Abstract
Previous studies of HIV-infected women with high risk behavior have indicated that neither neutralizing antibody nor cellular immunity elicited by an initial HIV-1 infection is associated with protection against superinfection with a different HIV-1 strain. Here, we measured antibody-dependent cell-mediated virus inhibition (ADCVI) antibody activity in the plasma of 12 superinfected cases and 36 singly infected matched controls against 2 heterologous viruses. We found no association between plasma ADCVI activity and superinfection status. ADCVI antibody activity against heterologous virus elicited by the original infection may not contribute to preventing a superinfecting HIV-1.
Keywords: antibody-dependent cell-mediated virus inhibition, ADCVI, HIV-1, superinfection, primary infection, clade A
Individuals with chronic HIV-1 infection can become infected with additional strains of HIV-1 upon sexual exposure [1]. In some high-risk populations, such superinfections occur at a rate approaching that of primary infection, calling into question the ability of virus-elicited immunity to prevent further infection [2-5]. This question has been directly investigated in a comparison of neutralizing antibody activity in plasma of superinfected and singly infected women from Mombasa, Kenya [6, 7]. In that study, no significant differences were found in the breadth or potency of HIV-1-specific neutralizing antibody responses between the singly and superinfected women in specimens obtained just prior to the time of superinfection. Further investigation in this same cohort of women found that cellular immune responses, measured by the frequency of HIV-1-specific cytokine-producing or cytolytic CD8+ or CD4+ T cells were not significantly associated with the odds of superinfection [8].
To explore further the possible role of immunity induced by the initial infection on acquiring a second infection, we measured antibody-dependent cell-mediated virus inhibition (ADCVI) activity in plasma from the Mombasa cohort. ADCVI is a measure of Fcγ receptor (FcγR)-mediated antiviral activity and occurs when antibody bound to virus-infected target cells engages FcγR-bearing effector cells, such as natural killer cells, monocytes or macrophages. The virus inhibition that results is due in part to target cell death (antibody-dependent cellular cytotoxicity [ADCC]) and in part to non-cytolytic mechanisms such as β-chemokine release from the effector cells. ADCVI antibody activity has been associated with protection from lentivirus infections in macaques [9]. In addition, higher ADCVI activity in serum from healthy individuals vaccinated with recombinant gp120 in the Vax004 trial was associated with a lower rate of sexually acquired HIV infection [10].
The cohort of subjects for this study has been described in detail elsewhere and consisted of 12 women with superinfection (cases) and three singly infected women matched to each superinfected case (controls; n = 36) according to HIV-1 subtype, timing of samples in relation to initial infection, and viral load [6, 8]. The median estimated time between sample collection and superinfection was 102 days (range range 13 - 573). All subjects were from Mombasa, Kenya and were infected through heterosexual contact; none used antiretroviral therapy during the study period. Women were classified as superinfected or singly infected based on phylogenetic analyses of env and gag sequences. The ethical review committees of the University of Nairobi, the University of Washington, the Fred Hutchinson Cancer Research Center, and the University of California, Irvine approved this study.
Because subtype A is the major subtype in the population [11], two subtype A HIV-1 variants were used to infect target cells for the ADCVI assays. The two replication-competent viruses were derived from proviral clones that were isogenic in all genome regions except envelope. Q23XhoΔXho is a full-length molecular clone obtained from a sexually-infected woman at 142 days post-infection, and the BS208.B1 chimeric provirus was generated from Q23XhoΔXho by replacing the envelope with one derived from a vertically-infected infant at the first time of documented infection (6 weeks of life) [12]. These strains were chosen from a panel of clade A molecular clones based on their ability to grow well in our CEM.NKR-CCR5 target cells. In addition, ADCVI results using the Q23XhoΔXho and BS208.B1 strains correlated well with results using other strains, and thus was felt to be the most representative (data not shown).
