Abstract
Ustilago coicis causes serious smut on Coix lacryma-jobi in Dayang Town, Jinyun County, Zhejiang Province of China. In this paper, ultrastructural assessments on fungus-host interactions and teliospore development are presented, and molecular phylogenetic analyses have been done to elucidate the phylogenetic placement of the taxon. Hyphal growth within infected tissues was both intracellular and intercellular and on the surface of fungus-host interaction, and the fungal cell wall and the invaginated host plasma membrane were separated by a sheath comprising two distinct layers between the fungal cell wall and the invaginated host plasma membrane. Ornamentation development of teliospore walls was unique as they appeared to be originated from the exosporium. In addition, internal transcribed spacer (ITS) and large subunit (LSU) sequence data showed that U. coicis is closely related to Ustilago trichophora which infects grass species of the genus Echinochloa (Poaceae).
Keywords: Fungus-host interaction, Molecular phylogenetics, Smut, Teliospore wall development
1. Introduction
Job’s tears (Coix lacryma-jobi), also called as Chinese pearl barley or adley, is a grass (Poaceae) cultivated as a nourishing food eaten in the same way as rice and is believed to have medicinal value (Chang, et al., 2003). Job’s tears smut is one of the most important diseases affecting this plant and occurs in many countries (Titatarn et al., 1983). The causal agent of Job’s tears smut was named as Ustilago coicis Bref. by Small (1927). Although subsequently Mundkur (1940) named the smut fungus infectious on Job’s tears as C. lacryma-jobi Mundkur, Chowdhury (1946) reported it as a synonym with U. coicis.
Smut genera Ustilago and Sporisorium (Ustilaginales) show a great diversity on grasses (Poaceae) (Stoll et al., 2005). Generic delimitation can be characterized by single teliospores/teliospore balls, sori with or without columella and peridium, and sterile cells between the teliospores (Vánky, 1987; Stoll et al., 2003). However, intermediate character combinations have made it difficult for consistent delimitation of these two closely related genera (Vánky, 1985; 1998; Stoll et al., 2003; 2005). Supplementary ultrastructural characters of teliospore wall development of smut fungi have been reported and an attempt is made to research their taxonomic relationships (Piepenbring et al., 1998; Stoll et al., 2003), but these studies have not included U. coicis. Molecular data have been used to delimit genera and species in Ustilaginaceae (Bauer et al., 1997; Begerow et al., 1997; Stoll et al., 2003; 2005). Such studies have led to the revisions of the taxonomic position of several species but did not involve U. coicis.
In this study, we systemically described interactions between U. coicis and C. lacryma-jobi, and the teliospore development at the ultrastructural level. In addition, we investigated the phylogenetic relationships of U. coicis with closely related taxa based on the internal transcribed spacer (ITS) and large subunit (LSU) region sequence analysis.
2. Materials and methods
2.1. Plants
Samples were collected from naturally infected leaves and inflorescences of Job’s tears at different development stages in a field in Dayang Town (Jinyun County, Zhejiang Province, China) in August 2010.
2.2. Electron microscopy
Tissue samples (1 mm×2 mm) with mycelia of U. coicis and also with teliospore sori were fixed in glutaraldehyde (2.5%) in 0.1 mol/L sodium phosphate buffer (pH 7.0), overnight at 4 °C, including 5 min vacuum-infiltration. Samples were washed in the same buffer, and postfixed in osmium tetroxide (0.01 g/ml) in buffer for 2 h at room temperature. Tissues were dehydrated in a graded ethanol series and were then embedded in Spurr’s epoxy resin, and polymerized at 70 °C for 9 h. Infection hyphae within or between cells at precise stages of development were identified by light microscopy and ultrathin sections were cut on a Reichert-Jung Ultracut-E ultramicrotome, collected onto Formvar-coated slot copper grids, and stained with uranyl acetate and lead citrate. Observations were made using a transmission electron microscope at 80 kV and a Gatan 832 CCD camera (Hitachi H-7650, Tokyo, Japan).
2.3. DNA extraction, amplification, and sequencing
The teliospores were collected from samples germinated at 28 °C on potato sucrose agar (PSA) with chloramphenicol and the single sporidia were separated as abundant sporidia were produced on PSA. The single-spore cultures were inoculated in 100 ml of liquid growth media (potato sucrose both (PSB)) in 250-ml flasks, and shaken at 150 r/min at 25 °C for 48 h. Fungal bodies were harvested by filtration, freeze-dried, ground to a final powder in liquid nitrogen, and then stored at −70 °C. About 50 mg of fungal powder was removed into a sterile 1.5 ml microcentrifuge tube, rehydrated in 600 μl of 2× CTAB buffer (100 mmol/L Tris (pH 8.0), 1.4 mol/L NaCl, 30 mmol/L ethylenediaminetetraacetic acid (EDTA), 2% hexadecyltrimethylammonium bromide) and incubated in a water bath at 65 °C for 30–60 min. Following a phenol/chloroform extraction, the genomic DNA was precipitated by isopropanol in the presence of sodium acetate. Genomic DNA was visualized in 1% agrose gels (0.01 g/ml) after ethidium bromide staining (Xie et al., 2010).
The ITS and LSU regions were amplified using ITS1 and ITS4 as well as NL1 and NL4, respectively (O′Donnell, 1993). The purified polymerase chain reaction (PCR) products were inserted into pMDTM19-T vector (TaKaRa Co., Japan) and transformed into Escherichia coli DH5α competent cells. The positive clone was propagated and the recombinant plasmids were extracted using AxyPrep plasmid miniprep kit according to the manufacturer’s instructions (Axygen, USA). In the meantime, they were identified by PCR and restriction endonuclease enzyme digestion. The sequence determination of recombinant plasmid was carried out by the Hangzhou Genomics Institute in both directions. The accession numbers of all sequences used for comparison are listed in Table 1.
