Abstract
We recently identified hundreds of transcripts with differential expression in rat zona glomerulosa (zG) and zona fasciculata. Although the genes up-regulated in the zG may be playing important roles in aldosterone production, the relationship between most of these genes and aldosterone production has not been uncovered. Because aldosterone, in the presence of a high sodium diet, is now considered a significant cardiovascular risk factor, in this review we performed gene ontology and pathway analyses on the same microarray data to better define the genes that may influence zG function. Overall, we identified a number of genes that may be involved in aldosterone production through transforming growth factor β (TGF-β), WNT, calcium, potassium, and ACTH signaling pathways. The list of genes we present in the current report may become an important tool for researchers working on primary aldosteronism and aldosterone-related cardiovascular diseases.
Keywords: Adrenal gland, Aldosterone, Transcriptome, Zona glomerulosa, Gene ontology, Pathway analysis
1. Introduction
Histologically the mammalian adrenal cortex consists of three concentric layers named the zona glomerulosa (zG), zona fasciculata (zF), and zona reticularis (zR) (Arnold, 1866). It is now accepted that these zones have distinct roles in steroid hormone production, with the zG synthesizing mineralocorticoids (aldosterone) and the zF producing glucocorticoids (Mitani et al., 1999; Vinson, 2003; Vinson and Ho, 1998). The role of zR varies between species but the primate zR is a source of the so-called adrenal androgens (Conley et al., 2004; Nakamura et al., 2009; Rainey et al., 2002). Thus, in addition to the zone-specific differences in histology, there is a functional zonation that allows the production of the adrenal zone-specific steroids.
In humans and rodents, functional zonation relies in part on the zone-specific expression of two cytochrome P450 isozymes termed aldosterone synthase (CYP11B2) and steroid 11β-hydroxylase (CYP11B1) that catalyze the last steps in the biosynthesis of aldosterone and glucocorticoids (i.e. corticosterone in rodents and cortisol in humans), respectively. In human children and rodents, CYP11B2 is expressed in the histological zG (Domalik, Chaplin, Kirkman et al., 1991; Aiba and Fujibayashi, 2011; Ogishima, Suzuki, Hata et al., 1992); whereas in human adults, in addition to the histological zG, CYP11B2 expression is found in subcapsular aldosterone-producing cell clusters (APCCs) (Aiba and Fujibayashi, 2011; Nishimoto et al., 2010).
Not only are the functional roles of APCC and molecular mechanisms of its formation unknown, but the exact molecular mechanisms causing the layered functional zonation remain poorly defined. The main regulators of aldosterone production are angiotensin II (AngII), K+, and adrenocorticotropic hormone (ACTH) while that for glucocorticoid is ACTH (Hattangady et al., 2011). In the zG cells, the three agonists acutely (minutes after stimulation) increase the protein accumulation and phosphorylation of steroidogenic acute regulatory protein (StAR) and activate the aldosterone synthesis cascade. On the other hand, only AngII and K+ can induce the chronic expression (hours to days) of zG-specific CYP11B2. As for glucocorticoid from the zF cells, ACTH is the main agonist that causes prompt expression of StAR and sustained induction of zF-specific CYP11B1. In the zG cells, AngII and K+ together increase intracellular calcium, which is essential for the continued aldosterone secretory response (Barrett et al., 1989; Ganguly and Davis, 1994), while in both the zG and zF, ACTH binds to its cell surface receptor (melanocortin receptor 2) and activates downstream signaling molecules including adenylate cyclases (Stocco et al., 2005). Thus, hormone productions in the zG and zF cells are controlled by partly overlapping pathways; however, the differences in particular genes involved in these cells are not fully explored.
In order to understand global differences between the zG and zF, we recently identified the transcripts that are differentially expressed in rat zG versus zF (Nishimoto et al., 2012). As described in the report, laser-capture microdissection (LCM) was employed to isolate tissues from the zG and zF layers in Sprague-Dawley rats. An enriched population of zG cells was carefully captured from cells immediately beneath the capsule, whereas the zF cells were taken from the lipid-rich cells in the middle of this histological zone. RNAs from the zG and zF were isolated, biotin-labeled, amplified, and used for microarray analysis. The microarray revealed the unique gene expression profile of the zG, with 234 zG transcripts that have at least 2-fold greater expression than that in the adjacent zF. These genes may have important roles in zG maintenance and/or aldosterone production.
In order to better understand the biological meaning of the observed differences in gene expression, in this report we analyzed the same rat microarray data using gene ontology (GO) and pathway analyses. In both analyses, we focused on zG-specific genes in accordance with zG function since the most potent mineralocorticoid, aldosterone, is now thought to be a cardiovascular risk factor in the presence of a modern high sodium diet (Briet and Schiffrin, 2010; Conlin, 2005; Pimenta and Calhoun, 2006). First, we performed GO analysis using the GeneSpring 12 software. GO is a major bioinformatics initiative which provides a systematic language (ontology) in three key biological domains: biological processes, cellular components, and molecular functions (Gene Ontology Consortium, 2008). Second, we performed pathway analysis on 921 pathways defined in the Biological Pathway Exchange (BioPAX) (Demir et al., 2010). The p-value of the pathway analysis was determined based on the number of genes differentially expressed in each pathway. In addition, we manually analyzed genes related to the aldosterone synthetic pathway since this pathway was not included in the BioPAX pathway set. From these observations, we identified dozens of genes which may potentially play a role in aldosterone production.
2. GO analysis of zonally expressed genes
GO analysis using the 234 zG-upregulated genes could not identify any GO term with statistical significance (p<0.05), but 2 terms showed an enrichment in the zG (p<0.1): ‘regulation of systemic arterial blood pressure by circulatory renin-angiotensin system’ in the biological process term (p=0.096, Table 1) and ‘proteinaceous extracellular matrix (ECM)’ in the cellular component term (p=0.084, Table 2). The main function of zG is to produce aldosterone under the control of the renin-angiotensin system, and therefore the up-regulation of transcripts in Table 1 is consistent with the function of zG.
