra R, Goodwin M, Zhang C, Yuan Y, Shiue L, Cline M, Scotti MM, Xia G, Kumar A, Ashizawa T, Clark HB, Kimura T, Takahashi MP, Fujimura H, Jinnai K, Yoshikawa H, Gomes-Pereira M, Gourdon G, Sakai N, Nishino S, Foster TC, Ares M, Jr, Darnell RB, Swanson MS. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron. 2012;75:437–450. doi: 10.1016/j.neuron.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nurnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nurnberg P, Striessnig J, Bolz HJ. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 104.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 105.Ishikawa K, Tanaka H, Saito M, Ohkoshi N, Fujita T, Yoshizawa K, Ikeuchi T, Watanabe M, Hayashi A, Takiyama Y, Nishizawa M, Nakano I, Matsubayashi K, Miwa M, Shoji S, Kanazawa I, Tsuji S, Mizusawa H. Japanese families with autosomal dominant pure cerebellar ataxia map to chromosome 19p13.1–p13.2 and are strongly associated with mild CAG expansions in the spinocerebellar ataxia type 6 gene in chromosome 19p13.1. Am J Hum Genet. 1997;61:336–346. doi: 10.1086/514867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 107.Soret J, Gabut M, Tazi J. SR proteins as potential targets for therapy. Prog Mol Subcell Biol. 2006;44:65–87. doi: 10.1007/978-3-540-34449-0_4. [DOI] [PubMed] [Google Scholar]
- 108.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 109.Grosso AR, Martins S, Carmo-Fonseca M. The emerging role of splicing factors in cancer. EMBO Rep. 2008;9:1087–1093. doi: 10.1038/embor.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bracco L, Kearsey J. The relevance of alternative RNA splicing to pharmacogenomics. Trends Biotechnol. 2003;21:346–353. doi: 10.1016/S0167-7799(03)00146-X. [DOI] [PubMed] [Google Scholar]
- 111.Simmons DL. Variants of cyclooxygenase-1 and their roles in medicine. Thromb Res. 2003;110:265–268. doi: 10.1016/s0049-3848(03)00380-3. [DOI] [PubMed] [Google Scholar]
- 112.Sazani P, Kole R. Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J Clin Invest. 2003;112:481–486. doi: 10.1172/JCI19547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Forte A, Cipollaro M, Cascino A, Galderisi U. Small interfering RNAs and antisense oligonucleotides for treatment of neurological diseases. Curr Drug Targets. 2005;6:21–29. doi: 10.2174/1389450053344920. [DOI] [PubMed] [Google Scholar]
- 114.van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, de Kimpe SJ, Ekhart PF, Venneker EH, Platenburg GJ, Verschuuren JJ, van Ommen GJ. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 115.Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107:222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Owen N, Zhou H, Malygin AA, Sangha J, Smith LD, Muntoni F, Eperon IC. Design principles for bifunctional targeted oligonucleotide enhancers of splicing. Nucleic Acids Res. 2011;39:7194–7208. doi: 10.1093/nar/gkr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Skordis LA, Dunckley MG, Yue B, Eperon IC, Muntoni F. Bifunctional antisense oli-gonucleotides provide a trans-acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts. Proc Natl Acad Sci U S A. 2003;100:4114–4119. doi: 10.1073/pnas.0633863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cartegni L, Krainer AR. Correction of disease-associated exon skipping by synthetic exon-specific activators. Nat Struct Biol. 2003;10:120–125. doi: 10.1038/nsb887. [DOI] [PubMed] [Google Scholar]
- 119.Thaler C, Gray AC, Lipscombe D. Cumulative inactivation of N-type CaV2.2 calcium channels modified by alternative splicing. Proc Natl Acad Sci U S A. 2004;101:5675–5679. doi: 10.1073/pnas.0303402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat Neurosci. 2007;10:285–292. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dirksen RT, Beam KG. Single calcium channel behavior in native skeletal muscle. J Gen Physiol. 1995;105:227–247. doi: 10.1085/jgp.105.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu H, Marban E. Isoform-specific inhibition of L-type calcium channels by dihydropyridines is independent of isoform-specific gating properties. Mol Pharmacol. 1998;53:902–907. [PubMed] [Google Scholar]
- 123.Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 125.Lin Y, McDonough SI, Lipscombe D. Alternative splicing in the voltage-sensing region of N-Type CaV2.2 channels modulates channel kinetics. J Neurophysiol. 2004;92:2820–2830. doi: 10.1152/jn.00048.2004. [DOI] [PubMed] [Google Scholar]
- 126.Klockner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned alpha1 subunits, alpha1G, alpha1H and alpha1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 127.Emerick MC, Stein R, Kunze R, McNulty MM, Regan MR, Hanck DA, Agnew WS. Profiling the array of Ca(v)3.1 variants from the human T-type calcium channel gene CACNA1G: alternative structures, developmental expression, and biophysical variations. Proteins. 2006;64:320–342. doi: 10.1002/prot.20877. [DOI] [PubMed] [Google Scholar]
- 128.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem. 2004;279:44335–44343. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- 129.Safa P, Boulter J, Hales TG. Functional properties of Cav1.3 (alpha1D) L-type Ca2+ channel splice variants expressed by rat brain and neuroendocrine GH3 cells. J Biol Chem. 2001;276:38727–38737. doi: 10.1074/jbc.M103724200. [DOI] [PubMed] [Google Scholar]