Table 1.
List of species studied
| Species | Host | Origin | GenBank acc. No. (ITS/LSU)1 | Source2 |
| Sporisorium aegypticum (A.A. Fisch. Waldh.) Vánky | Schismus arabicus | Iran | AY344970/AY740129 | Ust. Exs. 756 (M) |
| S. andropogonis (Opiz) Vánky | Bothriochloa saccharoides (as Andropogon saccharoides) | Ecuador | AY740042/AY740095 | 56588 (M) |
| S. andropogonis (Opiz) Vánky | Bothriochloa saccharoides | Bolivia | AY740043/AY740096 | Stoll et al., 2005 |
| S. andropogonis-micranthi (L. Ling) L. Guo (as S. capillipedii) | Capillipedium spicigerum | Australia | AY740047/AY740100 | 56595 (M) |
| S. anthracoideisporum Vánky & R.G. Shivas | Pseudoraphis spinescens | Papua; New Guinea | AY740044/AY740097 | Stoll et al., 2005 |
| S. apludae-aristatae (B.V. Patil & Thirum.) Vánky | Apluda mutica | India | AY740045/AY740098 | 56590 (M) |
| S. arthraxonis (Pat.) L. Guo | Arthraxon lanceolatus | China | AY740046/AY740099 | 56592 (M) |
| S. bursum (Berk.) Vánky | Themeda quadrivalvis | India | AY740154 | Ust. Exs. 844 (M) |
| S. cenchri (Lagerh.) Vánky | Cenchrus pilosus | Nicaragua | AY344972/AF453943 | MP 1974 (TUB) |
| S. chrysopogonis Vánky | Chrysopogon fulvus | Sri Lanka | AY344973/AY740131 | Ust. Exs. 407 (M) |
| S. consanguineum (Ellis & Everhart) Vánky | Aristida uruguayensis | Argentina | AY740048/AY740101 | H.U.V. 19145 (TUB) |
| S. cordobense (Spegazzini) Vánky | Digitaria insularis | Bolivia | AY740155 | Stoll et al., 2005 |
| S. cruentum (J.G. Kühn) Vánky | Sorghum halepense | USA | AY344974/AF453939 | Ust. Exs. 687 (M) |
| S. cruentum (J.G. Kühn) Vánky | Sorghum bicolor | Nicaragua | AY740156 | Stoll et al., 2005 |
| S. culmiperdum (J. Schröt.) Vánky | Andropogon gerardii | Honduras | AY344975/AF133580 | MP 2060 (TUB) |
| S. destruens (Schltdl.) Vánky | Panicum miliaceum | Romania | AY344976/AY747077 | Ust. Exs. 472 (M) |
| S. dimeriae-ornithopodae Vánky & C. Menge | Dimeria ornithopoda | India | AY344977/AY740132 | Ust. Exs. 848 (M) |
| S. elionuri (Henn. & A. Evans) Vánky | Elionurus muticus | Bolivia | AY740157 | Stoll et al., 2005 |
| S. enteromorphum (McAlpine) Vánky | Themeda triandra | South Africa | AY740158 | 56602 (M) |
| S. erythraeense (Syd. & P. Syd.) Vánky | Hackelochloa granularis | India | AY740049/AY740102 | Ust. Exs. 849 (M) |
| S. everhartii (Ellis & Galloway) M. Piepenbr | Andropogon virginicus | Cuba | AY740159 | Stoll et al., 2005 |
| S. fastigiatum Vánky | Andropogon angustatus | Nicaragua | AY344978/AY740133 | Stoll et al., 2005 |
| S. formosanum (Sawada) Vánky | Panicum repens | Taiwan, China | AY344979/AY740134 | Ust. Exs. 688 (M) |
| S. foveolati (Maire) Vánky | Eremopogon foveolatus | Canary Islands | AY740050/AY740103 | MP 2365 (TUB) |
| S. holwayi (G.P. Clinton & Zundel) Vánky | Andropogon bicornis | Panama | AY344980/AF453941 | Stoll et al., 2005 |
| S. hwangense Vánky & C. Vánky | Sporobolus panicoides | Zimbabwe | AY740051/AY740104 | 56607 (M) |
| S. lacrymae-jobi (Mundkur) Vánky | Coix lacryma-jobi | India | AY740052/AY740105 | 56611 (M) |
| S. lepturi (Thüm.) Vánky | Hemarthria uncinata | Australia | AY344981/AY740135 | Ust. Exs. 966 (M) |
| S. lepturi (Thüm.) Vánky | Hemarthria uncinata | Australia | AY740160 | 56613 (M) |
| S. loudetiae-pedicellatae Vánky & C. Vánky | Loudetia pedicellata | South Africa | AY740053/AY740106 | 56615 (M) |
| S. manilense (Syd. & P. Syd.) Vánky (as S. sacciolepidis) | Sacciolepis indica | India | AY740059/AY740112 | Ust. Exs. 854 (M) |
| S. mishrae Vánky | Apluda mutica | India | AY344983/AY740136 | Ust. Exs. 967 (M) |
| S. modestum (Syd.) H. Scholz | Enneapogon avenaceus | Australia | AY740054/AY740107 | 56617 (M) |
| S. monakai (Mishra) Vánky | Isachne globosa | India | AY740161 | 56618 (M) |
| S. moniliferum (Ellis & Everh.) L. Guo | Heteropogon contortus | Indonesia | AY344984/AF453940 | Ust. Exs. 851 (M) |