Table 1.
Transcripts in GO term 'regulation of systemic arterial blood pressure renin-angiotensin system' in biological process (p=0.096).
| symbol | gene # | fold change | gene definition |
|---|---|---|---|
| Cyp11b2 | 1 | 214.2 | aldosterone synthase |
| Agtr1b | 34 | 11.7 | angiotensin II receptor, type 1b |
| Agtr1a | 37 | 11.1 | angiotensin II receptor, type 1a |
The gene # is assigned in order of fold changes. See Supplemental Table 1.
Table 2.
Transcripts in GO term 'proteinaceous extracellular matrix protein' in cellular component (p=0.084).
| symbol | gene # | fold change | gene definition | related signaling pathway |
|---|---|---|---|---|
| Gpc3 | 11 | 30.6 | glypican 3 | Wnt/β-catenin |
| Fmod | 28 | 12.6 | fibromodulin | TGF-β |
| Col1a2 | 67 | 6.8 | collagen, type I, alpha 2 | others |
| Sparcl1 | 74 | 6.4 | secreted protein acidic and rich in cysteine (SPARC)-like 1 |
others |
| Lum | 86 | 5.7 | lumican | TGF-β |
| Ltbp1 | 107 | 4.9 | latent transforming growth factor beta binding protein 1 |
TGF-β |
| Fn1 | 110 | 4.7 | fibronectin 1 | others |
| Cpz | 172 | 3 | carboxypeptidase Z | Wnt/β-catenin |
| Vtn | 186 | 2.8 | vitronectin | others |
The gene # is assigned in order of fold changes. See Supplemental Table 1.
On the other hand, intriguingly, many transcripts belonging to the GO term ‘proteinaceous ECM’ have not been described in the zG (Table 2). For the purpose of reviewing these genes in this manuscript, we intuitively classified them into 3 groups based on their relationship with specific signaling pathways: transforming growth factor β (TGF-β) signaling, WNT signaling, and others. Fibromodulin (Fmod, 12.6x , gene #28 in Supplemental Table 1) and lumican (Lum, 5.7x, gene #86) are members of the structurally and functionally related family of small leucine-rich proteoglycans (Iozzo, 1999; Kresse et al., 1993). These proteins bind to the same region on type I collagen molecules, which consist of type I collagen α1 (Col1a1, 8.3x, p= 0.051) and α2 (Col1a2, 6.8x, gene #67) chains, and affect collagen fibrillogenesis in vitro (Rada et al., 1993; Svensson et al., 2000; Vogel et al., 1984). FMOD is known to interact with TGF-β and may silence TGF-β activities by sequestering TGF-β into the extracellular matrix (Hildebrand et al., 1994). Similarly, LUM is an endogenous inhibitor of TGF-β signaling (Nikitovic et al., 2011). In addition, latent TGF-β binding protein 1 (Ltbp1, 4.9x, gene #107) is a part of the TGF-β large latent complex, which binds TGF-β in the extracellular matrix (Horiguchi et al., 2012). It is reported that TGF-β signaling inhibits adrenal steroid production through inhibition of StAR, CYP11B1, and CYP11B2 (Brand et al., 2000; Liakos et al., 2003). Therefore, although the TGF-β binding proteins, Fmod, Lum, and Ltbp1, have not been described as genes related to the adrenal cortex, they may be involved in steroidgenesis in the zG by inhibiting TGF-β signaling pathways.
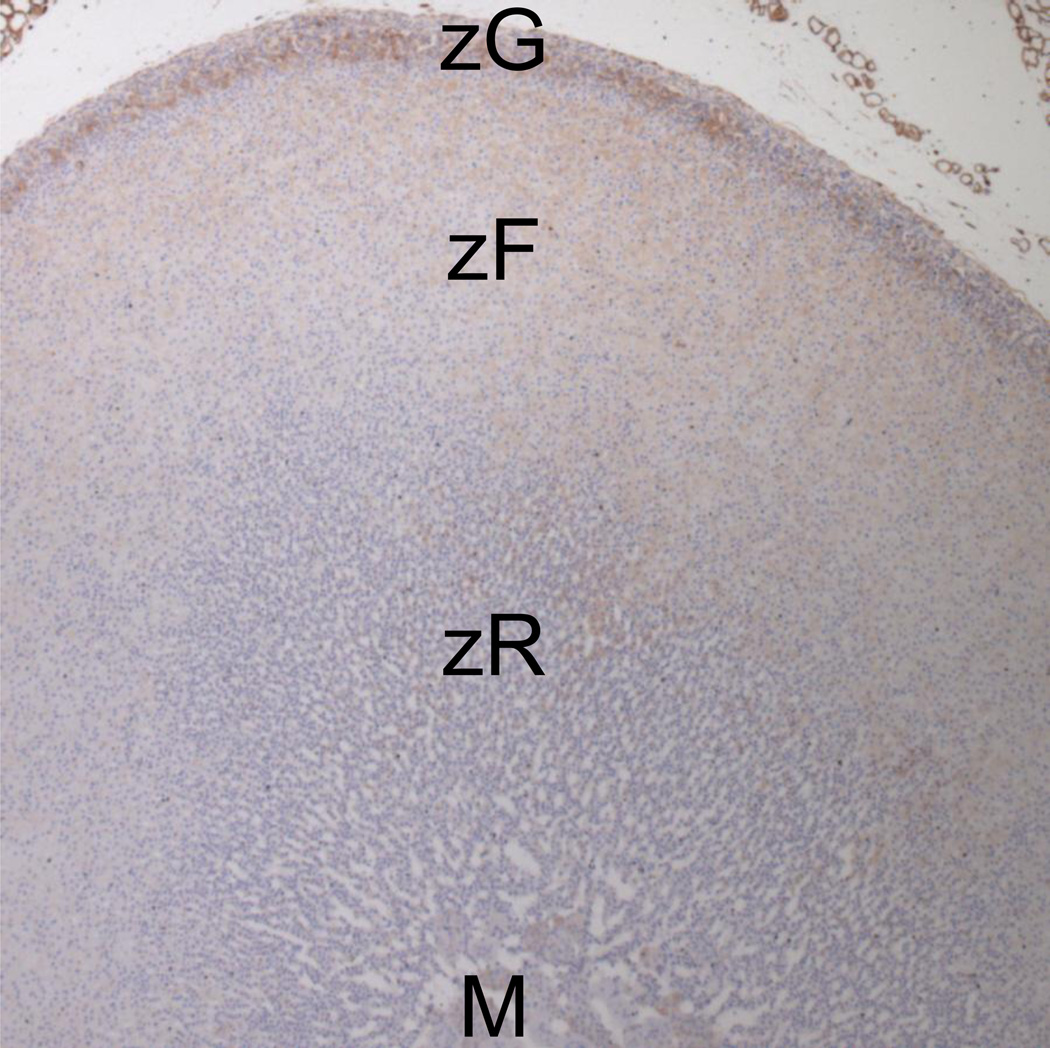
In addition, carboxypeptidase Z (Cpz, 3.0x, gene #172) and glypican 3 (Gpc3, 30.6x, gene #11) are known to modulate the WNT/β-catenin signaling pathway, which is involved in adrenal development (Heikkila et al., 2002; Mandel et al., 2008; Simon and Hammer, 2012). CPZ is a zinc-containing exopeptidase that catalyzes the removal of C-terminal amino acids from WNT4, thereby increasing WNT4 activity (Wang et al., 2009). In addition, GPC3 is a cell surface glycoprotein that has an ability to form complexes with WNTs and to stimulate the canonical WNT signaling pathway (Capurro et al., 2005). Although none of the 10 Wnt transcripts showed statistically significant up-regulation