Abstract
Objectives
• To quantify the impact of androgen deprivation therapy (ADT) in men with a high baseline risk of skeletal complications and evaluate the risk of mortality after a fracture.
Patients and methods
• We studied 75 994 men, aged ≥ 66 years, with localized prostate cancer from the Surveillance, Epidemiology and End Results–Medicare linked data.
• Cox proportional hazard models were employed to evaluate the risk.
Results
• Men with a high baseline risk of skeletal complications have a higher probability of receiving ADT than those with a low risk (52.1% vs 38.2%, P < 0.001).
• During the 12-year follow-up, more than 58% of men with a high risk and 38% of men with a low risk developed at least one fracture after ADT.
• The dose effect of ADT is stronger among men who received ADT only compared to those who received ADT with other treatments.
• In the high-risk group, the fracture rate increased by 19.9 per 1000 person-years (from 52.9 to 73.0 person-years) for men who did not receive ADT compared to those who received 18 or more doses of gonadotropin-releasing hormone agonist among men who received ADT only, and by 14.2 per 1000 person-years (from 45.2 to 59.4 person-years) among men who received ADT and other treatments.
• Men experiencing a fracture had a 1.38-fold higher overall mortality risk than those who did not (95% CI, 1.34–1.43).
Conclusions
• Men with a high baseline risk of skeletal complications developed more fractures after ADT.
• The mortality risk is 40% higher after experiencing a fracture.
• Consideration of patient risk before prescribing ADT for long-term use may reduce both fracture risk and fracture-associated mortality.
Keywords: ADT, baseline risk, fracture, prostate cancer
Introduction
Androgen deprivation therapy (ADT) has been used as a primary treatment for men with localized prostate cancer, despite limited evidence supporting a survival benefit [1]. Although the use of ADT has declined after 2005, one-third of men with newly-diagnosed prostate cancer continue to receive ADT within the first year of diagnosis every year [2].
Overall, older men with more adverse tumour features (i.e. higher Gleason grade, higher tumour stage and cancers associated with higher PSA levels) had a higher probability of receiving ADT [2]. However, many men with pre-existing health conditions receive ADT as their primary treatment because they are considered to be inappropriate candidates for attempted curative treatments (e.g. radical prostatectomy [RP] or radiation therapy [RT]).
The receipt of ADT has been associated with an increased risk of skeletal-associated complications [3–7], such as a decrease in bone mineral density [8] and an increase in fracture risk [4]. Additionally, several chronic health conditions, such as diabetes, rheumatoid disease and chronic liver disease, are strong predictors for osteoporosis and fractures [9,10]. However, it is unclear whether men who are have a high baseline risk for a skeletal complication have an even greater risk for fracture after ADT. The present study aimed to quantify the impact of treating men with ADT who carry known risk factors for skeletal complications. Furthermore, the study aimed to examine the risk of fractures associated with the long-term use of ADT in this population.
Patients and methods
Data source
The Surveillance, Epidemiology and End Results (SEER)–Medicare database links two population-based datasets together to provide information on patient demographics, tumour characteristics and treatment for incident cancer. The SEER regions encompass ≈25% of the US population [11]. Data were obtained from the most recent linkage for 1992–2007, for which a 93% match rate was achieved [11].
Study subjects
Data obtained from all men aged ≥66 years who were diagnosed as having localized prostate cancer from 1992 to 2007 were selected (n = 297 919). Patients who had any cancer diagnosis before their prostate cancer diagnosis (n = 31 791) were excluded. To ensure the completeness of claims data, we excluded patients who were not continuously enrolled in both Part A and Part B Medicare, as well as those who enrolled in health maintenance organizations 1 year after cancer diagnosis (n = 125 837). We further excluded men who had any ADT treatment before prostate cancer diagnosis and those who did not receive their first dose of ADT within 12 months after cancer diagnosis (n = 17 015). To avoid bias introduced by the survival time to the length of ADT [12], we adapted the landmark analysis and excluded patients who died within 48 months of cancer diagnosis (n = 47 282). After applying the exclusion criteria, 75 994 men with localized prostate cancer were included in the study cohort.
