Abstract
Acute upper respiratory tract infections (AURTIs) are the illnesses caused by an acute infection with various viruses and bacteria involving the upper respiratory tract. Shuanghuanglian (SHL) injection, a Chinese medicine intravenous preparation extracted from honeysuckle, Scutellaria baicalensis, and fructus forsythiae, is commonly used to treat AURTIs. Although it is used largely in Chinese hospitals, there is no substantial evidence to demonstrate its clinical effect on AURTIs. We conducted a systematic review to evaluate the effectiveness and safety of Shuanghuanglian injection for the treatment of acute upper respiratory tract infections.
1. Introduction
Acute upper respiratory tract infections (AURTIs) are the illnesses caused by an acute infection with various viruses and bacteria involving the upper respiratory tract. They include the common cold, laryngitis, pharyngitis/tonsillitis, acute rhinitis, acute rhinosinusitis, and acute otitis media, which are the commonest acute problem dealt with in primary care [1, 2]. Symptoms of AURTIs commonly include cough, sore throat, runny nose, nasal congestion, headache, low grade fever, facial pressure, and sneezing. Although the available evidence has shown that antibiotics probably provide little benefit for a large proportion of respiratory tract infections, antibiotics are still largely inappropriately used in clinic [3–5]. Antibiotic treatment to prevent suppurative and nonsuppurative complications may be inappropriate nowadays with generally low rates of major complications [3]. More effective approaches to control infections and relieve symptoms in AURTIs are in a great need.
Shuanghuanglian (SHL) injection, a Chinese medicine intravenous preparation extracted from honeysuckle, Scutellaria baicalensis, and fructus forsythiae [6], is commonly used to treat various kinds of infectius diseases caused by bacterium or viruses in respiratory traction [7]. The main chemical components of SHL injection are chlorogenic acid, baicalin, and forsythia glycosides [8], which have been found to have the ability of anti-inflammation, improving immunity, and inhibiting the growth of various viruses [9–11]. It has been reported that SHL injection could inhibit the respiratory syncytial viruse, parainfluenza I–IV [12], and 23 kinds of pathogenic bacteria such as Staphylococcus aureus [13] and Pseudomonas aeruginosa [14]. SHLI can also enhance the NK cell activity, promote the production of alpha-interferon, raise the rate of lymphocyte transformation [15], and decrease the level of CD4+ cells and the ratio of CD4+/CD8+ while increasing CD8+ [16].
SHL injection has been approved for treatment of acute respiratory tract infection since 1973 in China [17]. Although it is used largely in Chinese hospitals, there is no substantial evidence to demonstrate its clinical effect on AURTIs. We conducted a systematic review to evaluate the effectiveness and safety of Shuanghuanglian injection for the treatment of acute upper respiratory tract infection.
2. Methods
2.1. Inclusion Criteria
We included randomized controlled trials evaluating SHL injection for the treatment of AURTIs without language or publication status restriction. Any patients with AURTIs, including common cold, laryngitis, pharyngitis/tonsillitis, acute rhinitis, acute rhinosinusitis, and acute otitis media, without limitation on gender and age were included in the review. We defined the interventions as Shuanghuanglian injection in the form of liquid or power in the intravenous route of administration. The control group may have a placebo, nontreatment, or conventional treatment. Cointerventions such as supportive or symptomatic treatment were allowed as long as all arms of the randomized trial received the same cointervention(s). We excluded studies on other administration routes of Shuanghuanglian, comparing SHL injection with other Chinese herbal medicine, or SHL injection combined with other antibiotics or antivirus medication.
For trials to be eligible for this review, their results need to be extracted on at least one of the following primary outcomes: (1) severity of symptoms; (2) time to resolution of some common acute URTI-related symptoms (e.g., fever, cough, nasal discharge, cough, congestion, sneezing, and headache) and (3) one of the secondary outcomes: resolution of fever in five days, time off from school or work, antibiotic use, and adverse events associated with treatment.
