Abstract
Objective
Physical fitness is an important correlate of structural and functional integrity of the brain in healthy adults. In heart failure (HF) patients, poor physical fitness may contribute to cognitive dysfunction and we examined the unique contribution of physical fitness to brain structural integrity among patients with HF.
Methods
Sixty-nine HF patients performed the Modified Mini Mental State examination (3MS) and underwent brain magnetic resonance imaging. All participants completed the 2-minute step test (2MST), a brief measure of physical fitness. We examined the associations between cognitive performance, physical fitness, and three indices of global brain integrity: Total cortical gray matter volume, total white matter volume, and whole brain cortical thickness.
Results
Regression analyses adjusting for demographic characteristics, medical variables (e.g., left ventricular ejection fraction), and intracranial volume revealed reduced performance on the 2MST was associated with decreased gray matter volume and thinner cortex (p < .05). Follow up analyses showed that reduced gray matter volume and decreased cortical thickness were associated with poorer 3MS scores (p < .05).
Conclusions
Poor physical fitness is common in HF and associated with reduced structural brain integrity. Prospective studies are needed to elucidate underlying mechanisms for the influence of physical fitness on brain health in HF.
Keywords: Brain, cognitive function, heart failure, neuroimaging, physical fitness
1. Introduction
Heart failure (HF) affects nearly six million Americans [1] and its prevalence will likely increase in coming years due to the increasing proportion of older adults and the high rates of conditions such as obesity, hypertension, and type 2 diabetes mellitus [2,3]. Patients with HF are at high risk for many adverse outcomes, including elevated mortality risk, recurrent hospital readmission, and reduced quality of life [1,4,5]. HF patients are also at risk for cognitive dysfunction and cognitive impairment is a major contributor to poor outcomes in this population [6,7]. Indeed, patients with HF are at risk for dementia (i.e., Alzheimer’s disease, vascular dementia) [8,9] and as many as 75% of them exhibit impairment on neuropsychological tests of global cognition, attention, executive function, and memory [7,10,11].
White matter hyperintensities and gray matter atrophy are well documented in older adults with HF [11–16] and have been linked with reduced neuropsychological test performance [17]. Although the etiological underpinnings of structural brain changes in HF remain unclear, several contributing factors emerge from the extant literature. These putative contributors include advanced age [12], reduced left ventricular ejection fraction (LVEF) [12,16,19], depression and anxiety [16,17], and reduced cerebral perfusion [20,21], to name a few.
Another likely contributor to reduced brain integrity in HF is poor physical fitness. Reduced physical fitness is common in persons with HF [22] and it predicts poor outcomes [23–26]. Decreased physical fitness in HF patients is also associated with impairments in multiple cognitive domains [27–29]. In healthy elderly, reduced cognitive performance is associated with smaller regional brain volumes [30], and the association is moderated by concomitant vascular risk [31]. In healthy older adults, gross differences in cerebral morphology correlate with poor aerobic fitness [32–34]. Thus, it is highly plausible that cognitive impairments in HF may stem from deterioration in brain structure and poor physical conditioning. Extremely low levels of physical fitness in HF patients may result in reduced brain functioning and deterioration of brain structure, thus paving the way to persistent cognitive deficits.
Physical fitness provides many physiological benefits in cardiovascular disease populations [35], though its independent influence on brain structure in HF is unknown. The current study sought to determine whether physical fitness was associated with indicators of brain structural integrity in older adults with HF even after accounting for other factors linked to structural brain damage (e.g., HF severity). First, we investigated the effects of physical fitness on total cortical gray matter volume, white matter volume, and average cortical thickness. These indices were chosen because of the extant work highlighting the prevalence of atrophy and cortical thinning in cardiovascular disease and normal aging population with vascular risk factors [12,16,36,37]. We then examined the relationship between these MRI findings and global cognitive function to determine the possible impact of these structural brain differences on cognition.
2. Materials and Methods
2.1 Participants
The sample consisted of 69 consecutively enrolled persons with HF from an National Institutes of Health funded study examining neurocognitive function among HF patients. Strict inclusion/exclusion criteria were chosen for entry into the study to capture the independent contribution of HF on cognitive function. Specifically, the participants were between the ages of 50–85 years of age, native English speakers, and had an established diagnosis of New York Heart Association (NYHA) class II or III at the time of enrollment. Exclusion criteria included history of significant neurological disorder (e.g. dementia, stroke), head injury with more than 10-minutes loss of consciousness, severe psychiatric disorder (e.g. schizophrenia, bipolar disorder), history of substance abuse or dependence, renal failure, and sleep apnea. Participants were also excluded for any contraindications to magnetic resonance imaging (MRI) (e.g., pacemaker). Participants averaged 68.07 (SD = 8.02) years of age, 42.0% of them were women, and 84.0% Caucasian. Medical record review revealed that the current sample exhibited an average LVEF of 42.32 (SD = 14.11). Table 1 displays sample demographic and medical characteristics.