| S. nealii (Ellis & F.W. Anderson) Vánky | Heteropogon melanocarpus | India | AY740055/AY740108 | 56621 (M) |
| S. neglectum (Niessl) Vánky | Setaria pumila | Germany | AY740056/AY740109 | RB 2056 (TUB) |
| S. nervosum Vánky, C. Vánky & R.G. Shivas | Sehima nervosum | Australia | AY740057/AY740110 | 56622 (M) |
| S. occidentale (Seym.) Vánky & Snets | Andropogon gerardii | USA | AY344985/AY740137 | Ust. Exs. 758 (M) |
| S. ophiuri (Henn.) Vánky | Rottboellia cochinchinensis | Unknown | AY740019/AJ236136 | HB 20 |
| S. ovarium (Griffiths) Vánky | Urochloa fasciculata (Sw.) R. Webster | Mexico | AY740020/AJ236137 | Stoll et al., 2005 |
| S. paspali-notati (Henn.) M. Piepenbr | Paspalum notatum | Cuba | AY344982/AF453944 | Stoll et al., 2005 |
| S. penniseti (Maire) Vánky (as S. catharticum) | Pennisetum setaceum | Canary Islands | AY344971/AY740130 | MP 2367 (TUB) |
| S. polliniae (Magnus) Vánky | Andropogon distachyos | Greece | AY344987/AY740138 | Ust. Exs. 690 (M) |
| S. provinciale (Ellis & Galloway) Vánky & Snets | A. gerardii | USA | AY344988/AY747076 | Ust. Exs. 759 (M) |
| S. pseudechinolaenae Vánky & C. Menge | Pseudechinolaena polystachya | Indonesia | AY344989/AY740139 | Ust. Exs. 853 (M) |
| S. puellare (Syd. & P. Syd.) G. Deml | Hyparrhenia hirta | Canary Islands | AY740058/AY740111 | MP 2372 (TUB) |
| S. pulverulentum (Cooke & Massee) Vánky | Saccharum strictum | Yugoslavia | AY740162 | 56627 (M) |
| S. reilianum (J.G. Kühn) Langdon & Full. | Sorghum halepense | Greece | AY740163 | Ust. Exs. 527 (M) |
| S. scitamineum (Syd.) M. Piepenbr., M. Stoll & Oberw (as Ustilago scitaminea) | Saccharum sp. | Cuba | AY345007/AY740147 | Stoll et al., 2005 |
| S. scitamineum (Syd.) M. Piepenbr., M. Stoll & Oberw (as U. scitaminea) | Saccharum sp. | Costa Rica | AY740070/AJ236138 | Stoll et al., 2005 |
| S. sorghi Ehrenb. ex Link | Sorghum bicolor | Nicaragua | AY740021/AF009872 | Stoll et al., 2005 |
| S. themedae-arguentis Vánky | Themeda arguens | Indonesia | AY344991/AY740140 | Ust. Exs. 855 (M) |
| S. trachypogonicola Vnky & C. Vnky | Trachypogon plumosus | Cuba | AY344992/AY740141 | Stoll et al., 2005 |
| S. trachypogonis-plumosi Vánky | T. plumosus | Venezuela | AY740060/AY740113 | 56635 (M) |
| S. tumefaciens (McAlpine) Vánky (as Sorosporium tumefaciens) | Chrysopogon aciculatus | Sri Lanka | AY344969/AY740128 | Ust. Exs. 231 (M) |
| S. veracruzianum (Zundel & Dunlap) M. Piepenbr | Panicum viscidellum | Costa Rica | Y344993/AY740114 | MP 960 |
| S. veracruzianum (Zundel & Dunlap) M. Piepenbr | P. viscidellum | Costa Rica | AY747075/AY740142 | MP 735 (USJ) |
| Ustilago affinis Ellis & Everh | Stenotaphrum secundatum | Costa Rica | AY344995/AF133581 | Stoll et al., 2005 |
| U. alcornii Vánky | Tripogon loliiformis | Australia | AY740165 | 56514 (M) |
| U. altilis Syd. | Triodia pungens | Australia | AY740166 | Ust. Exs. 418 (M) |
| U. austro-africana Vánky & C. Vánky | Enneapogon cenchroides | Zimbabwe | AY740061/AY740115 | 56516 (M) |
| U. avenae (Pers.) Rostr. | Arrhenaterum elatius | Germany | AY740063/AY740117 | DB 559 (TUB) |
| U. avenae (Pers.) Rostr. | A. elatius | Germany | AY740062/AY740116 | RB 3092 (TUB) |
| U. avenae (Pers.) Rostr. | Avena barbata | Canary Islands | AY344997/AF453933 | MP 2362 (TUB) |
| U. avenae (Pers.) Rostr. | A. barbata | Italy | AY344996/AJ236140 | F 946/GD 1292 (TUB) |
| U. bouriquetii Maubl. & Roger | Stenotaphrum dimidiatum | Réunion | AY740167 | 56517 (M) |
| U. bromivora (Tul. & C. Tul.) A.A. Fisch. Waldh | Bromus catharticus | Argentina | AY740064/AY740118 | H.U.V. 19322 |
| U. bullata Berk | B. diandrus | Canary Islands | AY344998/AF453935 | MP 2363 (TUB) |
| U. calamagrostidis (Fuckel) G.P. Clinton | Calamagrostis epigeios | Bulgaria | AY740065/AY740119 | 56518 (M) |
| U. coicis Bref | Coix lacryma-jobi | China | JX219371/JX219374 | In this study |
| U. crameri Körnicke | Setaria italica | India | AY344999/AY740143 | Ust. Exs. 995 (M) |