2.2. Databases and Search Strategies
We searched the following electronic databases: Medline (1950 to 2012), Embase (1980 to 2012), the Cochrane Central Register of Controlled Trials (Issue 10, 2012), AMED (Allied and Complementary Medicine Database; 1985 to 2012), CMCC (Chinese Medical Current Contents, 1994 to 2012), China National Knowledge Infrastructure (CNKI) (1979 to 2012), VIP Database for Chinese Technical Periodicals (VIP) (1989 to 2012), and Wanfang Med Database (1994 to 2012). We employed highly sensitive strategies in which adapted subject headings and text words were developed around Shuanghuanglian and upper respiratory infection. Within these text words they were combined with “or,” and then the two kinds of searching terms were combined with “and.” For Chinese databases searching, additional limit on the study type of randomized controlled trial was added. Reference lists of included studies and significant reviews were also checked.
2.3. Data Extraction and Quality Assessment
Two authors (W. Zhou and S. Gao) independently screened the titles and abstracts of the search results to identify potential relevant studies. If necessary, their full texts were obtained for further evaluation on inclusion criteria. These two authors independently used self-developed data extraction form to extract data regarding study methods, participants, interventions, outcomes, and results. Any discrepancies were resolved by discussion between the two reviewers.
To assess the study quality, we used risk of bias assessment tool recommended by the Cochrane Collaboration to address the following six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and “other issues” [18]. The baseline comparability was considered in the “other issues.” The risk of bias for each outcome within and across the included studies was summarized into three levels: low, unclear, and high risk of bias. We used GRADE system to further assess the quality of the evidence for each individual outcome across included studies. Besides within-study risk of bias (methodological quality), the GRADE approach incorporates considerations of directness of evidence, inconsistency or heterogeneity, precision of effect estimates, and risk of publication bias [18, 19].
2.4. Data Analysis and Synthesis
We used risk ratio (RR) with 95% confidence intervals (CI) to summarize dichotomous outcome data of individual studies and used Mantel-Haenszel random-effects model to pool the results across all included studies. We used the mean difference (MD) to summarize continuous outcome data at the end of treatment or followup within studies and used the inverse-variance random-effects model to pool the results across studies. For meta-analysis, we used random-effects model because of the expected heterogeneity of the interventions. We examined forest plot visually first to detect heterogeneity and then used chi-squared test with an alpha of 0.1 for statistical significance and I 2 test to analyze heterogeneity across the included studies. We conducted funnel plot to investigate the possibility of publication bias.
3. Results
3.1. Search Results and Trial Characteristics
The flow chart in Figure 1 depicts the search process and study selection. We screened the title or abstract of 616 studies and assessed the full texts of 68 papers in Chinese or English. A total of 8 trials were finally included in the systematic review involving 857 participants, of whom 497 (58%) were males and 360 (42%) were females [20–27]. All these trials were conducted in China and published unanimously in Chinese.
Figure 1.
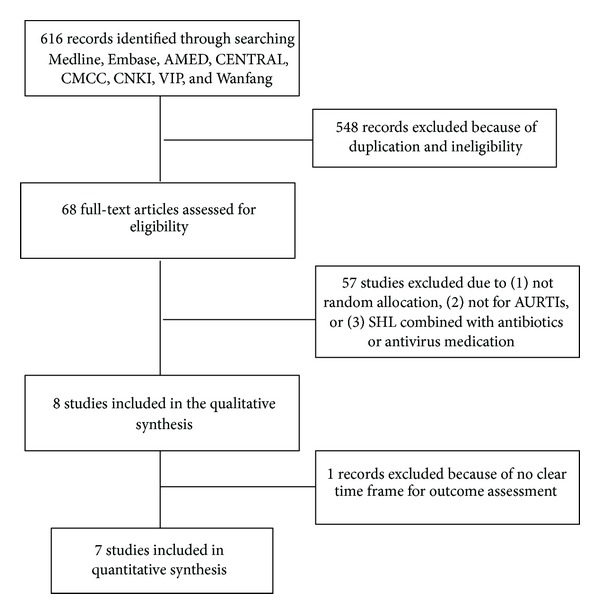
Flow chart showing the search process and study selection.
