Abstract
Despite recent advances in therapy, breast cancer remains the second most common cause of death from malignancy in women. Chemotherapy plays a major role in breast cancer management, and combining chemotherapeutic agents with non-chemotherapeutic agents is of considerable clinical interest. Cucurbitacins are triterpenes compounds found in plants of the Cucurbitaceae family, reported to have anti-cancer and anti-inflamatory activities. Previously, we have shown antiproliferative activity of cucurbitacin B (CuB) in breast cancer, and we hypothesized that combining CuB with chemotherapeutic agents can augment their anti-tumor effect. Here, we show that a combination of CuB with either docetaxel (DOC) or gemcitabine (GEM) synergistically inhibited the proliferation of MDA-MB-231 breast cancer cells in vitro. This antiproliferative effect was accompanied by an increase in apoptosis rates. Furthermore, in vivo treatment of human breast cancer orthotopic xenografts in immunodeficient mice with CuB at either low (0.5 mg/kg) or high (1 mg/kg) doses in combination with either DOC (20 mg/kg) or GEM (12.5 mg/kg) significantly reduced tumor volume as compared to monotherapy of each drug. Importantly, no significant toxicity was noted with low dose CuB in combination with either DOC or GEM. In conclusion, combination of CuB at a relatively low concentration with either of the chemotherapeutic agents, DOC or GEM, shows prominent antiproliferative activity against breast cancer cells without increased toxicity. This promising combination should be examined in therapeutic trials of breast cancer.
Keywords: Breast cancer, cucurbitacin B, chemotherapy
Introduction
Breast cancer is the second most common cause of cancer-related deaths in women (1). The goal of cancer therapy is to prolong survival while minimizing unwanted side effects. Chemotherapy continues to be an essential component of breast cancer regimens; however, resistance and toxicity represent major challenges, underscoring the need for new treatment options. Combining chemotherapeutic drugs with new agents may offer novel treatment options to increase survival and improve quality of life for breast cancer patients.
Docetaxel (DOC) and gemcitabine (GEM) are two chemotherapeutic drugs commonly used to treat breast cancer. The taxane agent DOC, is a microtubule inhibitor and has been commonly used either as a single agent in metastatic disease or in combination with other chemotherapeutic agents in early stages of breast cancer (2,3). GEM is a nucleoside analogue with proven activity in the advanced, metastatic stages of this disease (4,5). Cucurbitacins are triterpenes mainly produced by Cucurbitaceae Juss plants, comprising numerous traditional medicinal Chinese herbs. Cucurbitacin B (CuB) has antiproliferative activity against many cancer types including breast (6,7), pancreas (8–10), liver (11,12), brain (13), bone (14), skin (15) and head and neck (16), as well as, leukemia (17–19). In a human breast cancer orthotopic model, CuB reduced tumor size by 50% with no significant toxicities (7). Moreover, CuB in combination with chemotherapeutic agents in pancreatic, skin and head and neck cancers had additive antitumor activity without increased toxicity (8,9,15,16). This promising activity of CuB prompted us to investigate the effect and feasibility of combining CuB with chemotherapeutic agents, to achieve better tumor control with tolerable toxicity in breast cancer.
Materials and Methods
Cell culture and compounds
The human breast cancer cell line MDA-MB-231 was obtained from the American Type Culture Collection and cultured in DMEM(Life Technologies), supplemented with 10% Fetal Bovine Serum (FBS) (HyClone,). CuB was generously provided by CK Life Sciences International (Holdings) Inc. (CKBP002) and also purchased from Sigma-Aldrich (C8499). The two sources of CuB showed similar efficacy and were used interchangeably. DOC and GEM were from Sigma-Aldrich and Eli Lilly, respectively.
Measurement of cellular proliferation
MDA-MB-231 cells (104 cells per well) were placed into 96-well plates and treated with either diluent control(PBS) or drugs as indicated in Fig. 1 legend. Cell proliferation was measured by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays according to the manufacturer’s instructions. The obtained values of 50% effective dose (ED50) were expressed as the concentrations that corresponded to a reduction of cellular growth by 50% compared to control cells. Drug synergy was determined by the isobologram and combination index (CI) methods (CalcuSyn software, version 1.1.1 1996; Biosoft)(20). The isobologram method is a graphical representation of the pharmacologic interaction formed by selecting a desired fractional cell kill and plotting the required individual drug doses on their respective x and y axes. A straight line connects the points; and the observed dose combination of the two agents that achieves a particular fractional cell kill is plotted. Combination data points that fall on the line represent an additive drug-drug interaction, while data points that fall below or above the line represent either synergism or antagonism, respectively. A CI of 1 indicates an additive effect between two drugs, whereas a CI < 1 or CI > 1 indicates synergism or antagonism, respectively.