ADT
ADT is defined as medical (i.e. gonadotropin-releasing hormone [GnRH] agonists) or surgical (i.e. orchidectomy) castration, and includes the codes: Healthcare Common Procedure Coding System J9202, J9217, J9218, J9219, J9225, J1950, J3315; Common Procedure Terminology 54520, 54521, 54522, 54530, 54535; and International Classification of Diseases (9th Revision) (ICD-9) codes 62.3 and 62.4. We estimated the cumulative dose of GnRH agonist exposure by summing the number of 1-month equivalent doses [3,13,14]. For men with a fracture event, the cumulative dose was estimated from the beginning of the treatment until the date of the event. For men without fracture, the cumulative dose was estimated until 48 months. We stratified patients as ‘ADT only’ for those who received only ADT (primary ADT) within the first year of diagnosis and ‘ADT with other attempted curative treatment’ for those who received ADT as adjunctive treatment with RT or RP within the first year of diagnosis.
Patient characteristics and risk factors
Patient demographic and tumour characteristics were derived from the SEER data. The study population was divided into age cohorts: 66–69, 70–74, 75–79 and ≥80 years at cancer diagnosis. Clinical stage was categorized into T1c, other T1 (which included stages T1a and T1b) and T2 using the American Joint Committee on Cancer classification system [15,16]. For cancer grade, Gleason score 2– 4, 5–7 and 8–10 corresponded to well differentiated, moderately differentiated and poorly differentiated cancers, respectively. Education level, income, race and geographical location were obtained from SEER–Medicare linkages to the 2000 US census data.
A baseline risk factor index for skeletal complications was developed to summarize the occurrence of certain conditions within 12 months before cancer diagnosis: age ≥ 80 years, diabetes (ICD-9 code 250.x), alcoholism (codes 291.x and 303.x), cigarette smoking (code 305.1), rheumatoid disease (codes 714.81, 725, 710.0, 710.1, 710.4, 714.0–714.2), moderate–severe liver disease (codes 572.2–572.8 456.0–456.1, 456.2, 456.20, 456.21 ), paralysis (codes 342.x and 344.1) and a history of osteoporosis and fracture (codes 733.1, 800–829) [9,10]. We performed sensitivity analyses in men who carried different numbers of skeletal complications and found that the effect of ADT on the risk of fracture was similar among men who had more than one risk factor. As a result of the small sample size in men who had a higher number of skeletal complications, we grouped men who had one or more skeletal complications as the high-risk group and men who had no baseline skeletal complications as the low-risk group. We also adapted the approach of the Charlson score [17] and generated a co-morbidity index by totalling the occurrence of conditions that were not included in the risk factor index for skeletal complications to avoid over adjustment. The modified co-morbidity index included myocardial infarction, old myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease and renal disease. For both the baseline risk factor index for skeletal complications and other co-morbidities, we searched the inpatient, outpatient and physician files for any of the diagnoses in the 12 months before cancer diagnosis.
Use of intravenous bisphosphonates was ascertained from the Medicare claims. The Healthcare Common Procedure Coding System for pamidronate (J2430) and zoledronic acid (J3487) was used to identify patients who received at least one injection of the bisphosphonate pamidronate disodium or zoledronic from the inpatient, outpatient and physician files during the follow-up period.
Fractures and mortality
The study outcome was diagnosis of fracture. We used ICD-9 diagnosis codes to determine the variable of interests. Fracture cases were identified using an algorithm that has been reported previously [3]. The primary outcome of interest was the first fracture during the study period (ICD-9 codes 733.1, 800–829). Any fracture that occurred before ADT or within 1 year of diagnosis was not included in the analysis. We only included the first fracture claim in the analyses. The occurrence of fracture and overall survival in men diagnosed with prostate cancer were observed until 31 December 2009. The underlying cause of death was determined from SEER data. Cause of death in the SEER data confirmed the information available in the medical records in 87–88% of cases [18].