| U. cynodontis (Pass.) P. Henn | Cynodon dactylon | Mexico | AY345000/AF009881 | Stoll et al., 2005 |
| U. cynodontis (Pass.) P. Henn | C. dactylon | Taiwan, China | AY740168 | MS 1 (TUB) |
| U. davisii Liro | Glyceria multiflora | Argentina | AY740169 | H.U.V. 19252 |
| U. drakensbergiana Vánky | Digitaria tricholaenoides | South Africa | AY740170 | 56523 (M) |
| U. echinata J. Schröt. | Phalaris arundinacea | Germany | AY345001/AY740144 | Ust. Exs. 540 (M) |
| U. esculenta P. Henn | Zizania latifolia | Taiwan, China | AY345002/AF453937 | Ust. Exs. 590 (M) |
| U. esculenta P. Henn | Zizania latifolia | China | JX219372/JX219375 | In this study |
| U. esculenta P. Henn | Zizania latifolia | China | JX219373/JX219376 | In this study |
| U. filiformis (Schrank) Rostr | Glyceria fluitans | Germany | AY740066/AY740120 | RB 3011 (TUB) |
| U. hordei (Pers.) Lagerh (as U. kolleri) | Avena sativa | Spain | AY740068/AY740122 | F 947/GD 1300 |
| U. hordei (Pers.) Lagerh | Hordeum vulgare | Iran | AY345003/AF453943 | Ust. Exs. 784 (M) |
| U. ixophori Durán | Ixophorus unisetus | Costa Rica | AY740067/AY740121 | Stoll et al., 2005 |
| U. maydis (Link) Unger | Zea mays L. | Germany | AY345004/AF453938 | RB 3093 (TUB) |
| U. nuda (Jens.) Rostr | Hordeum leporinum | Unknown | AY740069/AJ236139 | H.U.V. 17782 |
| U. pamirica Golovin | Bromus gracillimus | Iran | AY345005/AY740145 | Ust. Exs. 789 (M) |
| U. schroeteriana Henn | Paspalum paniculatum | Costa Rica | AY345006/AY740146 | Ust. Exs. 887 (M) |
| U. spermophora Berk. & M.A. Curtis | Eragrostis ferruginea | Namibia | AY740171 | F 565/H.U.V. 13634 |
| U. striiformis (Westend.) Niessl | Alopecurus pratensis | Germany | AY740172 | H.U.V. 18286 |
| U. syntherismae (Schwein.) Peck | Digitaria ternata | India | AY740071/AY740123 | Ust. Exs. 998 (M) |
| U. tragana Zundel | Tragus berteronianus | Zimbabwe | AY740072/AY740124 | 56562 (M) |
| U. trichophora (H.F. Link) F. Körnicke | Echinochloa colona | Cuba | AY345009/AY740148 | Stoll et al., 2005 |
| U. trichophora (H.F. Link) F. Körnicke | E. colona | Mexico | AY740023/AJ236141 | Stoll et al., 2005 |
| U. trichophora (H.F. Link) F. Körnicke | E. colona | India | AY740073/AY740125 | 56564 (M) |
| U. triodiae Vánky | Triodia microstachya | Australia | AY740074/AY740126 | H.U.V. 17662 |
| U. triodiae Vánky | T. microstachya | Australia | AY740075/AY740127 | 56566 (M) |
| U. turcomanica Tranzschel | Eremopyrum distans | Iran | AY345011/AF453936 | F 585/H.U.V. 23 |
| U. vetiveriae Padwick | Vetiveria zizanioides | Unknown | AY345011/AY740149 | H.U.V. 17954 |
| U. xerochloae Vánky & R.G. Shivas | Xerochloa imberbis | Australia | AY345012/AY740150 | Ust. Exs. 1000 (M) |
ITS/LSU: ITS sequence and LSU sequence or single accession numbers with contiguous sequences (ITS and LSU)
GD: Günter Deml; RB: Robert Bauer; Ust. Exs.: Ustilaginales Exsiccata; H.U.V.: Herbarium Ustilaginales Vánky; M: München, Germany; TUB: Tübingen, Germany; MP: Meike Piepenbring; HB: Hansjörg Prillinger; F: Franz Oberwinkler; MS: Matthias Stoll
2.4. Phylogenetic analyses
Phylogenetic analyses were performed using PAUP* 4.0b10 (Swofford, 2002). Phylogenetic trees were built using the maximum parsimony (MP) method. In the MP analyses, trees were inferred using the heuristic search option with tree bisection and reconnection (TBR) branch swapping and 1 000 random sequence additions. Gaps were treated as missing data and characters were equally weighted. Maxtrees were unlimited, branches of zero length were collapsed, and all parsimonious trees were saved. Bootstrap analyses were based on 1 000 replications, each with 10 replicates of random stepwise addition of taxa. Kishino-Hasegawa (KH) tests were performed in order to determine whether trees were significantly different (Kishino and Hasegawa, 1989). Trees were figured using TreeView (Page, 1996).