in the zG, Wnt4 showed a fold change of 4.22 (p= 0.101). In fact, at the protein level, anti-WNT4 immunostaining was clearly localized in the zG layer in rats (Fig. 1, primary antibody purchased from LifeSpan BioSciences, Inc. Seattle, WA, #LS-C122550). As for β-catenin, a downstream protein of WNT signaling, it is predominantly expressed in the zG layer in mice (El Wakil and Lalli, 2011). In humans, β-catenin is equally expressed in the zG and zF, but activated nuclear β-catenin is mainly present in the zG (Boulkroun et al., 2011). Thus, the expression patterns of β-catenin differ between species. In rats our microarray results indicated that β-catenin (Ctnnb1) expression was not different between the zG and zF. However, further investigation will be needed to localize the activated form of β-catenin in rat adrenal cortex. Taken together, the WNT/β-catenin signaling pathway may be up-regulated and play an important role in the zG along with Cpz and Gpc3.
Figure 1.
Immunohistochemistal staining for WNT4 (brown) in rat adrenal gland. Anti-WNT4 antibody (Lifespan Biosciences, Seatle, WA, LS-C122550) was used as a primary antibody (1:300 dilution), and immunoreactivity was visualized with diaminobenzidine. As shown, WNT4 is predominantly expressed in the zG.
Regarding the other ECM proteins, fibronectin I (Fn1, 4.7x, gene #110) and type I collagen expression has been reported in the zG, but secreted protein acidic and rich in cysteine (SPARC)-like 1 (Sparcl1 also known as hevin, 6.4x, gene #74) and vitronectin (Vtn, 2.8x, gene #186) are not. FN1 has a remarkably wide variety of functional activities and it binds to a number of biologically important molecules including type I collagen (Pankov and Yamada, 2002). Both FN1 and type I collagen encase individual zG cells in rats and are able to increase aldosterone production in primary culture rat zG cells, suggesting important roles of these proteins in zG maintenance and function (Otis et al., 2007). VTN is a glycoprotein abundant in ECM (Schvartz et al., 1999), which interacts with insulin-like growth factor (IGF) signaling and enhances migration of cells (Beattie et al., 2010; Noble et al., 2003). SPARCL1 is a unique glycoprotein highly expressed in astrocytes in the brain, which has a counter-adhesive activity (Brekken and Sage, 2001) and can induce synapse formation (Kucukdereli et al., 2011). Together, these ‘proteinaceous ECM proteins’ in Table 2 may have roles in the maintenance and function of the zG.
3. Pathway analysis
Pathway analysis identified 38 pathways that showed significant up-regulation in the zG (p<0.05, Table 3). Based on up-regulated genes, we roughly classified them into 3 categories: (i) ECM-related pathways, (ii) cell cycle-related pathways, and (iii) others. Similar to GO analysis where many ‘proteinacious ECM’ genes were detected, 3 ECM-related genes were identified in the pathway analysis. Among the 3 genes, 2 overlapped with the GO analysis, Fn1 and Vtn, but syndecan 2 (Sdc2, 12.3x, gene #31) was detected only in this analysis. SDC2 is a heparan sulfate transmembrane proteoglycan with glycosaminoglycan chains attached to the extracellular domain (ectodomain) of the protein (Couchman, 2010). The glycosaminoglycan ectodomain is known to control both cell-matrix and cell-cell interactions as well as to serve as a co-receptor for plateletderived growth factor, fibroblast growth factor, vascular endothelial growth factor, and TGF-β receptors (Alexopoulou et al., 2007; Chen et al., 2004; Tkachenko et al., 2005). Therefore, Sdc2 may play a role in modulating zG cell proliferation through TGF-β signaling together with Fmod, Lum, and Ltbp1.