Statistical analysis
The proportion of men receiving ADT was calculated according to different baseline characteristics. The probability of the first fracture was estimated by cumulative incidence for all patients, as well as according to ADT treatment and the baseline risk of skeletal complications. The incidence of the first fracture was estimated as the number of total first claims for fracture divided by the number of person-years of observation per group. The incidence of fracture was estimated for patients by ADT dose and by patients’ status with or without other curative treatments in person-years (per 1000). Poisson models were used to model person-year rates of any fracture. A Cox proportional hazard model was used to analyze the data with time to fracture as the response variable. The Cox proportional hazard model for cause-specific hazard was used to assess the effects of covariates on time to fracture. Hazard ratios (HRs) were also obtained to compare the difference in doses of ADT in the skeletal risk stratum. Time zero is 1 year after diagnosis. The cumulative doses of ADT were treated as a covariate to examine any relationship with fracture risk. Finally, we used the landmark analysis method to examine the mortality risk associated with any fracture developed within the first 48 months, adjusting for other covariates, such as age at diagnosis, year of diagnosis, race, tumour grade and stage, risk factor index, other co-morbidities, ADT dose and curative treatment received within 1 year in a Cox proportional hazard model. Note that, using this method, patients who die before 48 months (i.e. the landmark time point) were excluded from the analysis. The Kaplan–Meier method was used to generate the survival curve by fracture. All analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC, USA) and R, version 2.13 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and the proportion of men receiving ADT according to the baseline risk of skeletal complications
Of 75 994 patients with prostate cancer diagnosed at the age of ≥ 66 years, 20 389 (26.83%) developed at least one fracture during the course of follow-up and 6687 (8.8%) required hospitalization. In addition, 4544 (22.3%) of these fractures comprised a hip fracture. Table 1 presents the baseline characteristics of patients according to their risk of fracture (low vs high). From the entire cohort, 25 488 (33.5%) men had at least one risk factor for a skeletal complication (high fracture risk) and 50 506 (66.9%) men had no risk factors for a skeletal complication (low fracture risk) in the 12 months before cancer diagnosis. The proportion of men receiving ADT within 1 year after cancer diagnosis was higher in the high-risk group than in the low-risk group (52.1% vs 38.2%, P < 0.001). Men diagnosed with prostate cancer at an older age, who were not white or black, who resided in the north-east area, who had higher staged or poorly differentiated cancers, who had other co-morbidities, and who received RT and bisphosphonate, had a higher probability of receiving ADT. However, men who received RP and were married had a lower probability of receiving ADT.
TABLE 1.