Table 1.
Demographic, Medical, and Cognitive Characteristics of 69 Older Adults with Heart Failure
| Demographic Characteristics | |
| Age, mean (SD) | 68.07(8.02) |
| Education, mean (SD) | 13.83(2.73) |
| Female (%) | 42.0 |
| Race (% Caucasian) | 84.0 |
| Medical Characteristics | |
| BDI-II, mean (SD) | 5.94(6.37) |
| LVEF, mean (SD) | 42.32(14.11) |
| Diabetes (% yes) | 29.0 |
| Hypertension (% yes) | 72.5 |
BDI-II = Beck Depression Inventory-II; LVEF = Left Ventricular Ejection Fraction
2.2 Measures
2.2.1 Neuroimaging
Whole-brain, high-resolution 3D T1-weighted images (Magnetization Prepared Rapid Gradient-Echo, MPRAGE) were acquired on a Siemens Symphony 1.5Tesla scanner for morphologic analysis. Twenty-six slices were acquired in the sagittal plane with a 230 × 100 mm field of view. The acquisition parameters were as follows: Echo time (TE) = 17, repetition time (TR) = 360, acquisition matrix = 256×100, slice thickness = 5 mm, and flip angle = 120°.
Morphological analysis of brain structure was completed with FreeSurfer Version 5.1 (http://surfer.nmr.mgh.harvard.edu). Detailed methodology for cortical thickness and regional and total volume derivation has been described in detail previously [38–41]. Briefly, FreeSurfer was used to preprocess images (e.g. intensity normalization, skull stripping) then provide an automated parcellation of cortical and subcortical structures via an automated processing stream. Freesurfer performs this parcellation by registering images to a probabilistic brain atlas, built from a manually labeled training set, and uses this probabilistic atlas to assign a neuroanatomical label to each voxel in an MRI volume. As part of the parcellation process, FreeSurfer derives cortical thickness measurements in various regions-of-interest (ROIs) throughout the brain. Parcellations were visually inspected for accuracy. For the purpose of this study, an average cortical thickness measurement was derived by taking the arithmetic mean of cortical thickness measurements from the component ROIs. Total gray matter, white matter, and intracranial volume measurements are derived automatically. Summary composites of volume and the mean of cortical thickness of each brain region including frontal, temporal, parietal, and occipital were also calculated using the organization schema as described in Desikan et al. (2006) [42].
2.2.2 Physical Fitness
Physical fitness was assessed with the 2MST [43]. The 2MST asks individuals to march in place for 2 minutes, lifting his/her knees to a marked target set on the wall set at the midpoint between the kneecap and crest of the iliac. The number of times the right knee met the marked target was counted. A greater step count within the time limit indicates greater physical fitness [43]. The average step count for women and men between the ages of 50–85 ranges from 71–115 and 60–107, respectively [43]. Poorer performance on 2MST has been previously linked with reduced cognitive function in HF populations [27].
2.2.3 Cognitive Function
The Modified Mini-Mental State Examination (3MS), a widely used brief cognitive screening tool, served as measure of the global cognitive function. The 3MS provides an estimate of global cognitive function, assessing aspects of attention, memory, language, and spatial abilities [44,45]. Lower scores on the 3MS indicate worse performance.
2.2.4 Depressive Symptoms
The BDI-II is a commonly used checklist that assesses depressive symptoms with strong psychometric properties in medical populations [46,47].
2.2.5 Demographic and Medical Characteristics
Participant demographic and medical characteristics were ascertained through self-report and corroborated through medical record review.
2.3 Procedures
The local Institutional Review Board (IRB) approved the study procedures and all participants provided written informed consent prior to study enrollment. A medical chart review was performed and participants completed demographic, medical and psychosocial self-report measures. Participants were then administered the 3MS to examine global cognitive function followed by the completion of the 2MST under the supervision of a trained research assistant. Finally, all HF patients underwent a brain MRI within two weeks of baseline assessment.