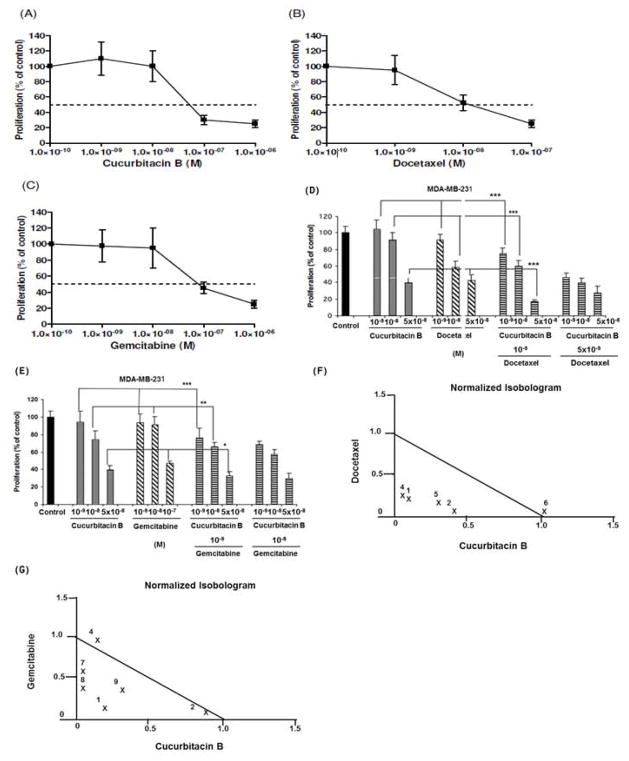
Figure 1. Effect of CuB, DOC and GEM on proliferation of MDA-MB-231 cells in vitro.
MTT-proliferation assays of MDA-MB-231 cells treated for 96 hrs with different concentrations of the following drugs: Panels (A) CuB, (B) DOC, (C) GEM, (D) CuB/DOC or (E) CuB/GEM. (F, G) Normalized isobolograms. Numbers represent different concentrations of Panel (F) CuB/DOC, Panel (G) CuB/GEM. Panel (F) #1: DOC 10−9 mol/L+ CuB 10−9 mol/L; #2: DOC 10−9 mol/L + CuB 10−8 mol/L; #4: DOC 5 × 10−9 mol/L + CuB 10−9 mol/L; #5: DOC 5 × 10−9 mol/L + CuB 10−8 mol/L; #6: DOC 10−8 mol/L + CuB 5 x 10−8 mol/L. Panel (G) #1: GEM 10−9 mol/L + CuB 10−9 mol/L; #2: GEM 10−9 mol/L + CuB 10−8 mol/L; #4: GEM 10−8 mol/L + CuB 10−9 mol/L; #7: GEM 10−7 mol/L + CuB 10−9 mol/L; #8: GEM 10−7 mol/L + CuB 10−8 mol/L; #9: GEM 10−7 mol/L + CuB 5 x 10−9 mol/L; *: p < 0.05; **: p < 0.01; ***: p< 0.001.
Apoptosis analysis
Apoptosis was measured using Annexin V-FITC apoptosis detection kit (BD Biosciences) according to the manufacturer’s protocol.
Murine orthotopic model and tumor treatment
All animal experiments were in accordance with the guidelines of Cedars-Sinai Research Institute and the National Institute of Health. Five-week-old female nu/nu athymic mice were obtained from Harlan Sprague-Dawley. MDA-MB-231 cells (106)in 0.2 mL Matrigel (Basement Membrane Matrix, High Concentration; BD Biosciences) were injected orthotopically in two mammary fat pads of each nude mouse (n = 5 mice/group). Treatments were initiated one day after cell implantation and were given intraperitoneally (i.p.). Tumors were measured with Vernier calipers every 3 days, and tumor volumes were calculated using the formula: (14). Mice were weighed twice a week. At sacrifice, tumors were harvested, weighed,, fixed in 10% neutral-buffered formalin, washed and placed in 70% ethanol embedded in paraffin wax and stained with hematoxylin and eosin (H&E), TUNEL (apoptotic index) and ki-67 (proliferation index). Inhibition rate of tumor growth was calculated using the formula: , where MT and MC are the mean normalized tumor masses of treatment and control groups, respectively.
Toxicity studies
Serum chemistry and blood count analysis
Heparinized blood samples (40 μl/mouse) were obtained on day 0 and at the end of treatment (day 36) by submandibular bleeding. Final blood results were obtained by the Hemagen Analyst® Benchtop Chemistry System (Hemagen Diagnostics, Inc. Columbia, USA).