Distribution of baseline patient characteristics according to the risk of skeletal complications at baseline: Surveillance, Epidemiology and End Results (SEER)
| Variable | Baseline risk of skeletal complication
|
|||||
|---|---|---|---|---|---|---|
| Low | High | |||||
|
| ||||||
| n | % | Received ADT (%) | n | % | Received ADT (%) | |
| All | 50 506 | 38.2 | 25 488 | 52.1 | ||
| Age at diagnosis (years) | ||||||
| 65–69 | 13 729 | 27.2 | 28.8 | 3212 | 12.6 | 36.7 |
| 70–74 | 20 969 | 41.5 | 37.5 | 5450 | 21.4 | 45.4 |
| 75–79 | 15 808 | 31.3 | 47.3 | 4519 | 17.7 | 53.4 |
| ≥80 | – | – | – | 12 307 | 48.3 | 58.6 |
| Race | ||||||
| White | 43 062 | 85.3 | 37.4 | 20 760 | 81.5 | 51.8 |
| Black | 4266 | 8.5 | 40.5 | 2536 | 10.0 | 48.6 |
| Other | 3178 | 6.3 | 45.8 | 2192 | 8.6 | 58.8 |
| SEER regions | ||||||
| North Central | 11 703 | 23.2 | 34.1 | 6045 | 23.7 | 62.2 |
| North-east | 8725 | 17.3 | 52.6 | 4720 | 18.5 | 53.6 |
| South | 5905 | 11.7 | 40.2 | 2709 | 10.6 | 50.2 |
| West | 24 173 | 47.9 | 34.5 | 12 014 | 47.1 | 55.5 |
| Married at diagnosis | ||||||
| Yes | 37 649 | 74.5 | 37.3 | 17 011 | 66.7 | 50.5 |
| No | 12 857 | 25.5 | 40.8 | 8477 | 33.3 | 55.5 |
| Zip code level with college education (%)* | ||||||
| 1st quartile | 13 308 | 26.4 | 34.8 | 6464 | 25.4 | 50.2 |
| 2nd quartile | 12 722 | 25.2 | 37.1 | 6343 | 24.9 | 52.3 |
| 3rd quartile | 12 192 | 24.1 | 38.5 | 6315 | 24.8 | 50.8 |
| 4th quartile | 12 284 | 24.3 | 42.7 | 6366 | 25.0 | 55.3 |
| Zip code level income (US$)† | ||||||
| 1st quartile (≤ $35 917) | 11 452 | 22.7 | 40.8 | 6556 | 25.7 | 53.4 |
| 2nd quartile ($35 918– 46,816) | 12 618 | 25.0 | 37.5 | 6343 | 24.9 | 52.0 |
| 3rd quartile ($46 817– 60 978) | 12 794 | 25.3 | 37.2 | 6392 | 25.1 | 51.0 |
| 4th quartile (≥$60 979) | 13 642 | 27.0 | 37.5 | 6197 | 24.3 | 52.1 |
| Cancer grade (Gleason score) | ||||||
| Well differentiated | 3762 | 7.5 | 20.8 | 1850 | 7.3 | 25.6 |
| Moderately differentiated | 34 218 | 67.8 | 34.6 | 15 257 | 59.9 | 46.7 |
| Poorly differentiated | 10 936 | 21.7 | 56.6 | 7352 | 28.8 | 71.5 |
| Unknown | 1590 | 3.2 | 30.8 | 1029 | 4.0 | 41.3 |
| Cancer stage | ||||||
| T1c | 16 983 | 33.6 | 39.3 | 7496 | 29.4 | 53.6 |
| T1other | 3695 | 7.3 | 17.6 | 2823 | 11.1 | 21.7 |
| T2 | 29 828 | 59.1 | 40.1 | 15 169 | 59.5 | 57.1 |
| Other co-morbidities‡ | ||||||
| 0 | 45 148 | 89.4 | 37.4 | 20 416 | 80.1 | 51.7 |
| ≥1 | 5358 | 10.6 | 44.7 | 5072 | 19.9 | 53.9 |
| Received bisphosphonate therapy during follow-up | ||||||
| Yes | 908 | 1.8 | 79.5 | 625 | 2.5 | 51.2 |
| No | 49 598 | 98.2 | 37.4 | 24 863 | 97.6 | 88.6 |
| Radiation therapy within 1 year | ||||||
| Yes | 28 516 | 56.5 | 47.6 | 11 664 | 45.8 | 55.3 |
| No | 21 990 | 43.5 | 26.0 | 13 824 | 54.2 | 49.5 |
| Radical prostatectomy within 1 year | ||||||
| Yes | 12 256 | 56.5 | 14.3 | 2502 | 45.8 | 17.7 |
| No | 38 250 | 43.5 | 45.8 | 22 986 | 54.2 | 55.9 |
ADT, androgen-deprivation therapy.
Lived in ZIP code tabulation area in which 25% or more of adults had a college education.
Median annual income of ZIP code tabulation area.
Other co-morbidities include myocardial infarction, old myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease and renal disease.