Table 3.
Up-regulated pathways in rat zG.
| significant pathways | p-value | number of genes in pathways |
up-regulated genes | ||||
|---|---|---|---|---|---|---|---|
| ECM | FGFR1c and Klotho ligand binding and activation | 0.010 | 2 | Sdc2 | |||
| Klotho-mediated ligand binding | 0.010 | 2 | Sdc2 | ||||
| FGFR1 ligand binding and activation | 0.021 | 3 | Sdc2 | ||||
| FGFR1c ligand binding and activation | 0.021 | 3 | Sdc2 | ||||
| Integrin cell surface interactions | 0.026 | 24 | Fn1 | Vtn | |||
| Signal Transduction | 0.030 | 68 | Sdc2 | Fn1 | Vtn | ||
| FGFR2 ligand binding and activation | 0.031 | 3 | Sdc2 | ||||
| FGFR2b ligand binding and activation | 0.031 | 3 | Sdc2 | ||||
| FGFR2c ligand binding and activation | 0.031 | 3 | Sdc2 | ||||
| FGFR3b ligand binding and activation | 0.031 | 3 | Sdc2 | ||||
| FGFR3c ligand binding and activation | 0.031 | 3 | Sdc2 | ||||
| FGFR4 ligand binding and activation | 0.031 | 3 | Sdc2 | ||||
| FGFR ligand binding and activation | 0.041 | 5 | Sdc2 | ||||
| FGFR3 ligand binding and activation | 0.041 | 4 | Sdc2 | ||||
| Signaling by FGFR | 0.041 | 5 | Sdc2 | ||||
| cell cycle | S Phase | 0.004 | 10 | Wee1 | Ccnd1 | ||
| Mitotic G1-G1/S phases | 0.005 | 11 | Wee1 | Ccnd1 | |||
| G2/M DNA replication checkpoint | 0.010 | 1 | Wee1 | ||||
| Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex |
0.021 | 2 | Wee1 | ||||
| Polo-like kinase mediated events | 0.021 | 2 | Wee1 | ||||
| Ubiquitin-dependent degradation of Cyclin D | 0.021 | 2 | Ccnd1 | ||||
| Ubiquitin-dependent degradation of Cyclin D1 | 0.021 | 2 | Ccnd1 | ||||
| G2/M DNA damage checkpoint | 0.031 | 3 | Wee1 | ||||
| Cell Cycle, Mitotic | 0.032 | 31 | Wee1 | Ccnd1 | |||
| G2/M Checkpoints | 0.041 | 4 | Wee1 | ||||
| others | Chemokine receptors bind chemokines | 0.013 | 17 | Cxcl12 | Cxcl16 | ||
| Class A/1 (Rhodopsin-like receptors) | 0.024 | 109 | Cxcl12 | Cxcl16 | Agtr1a | Ptafr | |
| Peptide ligand-binding receptors | 0.027 | 64 | Cxcl12 | Cxcl16 | Agtr1a | ||
| GPCR ligand binding | 0.043 | 131 | Cxcl12 | Cxcl16 | Agtr1a | Ptafr | |
| Ion channel transport | 0.021 | 2 | Atp2a2 | ||||
| Ion transport by P-type ATPases | 0.021 | 2 | Atp2a2 | ||||
| Platelet calcium homeostasis | 0.031 | 3 | Atp2a2 | ||||
| Reduction of cytosolic Ca++ levels | 0.031 | 3 | Atp2a2 | ||||
| Eicosanoids | 0.031 | 3 | Ptgis | ||||
| Prostanoid metabolism | 0.031 | 3 | Ptgis | ||||
| Association of TriC/CCT with target proteins during biosynthesis |
0.010 | 1 | Sphk1 | ||||
| Chaperonin-mediated protein folding | 0.031 | 3 | Sphk1 | ||||
| Notch receptor binds with a ligand | 0.010 | 1 | Jag1 | ||||
FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; GPCR, G protein coupled receptor; Sdc2, syndecan 2 (gene #31) ; Fn1, fibronectin 1 (#110); Vtn, vitronectin (#186); Wee1, WEE1 homolog (#106); Ccnd1, cyclin D1 (#36); Cxcl12, chemokine (C-X-C motif) ligand 12 (#54); Cxcl16, chemokine (C-X-C motif) ligand 16 (#222); Agtr1a, angiotensin II receptor, type 1a (#37); Ptafr, platelet-activating factor receptor (#102); Atp2a2, ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2 (#175); Ptgis, prostaglandin I2 (prostacyclin) synthase (#24); Sphk1, sphingosine kinase 1 (#124); Jag1, jagged 1 (#91). The detail of genes are shown in Supplemental Table 1.
In the cell cycle-related pathways, cyclin D1 (Ccnd1, 11.3x, gene #36) and WEE-1 homolog (Wee1, 5.0x, gene #106) were up-regulated in the zG. CCND1 regulates cell-cycle transition from the G1 phase to the S-phase (Alberts et al., 2008) and is essential for shortening G1 phase progression (Jiang et al., 1993; Quelle et al., 1993). Interestingly, AngII, a key aldosterone regulator, induces adrenal cell expression of Ccnd1 through transcriptional activation of its promoter (Watanabe et al., 1996). On the other hand, WEE1 is a tyrosine kinase, the primary function of which is to inactivate M phase-specific cyclin-dependent kinase 1, thereby preventing cells from entering mitosis (Alberts et al., 2008). Thus, CCND1 and WEE1 may together be regulating zG cell proliferation.