Colony formation assay
Bone marrow cells from femurs of all groups (3 mice/group) were plated in triplicates in a 6-well plate at 2×104 per well in semi-solid 1% methylcellulose medium (MethoCult GF M3434, StemCell Technologies) containing Stem Cell Factor, IL-3, IL-6, and erythropoietin. Number of colonies (BFU-E: Blast-forming unit-erythroid; CFU-GM: Colony-forming unit-granulocyte/macrophages; CFU-GEMM: Colony-forming unit granulocyte/ erythrocyte/ monocyte/megakaryocyte) were counted on day 10.
Exploration of internal organs
After sacrifice, internal organs were grossly examined for metastatic disease and suspicious lesions were excised, and examined for histopathology as described above for the primary tumors.
Statistical analysis
All in vitro experiments were repeated at least three times. Statistical significance was determined by Student’s t test (two-tailed) when comparing two groups or ANOVA when comparing more than two groups of data set. P values < 0.05 were considered statistically significant.
Results
CuB in combination with either DOC or GEM synergistically inhibits proliferation of MDA-MB-231 cells
The effect of CuB, DOC and GEM on the proliferation of MDA-MB-231 cells is shown in Figs. 1A–C. The calculated ED50s were 5 × 10−8 mol/L for CuB, 10−8 mol/L for DOC, and 10−7 mol/L for GEM. Combinatorial treatment of CuB with either DOC or GEM showed significant growth inhibitory activity (Figs. 1D, E). Synergistic growth inhibition (CI < 0.9) was observed with the combination of DOC (10−9 and 5 × 10−9 mol/L) and CuB (10−9 or 10−8 mol/L), or with the combination of GEM (10−9 mol/L or 10−7 mol/L) with CuB (10−9 mol/L, 10−8 mol/L, or 5 × 10−8 mol/L) (Figs. 1F, G).
Effect of CuB in combination with either DOC or GEM on apoptosis in MDA-MB-231 cells
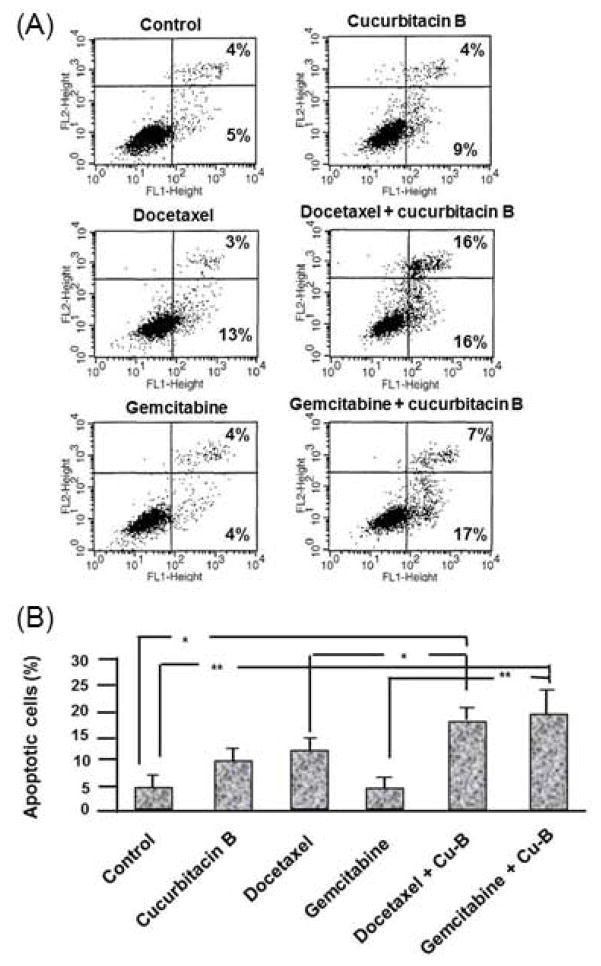
Annexin V/PI staining was used to measure the percent of apoptotic cells in response to the drug treatments (Fig. 2). After 24 hrs of exposure to either CuB, DOC or GEM, 9%, 13% and 4% of the cells were in the early stages of apoptosis, respectively, compared to 5% of control cells. Apoptosis increased in the cells exposed to the combination of CuB with either DOC (16%; p = 0.049) or GEM (17%; p = 0.01). The percent of apoptotic cells increased with prolonged (48 hrs) exposure to GEM in combination with CuB (71 % of the cells, data not shown).
Figure 2. Effect of CuB, DOC and GEM on apoptosis in MDA-MB-231 cells.
(A) Display of the percentage of cells in early (lower right box) and in late (upper right box) apoptosis. (B) Graphic display of the percentage of cells in early apoptosis. *: p < 0.05; **: p < 0.01.