Incidence of fracture
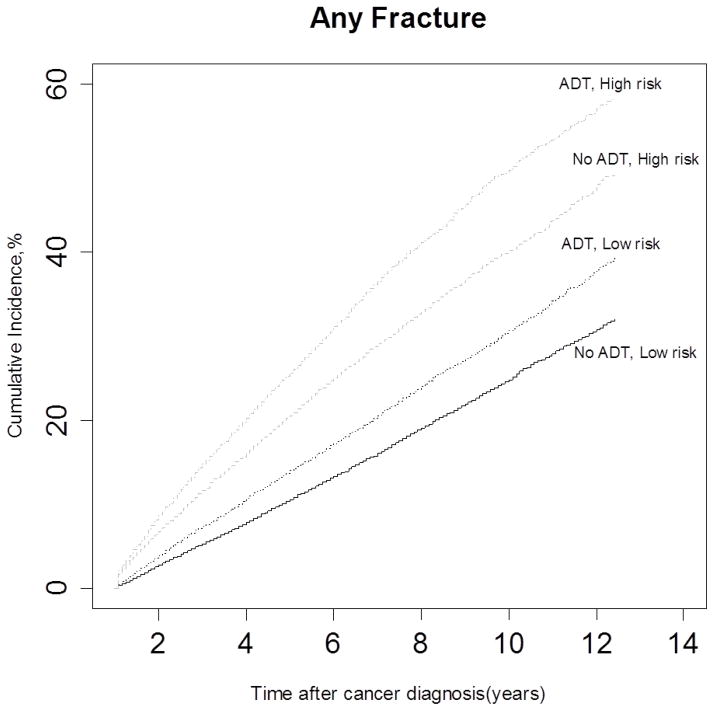
Estimates of cumulative incidence of fracture rate are presented in Fig. 1, according to fracture risk group and whether or not ADT was received. Not unexpectedly, men who with a at high baseline fracture risk and who received ADT within 1 year of cancer diagnosis had a higher incidence rate of fractures compared to other groups. During the 12-year follow-up, more than 58% of men in the high-risk group and 38% of men in the low-risk group developed a fracture, whereas 31% of men in the low-risk group that did not receive ADT sustained a fracture.
FIG. 1.
Unadjusted cumulative incidence of fractures among patients with prostate cancer, according to androgen-deprivation therapy (ADT) and the baseline risk of skeletal complications.
The incidence of fracture was estimated as the event rate per 1000 among the groups that did or did not receive ADT, and did or did not have attempted curative treatment, as well as the risk to fracture at baseline (Table 2) ADT was divided into those who underwent orchidectomy and those who received GnRH agonist treatment according to the number of doses received (1–5, 6–17 or ≥ 18). An increasing number of ADT doses was associated with a marked increase in the risk of fracture in all men. The absolute increases in fracture rate were particularly high among men who had ADT as their only treatment and had a high fracture risk at baseline. In the group who received ADT as their only treatment, the fracture rate increased by 32.9 per 1000 (from 52.9 to 85.8) for men who did not receive ADT compared to those who underwent orchidectomy in the high-risk group vs 28.5 per 1000 (from 28.9 to 57.4) in the low-risk group. In the group who had ADT along with RT or RP, the fracture rate increased by 14.8 per 1000 (from 45.2 to 60.0) for men who did not receive ADT compared to those who underwent orchidectomy in the high-risk group vs 15.8 per 1000 (from 25.9 to 41.7) in the low-risk group.
TABLE 2.
Fracture rate stratified by dose of androgen-deprivation therapy (ADT), attempted curative treatment and the baseline risk of skeletal complications
| Baseline risk ADT dose | Low risk | High risk | ||
|---|---|---|---|---|
|
| ||||
| Person years | Event rate per 1000 | Person years | Event rate per 1000 | |
| ADT only | ||||
| No ADT | 61 820 | 28.9 | 35 150 | 52.9 |
| 1–5 | 3274 | 35.4 | 2377 | 48.4 |
| 6–17 | 5544 | 30.3 | 5565 | 67.9 |
| ≥18* | 20 418 | 50.1 | 28 182 | 73.0 |
| Orchidectomy | 3870 | 57.4 | 4664 | 85.8 |
| ADT + attempted curative treatment† | ||||
| No ADT | 234 983 | 25.9 | 51 599 | 45.2 |
| 1–5 | 50 329 | 27.4 | 15 899 | 48.7 |
| 6–17 | 43 506 | 30.5 | 16 787 | 54.4 |
| ≥18* | 23 746 | 38.7 | 10 768 | 59.4 |
| Orchidectomy | 1630 | 41.7 | 550 | 60.0 |
The incidence rate of fracture was significantly associated with the risk of skeletal complications and the dose of ADT. Tests were examined by Poisson regression.