3. Results
3.1. Interaction between Ustilago coicis and Coix lacryma-jobi
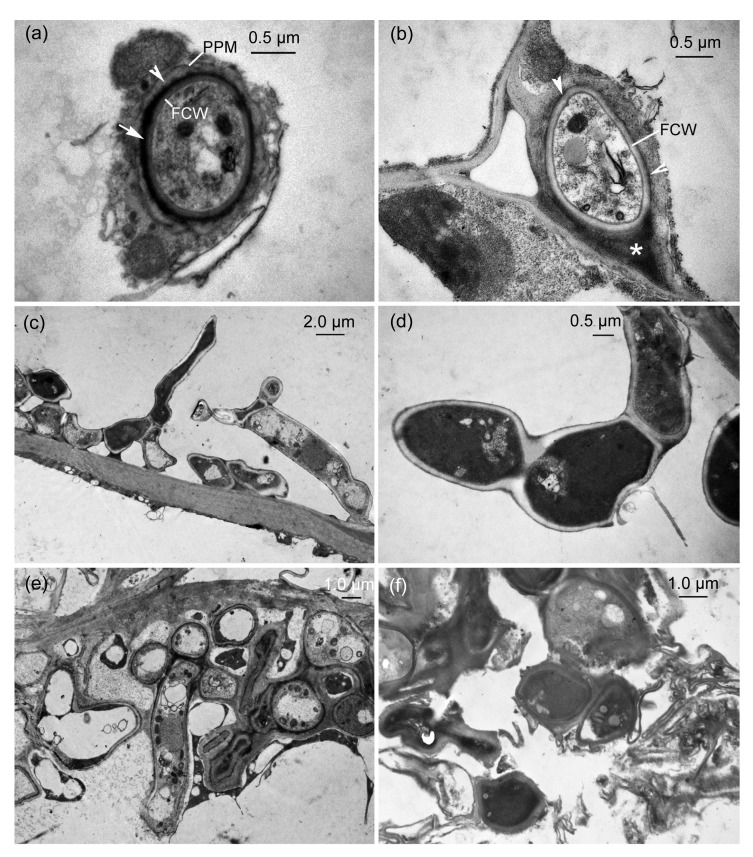
Before teliospore formation, transmission electron micrographs (TEMs) showed that hyphae of U. coicis were present between and within plant cells in parenchyma tissue of an ovary. The intracellular hypha growing in a cell was surrounded by a sheath comprising two distinct layers, which separated the fungal cell wall from the invaginated host plasma membrane (Fig. 1a). The plant plasma membrane was continuous but occasionally it was convoluted at some sites. The outer layer of a sheath was an electron-opaque layer surrounded by the plant plasma membrane and an inner electron-dense layer covering the hyphal wall. However, the sheaths were variable between and within parenchyma tissue. The intercellular hypha development caused intercellular space enlargement and elongation of plant cell walls but intercellular hyphal walls were occasionally only coated by an inconspicuous electron-dense layer (Fig. 1b).
Fig. 1.
Transmission electron micrographs (TEMs) of the interaction between Ustilago coicis and ovarian parenchyma of Coix lacryma-jobi
(a) Transverse section of an intracellular hypha. A hypha is surrounded by a sheath comprising electron-opaque layer (arrowhead) and electron-dense layer (arrow), which is located between the plant plasma membrane (PPM) and the fungal cell wall (FCW). (b) Transverse section of an intercellular hypha. Hyphae induce intercellular space enlargement (*) and FCW is covered by an inconspicuous electron-dense layer in some areas (arrowheads). (c) Sporogenous hyphae. (d) A magnified sporogenous hyphae. (e) Abundant hyphae in a host cell. (f) Disintegrated host tissues with fungal hyphae
In some cells with intracellular fungal development, the hyphae produced short or lateral branches (Fig. 1c). In this case the host protoplast appeared to be disintegrated and these hyphae were not surrounded by a sheath (Figs. 1c and 1d). They grew and branched extensively until individual host cells became filled with contorted and coiled hyphae, which constituted the hyphal aggregations (Fig. 1e). These hyphae were sporogenous hyphae and their hyphal aggregations were the sites of teliosporogenesis. Subsequently, the aggregations caused host cells to disintegrate, resulting in large intercellular spaces in the parenchyma tissue of an affected ovary.
3.2. Teliospore development
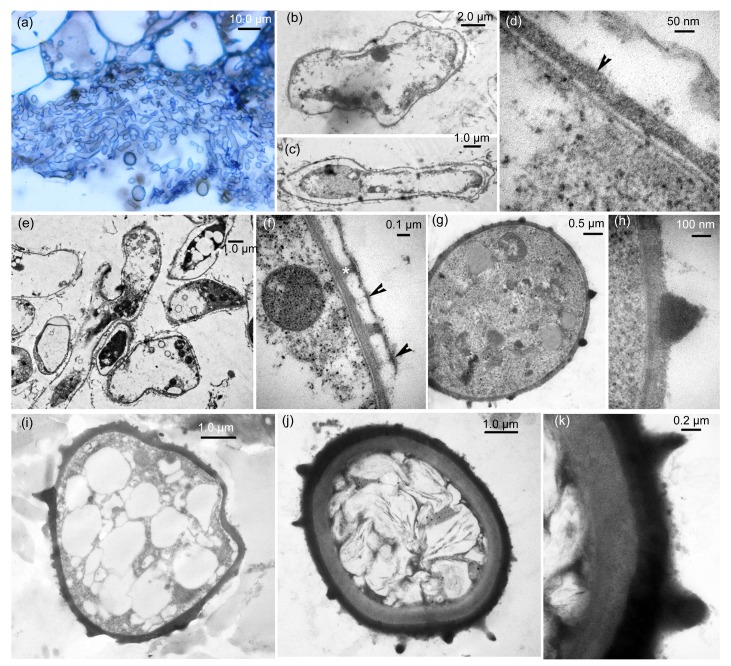
In a large intercellular space, abundant hyphal fragments were seen under the light microscope (Fig. 2a) and they had originated from the sporogenous hyphae. Under TEM, the hyphal fragments began to plasmolyze (Figs. 2b and 2c) and the initial exosporia of the young teliospores were produced on the plasma membranes of the plasmolyzed hyphal fragments (Fig. 2d). In this case, a young teliospore was in the completely plasmolyzed cell wall, but the fungal cell wall was still intact. With teliospore development, the electron-opaque warty ornamentation produced from the exosporium of a young teliospore (Fig. 2e) but it appears to be homogenous with the exosporium. Fungal cell walls began to disintegrate but some remnants of fungal cell wall were retained on top of warts (Fig. 2f) and young teliospores were irregular. With further development, the warty ornamentation became electron-dense and young teliospores were regularly spherical or subspherical with a few lipid globules (Figs. 2g and 2h). At this time, the exosporium also became electron-dense and was covered by spiny ornamentation, and young teliospores exhibited more lipid globules (Fig. 2i). Subsequently, an electron-opaque endosporium in a young teliospore developed beneath the exosporium (Fig. 2j). The mature teliospore wall was comprised of an endosporium of mostly constant thickness and an exosporium covered by warts (Fig. 2k).