2.4 Statistical Analyses
A series of multiple linear hierarchical regression analyses examined whether performance on the 2MST was associated with total gray matter volume, total cortical white matter volume, and whole brain cortical thickness. For each analysis, medical and demographic characteristics were entered in block 1, including age, sex (1 = male; 2 = female), number of years of education, depressive symptoms (as assessed by the BDI-II), LVEF, diagnostic history of vascular risk factors, such as hypertension and diabetes (1 = positive diagnostic history; 0 = negative diagnostic history) and intracranial volume. The 2MST entered in block 2 to determine its independent effect on each imaging index. Follow-up partial correlations also examined the differential influence of 2MST performance on the frontal, temporal, parietal, and occipital brain regions.
Finally, follow up regression analyses controlling for the same demographic and medical characteristics were then conducted to determine the relationship between neuroimaging indices and cognitive function as measured by the 3MS.
3. Results
Physical Fitness and Cognitive Function
When compared to normative data accounting for age and gender, both men and women in this sample of HF patients exhibited low average physical fitness. Specifically, when using established cutoffs that suggest below average levels of physical fitness for males and females, 40.6% of the sample had a step count less than 60, and 60.9% of the sample had a step count less than 71.
Deficits in cognitive performance were also common as the sample averaged 92.96 (SD = 5.55) on the 3MS. Specifically, 20.3% of the sample scored below a 90 on the 3MS, 36.2% between 90 and 95, and 43.5% scored above a 95. See Table 2 for a descriptive summary of indicators of physical fitness and cognitive function. Partial correlations adjusting for demographic and medical characteristics revealed that poorer performance on the 2MST was associated with decreased scores on the 3MS (r(60) = .30, p = .02). Table 3 displays a partial correlation matrix adjusting for intracranial volume between key demographic variables (e.g., age, education, gender), brain structural indices, and physical fitness.
Table 2.
Physical Fitness Levels, Cognitive Function, and Neuroimaging Characteristics of 69 Older Adults with Heart Failure
| Physical Fitness, mean (SD) | |
| 2-Minute Step Test Men | 70.30 (22.17) |
| 2-Minute Step Test Women | 57.34 (18.37) |
| 2-Minute Step Test Overall Sample | 64.86 (21.50) |
| Neuroimaging, mean (SD) | |
| Gray Matter Volume | 547653.90(65794.95) mm3 |
| Cortical White Matter Volume | 513960.50(115118.86) mm3 |
| Cortical Thickness | 2.36(.23) mm |
| Cognitive Function, mean (SD) | |
| 3MS | 92.96(5.55) |
Note.
3MS = Modified Mini Mental State Examination
Table 3.
Partial Correlations Between Demographics, Physical Fitness, and Brain Structural Indexes (N= 69)
| EF | Age | Gender | Edu | 2MST | GM | WM | Thick | |
|---|---|---|---|---|---|---|---|---|
| EF | -- | .09 | .16 | .11 | .04 | .14 | − .03 | .14 |
| Age | -- | -- | .06 | .00 | − .24* | − .16 | − .02 | − .08 |
| Gender | -- | -- | -- | −.29 | − .04 | − .03 | − .13 | .18 |
| Education | -- | -- | -- | -- | .30* | .12 | .03 | .10 |
| 2MST | -- | -- | -- | -- | -- | .41* | − .14 | .34* |
| Gray Matter | -- | -- | -- | -- | -- | -- | −.54* | .84* |
| White Matter | -- | -- | -- | -- | -- | -- | -- | −.56* |
| Thickness |
Note.