Table 1 summarizes the baseline characteristics of the patients. Of the 8 trials, 3 compared SHL injection with penicillin [22, 23, 25], another 3 compared SHL injection with ribavirin [20, 21, 26], and the other 2 studies compared SHL injection with penicillin and ribavirin [24, 27]. The treatment duration was generally 3 or five days except one. It was stated that treatment was provided for 3 to 7 days [27], so it was not included into the meta-analysis.
Table 1.
Characteristics of included studies.
| Study ID | Number (male/female) |
Treatment/control | Disease | SHL intravenous injection, dosage |
Treatment duration |
Control | Outcome measure |
|---|---|---|---|---|---|---|---|
| Li 1996 [23] | 103/67 | 86/84 | Acute tonsillitis | 60 mg/kg/d (injection powder) |
3 d | Penicillin | Fever resolution |
| Li and Jia 2000 [24] | 35/53 | 50/38 | Acute upper respiratory infection | 1 mL/kg/d (injection) |
3 d | Ribavirin and lincomycin | Fever resolution after treatment; time to resolution of fever |
| Li 2002 [25] | 53/42 | 48/47 | Acute tonsillitis | 60 mg/kg/d (injection powder) |
5 d | Penicillin | Fever resolution |
| Hong et al. 2003 [22] | 73/50 | 62/61 | Acute tonsillitis | 60 mg/kg/d | 5 d | Penicillin | Fever resolution |
| Zhang and Hu 2003 [27] | 45/37 | 52/30 | Acute upper respiratory infection | 1 mL/kg/d, 20 mL (maximum) (injection) | 3–7 d | Penicillin and ribavirin | No clear description |
| Liang 2007 [26] | 54/36 | 49/41 | Acute upper respiratory infection | 60 mg/kg/d (injection powder) |
5 d | Ribavirin | Fever resolution |
| Gao and Xu 2010 [21] | 77/53 | 68/62 | Acute upper respiratory infection | 60 mg/kg/d (injection powder) | 5 d | Ribavirin | Fever resolution after treatment; time to resolution of fever, cough, and nasal congestion and discharge |
| Cui et al. 2012 [20] | 57/22 | 41/38 | Acute upper respiratory infection | 60 mg/kg/d (injection powder) |
5 d | Ribavirin | Fever resolution after treatment; time to resolution of fever, cough, and sore throat |
3.2. Trial Quality
We used the Cochrane Collaboration's tool for assessing risk of bias and GRADE system to assess the quality of evidence pertaining to each individual outcome. The randomized allocation was stated all the included studies. After contacting trial authors by telephone, it was confirmed that 4 studies used random number table or computer to generate random numbers [21–23, 25]. In other 4 studies, the authors could not be accessed to obtain further information about the method for randomization [20, 24, 26, 27]. Allocation concealment was not mentioned in all studies.
No measures for blinding were ever mentioned in the included studies. The outcome measures were collected and recorded immediately after 3 or five days of treatment. No missing data was found in all the included studies. Selective reporting was generally unclear in the included studies because no information about the protocol was available. The baseline characteristics were in general comparable within each of the studies except the imbalance in the numbers between treatment and control group in two studies [24, 27].
Based on the summarization of six domains on methodological evaluation, the risk of bias within studies was unclear in 4 studies [21–23, 25] and high in the other 4 studies [20, 24, 26, 27].
3.3. The Effect of the Interventions
3.3.1. Primary Outcomes
Time to Resolution of Some Common Acute URTI-Related Symptoms. SHL injection showed significant effect on reducing the time to resolution of fever (3 trials, 297 patients; MD 0.82 day, 95% CI 0.6 to 1.04) and the resolution time of cough (2 trials, 209 patients; MD 0.9 day, 95% CI 0.58 to 1.23), when compared with ribavirin and/or lincomycin (Figures 2 and 3).
Figure 2.
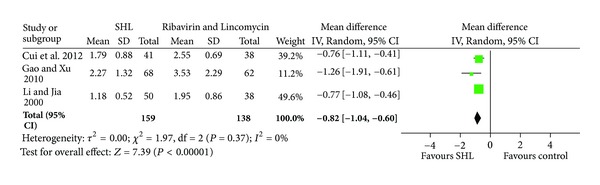
Effects of SHL injection on the time to resolution of fever when compared with Ribavirin and/or Lincomycin.
Figure 3.