CuB in combination with either DOC or GEM markedly inhibited the growth of MDA-MB-231 breast tumors in an orthotopic murine model
Next, the ability of the combination of CuB with either DOC or GEM to enhance the growth suppression compared to monotherapy of either DOC or GEM was examined in vivo. MDA-MB-231 cells were orthotopically implanted into the mammary fat pads of nude mice and mice were treated with either single agents, drug combinations or diluent control. The experiment ended on day 36 due to excessive tumor size in the control group.
CuB and docetaxel in vivo studies
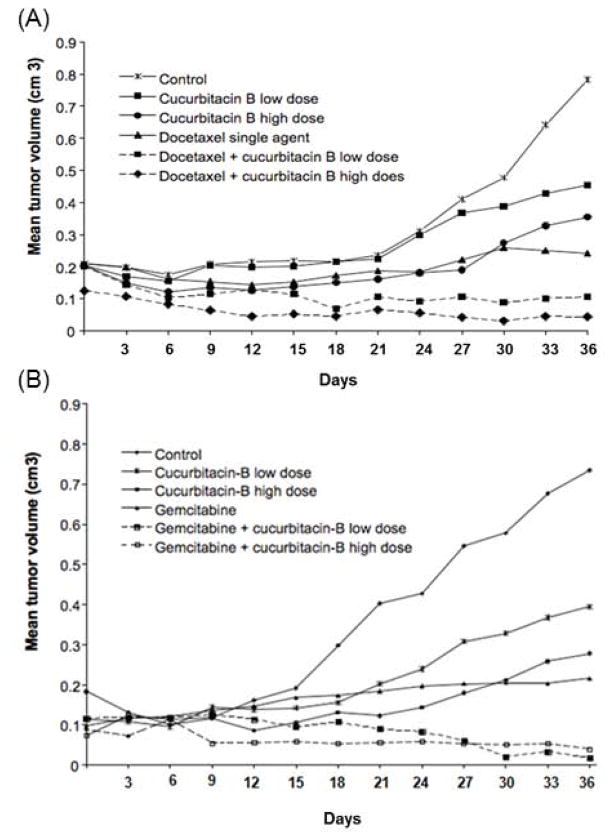
The mean tumor volume was reduced in all treatment groups as compared to control mice: 42% with CuB (low dose), 55% with CuB (high dose), 69% with DOC single agent, and by 90% and 94% with the combination of DOC and either low dose or high dose CuB, respectively (P < 0.01) (Fig. 3A). DOC in combination with CuB either at high or low dose caused significant reduction of tumor size as compared to single agent treatment groups of either CuB low dose, CuB high dose or DOC (P < 0.05). Interestingly, the inhibitory effect of DOC in combination with either high dose CuB or low dose CuB was not statistically different (p = 0.3).
Figure 3. CuB augmented the anti-proliferative activity of DOC and GEM in MDA-MB-231 orthotopic xenografts.
MDA-MB-231 cells (106) were subcutaneously injected in two mammary fat pads of each nude mouse. Mice were treated as follow: Panel (A) CuB/DOC combination: (1) diluent control (PBS, three times/week); (2) low dose CuB (0.5 mg/kg three times/week); (3) high dose CuB (1.0 mg/kg three times/week); (4) single agent DOC (20 mg/kg two times/week); (5) combination CuB (0.5 mg/kg three times/week) and DOC (20 mg/kg two times/week); (6) combination CuB (1 mg/kg three times/week) and DOC (20 mg/kg i.p. two times/week). Panel (B) CuB/GEM combination: (1) diluent control (PBS, three times/week); (2) low dose CuB (0.5 mg/kg three times/week); (3) high dose CuB (1.0 mg/kg three times/week); (4) single agent GEM (12.5 mg/kg two times/week); (5) combination CuB (0.5 mg/kg three times/week) and GEM (12.5 mg/kg i.p. two times/week); (6) combination CuB (1 mg/kg three times/week) and GEM (12.5 mg/kg two times/week). Results represent the mean tumor volume (cm3) of 10 tumors (5 mice) over 36 days of treatment.
CuB and gemcitabine in vivo studies
The mean tumor volume was reduced in all treatment groups as compared to diluent control mice, by 46% with CuB (low dose), 62% with CuB (high dose), 71% with GEM single agent, 97% and 94% with the combination of GEM and either low or high dose CuB, respectively (P < 0.01) (Fig. 3B). Gemcitabine in combination with CuB either at high or low dose caused significant reduction of tumor size as compared to single agent treatment groups of CuB (low dose), CuB (high dose) or GEM (P < 0.05). The inhibitory effect of GEM in combination with either high-dose or low-dose CuB was not statistically different (p = 0.27).