The ADCVI assay has been described previously [10, 13]. Briefly, target cells, consisting of CEM.NKR-CCR5 cells (NIH AIDS Research and Reference Reagent Program), were infected with either of the viral strains for 72 hours and then washed to remove cell-free virus. Next, effector cells (PBMCs from healthy donors) and a 1:100 dilution of test plasma from cases or controls were added to the infected target cells. The effector:target ratio was 10:1. After three days, supernatant fluid bathing the target and effector cells was replaced with fresh medium after washing 5 times. After an additional 4 days (total of 10 days after infecting target cells), the supernatant fluid was assayed for p24 by ELISA (Zeptometrix, Minneapolis, MN). The percent virus inhibition was calculated by comparison with negative control samples as previously described. The ADCVI assay was conducted with two different PBMC effector cell donors for each virus strain. Thus, a total of four different donors were used; for each donor, the ADCVI assay was run in triplicate and mean percent virus inhibition is reported.
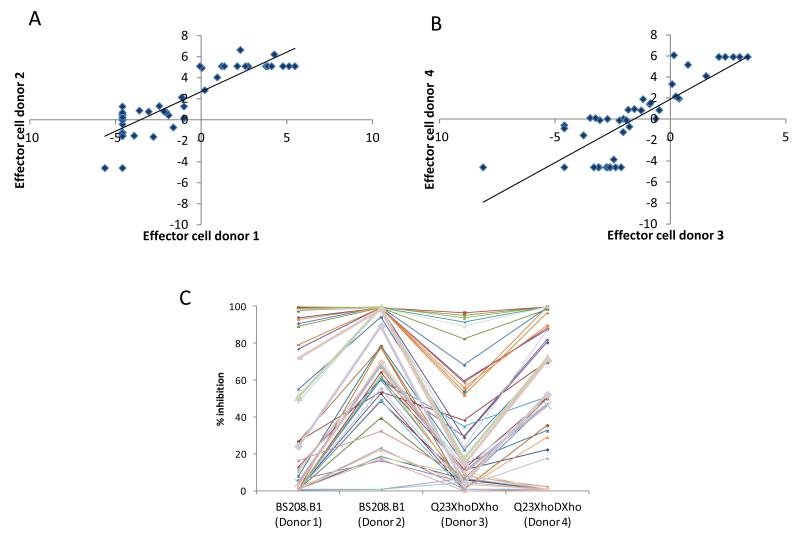
Plasma ADCVI antibody activity ranged from 0 to >95% irrespective of virus strain or effector cell donor. Although absolute ADCVI activity for each virus differed between experiments with different effector cell donors, there was a strong correlation between the results from the two individual donor effector cells when assayed with the same virus (Spearman rho = 0.8; p <0.0001 for both viruses; figure 1). Thus, despite effector cell donor-to-donor variability, the relative antibody activity of plasma stayed similar in repeated assays.
Figure 1.
Correlation between ADCVI assays run on separate days with different effector cell donors. Plasma samples were tested against CEM.NKR-CCR5 cells infected with either HIV-1BS208.B1 (A) or HIV-1Q23XhoΔXho (B) on two separate occasions and with different effector cell donors. Data are shown as logit-transformations of mean % virus inhibition. ADCVI activity for each subject (represented by a unique symbol and line) with each effector cell donor is also shown (C). Each plasma sample was run in triplicate each of the four assays depicted.
These results demonstrate the marked variability in the ADCVI antibody response among infected individuals. We have shown previously that vaccination of healthy individuals with recombinant gp120 also elicits a wide range of ADCVI antibody responses [10]. Although the factors underlying this variability are not clear, Fc-FcγR interactions, which are required for ADCVI, are highly dependent on IgG subclass and on the pattern of Fc glycosylation. Thus, genetic or environmental factors that determine IgG subclass responses to infection and vaccination and that influence Fc glycans are likely to be important.