4. Aldosterone synthetic pathway
Since the aldosterone synthetic pathway was not included in the BioPAX pathway set, we manually listed the genes in this pathway based on 3 articles (Guagliardo et al., 2012; Hattangady et al., 2011; Spat and Hunyady, 2004) and analyzed their differential expression. We classified these genes into 4 categories: renin-angiotensin system-related genes (discussed in the GO analysis), calcium-related genes, potassium-related genes, and others. The zG up-regulated genes with statistically significant up-regulation (p<0.05) or an enrichment (p<0.1) are listed in Table 4, and a complete list of analyzed genes is shown in Supplemental Table 2.
Table 4.
Up-regulated genes in rat zG in the aldosterone synthetic pathways
| gene | gene # | p-value | fold change |
gene definition |
|---|---|---|---|---|
|
renin angiotensin system related genes |
||||
| Agtr1a | #37 | <0.05 | 11.1 | angiotensin II receptor, type 1a |
| Agtr1b | #34 | <0.05 | 11.7 | angiotensin II receptor, type 1b |
| Agtr2 | n.a. | 0.094 | 2.1 | angiotensin II receptor, type 2 |
| Cyp11b2 | #1 | <0.05 | 214.2 | cytochrome P450, family 11, subfamily B, polypeptide 2 (aldosterone synthase) |
| potassium-related genes | ||||
| potassium channels | ||||
| Kcnn2 | #19 | <0.05 | 17.5 | potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 |
| potassium transporting ATPase | ||||
| Atp1b1 | n.a. | 0.052 | 3.5 | ATPase, Na+/K+ transporting, beta 1 polypeptide |
| Atp1b2 | #118 | <0.05 | 4.5 | ATPase, Na+/K+ transporting, beta 2 polypeptide |
| calcium-related genes | ||||
| calcium channels | ||||
| Cacna1c | #176 | <0.05 | 3.0 | calcium channel, voltage-dependent, L type, alpha 1C subunit |
| Cacna1d | #218 | <0.05 | 2.3 | calcium channel, voltage-dependent, L type, alpha 1D subunit |
| Cacna1h | n.a. | 0.082 | 2.6 | calcium channel, voltage-dependent, T type, alpha 1H subunit |
| Cacnb3 | #119 | <0.05 | 4.5 | calcium channel, voltage-dependent, beta 3 subunit |
| calmodulins | ||||
| Calm1 | n.a. | 0.069 | 2.7 | calmodulin 1 |
| Calm4 | n.a. | 0.100 | 2.2 | calmodulin 4 |
| calcium transporting ATPase | ||||
| Atp2a2 | #175 | <0.05 | 3.0 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 |
| Atp2a3 | n.a. | 0.063 | 4.1 | ATPase, Ca++ transporting, ubiquitous |
| Atp2c1 | #81 | <0.05 | 5.9 | ATPase, Ca++ sequestering |
|
inositol 1,4,5-triphosphate receptors |
||||
| Itpr1 | n.a. | 0.095 | 2.8 | inositol 1,4,5-triphosphate receptor 1 |
| others | ||||
| adenylate cyclases | ||||
| Adcy2 | n.a. | 0.056 | 3.8 | adenylate cyclase 2 |
| Adcy3 | #159 | 0.021 | 3.4 | adenylate cyclase 3 |
| Adcy4 | #53 | 0.022 | 7.8 | adenylate cyclase 4 |
| protein kinases | ||||
| Prkcz | n.a. | 0.090 | 2.6 | protein kinase C, zeta |
The gene # is assigned in order of fold changes. See Supplemental Table 1. n.a.: not available in Supplemental Table 1 due to p>0.05. P-values were calculated using a modified t test along with multiple testing corrections with the Benjamini and Hochberg false discovery rate.
It is well known that circulating potassium is a major regulator of zG aldosterone production. The ability of zG cells to respond to small changes in circulating potassium is due to the ability of K+ to depolarize the zG cells and the fact that the zG cells are selectively permeable to K+, making them essentially K+ electrodes (Guagliardo et al., 2012). ZG cell polarity can be influenced by numerous factors including potassium channels, and it has been suggested that each species may utilize a different set of potassium channels to maintain membrane potential in zG cells (Guagliardo et al., 2012). In our analysis, among the 82 potassium channels found on the rat microarray (Supplemental Table 2), only potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 (Kcnn2, 17.5x, #19) was up-regulated in the rat zG. This voltage-independent and calcium-activated K+ channel, also known as SK2, has been identified in human adrenal glands as well (Chen et al., 2004). SK2 in neurons plays a role in the after-hyperpolarization phase, a refractory period of membrane hyperpolarization following an action potential. Although it is not clear if the channel’s role is identical in the zG cells, it seems likely that this channel could help to return the zG membrane potential to its normally very negative value following increases in intracellular calcium levels induced by aldosterone agonists (see below). It has been reported that the potassium channel, subfamily K, member 9 (Kcnk9 also known as TASK3) mRNA is up-regulated in mouse zG and rat outer adrenal cortex (Czirjak et al., 2000; Davies et al., 2008). However, to our surprise, the expression of Kcnk9 was not different between the zG and zF in the current study. In addition, the probe sequence for potassium inwardly-rectifying channel, subfamily J, member 5 (Kcnj5) did not align to the rat Kcnj5 genomic sequence and therefore this gene was not included in the analysis. Interestingly, ATPase, Na+/K+ transporting, β2 polypeptide (Atp1b2, 4.5x, gene #118) was also up-regulated and ATPase, Na+/K+ transporting, β1 polypeptide (Atp1b1, 3.5x, p=0.052) showed an enrichment in the zG. These ATPase genes encode Na+/K+-ATPases which generate the transmembrane Na+ and K+ gradients required for maintenance of cellular homeostasis and membrane potential (Stahl, 1984). Overall, we identified 3 genes that may be involved in K+ signaling in the rat zG.