In vivo toxicity of therapies
At the conclusion of the study, all murine cohorts were sacrificed and autopsies were performed; livers, spleens and kidneys were excised. After histological review of all tissue samples, none of organs showed signs of toxicity. All treatment groups continued to gain weight during the period of the study (median 13%, range 10–40%) except for the DOC monotherapy (lost 12% body weight). The latter appeared to lose weight secondary to gastrointestinal toxicity (colon dilation). Toward the end of the study, two mice in the DOC monotherapy were sacrificed secondary to failure to thrive. All treatment groups, which received DOC either as monotherapy or in combination, were found to have dilated colons on autopsy. The degree of colon dilation was less severe in the combination treatment groups.
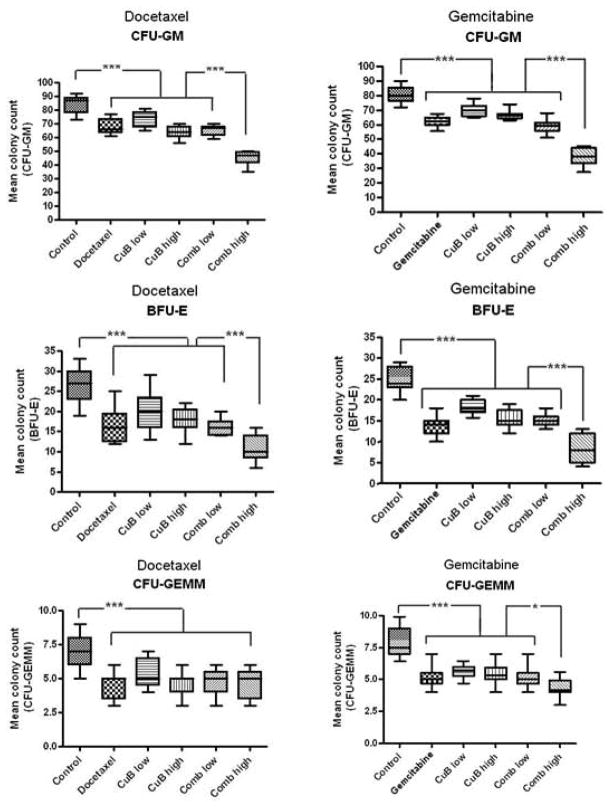
Myelosuppression was the most common side-effect. Leukopenia occurred in the GEM combined with high-dose but not with low-dose CuB. Liver toxicity occurred with both DOC and GEM when combined with high-dose but not with the low-dose CuB (Table 1). In order to examine underlying bone marrow toxicities, we preformed colony formation assays in semi-solid methylcellulose medium (Fig. 4). Colony numbers of all treated animals, either with mono- or combination regimens, showed significantly decreased colony counts compared to control mice (p<0.001). Interestingly, BFU-E, CFU-GM and CFU-GEMM colony numbers from mice treated either with single agent DOC, GEM or CuB were similar to those from mice treated with the low dose combination (all p ≥ 0.18). In contrast, counts for BFU-E and CFU-GM were significantly decreased in mice treated with the high dose CuB combined with either DOC or GEM compared to mice who received either the single compound or the low dose CuB combined with either chemotherapeutic drug (BFU-E: p = 0.009; CFU-GM: p < 0.001). In addition, CFU-GEMM colony numbers were significantly decreased (p < 0.05) in mice treated with the CuB/GEM high combination, while in all other treatment groups CFU-GEMM colony numbers were comparable.
Table 1.
| AST | ALT | BUN | WBC | Hgb | PLT | |
|---|---|---|---|---|---|---|
| Normal value | 54–298 (U/L) | 31–46 (U/L) | 0–140 (mg/dL) | 1.8–10.7 (K/μl) | 11–15.1 (g/dL) | 592–2972 (k/μL) |
| Control | 125 +/−12 | 45+/−10 | 30+/−9 | 2 | 12+/−1 | 829+/−42 |
| Cucurbitacin B low dose | 116 +/−13 | 43+/−3 | 29+/−5 | 5+/−1 | 11+/− 1 | 811+/−57 |
| Cucurbitacin B high dose | 83 +/−58 | 40+/−9 | 108+/−38 | 5+/−2 | 12+/−1 | 847+/−259 |
| Docetaxel | 109 +/−96 | 40+/−8 | 49+/−43 | 6+/−4 | 10+/−2 | 1107+/− 347 |
| Docetaxel + Cucurbitacin B low dose | 101 +/−96 | 46+/−2 | 60+/− 55 | 2+/−1 | 9+/−2 | 559+/−303 |
| Docetaxel + Cucurbitacin B high dose | 423 +/−244* | 50+/−9 | 77+/−28 | 1+/−1* | 11+/13 | 674+/−404 |
| Gemcitabine | 81 +/−24 | 39+/−6 | 33+/−28 | 2+/−1 | 11+/−2 | 470+/−412 |
| Gemcitabine + Cucurbitacin B low dose | 124 +/−88 | 51+/−13 | 36+/−20 | 1 | 9+/−4 | 607+/−341 |
| Gemcitabine + Cucurbitacin B high dose | 241+/−56* | 36+/−9 | 26+/−14 | 4+/−5 | 8 | 490+/−240 |
Mean and standard deviation of 3 mice per treatment group at the end of the study. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; BUN: blood urea nitrogen; WBC: White blood cells; Hgb: hemoglobin; PLT: Platelets;
indicate p <0.05.