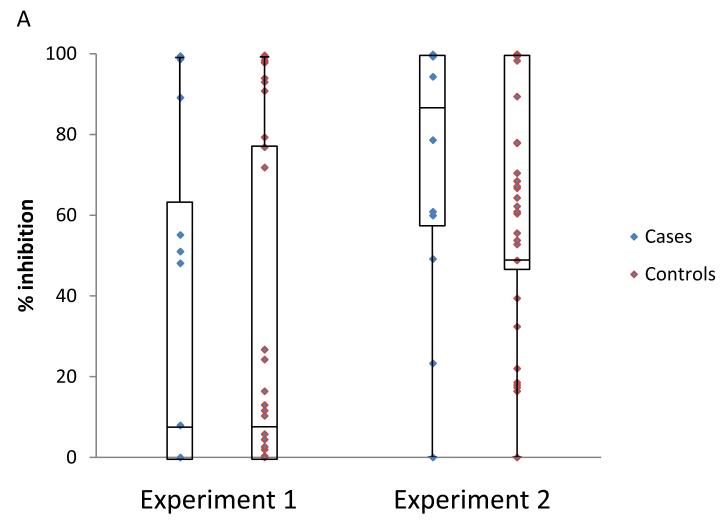
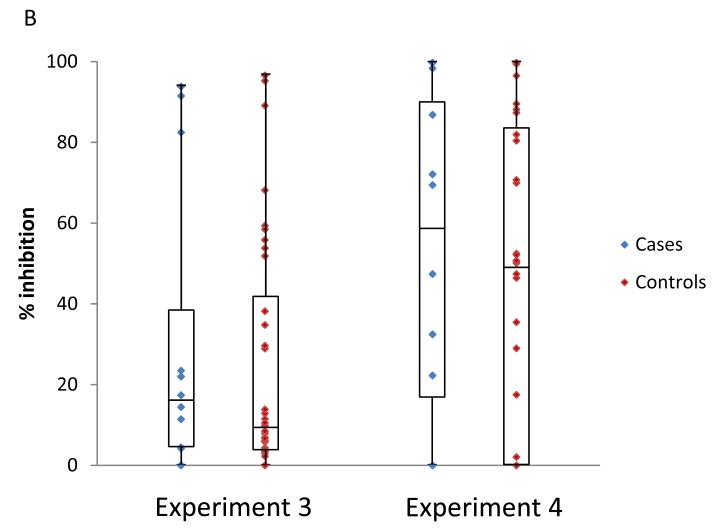
We compared ADCVI activity, as a continuous variable, between superinfected cases and singly infected controls. For target cells infected with the BS208.B1 virus, cases did not differ from controls in either of the two experiments using different effector cell donors (p = 0.98 and p = 0.48; Friedman test; figure 2A). Similarly, there were no differences between cases and controls in either of the experiments against target cells infected with the Q23XhoΔXho virus (p = 0.17 and p = 0.61; Friedman test; figure 2B).
Figure 2.
Association between ADCVI antibody activity and superinfection status. Plasma samples were tested at a dilution of 1:100 against CEM.NKR-CCR5 cells infected with either HIV-1BS208.B1 (A) or HIV-1Q23XhoΔXho (B) in two separate experiments and with different effector cell donors. Each plasma sample was run in triplicate each of the four assays depicted. There are no significant differences between superinfected subjects (cases) and singly infected subjects (controls).
We had previously shown that individuals with the highest quartile of ADCVI responses (≥90% virus inhibition) to recombinant gp120 vaccination in the Vax004 trial had almost ½ the infection rate as those in the lowest quartile [10]. Therefore, we conducted separate analyses using ADCVI data bifurcated at 90%, 80% or 70% virus inhibition. We also quartilized virus inhibition. In all of these Generalized Estimating Equations logistic models, there was no association between ADCVI activity and the odds of a superinfection (data not shown).
These results indicate that the ADCVI antibody response to a primary infection with HIV-1 may not prevent infection after exposure to additional strains. Of particular note, three of the 12 superinfected women had high ADCVI activity (ranging from 82 to >99% virus inhibition) against both viruses tested. Because a study of this size required that we use heterologous virus as a surrogate for measure of protective ADCVI responses, it remains possible that the viruses that established superinfection were not recognized by the relatively potent heterologous responses that we measured. We also cannot rule out the possibility that some of the women who were not superinfected do, in fact, have effective immune responses against strains that were successfully inhibited. In addition, in vivo ADCVI activity depends on the presence of well-functioning FcγR-bearing effector cells presumably at or near sites of exposure. We did not measure effector cell function in this cohort, and individuals who are chronically infected are likely to have defects in FcγR-mediated immune functions [7] [14-16]. Thus, despite broad and potent ADCVI antibody activity, anti-viral activity might be ineffective in vivo. Nonetheless, our results, together with those from some [6, 8, 17] but not other studies [18, 19] provide further evidence that immunity engendered by a primary HIV infection may do little to prevent a subsequent infection upon sexual exposure to another strain.