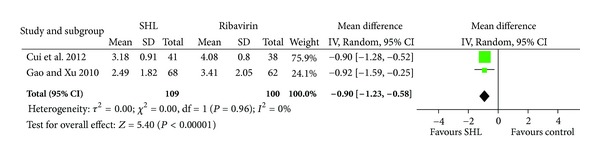
Effects of SHL injection on the resolution time of cough when compared with Ribavirin.
It was also reported that SHL injections had significant effect on reducing the resolution time of sore throat (1 trial, 79 patients; mean difference 1.39 day, 95% CI 0.88 to 1.9) and nasal congestion and discharge (1 trial, 130 patients; mean difference 0.74 day, 95% CI 0.11 to 1.37).
3.3.2. Secondary Outcomes
Fever Resolution in Five Days. We also found a significant effect of SHL injection on reducing the incidence of fever resolution in five days (7 trials, 775 patients; relative risk 1.44, 95% CI 1.18 to 1.76), when compared with ribavirin and/or penicillin. A moderate heterogeneity was found in this analysis (I 2 = 68%) (Figure 4). Different treatment duration, control interventions, and disease severity of patients may contribute substantially to this heterogeneity. Subgroup analysis on different control interventions generated similar results.
Figure 4.
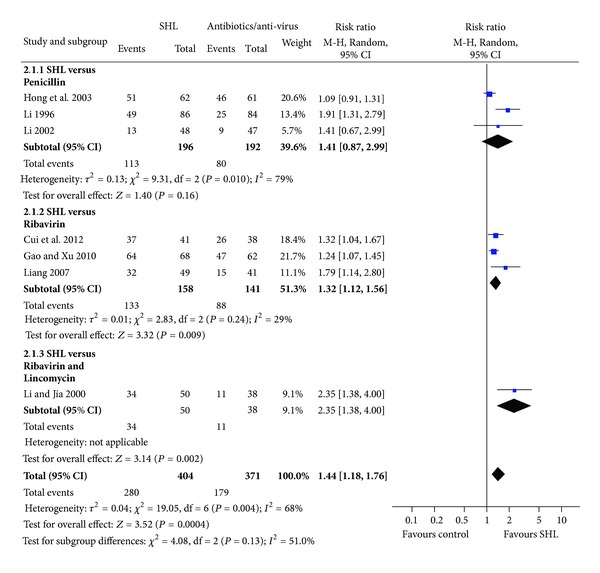
Effects of SHL injection on the fever resolution when compared with antibiotics/anti-virus.
Although publication bias was not detected by funnel plot analysis because of no sufficient number of included studies, it should be noticed during the interpretation of meta-analysis results.
Adverse Effects. Adverse effects were reported in 5 included studies and were not described in the other 3 studies [22, 23, 25]. Abdominal distension, diarrhea, nausea, and vomiting was reported in 4 studies in the treatment group and relieved after symptomatic treatment. Skin rash was found in 6 among 50 patients in the treatment group after receiving the first SHL injection treatment and soon relieved after antihistamine treatment [24].
4. Discussion
We found in this systematic review that Shuanghuanglian injection showed better effect than common antibiotics on helping relieve some symptoms, such as fever, cough, sore throat, and nasal congestion and discharge and decrease the course of acute upper respiratory tract infections. However, due to the limited number of reports on primary outcomes and generally low methodological quality of included studies, no definite conclusion can be drawn on the effect of SHL injection on AURTIs.
According to Chinese medicine, Shuanghuanglian has the effect of clearing away heat and toxic material and is suitable for diseases caused by heat and toxins. Most AURTIs are differentiated in Chinese medicine as syndrome of heat; however, some complications such as insufficiency in Qi, Yin, or Yang may exist. In this paper, there was no description of syndrome of AURTIs patients in all the included studies. Therefore, it is difficult for us to further explore the effect of SHL for different syndromes of AURTIs. As a common medicine for AURTIs, Shuanghuanglian was prepared in different forms including oral tablet or granule; the injection administered intravenously provides us an approach to deliver the medicine more quickly. However, safety issues about SHL injection should be paid much attention. Some adverse effects related to SHL injection may be due to impurities from the production procedure. The difference in oral SHL or SHL injection may be examined further to explore a more safe, effective, and efficient treatment approach.