EF = Ejection fraction; 2MST = 2-Minute Step Test; Edu = Education; 2MST = 2-Minute Step Test; GM = Gray Matter; WM = White Matter; Thick = Cortical Thickness
p < .05
Physical Fitness and Neuroanatomical Measures
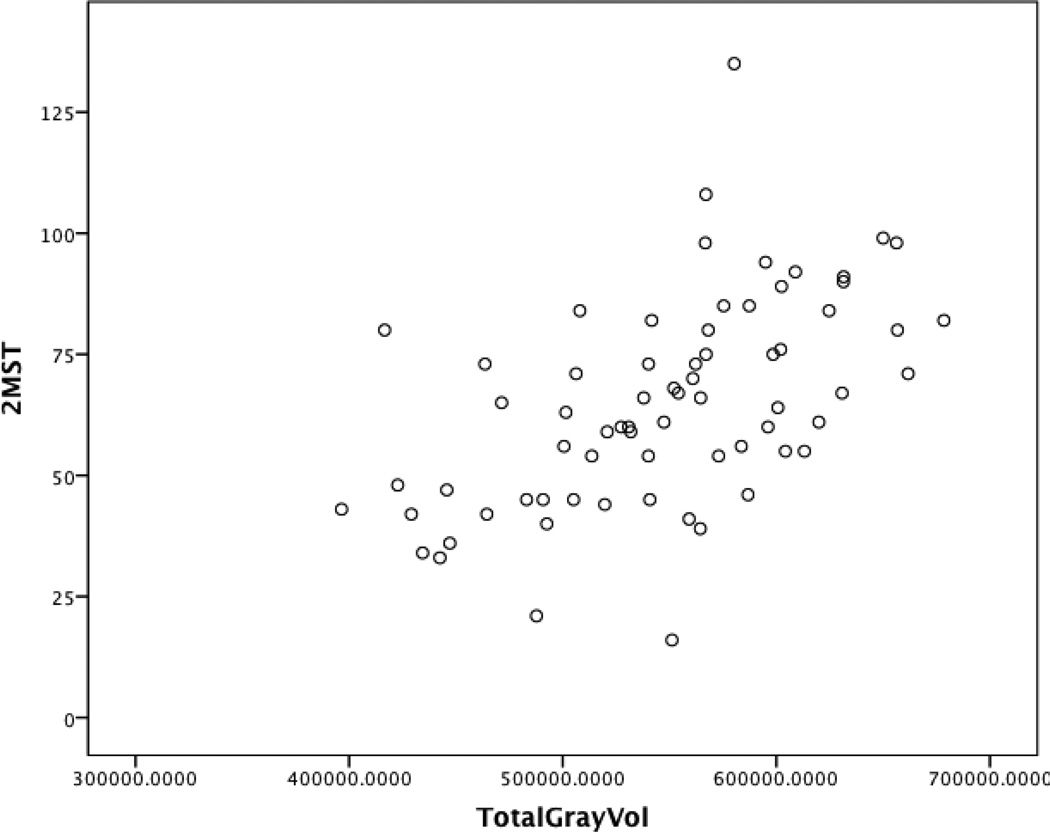
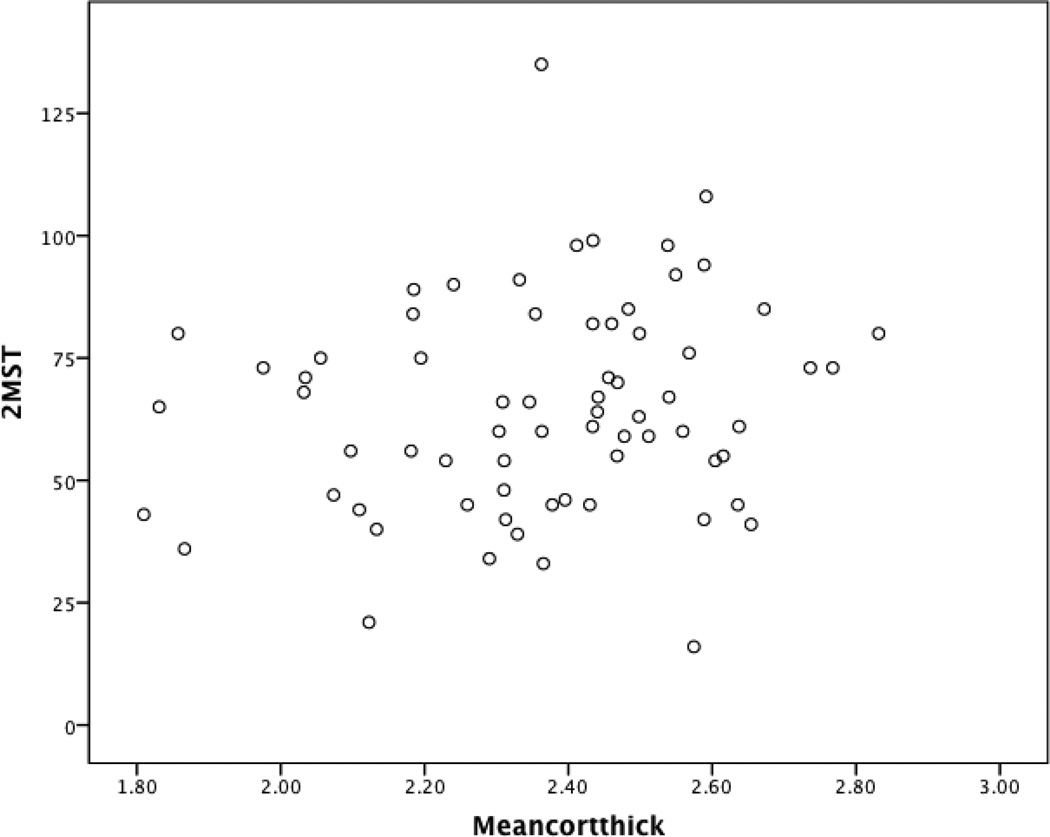
Demographic and medical characteristics were associated with total gray matter volume (F(8, 60) = 6.42, p < .001), total cortical white matter volume (F(8, 60) = 6.14, p < .001), and average cortical thickness (F(8, 60) = 2.28, p = .03). After accounting for intracranial volume and demographic and medical factors known to be associated with structural brain injury, reduced step count on the 2MST demonstrated incremental predictive validity for reduced total gray matter volume (β = .38, p < .01) and decreased whole brain cortical thickness (β = .34, p = .01). Figures 1 and 2 present an unadjusted scatter plot between the 2MST with total gray matter volume and whole brain cortical thickness. No such pattern emerged for total white matter volume (p > .05 for all). See Table 4.