Fig. 2.
Development of teliospore walls as seen by light microscopy and transmission electron micrograph (TEM)
(a) Light microscopy. Abundant hyphal segments produced from sporogenous hyphae in a large intercellular space. (b–k) TEM. (b, c) Plasmolyzed process of hyphal segments: (b) a hyphal segment being plasmolyzed; (c) a completely plasmolyzed hyphal segment. (d) Exosporium (arrowhead) of a young teliospore produced on plasma membrane in plasmolyzed process. (e) Warty ornamentation formation and fungal cell walls being degraded. (f) A magnified view of warty ornamentation (*) with the remnant (arrowheads) of fungal cell wall in (e). (g) A regularly young teliospore with electron-dense warty ornamentation. (h) Magnified view of a conical wart in (g). (i) A young teliospore with electron-dense exosporium and spines. (j) A well-developed wall of teliospore with endosporium, exosporium, and warts. (k) Magnified view of a teliospore wall in (j)
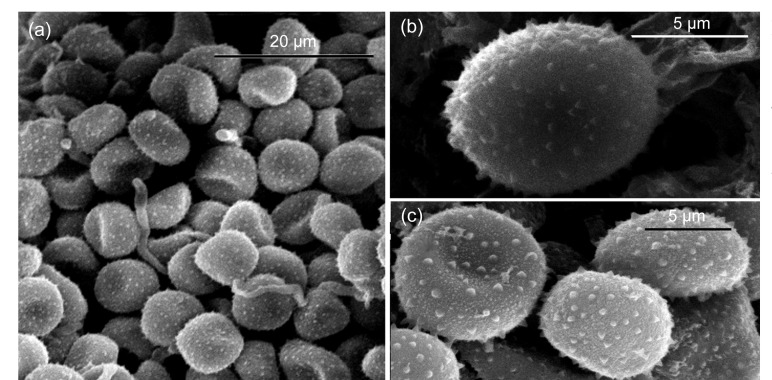
As teliospores of U. coicis matured, sori became darker and eventually black. SEM of sori revealed masses of matured teliospores (Fig. 3a). Individual teliospores were fine spines or warts (Figs. 3b and 3c) and usually between 9 and 11 μm in diameter.
Fig. 3.
Scanning electron micrographs (SEMs) of mature teliospores of Ustilago coicis in cupules of Coix lacryma-jobi
(a) Sorus. (b, c) Mature teliospores clearly showing the surface spines or warts
3.3. Phylogenetic analysis
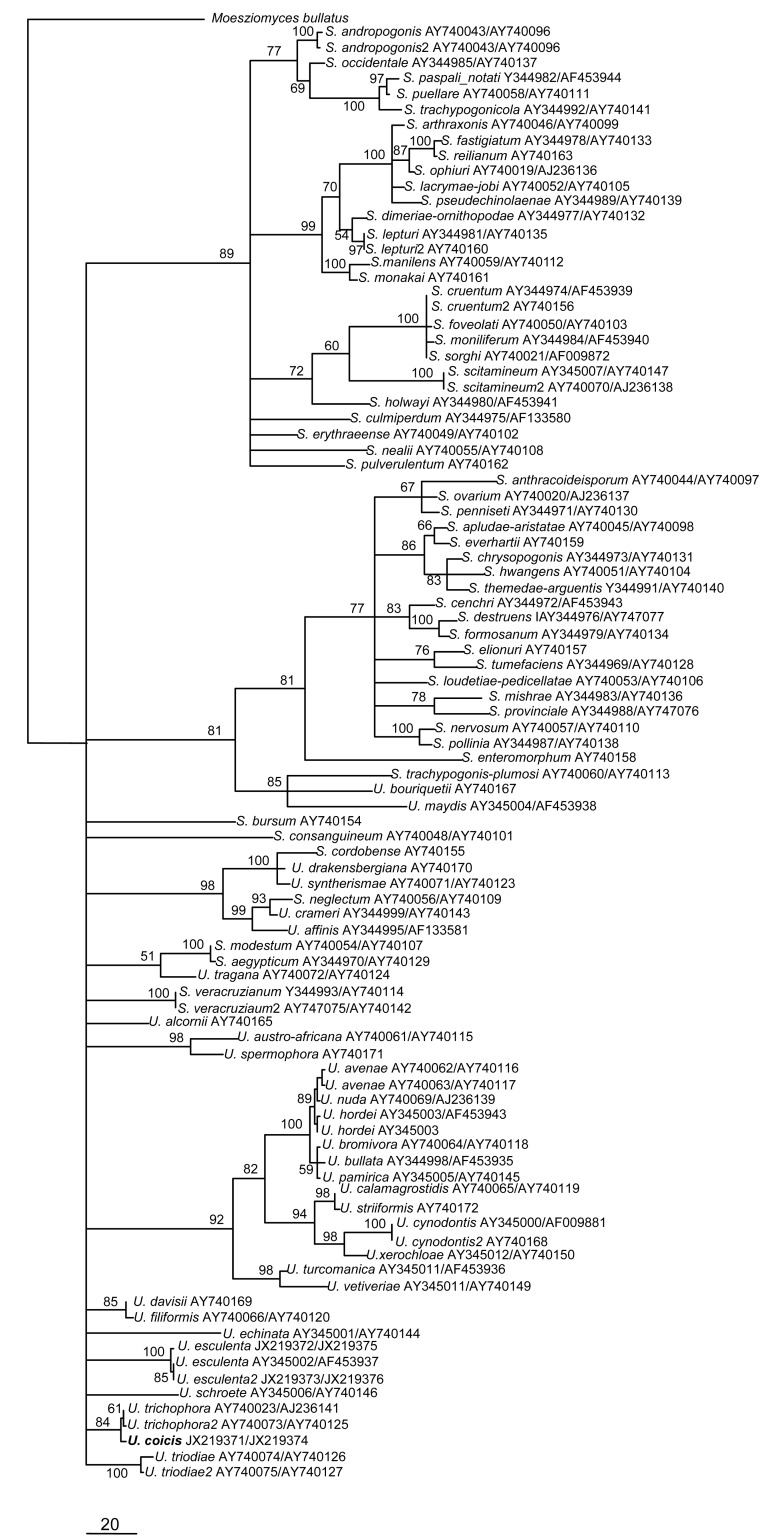
Sequence analysis showed that ITS/5.8S and LSU regions from three isolates of U. coicis were identical and one of them, isolate UCDY01, had been submitted to the GenBank database (Table 1). The final ITS/5.8S and LSU dataset included 95 sequences with 1 316 characters after alignment. Parsimony analysis resulted in 52 equally parsimonious trees. The KH test showed that these trees were not significantly different. One of these trees is shown in Fig. 4. The taxa name and GenBank number of each sequence are given on the tree. One isolate of U. coicis formed a monophyletic lineage and clustered together with U. trichophora with 84% bootstrap support when Moesziomyces bullatus (AB369259) was used as the outgroup taxon. Teliospores of U. trichophora are globose and ornamented with spines, and are similar to those of U. coicis (7–14 μm wide compared with 6–14 μm wide in U. trichophora) (Fullerton and Langdon, 1968; Titatarn et al., 1983). U. trichophora is a pathogen infecting the ovaries and vegetable parts of Echinochloa grass species and exhibits intermediate morphlogical characters between Ustilago and Sporisorium.
Fig. 4.
Phylogram generated from parsimony analysis based on combined ITS and LSU sequences in Ustilago spp. and Sporisorium spp.
Bootstrap values ≥50% are shown above or below branches
4. Discussion
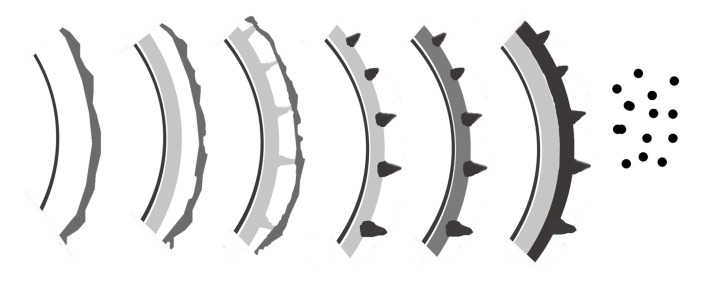