For the outcome reporting, most of the studies published in China used the national evaluation criteria for Chinese medicine research. A composite outcome measure, effective rate or noneffective rate, which combines the temperature, several clinical symptoms or signs such as pharyngeal check-up, and blood counting, was reported in some of the included studies. It is somewhat difficult for interpretation. Therefore, the outcome of fever resolution in five days was chosen from the composite measurement. However, it should be noted that in some studies this outcome was reported in three days and not measured separately. It may be somewhat different from the detection of single symptom. In addition, the measure of time to resolution of some common acute URTI-related symptoms, such as fever, cough, sore throat, and nasal congestion and discharge, was reported in some studies. However, no detailed information on how to measure them was reported. The subjective influence, especially when no blinding measurement was taken, cannot be ignored when interpreting these results.
In some included studies, cointerventions such as supportive or symptomatic treatments were provided in both treatment and control groups. Some analgesics and antipyretics drugs, cough suppressants, were administered as cointerventions in the two groups. In the situation of unblinding during the trial, it could be excluded that the cointerventions may be different between groups.
In conclusion, Shuanghuanglian injection may have potential effect on relieving some symptoms such as fever, cough, and sore throat and reduce the disease course in acute upper respiratory tract infections. Due to the methodology weakness of the included studies and often poor reporting, this paper cannot make any definite conclusions regarding the clinical effectiveness of SHL injection for AURTIs.
The available clinical evidence and related pharmacological findings so far highlight the need for further research on SHL injection for AURTIs. In particular, the randomized controlled trials with scientifically rigorous methodology are badly needed. To make a more comprehensive understanding on the potential effect of SHL injection, the outcomes, such as severity of symptoms, time to resolution of some common acute URTI-related symptoms, or antibiotic use, should be measured. Furthermore, possible adverse effect associated with the use of SHL injection should also be an important issue of investigation.
Authors' Contribution
H. Zhang and Q. Chen contributed equally to the paper.
References
- 1.Mitra A, Hannay D, Kapur A, Baxter G. The natural history of acute upper respiratory tract infections in children. Primary Health Care Research & Development. 2011;12(4):329–334. doi: 10.1017/S1463423611000193. [DOI] [PubMed] [Google Scholar]
- 2.Jain N, Lodha R, Kabra SK. Upper respiratory tract infections. Indian Journal of Pediatrics. 2001;68(12):1135–1138. doi: 10.1007/BF02722930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan T, Little P, Stokes T. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. British Medical Journal. 2008;337, article a437 doi: 10.1136/bmj.a437. [DOI] [PubMed] [Google Scholar]
- 4.Carranza-Martinez MI, Newton-Sanchez O, Franco-Paredes C, Villaseñor-Sierra A. Clinical outcomes in Mexican children with febrile acute upper respiratory tract infections: no impact of antibiotic therapy. International Journal of Infectious Diseases. 2010;14(9):e759–e763. doi: 10.1016/j.ijid.2010.02.2250. [DOI] [PubMed] [Google Scholar]
- 5.Franck AJ, Smith RE. Antibiotic use for acute upper respiratory tract infections in a veteran population. Journal of the American Pharmacists Association. 2010;50(6):726–729. doi: 10.1331/JAPhA.2010.09103. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Cheng L, Yuan Q, et al. Adverse drug reactions of Shuanghuanglian injection: a systematic review of public literatures. Journal of Evidence-Based Medicine. 2010;3(1):18–26. doi: 10.1111/j.1756-5391.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang HS, Cheng F, Shi YQ, et al. Hypotensive response in rats and toxicological mechanisms induced by shuanghuanglian, an herbal extract mixture. Drug Discoveries & Therapeutics. 2010;4(1):13–18. [PubMed] [Google Scholar]