Acknowledgements
This work was supported by NIH R37-AI038518. DNF drafted the paper and designed the ADCVI experiments; GL conducted the ADCVI experiments; BC identified cases of superinfection and the timing of superinfection; BAR provided statistical analyses; CB and JO designed the case-control study; RSM and WJ provided clinical data and samples; JO developed the overall concept and oversaw the study.
Funding: This work was supported by NIH R37-AI038518.
Footnotes
Conflict of interest: There are no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chohan BH, Piantadosi A, Overbaugh J. HIV-1 superinfection and its implications for vaccine design. Curr HIV Res. 2010;8:596–601. doi: 10.2174/157016210794088218. [DOI] [PubMed] [Google Scholar]
- 2.Smith DM, Wong JK, Hightower GK, Ignacio CC, Koelsch KK, Daar ES, et al. Incidence of HIV superinfection following primary infection. JAMA. 2004;292:1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- 3.Chohan B, Lavreys L, Rainwater SM, Overbaugh J. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J Virol. 2005;79:10701–10708. doi: 10.1128/JVI.79.16.10701-10708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piantadosi A, Chohan B, Chohan V, McClelland RS, Overbaugh J. Chronic HIV-1 infection frequently fails to protect against superinfection. PLoS Pathog. 2007;3:e177. doi: 10.1371/journal.ppat.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, et al. The rates of HIV superinfection and primary HIV incidence in a general population in Rakai, Uganda. J Infect Dis. 2012;206:267–274. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blish CA, Dogan OC, Derby NR, Nguyen MA, Chohan B, Richardson BA, et al. Human immunodeficiency virus type 1 superinfection occurs despite relatively robust neutralizing antibody responses. J Virol. 2008;82:12094–12103. doi: 10.1128/JVI.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner BG, Gryllis C, Wainberg MA. Role of antibody-dependent cellular cytotoxicity and lymphokine-activated killer cells in AIDS and related diseases. J Leukoc Biol. 1991;50:628–640. doi: 10.1002/jlb.50.6.628. [DOI] [PubMed] [Google Scholar]
- 8.Blish CA, Dogan OC, Jaoko W, McClelland RS, Mandaliya K, Odem-Davis KS, et al. Cellular immune responses and susceptibility to HIV-1 superinfection: a case-control study. AIDS. 2012;26:643–646. doi: 10.1097/QAD.0b013e3283509a0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 10.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol. 2007;178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 11.Rainwater S, DeVange S, Sagar M, Ndinya-Achola J, Mandaliya K, Kreiss JK, et al. No evidence for rapid subtype C spread within an epidemic in which multiple subtypes and intersubtype recombinants circulate. AIDS Res Hum Retroviruses. 2005;21:1060–1065. doi: 10.1089/aid.2005.21.1060. [DOI] [PubMed] [Google Scholar]
- 12.Provine NM, Cortez V, Chohan V, Overbaugh J. The neutralization sensitivity of viruses representing human immunodeficiency virus type 1 variants of diverse subtypes from early in infection is dependent on producer cell, as well as characteristics of the specific antibody and envelope variant. Virology. 2012;427:25–33. doi: 10.1016/j.virol.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad A, Morisset R, Thomas R, Menezes J. Evidence for a defect of antibody-dependent cellular cytotoxic (ADCC) effector function and anti-HIV gp120/41-specific ADCC-mediating antibody titres in HIV-infected individuals. J Acquir Immune Defic Syndr. 1994;7:428–437. [PubMed] [Google Scholar]
- 15.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 16.Forthal DN, Landucci G, Keenan B. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res Hum Retroviruses. 2001;17:553–561. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 17.Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420:434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- 18.Basu D, Kraft CS, Murphy MK, Campbell PJ, Yu T, Hraber PT, et al. HIV-1 subtype C superinfected individuals mount low autologous neutralizing antibody responses prior to intrasubtype superinfection. Retrovirology. 2012;9:76. doi: 10.1186/1742-4690-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T, et al. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355:1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]