Figure 1.
Scatter Plot Examining the Association Between the 2MST and Total Gray Matter Volume in Heart Failure
Note. This graphical depiction does not adjust for key medical and demographic variables; 2MST = 2-Minute Step Test
Figure 2.
Scatter Plot Examining the Association Between the 2MST and Whole Brain Cortical Thickness in Heart Failure
Note. This graphical depiction does not adjust for key medical and demographic variables; 2MST = 2-Minute Step Test; Meancortthick = Whole brain cortical thickness
Table 4.
Physical Fitness Independently Predicts Brain Structure in Older Adults with Heart Failure (N = 69)
| Gray Matter | White Matter | Cortical Thickness | |
|---|---|---|---|
| Variable | β(SE b) | β (SE b) | β(SE b) |
| Block 1 | |||
| Age | −.18(794.67) | −.01(1404.32) | −.13(.00) |
| Sex | .00(17783.29) | −.13(31426.13) | .28(.07) |
| Education | .03(2519.83) | .00(4452.98) | .10(.01) |
| LVEF | .12(456.81) | −.01(807.27) | .09(.00) |
| BDI-II | −.09(1049.91) | −.04(1855(.38) | −.17(.00) |
| Hypertension | − .12(14546.91) | .12(25706.90) | −.14(.06) |
| Diabetes | −.27(14317.61)* | −.06(25301.68) | −.16(.06) |
| Volume | .52(.05)* | .58(.09)* | −.20(.00) |
| R2 | .46 | .45 | .23 |
| F | 6.42* | 6.14* | 2 .28* |
| Block 2 | |||
| 2MST | .38 (317.07)* | −.14(611.81) | .34(.00)* |
| R2 | .56 | .46 | .31 |
| F for ΔR2 | 13 .08* | 1.46 | 6.95* |
Note.
denotes p < 0.05
Abbreviations: β – standardized regression coefficients, SE – standard error; LVEF = Left Ventricular Ejection Fraction; BDI-II = Beck Depression Inventory-II; Volume = Intracranial Volume; 2MST = 2-minute step test
Partial Correlations between Fitness and 3MS Performance with Specific Brain Regions
To identify possible differential effects of fitness on brain structure, partial correlations controlling for age, sex, number of years of education, BDI-II, LVEF, history of hypertension and diabetes and intracranial volume examined the association between physical fitness and specific brain lobes. Analyses showed that decreased step count was associated with reduced frontal (r(59) = .39, p < .01), temporal (r(59) = .38, p < .01), parietal (r(59) = .42, p < .01), and occipital lobe volume (r(59) = .45, p < .01). A similar pattern also emerged for cortical thickness of the frontal (r(59) = .32, p = .01), temporal (r(59) = .36, p < .01), parietal (r(59) = .28, p = .03), and occipital lobe (r(59) = .23, p = .08).
Neuroimaging and Cognitive Function
Follow up regression analyses also controlling for the same demographic and medical characteristics showed that reduced gray matter volume (F(1, 59) = 3.04, β = .37, p = .01) and decreased cortical thickness (F(1, 59) = 2.76, β = .27, p = .04) were associated with poorer 3MS scores. The 3MS was not associated with total white matter volume (F(1, 59) = 2.22, β = −.14, p = .35).
4. Discussion
Consistent with past work, reduced physical fitness was common and associated with lower cognitive function in this sample of HF patients. The current study extends these findings by showing that physical fitness is independently associated with brain morphology in HF patients. Several aspects of these findings warrant discussion.