Figure 4. Analysis of bone marrow toxicity after drug treatment measured by clonogenic assays.
Bone marrow cells isolated from mice treated either with PBS (control), low dose CuB, high dose CuB, DOC, GEM, or combinations of either DOC or GEM with either low dose or high dose CuB (Comb low, Comb high, respectively; see Fig. 4 for in vivo treatment details, before harvest of bone marrow) were plated in triplicates in semi-solid methylcellulose medium. Box and whisker plots display mean colony counts +/−SD of CFU-GEMM, CFU-GM or BFU-E of three mice per treatment group. *, p < 0.05; **, p = 0.009; ***, p < 0.001.
Discussion
DOC and GEM are among the most commonly used chemotherapic agents in breast cancer treatment. As monotherapies, these drugs show varying overall responses; and better outcome can be achieved by combination therapies, although this is often associated with increased toxicity (21–29). Here, we show clear in vitro anti-proliferative synergism of CuB in combination with either DOC or GEM. Furthermore, in human breast cancer xenografts grown orthotopically in immunodeficient mice, superior anti-tumor activity occurred with the combination therapies, without increase in toxicity.
Although the structure of CuB and other cucurbitacins is known, the cellular and molecular mechanisms by which these compounds exert their anticancer effects are not well understood. Studies suggest that some cucurbitacin compounds act by inhibiting precursor incorporation into DNA, RNA, and proteins as well as by inhibiting NF-kB activation and iNOS gene transcription (30). Recent investigations using breast cancer cells revealed a number of cellular pathways impacted by CuB including down-regulation of hTERT and c-Myc (31), as well as inhibition of Wnt signaling with reduced nucleus translocation of β-catenin (32). In addition, CuB causes increased radiation sensitivity associated with a G2/M cell cycle arrest in breast cancer cells (33).
Apoptosis is a major mechanism accounting for cell death in response to chemotherapeutic agents (34). We found that both combinations of CuB/DOC and CuB/GEM increased apoptosis in MDA-MB-231 cells compared to single agent treatment. The mechanism by which CuB enhances DOC and GEM induced apoptosis is unknown. STAT3 and NF-κB, have been reported to be inhibited by CuB (8,10,11,35) and may contribute to the induction of apoptosis by CuB. Indeed, a combination of CuB and DOC strongly inhibited STAT3 activation, as well as reduced Bcl-2 expression in head and neck cancer cells (16). In several studies the antitumor effects of CuB were associated with Erk phosphorylation (12,8,16). Erk has been reported to promote apoptosis in response to GEM, in part by increasing Bcl-2 activation, through a p53-independent pathway (36). Additional studies will be required to explore how CuB and either DOC or GEM induce apoptosis.
Similar to our in vitro results, our in vivo study using orthotopic xenografts of human breast cancer cells in nude mice showed increased anti-tumor effectiveness with the combination therapies. Importantly, we observed no significant additive toxicity when either of the chemotherapeutic agents were combined with CuB (low dose). Neurosensory and neuromotor toxicities are common in patients treated with DOC and other taxanes. Interestingly, when the DOC was combined with CuB, we noted less neurotoxicity in the colon as manifested by dilated colon compared to DOC monotherapy. CuB may have a protective affect against neurotoxicity of taxanes, but further investigation is warranted. Leukopenia occurred in the combination treatment of CuB high/GEM. However, colony formation assays with bone marrow cells from the treated mice showed lower colony numbers for all treated groups. The discrepancy could be due to modulation in endogenous cytokine levels caused by each agent, which in turn affect the number of white blood cells. An alternative explanation is that CuB high/GEM treatment, but not the other treatments, induced specific changes in the bone marrow microenvironment resulting in leukopenia in the CuB high/GEM treated mice.