Upon stimulation, influx of calcium ions from extracellular interstitial fluid plays an important role in controlling aldosterone production in response to various agonists (Barrett et al., 1989; Ganguly and Davis, 1994). Calcium ion influx is controlled in part by 2 types of calcium channels in the zG: transient (T) -type and long-lasting (L) -type voltage-gated calcium channels (TTCC and LTCC) (Spat and Hunyady, 2004). These channels biochemically consist of a pore-forming α1 subunit; a transmembrane, disulfide-linked complex of α2 and δ subunits; an intracellular β subunit; and a transmembrane γ subunit (Catterall, 2000). In the current study, calcium channel, voltage-dependent, L type, α1C (Cacna1c, 3.0x, gene #176) and α1D (Cacna1d, 2.3x, gene #218) subunits of LTCC as well as calcium channel, voltage-dependent, β3 subunit (Cacnb3, 4.5x, gene #119) for both TTCC and LTCC were up-regulated in the zG. In addition, calcium channel, voltage-dependent, T type, α1H (Cacna1h) showed an enrichment in the zG (2.6x, p=0.082). It is known that the channels comprised by these 4 genes have important roles in calcium influx into the zG cells, and their differential expression suggests their significance to aldosterone production.
Intracellular calcium is also increased by its release from the endoplasmic reticulum (ER). Under AngII stimulation, phosphatidylinositol 4,5-bisphosphate (PIP2) is hydrolyzed to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 binds to the IP3 receptor (IP3R) on the ER, and ER calcium is released. It has been reported that IP3R type 1 (ITPR1) is the most abundant IP3 receptor in rat zG cells (Enyedi et al., 1994). In line with the report, only Itpr1 showed an enrichment in the current study (2.8x, p=0.095). Increased intracellular calcium activates calcium/calmodulin-dependent protein kinases I/II through calmodulins, which also showed an enrichment: calmodulin 1 (Calm1, 2.7x, p=0.069) and 4 (Calm4, 2.2x, p=0.100). Meanwhile, DAG stimulates protein kinase C (PKC) and protein kinase D, which are thought to promote aldosterone production (Hattangady et al., 2011). In the current study, only PKC δ (Prkcz, 2.6x, p=0.090), an atypical DAG-insensitive PKC isoform, showed an enrichment in the zG among 12 protein kinase genes belonging to these families. Nonetheless, it should be noted that many of these enzymes are regulated at the level of activity in addition to that of transcription, therefore, increased expression of upstream genes (e.g., for the AngII type 1 receptors) may enhance activity independent of changes in expression. Our results suggest that Itpr1, Calm1, Calm4, and Prkcz may play roles in increasing or responding to intracellular calcium levels in the zG cells, in concert with the aforementioned calcium channels.
On the other hand, there are also proteins that eliminate calcium from the cytoplasm, including the plasma membrane Ca2+ ATPases, the sarco/endoplasmic Ca2+ ATPases (SERCAs), and the Na+/Ca2+ antiporters (Spat and Hunyady, 2004). Furthermore, cytoplasmic calcium can be decreased by secretory pathway Ca2+ ATPases (SPCAs) through transporting calcium from the cytosol to the Golgi apparatus (Van Baelen et al., 2004). As for the SERCAs, ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2 (Atp2a2, 3.0x, gene #175) was up-regulated, and ATPase, Ca2+ transporting, ubiquitous (Atp2a3, 4.6x, p=0.06) showed an enrichment in the zG. Among the SPCAs, ATPase, Ca2+ sequestering (Atp2c1, 5.9x, gene #81) was up-regulated in the zG. The products of these genes may have important roles in controlling intracellular calcium homeostasis, along with the proteins that raise or respond to changes in intracellular calcium.
Among other aldosterone-regulating proteins, adenylate cyclase 3 and 4 (Adcy3, 3.4x, gene #159; Adcy4, 7.8x, gene #53) were up-regulated and Adcy2 (3.8x, p=0.056) showed an enrichment in the zG. ACTH is able to stimulate aldosterone production acutely by binding to the melanocortin receptor 2, and this stimulation activates adenylate cyclase via the heterotrimeric G protein. Therefore, the up-regulation of Adcys may also be involved in aldosterone production via this pathway. In summary, 21 genes showed an up-regulation or enrichment in the aldosterone synthetic pathway.
In our original study we were surprised by the rather modest difference in transcript expression in zG vs. zF(234 transcripts) (Nishimoto et al., 2012). Similarly in this study we found very few zG pathway differences in this follow-up analysis. This may suggest that the zG and zF share most of their molecular pathways and genes despite their distinct functions, a result which may be in line with the current paradigm that zG cells trans-differentiate into zF cells in the adrenal cortex. This finding also suggests that the pathways/genes identified by the microarrays are the key pathways/genes that distinguish these two cell types, even though their differences may only be marginal.
In conclusion, this microarray analysis identifies several pathway genes that are differentially expressed in the zG versus zF cells and therefore may be important in regulating aldosterone production. Some of these, e.g. Cyp11b2 (214.2x) and the genes encoding the AngII receptor type 1a and 1b (Agtr1a, 11.1x, gene #37; Agtr1b, 11.7x, gene #34), are known to play key roles for aldosterone biosynthesis. The role of others is less clear, but we speculate that future studies will demonstrate their involvement in aldosterone production as well as aldosterone-related cardiovascular pathologies.
5. Perspective
In recent years, aldosterone has been recognized as an important cardiovascular risk factor. In order to control pathological secretion of this hormone, a better understanding of the molecular mechanisms underlying aldosterone production is needed. We are confident that the list of genes we present in the current study will become an important tool for researchers working on primary aldosteronism and aldosterone-related cardiovascular diseases.