Our findings suggest that poor physical fitness is associated with decreased gray matter volume and cortical thickness independent of the effects of HF severity and other medical and demographic characteristics. The observed association between poor physical fitness and reduced indicators of brain integrity is consistent with the extant evidence linking aerobic fitness with brain volume in patients with Alzheimer’s disease and healthy adults [34,48–51]. There are several possible explanations for the unique relationship between physical fitness and brain structure. Better physical fitness may preserve cerebral structure through its beneficial effects on basic biological processes, including cell proliferation and survival [52], synaptic plasticity [53], neurogenesis [52], and protection against brain insult [54]. In addition, better fitness levels may lead to vascular benefits such as improved endothelial functioning and cerebral perfusion [55–61]. The effect of fitness on cerebral perfusion is noteworthy, as cerebral hypoperfusion and subsequent ischemia is believed to underlie neuropathological insult in HF patients [62,63]. Thus, increased fitness may attenuate vascular-related pathology such as white matter hyperintensities that also affect brain atrophy [64]. Because aerobic exercise increases cerebral blood volume [65], prospective studies should examine whether exercise-training programs might preserve brain integrity in HF patients by improving physical fitness levels.
Notably, the magnitude of differences was uniform across all cerebral lobes. Such pattern of a uniform decline is in contrast to a typical differential patter of cortical volume [66] and cortical thickness [67] reduction observed in normal elderly. It is, however, similar to the pattern of expansion of cortical shrinkage associated with vascular risk [68]. Viewed in conjunction with the global reduction in cerebral perfusion, this global pattern of structural decline appears characteristic of HF. A longitudinal study is needed to establish the temporal order of structural and hemodynamic changes in patients with HF.
In addition, the association between physical fitness and brain structure has implications for clinicians working with HF patients. An important aspect of the current study is the additional evidence for the role of physical activity and fitness levels in neurocognitive outcomes in persons with HF. Past work has shown that the structured exercise of cardiac rehabilitation improves cognitive function in persons with CVD [69] and future studies should examine whether similar benefits are found for HF patients. Cognitive impairment in HF is associated with reduced quality of life, poor treatment adherence, and increased mortality risk in HF [70,71] and it is possible that improved fitness levels may delay or even prevent some of these adverse outcomes in this high risk population.
Interestingly, LVEF was not associated with structural brain indices in the current study. Although past studies have also failed to show a linear association between LVEF and cognitive function [72], the exact reason for this lack of association is not entirely clear. One possible explanation may involve cerebral perfusion, as past work has failed to find a significant relationship between LVEF and cerebral blood flow suggesting that cardiac function may only partially account for cerebral hypoperfusion in HF patients [73]. Such findings may also help to explain the inconsistencies in the literature regarding the association between LVEF and cognitive test performance. It is possible that physical fitness level may prove to be a more promising assessment of neurocognitive outcomes than LVEF as it not only reflects the ability of the heart to deliver blood to the muscles, but is also associated with endothelial functioning, blood flow levels, among many others [22]. Future studies clarifying the relationship between systemic perfusion and neurocognitive outcomes are much needed.
The current findings also revealed a positive association between gray matter volume and cortical thickness with global cognitive function. Serber and colleagues (2008) [18] also showed that reduced neuropsychological test performance on measures assessing executive function predicted corresponding structural injury of the frontal cortex. Such findings suggest that cognitive impairment in HF is at least partly attributable to underlying abnormalities in brain morphology. The 3MS is a brief measure sensitive to cognitive impairment [44] and prospective studies should evaluate whether change in 3MS scores correspond with subsequent changes in brain morphology, particularly as these factors relate to physical fitness.
The current study is limited in several ways. First, our findings were based on cross-sectional data and prospective studies are needed to elucidate changes and contributors to brain structure over time in HF. The present study also assessed physical fitness using the 2MST, which offers several advantages, as it is brief and administered in the confines of the examination room. However, future studies should investigate the effect of physical fitness on brain structure in HF using more detailed measures of cardiorespiratory fitness, such as VO2 max from stress testing. The current study controlled for prevalent comorbid conditions in HF that influence cognitive test performance and physical capacity in this population, though it is also possible that other comorbidities (e.g., respiratory disorders, medication effects) could have influenced performance on the 2MST. Prospective studies that account for such factors are needed to confirm the association between physical fitness and brain structure in HF. Future studies should also examine the association between cognitive test performance and brain morphology in HF patients using comprehensive neuropsychological test batteries. Similarly, future studies that compare fitness levels and brain morphology in HF vs. age-matched controls are needed to confirm the deleterious effects of poor fitness on neurocognitive outcomes in a HF population. Finally, fMRI studies are also needed to help clarify the effect of physical fitness on brain function, including regional examination, as specific brain structures (e.g., medial temporal lobe) have been shown to be particularly sensitive to the effects of exercise training and physical activity [74–76].