Despite recent advances in treatment of metastatic breast cancer, it is still usually an incurable disease promoting the ongoing search for new drug combinations with effective cytotoxicity and good tolerability. In the present study, we show for the first time that the combination of either DOC or GEM with CuB potentiates anti-breast cancer activity without increased side-effects; these findings suggest new combination regimens with potential clinical application for breast cancer.
Novelty & Impact Statements.
Current strategies combining new anticancer drugs with chemotherapy provide clinical benefit for cancer patients. We show that combination of the triterpenoid compound, cucurbitacin B with traditional agents synergistically inhibited proliferation of breast cancer cells in vitro. Furthermore, combination of cucurbitacin B with chemotherapy drugs markedly inhibited tumor growth without increasing toxicity in a murine model of human breast cancer orthotopic xenografts. This promising combination has potential clinical application for breast cancer treatment.
Acknowledgments
supported in part by East Meets West Institutional Funds (Cedars-Sinai Medical Center), NIH grants R01CA026038-32, U54CA143930 and A* STAR Grant.
Footnotes
Conflict of interest: At the time of the preparation of this manuscript M Toh and KT Chan were employees of CK Life Sciences, Inc.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–39. doi: 10.1016/S1470-2045(05)70094-2. Review. [DOI] [PubMed] [Google Scholar]
- 3.Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M, Cold S. Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol. 2006;24:4963–70. doi: 10.1200/JCO.2005.05.0294. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, Walling J, Harris AL. Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol. 1995;13:2731–6. doi: 10.1200/JCO.1995.13.11.2731. [DOI] [PubMed] [Google Scholar]
- 5.Chew HK, Doroshow JH, Frankel P, Margolin KA, Somlo G, Lenz HJ, Gordon M, Zhang W, Yang D, Russell C, Spicer D, Synold T, et al. Phase II studies of gemcitabine and cisplatin in heavily and minimally pretreated metastatic breast cancer. J Clin Oncol. 2009;27:2163–9. doi: 10.1200/JCO.2008.17.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73:56–67. doi: 10.1016/j.bcp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Wakimoto N, Yin D, O’Kelly J, Haritunians T, Karlan B, Said J, Xing H, Koeffler HP. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008;99:1793–7. doi: 10.1111/j.1349-7006.2008.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, Said JW, Koeffler HP. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009;69:5876–84. doi: 10.1158/0008-5472.CAN-09-0536. [DOI] [PubMed] [Google Scholar]
- 9.Iwanski GB, Lee DH, En-Gal S, Doan NB, Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH, Koeffler HP. Cucurbitacin B, a novel in vivo potentiator of gemcitabine with low toxicity in the treatment of pancreatic cancer. Br J Pharmacol. 2010;160:998–1007. doi: 10.1111/j.1476-5381.2010.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Sun C, Shan X, Yang X, Li-Ling J, Deng Y. Inhibition of pancreatic cancer cell growth by cucurbitacin B through modulation of signal transducer and activator of transcription 3 signaling. Pancreas. 2010;39:923–9. doi: 10.1097/MPA.0b013e3181ce719e. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Zhang H, Sun C, Shan X, Yang X, Li-Ling J, Deng Y. Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol. 2009;63:635–42. doi: 10.1007/s00280-008-0780-0. [DOI] [PubMed] [Google Scholar]
- 12.Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, Lee WH, Li M, Chu KH, Toh M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL–7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010;294:118–24. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Yin D, Wakimoto N, Xing H, Lu D, Huynh T, Wang X, Black KL, Koeffler HP. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int J Cancer. 2008;123:1364–75. doi: 10.1002/ijc.23648. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Thoennissen NH, Goff C, Iwanski GB, Forscher C, Doan NB, Said JW, Koeffler HP. Synergistic effect of low-dose cucurbitacin B and low-dose methotrexate for treatment of human osteosarcoma. Cancer Lett. 2011;306:161–70. doi: 10.1016/j.canlet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Leiter A, Yin D, Meiring M, Louw VJ, Koeffler HP. Cucurbitacin B inhibits growth, arrests the cell cycle, and potentiates antiproliferative efficacy of cisplatin in cutaneous squamous cell carcinoma cell lines. Int J Oncol. 2010;37:737–43. doi: 10.3892/ijo_00000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Zhang M, Zhang H, Sun C, Yang X, Deng Y, Ji W. Combined antitumor activity of cucurbitacin B and docetaxel in laryngeal cancer. Eur J Pharmacol. 