Supplementary Material
We performed gene ontology and pathway analyses of rat adrenal zona glomerulosa genes.
TGF-beta, WNT, calcium, potassium, and ACTH signaling-related genes were identified.
The list of genes may become an important tool for adrenal researchers.
Acknowledgements
This work was supported by Grant DK43140 from the National Institute of Diabetes and Digestive and Kidney Diseases (to T.S. and W.E.R)., VA Merit Award (to W.B.B), and fellowships from the Federation of National Public Service Personnel Mutual Aid Associations and the Tachikawa Hospital, Tokyo, Japan (to K.N.).
Abbreviations
- zG
zona glomerulosa
- zF
zona fasciculata
- zR
zona reticularis
- Cyp11b1
steroid 11β-hydroxylase
- Cyp11b2
aldosterone synthase
- TGF-β
transforming growth factor β
- Gpc3
glypican 3
- Kcnn2
potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2
- Fmod
fibromodulin
- Sdc2
syndecan 2
- Agtr1a
angiotensin II receptor, type 1a
- Adcy4
adenylate cyclase 4
- Agtr1b
angiotensin II receptor, type 1b
- Ccnd1
cyclin D1
- Col1a1
type I collagen α1 chain
- Col1a2
type I collagen α2 chain
- Sparcl1
secreted protein acidic and rich in cysteine (SPARC)-like 1
- Atp2c1
ATPase, Ca2+ sequestering
- Lum
lumican
- Wee1
WEE-1 homolog
- Ltbp1
latent TGF-β binding protein 1
- Fn1
fibronectin I
- Atp1b2
ATPase, Na+/K+ transporting, β2 polypeptide
- Cacnb3
calcium channel, voltage-dependent, β3 subunit
- Adcy3
adenylate cyclase 3
- Cpz
carboxypeptidase Z
- Atp2a2
ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2
- Cacna1c
calcium channel, voltage-dependent, L type, α1C
- Vtn
vitronectin; calcium channel
- Cacna1d
voltage-dependent, L type, α1D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have declared no conflict of interest.
Contributor Information
Koshiro Nishimoto, Email: sweet.marino@gmail.com.
William E. Rainey, Email: WER@umich.edu.
Wendy B. Bollag, Email: wbollag@georgiahealth.edu.
Tsugio Seki, Email: tseki@georgiahealth.edu.
References
- Aiba M, Fujibayashi M. Alteration of subcapsular adrenocortical zonation in humans with aging: the progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. The journal of histochemistry and cytochemistry. 2011;59:557–564. doi: 10.1369/0022155411404071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of The Cell. Fifth ed. New York: Garland Science, Taylor & Francis Group; 2008. [Google Scholar]
- Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. The international journal of biochemistry & cell biology. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Arnold J. Ein Beitrag zu der feiner Struktur und dem Chemismus der Nebennieren. Virchows Arch. 1866;35:64–107. [Google Scholar]
- Barrett PQ, Bollag WB, Isales CM, McCarthy RT, Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocrine reviews. 1989;10:496–518. doi: 10.1210/edrv-10-4-496. [DOI] [PubMed] [Google Scholar]
- Beattie J, McIntosh L, van der Walle CF. Cross-talk between the insulin-like growth factor (IGF) axis and membrane integrins to regulate cell physiology. Journal of cellular physiology. 2010;224:605–611. doi: 10.1002/jcp.22183. [DOI] [PubMed] [Google Scholar]
- Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, Lefebvre H, Louiset E, Jeunemaitre X, Meatchi T, Benecke A, Lalli E, Zennaro MC. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205. [DOI] [PubMed] [Google Scholar]
- Brand C, Nury D, Chambaz EM, Feige JJ, Bailly S. Transcriptional regulation of the gene encoding the StAR protein in the human adrenocortical cell line, H295R by cAMP and TGFbeta1. Endocr Res. 2000;26:1045–1053. doi: 10.3109/07435800009048637. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix biology : journal of the International Society for Matrix Biology. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nature reviews. Nephrology. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer research. 2005;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annual review of cell and developmental biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chen L, Klass C, Woods A. Syndecan-2 regulates transforming growth factor-beta signaling. J Biol Chem. 2004;279:15715–15718. doi: 10.1074/jbc.C300430200. [DOI] [PubMed] [Google Scholar]
- Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn-Schmiedeberg's archives of pharmacology. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Conlin PR. Interactions of high salt intake and the response of the cardiovascular system to aldosterone. Cardiology in review. 2005;13:118–124. doi: 10.1097/01.crd.0000148845.74124.c0. [DOI] [PubMed] [Google Scholar]
- Gene Ontology Consortium. The Gene Ontology project in 2008. Nucleic acids research. 2008;36:D440–D444. doi: 10.1093/nar/gkm883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR. Transmembrane signaling proteoglycans. Annual review of cell and developmental biology. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Molecular endocrinology. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U.S.A. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, Wu G, D'Eustachio P, Schaefer C, Luciano J, Schacherer F, Martinez-Flores I, Hu Z, Jimenez-Jacinto V, Joshi-Tope G, Kandasamy K, Lopez-Fuentes AC, Mi H, Pichler E, Rodchenkov I, Splendiani A, Tkachev S, Zucker J, Gopinath G, Rajasimha H, Ramakrishnan R, Shah I, Syed M, Anwar N, Babur O, Blinov M, Brauner E, Corwin D, Donaldson S, Gibbons F, Goldberg R, Hornbeck P, Luna A, Murray-Rust P, Neumann E, Ruebenacker O, Samwald M, van Iersel M, Wimalaratne S, Allen K, Braun B, Whirl-Carrillo M, Cheung KH, Dahlquist K, Finney A, Gillespie M, Glass E, Gong L, Haw R, Honig M, Hubaut O, Kane D, Krupa S, Kutmon M, Leonard J, Marks D, Merberg D, Petri V, Pico A, Ravenscroft D, Ren L, Shah N, Sunshine M, Tang R, Whaley R, Letovksy S, Buetow KH, Rzhetsky A, Schachter V, Sobral BS, Dogrusoz U, McWeeney S, Aladjem M, Birney E, Collado-Vides J, Goto S, Hucka M, Le Novere N, Maltsev N, Pandey A, Thomas P, Wingender E, Karp PD, Sander C, Bader GD. The BioPAX community standard for pathway data sharing. Nature biotechnology. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domalik LJ, Chaplin DD, Kirkman MS, Wu RC, Liu WW, Howard TA, Seldin MF, Parker KL. Different isozymes of mouse 11 beta-hydroxylase produce mineralocorticoids and glucocorticoids. Molecular endocrinology. 1991;5:1853–1861. doi: 10.1210/mend-5-12-1853. [DOI] [PubMed] [Google Scholar]