In summary, the current study found that reduced physical fitness was independently associated with brain structure among older adults with HF. These findings may offer as a possible explanation for the growing evidence linking poor fitness and cognitive function in persons with HF. Prospective studies are needed to elucidate the underlying mechanisms (i.e., cerebral perfusion) for the effects of physical fitness on brain health in HF.
Acknowledgments
Support for this work included National Institutes of Health (NIH) grants DK075119 and HLO89311. Dr. Naftali Raz is also supported by National Institutes of Health (NIH) grant R37 AG011230.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to report.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simon G, Gerguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Jencks SF, Williams MV, Coleman EA. Rehospitalization among patients in Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.Bennett SJ, Oldridge NB, Eckert G, Embree JL, Browing S, Hou N, et al. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Riegel B, Weaver TE. Poor sleep and impaired self-care: towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nurs. 2009:337–344. doi: 10.1016/j.ejcnurse.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pressler SJ, Subramanian U, Kareken D, Perksin SM, Gradus-Pizlo I, Suave MJ, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 9.Roman G. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20:91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 10.Suave MJ, Lewis WR, Blankenbiller M, Rickabuagh B, Pressler SJ. Cognitive impairments in chronic heart failure: a case controlled study. J Card Fail. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Vogels RL, van der flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM et al. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Woo M, Macey P, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 14.Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307:106–113. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogels RL, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24:418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 17.Almeida JR, Alves TC, Wajngarten M, Rays J, Castro CC, Cordeiro Q, et al. Late-life depression, heart failure and frontal white matter hyperintensity: A structural magnetic resonance imaging study. Braz J Med Biol Res. 2005;38:431–436. doi: 10.1590/s0100-879x2005000300014. [DOI] [PubMed] [Google Scholar]
- 18.Serber SL, Kumar, Woo MA, Macey PM, Fonarow GC, Harper RM. Cognitive test performance and brain pathology. Nurs Res. 2008;57:75–83. doi: 10.1097/01.NNR.0000313483.41541.10. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya P, Bao F, Shah M, Ramesh G, Madhavan R, Khan O. Left ventricular dysfunction is associated with cerebral grey matter injury: An in-vivo brain MRI segmentation study. J Neurol Sci. 2012 doi: 10.1016/j.jns.2012.07.051. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Alves TC, Rays J, Fraguas R, Jr, Wajngarten M, Meneghetti JC, Prando S, et al. Localized cerebral blood fl ow reductions in patients with heart failure: A study using 99mTc-HMPAO SPECT. J Neuroimaging. 2005;15:150–156. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- 21.Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Transcranial Doppler blood flow assessment in patients with mild heart failure: correlates with neuroimaging and cognitive performance. Congest Heart Fail. 2008;14:61–65. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- 22.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. AHA Scientific Statement. Exercise and Heart Failure. A Statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 23.Lucas C, Stevenson LW, Johnson W, Hartley H, Hamilton MA, Walden J, et al. The 6-min walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am Heart J. 1999;138:618–624. doi: 10.1016/s0002-8703(99)70174-2. [DOI] [PubMed] [Google Scholar]
- 24.Boxer R, Klepppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, et al. Modest increase in peak VO2 is related to better clinical outcomes in chornic heart failure patients: Results from Heart Failure and a Controlled Trial to Investigate Outcomes of Exercise Training (HF-ACTION) Circ Heart Fail. 2012 doi: 10.1161/CIRCHEARTFAILURE.111.965186. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutcher JR, Kahn J, Grines C, Franklin B. Comparison of left ventricular ejection fraction and exercise capacity as predictors of two- and five-year mortality following acute myocardial infarction. American Journal of Cardiology. 2007;99:436–441. doi: 10.1016/j.amjcard.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 27.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, et al. The 2-Minute Step Test is independently associated with cognitive function in older adults with heart failure. Aging Clin Exp Res. 2011 doi: 10.3275/8186. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg G, Lossnitzer N, Schellberg D, Mueller-Tasch T, Krueger C, Haas M, et al. Peak oxygen uptake and left ventricular ejection fraction, but not depressive symptoms, are associated with cognitive impairment in patients with heart failure. Int Gen Med. 2011;4:879–887. doi: 10.2147/IJGM.S23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldasseroni S, Mossello E, Romboli B, Orso F, Colombi C, Fumagalli S, et al. Relationship between cognitive function and 6-minute walking test in older outpatients with chronic heart failure. Aging Clin Exp Res. 2010;22:308–313. doi: 10.1007/BF03324936. [DOI] [PubMed] [Google Scholar]