2008;10;587:78–84. doi: 10.1016/j.ejphar.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Haritunians T, Gueller S, Zhang L, Badr R, Yin D, Xing H, Fung MC, Koeffler HP. Cucurbitacin B induces differentiation, cell cycle arrest, and actin cytoskeletal alterations in myeloid leukemia cells. Leuk Res. 2008;32:1366–73. doi: 10.1016/j.leukres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Chan KT, Li K, Liu SL, Chu KH, Toh M, Xie WD. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010;289:46–52. doi: 10.1016/j.canlet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Zhu JS, Ouyang DY, Shi ZJ, Xu LH, Zhang YT, He XH. Cucurbitacin B induces cell cycle arrest, apoptosis and autophagy associated with G actin reduction and persistent activation of cofilin in Jurkat cells. Pharmacology. 2012;89:348–6. doi: 10.1159/000338757. [DOI] [PubMed] [Google Scholar]
- 20.Chou T, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, Sandbach J, Hyman WJ, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–7. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 22.Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan JP, Llombart AC, Pluzanska A, Rolski J, Melemed AS, Reyes-Vidal JM, Sekhon JS, Simms L, O’Shaughnessy J. Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26:3950–7. doi: 10.1200/JCO.2007.11.9362. [DOI] [PubMed] [Google Scholar]
- 23.Gudena V, Montero AJ, Glück S. Gemcitabine and taxanes in metastatic breast cancer: a systematic review. Ther Clin Risk Manag. 2008;4:1157–64. [PMC free article] [PubMed] [Google Scholar]
- 24.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, Wood WC, Davidson NE. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Schneeweiss A, Lluch A, Llombart A, du Bois A, Kreienberg R, Mayordomo JI, Antón A, et al. Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol. 2009;27:1753–60. doi: 10.1200/JCO.2007.15.8485. [DOI] [PubMed] [Google Scholar]
- 26.Roy V, LaPlant B, Gross G, Bane C, Palmieri F. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531) Ann Oncol. 2009;20:449–53. doi: 10.1093/annonc/mdn661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vici P, Giotta F, DI Lauro L, Brandi M, Gebbia V, Foggi P, Lorusso V, Vitucci C, Sergi D, Fattoruso SI, Giannarelli D, Viola G, et al. Multicenter phase II trial of first-line docetaxel/gemcitabine in advanced breast cancer pretreated with adjuvant anthracyclines. Anticancer Res. 2009;29:1841–5. [PubMed] [Google Scholar]
- 28.Gajria D, Seidman A, Dang C. Adjuvant taxanes: more to the story. Clin Breast Cancer. 2010;10:S41–9. doi: 10.3816/CBC.2010.s.011. Review. [DOI] [PubMed] [Google Scholar]
- 29.Ginés J, Sabater E, Martorell C, Grau M, Monroy M, Casado MA. Efficacy of taxanes as adjuvant treatment of breast cancer: a review and meta-analysis of randomised clinical trials. Clin Transl Oncol. 2011;13:485–98. doi: 10.1007/s12094-011-0686-x. Review. [DOI] [PubMed] [Google Scholar]
- 30.Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep. 2005;22:386–99. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 31.Duangmano S, Dakeng S, Jiratchariyakul W, Suksamrarn A, Smith DR, Patmasiriwat P. Antiproliferative Effects of Cucurbitacin B in Breast Cancer Cells: Down-Regulation of the c-Myc/hTERT/Telomerase Pathway and Obstruction of the Cell Cycle. Int J Mol Sci. 2010;11:5323–38. doi: 10.3390/ijms11125323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dakeng S, Duangmano S, Jiratchariyakul W, U-Pratya Y, Bögler O, Patmasiriwat P. Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: Reduction of Wnt associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus. J Cell Biochem. 2012;113:49–60. doi: 10.1002/jcb.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duangmano S, Sae-Lim P, Suksamrarn A, Patmasiriwat P, Domann FE. Cucurbitacin B Causes Increased Radiation Sensitivity of Human Breast Cancer Cells via G2/M Cell Cycle Arrest. J Oncol. 2012;2012:601682. doi: 10.1155/2012/601682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansilla S, Llovera L, Portugal J. Chemotherapeutic targeting of cell death pathways. Anticancer Agents Med Chem. 2012;12:226–38. doi: 10.2174/187152012800228805. [DOI] [PubMed] [Google Scholar]
- 35.Jin HR, Jin X, Dat NT, Lee JJ. Cucurbitacin B suppresses the transactivation activity of RelA/p65. J Cell Biochem. 2011;112:1643–50. doi: 10.1002/jcb.23078. [DOI] [PubMed] [Google Scholar]
- 36.Chang GC, Hsu SL, Tsai JR, Wu WJ, Chen CY, Sheu GT. Extracellular signal-regulated kinase activation and Bcl-2 downregulation mediate apoptosis after gemcitabine treatment partly via a p53-independent pathway. Eur J Pharmacol. 2004;502:169–83. doi: 10.1016/j.ejphar.2004.09.006. [DOI] [PubMed] [Google Scholar]