≥18 years: no more than 48 doses
Attempted curative treatments: men received radiation therapy or radical prostatectomy within 1 year of cancer diagnosis.
Fracture risk and ADT
The risk of fracture associated with an increasing ADT dose adjusted for other covariates is presented in Table 3. The HR of the occurrence of a fracture increased with the cumulative number of doses of a GnRH agonist received after prostate cancer diagnosis. After adjusting for the effect of other variables, the effect of ADT dose on fracture risk was stronger in men who had ADT as their only treatment compared to those who received ADT with other attempted curative treatments. Among men who received ADT only, the fracture risk (HR) of men receiving ≥ 18 doses of GnRH agonist was 1.53 (95% CI, 1.44–1.62) for the low-risk group and 1.27 (95% CI, 1.20–1.35) for the high-risk group compared to men who did not receive ADT. Among men who received ADT and other curative treatments, the fracture risk was 1.37 (95% CI, 1.27–1.49) for the low-risk group and 1.20 (95% CI, 1.09–1.33) for the high-risk group.
Table 3.
Adjusted hazard ratio (HR) of the risk of fracture associated with androgen deprivation therapy according to the baseline risk of skeletal complications and attempted curative treatment
| Baseline risk ADT dose | Low risk | High risk | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Adjusted HR | 95% CI | Adjusted HR | 95% CI | |||
| ADT only | ||||||
| No ADT | 1.00 | – | 1.00 | – | ||
| 1–5 | 1.08 | 1.02 | 1.15 | 1.08 | 1.00 | 1.17 |
| 6–17 | 1.18 | 1.11 | 1.26 | 1.28 | 1.19 | 1.36 |
| ≥18* | 1.53 | 1.44 | 1.62 | 1.27 | 1.20 | 1.35 |
| Orchidectomy | 1.64 | 1.45 | 1.87 | 1.52 | 1.36 | 1.70 |
| ADT + attempted curative treatment | ||||||
| No ADT | 1.00 | – | 1.00 | – | ||
| 1–5 | 1.03 | 0.97 | 1.10 | 1.05 | 0.96 | 1.15 |
| 6–17 | 1.15 | 1.07 | 1.22 | 1.17 | 1.08 | 1.27 |
| ≥18* | 1.37 | 1.27 | 1.49 | 1.20 | 1.09 | 1.33 |
| Orchidectomy | 1.37 | 1.07 | 1.76 | 1.40 | 1.00 | 1.96 |
HR was estimated from the Cox proportional hazard model. Other covariates included in the model were age at cancer diagnosis, diagnosis year, race, education, income, cancer grade, cancer stage, other co-morbidities, and the use of bisphosphonate therapy.
≥18 years: no more than 48 doses.
Mortality after a fracture
Fracture is associated with an increase in overall mortality. The mortality among men experiencing a fracture was 6.27% within 6 months and 9.87% within 12 months. Figure 2 presents the survival probability by fracture. Men who developed a fracture within 48 months of cancer diagnosis had a significantly lower survival than men who did not (log-rank test: P < 0.001). We also perform a Cox model to examine the risk of fracture with respect to overall mortality. Fracture was associated with a 1.38-fold increase in the rate of death (95% CI, 1.34–1.43), after adjusting for age at diagnosis, race, year of diagnosis, income, education, marital status, cancer stage, cancer grade, risk factor index, other co-morbidities, ADT dose and attempted curative treatments received within 1 year of diagnosis.
FIG. 2.