U. coicis can infect the ovaries and leaves of C. lacryma-jobi and cause Job’s tears smut. In infected tissues, hyphal distribution is both intercellular and intracellular. At the ultrastructural level, it can be seen that intracellular hypha at the interface of fungus-host interaction is encased by a sheath with an outer electron-opaque layer and an inner electron-dense layer, as shown in other species of Ustilago, such as U. esculenta (Zhang et al., 2012). The teliosporogenesis process in U. coicis is similar to those of many species of Ustilago-infecting members of Poaceae. Teliospores are covered by fine to coarse warts or by warts of different sizes on the same teliospore. However, ornamentation development in U. coicis is unique. During teliosporogenesis in almost all smut fungi, the outer layers are usually deposited first. At the beginning of ornamentation formation, the plasma membrane may be smooth or undulated, carrying the developing ornaments on its tips or in its depressions (Piepenbring et al., 1998). By contrast, the ornamentation of teliospores in U. coicis appeared to be produced on the exosporium of a young teliospore (Fig. 5), never from the plasma membrane, being different from developmental patterns of ornamentation of Ustilago (Piepenbring et al., 1998; Zhang et al., 2012). This shows that teliospore development in Ustilago is diverse.
Fig. 5.
Schematic drawing of teliospore wall development (from left to right) of Ustilago coicis
Parts of walls are shown in section, and ornamentation also in surface view. The process of a teliospore wall development is plasmolyzation of hyphal segment, exosporium deposition, formation of the ornamentation, and endosporium deposition
The phylogenetic relationship between U. coicis and its closely related species in Ustilago was analyzed based on combined ITS and LSU sequence data. The phylogenetic analysis clearly indicates that U. coicis is closely related to U. trichophora.
Although peridia, columella, spore balls, and sterile cells have all been used as morphological characters to distinguish Ustilago from Sporisorium, sori of U. trichophora contain a columella and are packed by a peridium (Fullerton and Langdon, 1968), which are typical Sporisorium characters (Piepenbring, 2003), so U. trichophora was considered to demonstrate intermediate morphological characters (Piepenbring et al., 1998) between these genera. In Ustilago spp., U. trichophora and U. coicis have the same ornamentation with spines and could be not well differentiated by overlapping teliospore size (Fullerton and Langdon, 1968; Titatarn et al., 1983). Piepenbring (2004) and Stoll et al. (2005) found that it was because morphological characters were non-homologous and had been misused as a taxonomic standard for defining the genera, resulting in the genus complex, as shown in Fig. 4. Therefore, additional molecular loci are needed to resolve the Ustilago-Sporisorium complex, and should include morphological characters.