- El Wakil A, Lalli E. The Wnt/beta-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011;332:32–37. doi: 10.1016/j.mce.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Enyedi P, Szabadkai G, Horvath A, Szilagyi L, Graf L, Spat A. Inositol 1,4,5-trisphosphate receptor subtypes in adrenal glomerulosa cells. Endocrinology. 1994;134:2354–2359. doi: 10.1210/endo.134.6.8194461. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Davis JS. Role of calcium and other mediators in aldosterone secretion from the adrenal glomerulosa cells. Pharmacological reviews. 1994;46:417–447. [PubMed] [Google Scholar]
- Guagliardo NA, Yao J, Hu C, Barrett PQ. Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology. 2012;153:3579–3586. doi: 10.1210/en.2012-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2011;352:151–162. doi: 10.1016/j.mce.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M, Ota M, Rifkin DB. Matrix Control of Transforming Growth Factor-beta Function. Journal of biochemistry. 2012;152:321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace AM, Infante AS, Doi S, Santella RM, Weinstein IB. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993;8:3447–3457. [PubMed] [Google Scholar]
- Kresse H, Hausser H, Schonherr E. Small proteoglycans. Experientia. 1993;49:403–416. doi: 10.1007/BF01923585. [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U.S.A. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakos P, Lenz D, Bernhardt R, Feige JJ, Defaye G. Transforming growth factor beta1 inhibits aldosterone and cortisol production in the human adrenocortical cell line NCI-H295R through inhibition of CYP11B1 and CYP11B2 expression. J Endocrinol. 2003;176:69–82. doi: 10.1677/joe.0.1760069. [DOI] [PubMed] [Google Scholar]
- Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-offunction mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. Development of functional zonation in the rat adrenal cortex. Endocrinology. 1999;140:3342–3353. doi: 10.1210/endo.140.7.6859. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord. 2009;10:19–26. doi: 10.1007/s11154-008-9092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN. Lumican regulates osteosarcoma cell adhesion by modulating TGFbeta2 activity. The international journal of biochemistry & cell biology. 2011;43:928–935. doi: 10.1016/j.biocel.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Rigsby CS, Wang T, Mukai K, Gomez-Sanchez CE, Rainey WE, Seki T. Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology. 2012;153:1755–1763. doi: 10.1210/en.2011-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A, Towne C, Chopin L, Leavesley D, Upton Z. Insulin-like growth factor-II bound to vitronectin enhances MCF-7 breast cancer cell migration. Endocrinology. 2003;144:2417–2424. doi: 10.1210/en.2002-221138. [DOI] [PubMed] [Google Scholar]
- Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y. Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011 beta in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology. 1992;130:2971–2977. doi: 10.1210/endo.130.5.1572304. [DOI] [PubMed] [Google Scholar]
- Otis M, Campbell S, Payet MD, Gallo-Payet N. Expression of extracellular matrix proteins and integrins in rat adrenal gland: importance for ACTH-associated functions. J Endocrinol. 2007;193:331–347. doi: 10.1677/JOE-07-0055. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. Journal of cell science. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Pimenta E, Calhoun DA. Aldosterone, dietary salt, and renal disease. Hypertension. 2006;48:209–210. doi: 10.1161/01.HYP.0000230482.82239.a0. [DOI] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes & development. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Experimental eye research. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Schvartz I, Seger D, Shaltiel S. Vitronectin. The international journal of biochemistry & cell biology. 1999;31:539–544. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- Simon DP, Hammer GD. Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol Cell Endocrinol. 2012;351:2–11. doi: 10.1016/j.mce.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiological reviews. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- Stahl WL. (Na+/K+)-ATPase: function, structure, and conformations. Annals of neurology. 1984;16(Suppl):S121–S127. doi: 10.1002/ana.410160718. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Molecular endocrinology. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Svensson L, Narlid I, Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS letters. 2000;470:178–182. doi: 10.1016/s0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circulation research. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochimica et biophysica acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Vinson GP. Adrenocortical zonation and ACTH. Microsc Res Tech. 2003;61:227–239. doi: 10.1002/jemt.10331. [DOI] [PubMed] [Google Scholar]
- Vinson GP, Ho MM. Origins of zonation: the adrenocortical model of tissue development and differentiation. Clin Exp Pharmacol Physiol Suppl. 1998;25:S91–S96. doi: 10.1111/j.1440-1681.1998.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shao YY, Ballock RT. Carboxypeptidase Z (CPZ) links thyroid hormone and Wnt signaling pathways in growth plate chondrocytes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:265–273. doi: 10.1359/jbmr.081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Lee RJ, Albanese C, Rainey WE, Batlle D, Pestell RG. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.