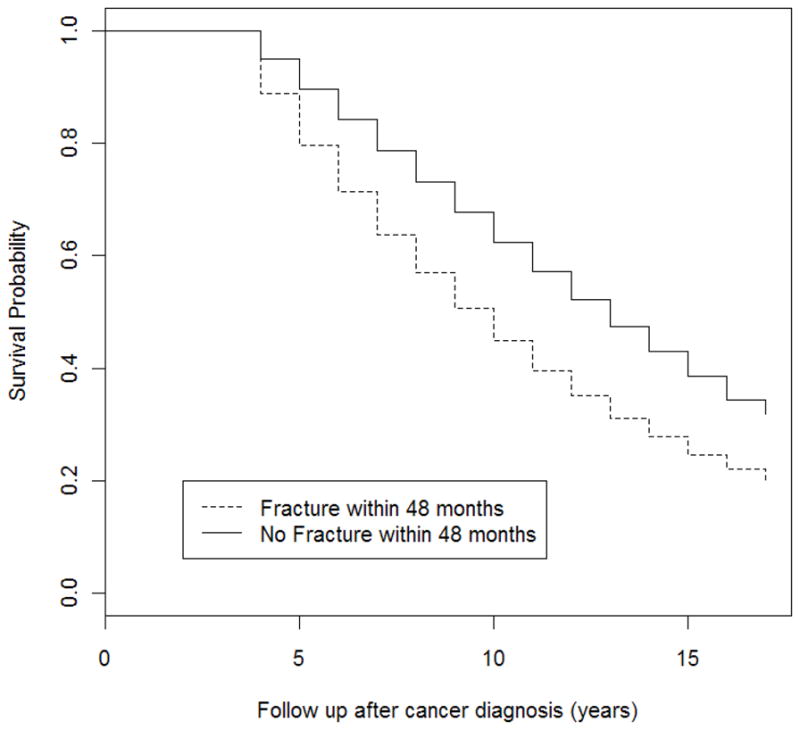
Survival among patients with prostate cancer, according to the status of fracture within 48 months of diagnosis. The survival curves start at 48 months after diagnosis. Men had to survive at least 48 months (i.e. the landmark time point) to be included in the present study. Note that this explains why the survival estimate remains at 1 for the first 48 months.
Discussion
In the present population-based cohort study, not unexpectedly, it was found that men with prostate cancer who had a higher fracture risk at baseline had a higher probability of receiving ADT than those who had at a lower fracture risk at baseline. Among these high-risk men, more than 58% develop at least one fracture after ADT within the 12 years of follow-up. Men who sustained a fracture within 48 months experienced an almost 40% higher risk of mortality than those who did not. Our findings suggest that treating men with a high fracture risk at baseline with long-term ADT may have serious adverse consequences. Because the use of ADT has not been shown to impact upon survival in most elderly men with localized prostate cancer, a consideration of a patient’s baseline risk of skeletal complications before initiating long-term ADT is crucial for preventing skeletal-associated complications and reducing mortality.
The present study identified a group of men with known risk factors for skeletal complications at prostate cancer diagnosis. To our knowledge, this is the first study to describe the use of ADT and its impact in men with high-risk skeletal complications. Men in the high-risk group had a higher probability of receiving ADT, with a resultant increase in the incidence of fracture. Furthermore, the long-term use of ADT is associated with a higher absolute increase in the incidence of fracture among prostate cancer men, particularly among men who received ADT as their only treatment. Men who received ADT in conjunction with primary RT or after RP appear to have a lower risk of fracture compared to those who received ADT only. This can be explained, at least in part, by the likelihood that men with more significant co-morbid conditions (including those with a higher baseline fracture risk) had a higher probability of receiving ADT only compared to men who were fit to undergo curative treatment with concomitant ADT. Finally, consistent with the findings reported in previous studies [19,20], we observed an increased risk of mortality after a fracture in the present study cohort. The results suggest that treating these men with long-term ADT may not be optimal.