5. Conclusions
In this article, we showed, for the first time, that there is a unique development of teliospore walls of U. coicis in Ustilaginaceae. In addition, phylogeny and fungus-host interactions of U. coicis were ultra-structurally assessed. Therefore, this research can provide more valuable information for taxonomy and biology in Ustilaginaceae.
Acknowledgments
The authors would like to thank Mrs. Zi-lan XIAO (Biotechnology Institute of Zhejiang University, China) for the manuscript formatting.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31070123) and the Special Fund for Agro-scientific Research in the Public Interest of China (No. 201003004)
Compliance with ethics guidelines: Jing-ze ZHANG, Pei-gang GUAN, Gang TAO, Mohammad Reza OJAGHIAN, and Kevin David HYDE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Bauer R, Oberwinkler F, Vánky K. Ultrastructural markers and systematics in smut fungi and allied taxa. Can J Bot. 1997;75(8):1273–1314. doi: 10.1139/b97-842. [DOI] [Google Scholar]
- 2.Begerow D, Bauer R, Oberwinkler F. Phylogenetic studies on nuclear large subunit ribosomal DNA sequences of smut fungi and related taxa. Can J Bot. 1997;75(12):2045–2056. doi: 10.1139/b97-916. [DOI] [Google Scholar]
- 3.Chang HC, Huang YC, Hung WC. Antiproliferative and chemopreventive effects of adlay seed on lung cancer in vitro and in vivo. J Agric Food Chem. 2003;51(12):3656–3660. doi: 10.1021/jf021142a. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury S. Some studies on the smut Ustilago coicis Bref. of Job’s tears millet. J Indian Bot Soc. 1946;25:123–130. [Google Scholar]
- 5.Fullerton RA, Langdon RFN. A study of some smuts of Echinochloa spp. Proc Linnean Soc New South Wales. 1968;93:281–293. [Google Scholar]
- 6.Kishino H, Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary. J Mol Evol. 1989;29(2):170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- 7.Mundkur BB. A second contribution towards a knowledge of Indian Ustilaginales: Fragments xxvi-l. Trans Br Mycol Soc. 1940;24(3-4):312–336. doi: 10.1016/S0007-1536(40)80031-4. [DOI] [Google Scholar]
- 8.O′Donnell K. Fusarium and its Near Relatives. In: Reynolds DR, Taylor JW, editors. The Fungal Holomorph. Mitotic, Meiotic and Pleiomorphic Speciation in Fungal Systematics. Wallingford: CAB International; 1993. pp. 225–233. [Google Scholar]
- 9.Page RDM. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12(4):357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 10.Piepenbring M. Flora Neotropica Monograph 86. New York: New York Botanical Garden Press; 2003. Smut Fungi (Ustilaginomycetes p.p. and Microbotryales, Basidiomycota) [Google Scholar]
- 11.Piepenbring M. Comparative Morphology of Galls Formed by Smut Fungi and Discussion of Generic Concepts. In: Agerer R, Piepenbring M, Blanz P, editors. Frontiers in Basidiomycote Mycology. Eching: IHW; 2004. pp. 117–164. [Google Scholar]
- 12.Piepenbring M, Bauer R, Oberwinkler F. Teliospores of smut fungi: teliospore connections, appendages, and germ pores studied by electron microscopy; phylogenetic discussion of characteristics of teliospores. Protoplasma. 1998;204(3-4):202–218. doi: 10.1007/BF01280324. [DOI] [Google Scholar]
- 13.Small W. Matter of phytopathological interest during 1926. Rev Appl Mycol. 1927;6:273–274. [Google Scholar]
- 14.Stoll M, Piepenbring M, Begerow D, Oberwinkler F. Molecular phylogeny of Ustilago and Sporisorium species (Basidiomycota, Ustilaginales) based on internal transcribed spacer (ITS) sequences. Can J Bot. 2003;81(9):976–984. doi: 10.1139/b03-094. [DOI] [Google Scholar]
- 15.Stoll M, Begerow D, Oberwinkler F. Molecular phylogeny of Ustilago, Sporisorium, and related taxa based on combined analyses of rDNA sequences. Mycol Res. 2005;109(3):342–356. doi: 10.1017/S0953756204002229. [DOI] [PubMed] [Google Scholar]
- 16.Swofford DL. Sunderland, USA: Sinauer Associates; 2002. PAUP*: Phylogenetic Analysis Using Parsimony and Other Methods Version 4b10. [Google Scholar]
- 17.Titatarn S, Chiengkul A, Unchalisangkas D, Chamkrachang W, Chew-Chin N, Chandrasrikul A. Occurrence of Ustilago coicis on Coix lachryma-jobi in Thailand. Plant Dis. 1983;67(4):434–435. doi: 10.1094/PD-67-434. [DOI] [Google Scholar]
- 18.Vánky K. Carpathian Ustilaginales. Symb Bot Upsal. 1985;24:1–309. [Google Scholar]
- 19.Vánky K. Stuttgart, New York: Gustav Fischer Verlag GmbH & Co. KG; 1987. Illustrated Genera of Smut Fungi. [Google Scholar]
- 20.Vánky K. A survey of the spore-ball-forming smut fungi. Mycol Res. 1998;102(5):513–526. doi: 10.1017/S0953756297005182. [DOI] [Google Scholar]
- 21.Xie L, Zhang JZ, Wan Y, Hu DW. Identification of Colletotrichum spp. isolated from strawberry in Zhejiang Province and Shanghai City, China. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(1):61–70. doi: 10.1631/jzus.B0900174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JZ, Chu FQ, Guo DP, Hyde KD, Xie GL. Cytology and ultrastructure of interactions between Ustilago esculenta and Zizania latifolia . Mycol Progr. 2012;11(2):499–508. doi: 10.1007/s11557-011-0765-y. [DOI] [Google Scholar]