- 30.Raz N, Dixon FM, Head DP, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural MRI. Neuropsychology. 1998;12:95–106. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;17:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 32.Colcombe SJ, Erickson KI, Raz N, Webb AH, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol: Bio Med Sci. 2003;53:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 33.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer’s disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure. HF- ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo SW, Lee JW, Im K, Park JS, Kim SH, Kim ST, et al. Cardiovascular risk factors cause cortical thinning in cognitively impaired patients: relationships among cardiovascular risk factors, white matter hyperintensities, and cortical atrophy. Alzheimer Dis Assoc Diord. 2012;26:106–112. doi: 10.1097/WAD.0b013e31822e0831. [DOI] [PubMed] [Google Scholar]
- 37.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Gach HM, et al. White matter lesions and brain gray matter volume in cognitive normal elders. Neurobiol Aging. 2012;33:e7–e16. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haseigrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 40.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 41.Fischl B, van der Kouwe A, Destrieux C, Haigren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 42.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 43.Jones CJ, Rikli RE. Measuring functional fitness of older adults. The Journal on Active Aging. 2002 March April;:24–30. [Google Scholar]
- 44.Teng E, Chui H. The Modified Mini-Mental (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 45.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- 46.Arnau R, Meagher M, Norris M, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20:112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 48.Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, et al. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011;25:545–553. doi: 10.1037/a0022733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: a brief review. Brain Behav Immun. 2004;18:214–220. doi: 10.1016/j.bbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Peters J, Dauvermann M, Mette C, Platen P, Franke J, Hinrichs T, et al. Voxel-based morphmoetry reveals and association between aeropic capacity and grey matter density in the right anterior insula. Neuroscience. 2009;163:1102–1108. doi: 10.1016/j.neuroscience.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Vidon ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorepiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol of Aging. 2012;33:1624–16632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Praag H, Shubert T, Zhao C, Gage FH. Exercise Enhances Learning and Hippocampal Neurogenesis in Aged Mice. Journal of Neuroscience. 2005;25:8680–8868. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: How physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- 54.Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 55.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.11.086. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Fu TC, Wang CH, Hsu CC, Cherng WJ, Huang SC, Wang JS. Suprression of cerebral hemodynamics is associated with reduced functional capacity in patients with heart failure. Am J physiol Heart Circ Physiol. 2011;300:H1545–H1555. doi: 10.1152/ajpheart.00867.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corvera-Tindel T, Doering L, Woo MA, Khan S, Dracup K. Effects of a home-walking exercise program on functional status and symptoms in heart failure. Am Heart J. 2004;147:339–346. doi: 10.1016/j.ahj.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Chicco AJ. Exercise training in prevention and rehabilitation which training mode is best? Minerva Cardioangiol. 2008;56:557–570. [PubMed] [Google Scholar]
- 59.Papathanasiou G, Tsamis N, Georgiadou P, Adamopoulos S. Beneficial effects of physical training and methodology of exercise prescription in patients with heart failure. Hellenic J Cardiol. 2008;49:267–277. [PubMed] [Google Scholar]
- 60.Davison K, Bircher S, Hill A, Coates AM, Howe PR, Buckley JD. Relationships between obesity, cardiorespiratory fitness, and cardiovascular function. J Obes. 2010:191253. doi: 10.1155/2010/191253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moser DJ, Hoth KF, Robinson RG, Paulsen JS, Sinkey CA, Benjamin ML. Blood vessel function and cognition in elderly patients with atherosclerosis. Stroke. 2004;35:e369–e372. doi: 10.1161/01.STR.0000145050.35039.51. [DOI] [PubMed] [Google Scholar]
- 62.Jefferson A, Poppas A, Paul R, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, et al. Relating Imaging Indices of White Matter Integrity and Volume in Healthy Older Adults. Cereb Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- 65.Pereira AC, Huddleston DR, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 68.Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 69.Stanek KM, Gunstad J, Spitznagel MB, Waechter D, Hughes JW, Luyster F, Josephson R, Rosneck J. Improvements in cognitive function following cardiac rehabilitation for older adults with cardiovascular disease. Int J Neurosci. 2011;121:86–93. doi: 10.3109/00207454.2010.531893. [DOI] [PubMed] [Google Scholar]
- 70.Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Cognitive function and treatment adherence in older adults with heart failure. Psychosom Med. 2012;74:965–973. doi: 10.1097/PSY.0b013e318272ef2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuccala G, Pedone C, Cesari M, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 72.Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, et al. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study) Am J Cardio. 2011;108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Transcranial Doppler blood fl ow assessment in patients with mild heart failure: Correlates with neuroimaging and cognitive performance. Congest Heart Fail. 2008;14:61–65. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- 74.Eickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]