Several limitations to the present study are worthy of note. First, studies have shown that bisphosphonates are effective in preventing bone loss in patients with prostate cancer who are receiving ADT [21]. However, as a result of the limitation of SEER–Medicare linked data, no information was available with respect to oral bisphosphonate usage. In the present study, we identified a total of 1533 men who used intravenous bisphosphonates, and more than 80% of these were given to men receiving ADT. We found that the risks of fracture among men who received bisphosphonate and those who did not were similar in the present study population. We performed an additional analysis excluding those patients who received bisphosphonate. The results from the population who did not receive bisphosphonate are not very different from the results that we report in the present study. Second, younger men aged < 65 years are not included in the SEER–Medicare database and therefore our findings may not be generalizable to the younger population. Finally, because the Medicare data are based on administrative claims, some risk factors for skeletal complications (e.g. height and weight) are not captured. Because of the potential for missing information, the risk index for skeletal complications would not be as discriminatory had more risk factors that are not available in the claims data been included in the present study.
In total, 241 740 men are diagnosed with prostate cancer every year [22] and one-third of them will receive ADT [2]. In the present study, 22.3% of the fractures that developed after ADT were hip fractures. Hip fractures are known to be associated with an increased risk of mortality [20]. Therefore, the potential burden of morbidity and mortality associated with ADT after a fracture needs to be well recognized because men diagnosed with localized prostate cancer in the PSA era had a higher probability of death from causes other than prostate cancer. The higher mortality after experiencing a fracture may contribute to the all-cause mortality among patients with prostate cancer. An alternative explanation is that a fracture may be an indicator of frailty among the elderly. Therefore, the increased mortality may not only be related to the use of ADT, but also reflect a selection bias. Given these findings, it is essential to consider the impact of the use of ADT on patient mortality and to reconsider its use in the setting of clinically localized prostate cancer, where a survival advantage has not been shown in the most men. The use of ADT is associated with an increased fracture and mortality risk, particularly in those patients with a high baseline risk of skeletal complications.
In conclusion, men who had a high risk of skeletal complications at baseline had a higher fracture incidence after ADT. Men who experienced a fracture experienced an almost twofold higher mortality risk compared to those who did not. Consideration of a patient’s risk of fracture before initiating ADT may reduce ADT-related fractures and the risk of mortality.
Acknowledgments
The present study was supported by: National Cancer Institute Grant # RO1 CA 116399, National Cancer Institute Cancer Center Support Grant # P30 CA072720-13 and Robert Wood Johnson Foundation Grant # 60624. All of the authors have participated directly in the planning, execution or analysis of this study. All authors have read and approved the final version submitted for publication.
Abbreviations
- ADT
androgen deprivation therapy
- GnRH
gonadotropin-releasing hormone
- HR
hazard ratio
- ICD-9
International Classification of Diseases (9th Revision)
- RP
radical prostatectomy
- RT
radiation therapy
- SEER
Surveillance, Epidemiology and End Results
Footnotes
All authors declare that they have no conflicts of interest.
References
- 1.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahinian VB, Kuo Y-F, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–32. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–9. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 6.Dickman PW, Adolfsson J, Astrom K, Steineck G. Hip fractures in men with prostate cancer treated with orchiectomy. J Urol. 2004;172:2208–12. doi: 10.1097/01.ju.0000143930.73016.c6. [DOI] [PubMed] [Google Scholar]
- 7.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–8. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadhwa VK, Weston R, Mistry R, Parr NJ. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int. 2009;104:800–5. doi: 10.1111/j.1464-410X.2009.08483.x. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–9. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71:392–7. doi: 10.1016/j.ejrad.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER–Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26:3913–5. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 13.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 14.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer. Manual for Staging of Cancer. 5. Philadelphia, PA: JB Lippincott; 1997. [Google Scholar]
- 16.Hamilton AS, Gloeckler Ries L. Cancer of Prostate. In: Ries L, Young J, Keel G, Eisner M, YDL, M-JH, editors. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. Chapt 22. Bethesda, MD: National Cancer Institute, SEER Program, NIH Publication; 2007. [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Albertsen PC, Walters S, Hanley JA. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163:519–23. [PubMed] [Google Scholar]
- 19.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 20.Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–90. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serpa Neto A, Tobias-Machado M, Esteves MA, et al. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012;15:36–44. doi: 10.1038/pcan.2011.4. [DOI] [PubMed] [Google Scholar]
- 22.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]