- 8.Zhou W, Di L, Bi X, Chen L, Du Q. Study on in situ intestinal absorption of active ingredients in Shuanghuanglian oral liquid in rats. Zhongguo Zhong Yao Za Zhi. 2011;36(13):1733–1738. [PubMed] [Google Scholar]
- 9.Wang GF, Shi LP, Ren YD, et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Research. 2009;83(2):186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Chu ZY, Chu M, Teng Y. Effect of baicalin on in vivo anti-virus. Zhongguo Zhong Yao Za Zhi. 2007;32(22):2413–2415. [PubMed] [Google Scholar]
- 11.Li H, Wu J, Zhang Z, et al. Forsythoside a inhibits the avian infectious bronchitis virus in cell culture. Phytotherapy Research. 2011;25(3):338–342. doi: 10.1002/ptr.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Douglas RM. Chinese herbal medicines in the treatment of acute respiratory infections: a review of randomised and controlled clinical trials. Medical Journal of Australia. 1998;169(11-12):579–582. doi: 10.5694/j.1326-5377.1998.tb123423.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Howard OM, Yang X, Wang L, Oppenheim JJ, Krakauer T. Effects of Shuanghuanglian and Qingkailing, two multi-components of traditional Chinese medicinal preparations, on human leukocyte function. Life Sciences. 2002;70(24):2897–2913. doi: 10.1016/s0024-3205(02)01541-2. [DOI] [PubMed] [Google Scholar]
- 14.Song ZJ, Johansen HK, Moser C, et al. Effects of Radix angelicae sinensis and Shuanghuanglian on a rat model of chronic Pseudomonas aeruginosa pneumonia. Chinese Medical Sciences Journal. 2000;15(2):83–88. [PubMed] [Google Scholar]
- 15.Wang YH, Xu KJ, Jiang WS. Experimental and clinical study of shuanghuanglian aerosol in treating acute respiratory tract infection. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15(6):347–350. [PubMed] [Google Scholar]
- 16.Lin G, Liu D, Zhu L. Clinical study on Shuanghuanglian powder in treating children viral myocarditis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18(10):601–602. [PubMed] [Google Scholar]
- 17.Kong XT, Fang HT, Jiang GQ, Zhai SZ, O’Connell DL, Brewster DR. Treatment of acute bronchiolitis with Chinese herbs. Archives of Disease in Childhood. 1993;68(4):468–471. doi: 10.1136/adc.68.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5. 1. 0. The Cochrane Collaboration; 2011. [Google Scholar]
- 19.Oxman AD. Grading quality of evidence and strength of recommendations. British Medical Journal. 2004;328(7454):1490–1494. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui ZP, Gu WJ, Bo L. Curative effect observation on ShuangHuangLian powder injection in treatment of acute upper respiratory tract infection. Gansu Science and Technology. 2012;28(11):125–127. [Google Scholar]
- 21.Gao GT, Xu XT. Curative effect observation on ShuangHuangLian powder injection treatment of children acute upper respiratory tract infection. Jilin Journal of Traditional Chinese Medicine. 2010;30(7):591–592. [Google Scholar]
- 22.Hong HF, Liu YZ, Chen LF. The curative effect observation of ShuangHuangLian and penicillin on acute tonsillitis. Hebei Medicine. 2003;9(7):630–631. [PubMed] [Google Scholar]
- 23.Li XP. Curative effect observation on ShuangHuangLian powder injection in the treatment of acute tonsillitis. Journal of Henan College of Traditional Chinese Medicine. 1996;11(3):47–48. [Google Scholar]
- 24.Li XW, Jia LR. Curative effect observation on ShuangHuangLian injection in treatment of acute upper respiratory tract infection 50 cases. Journal of Sichuan Continuing Education College of Medical Sciences. 2000;19(1):p. 52. [Google Scholar]
- 25.Li W. The curative effect observation of ShuangHuangLian and penicill in on acute tonsill it is. Journal of Clinical Otorhinolaryngology. 2002;16(9):475–476. [PubMed] [Google Scholar]
- 26.Liang YM. Clinical observation on ShuangHuangLian drip in treatment of acute upper respiratory tract infection. Modern Medicine & Health. 2007;23(8):p. 1207. [Google Scholar]
- 27.Zhang Y, Hu SM. Curative effect observation on ShuangHuangLian injection treatment of children acute upper respiratory tract infection in 52 cases. Public Medical Forum Magazine. 2003;7(9):852–853. [Google Scholar]


