Abstract
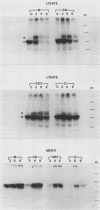
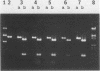
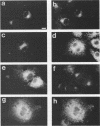
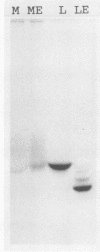
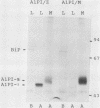
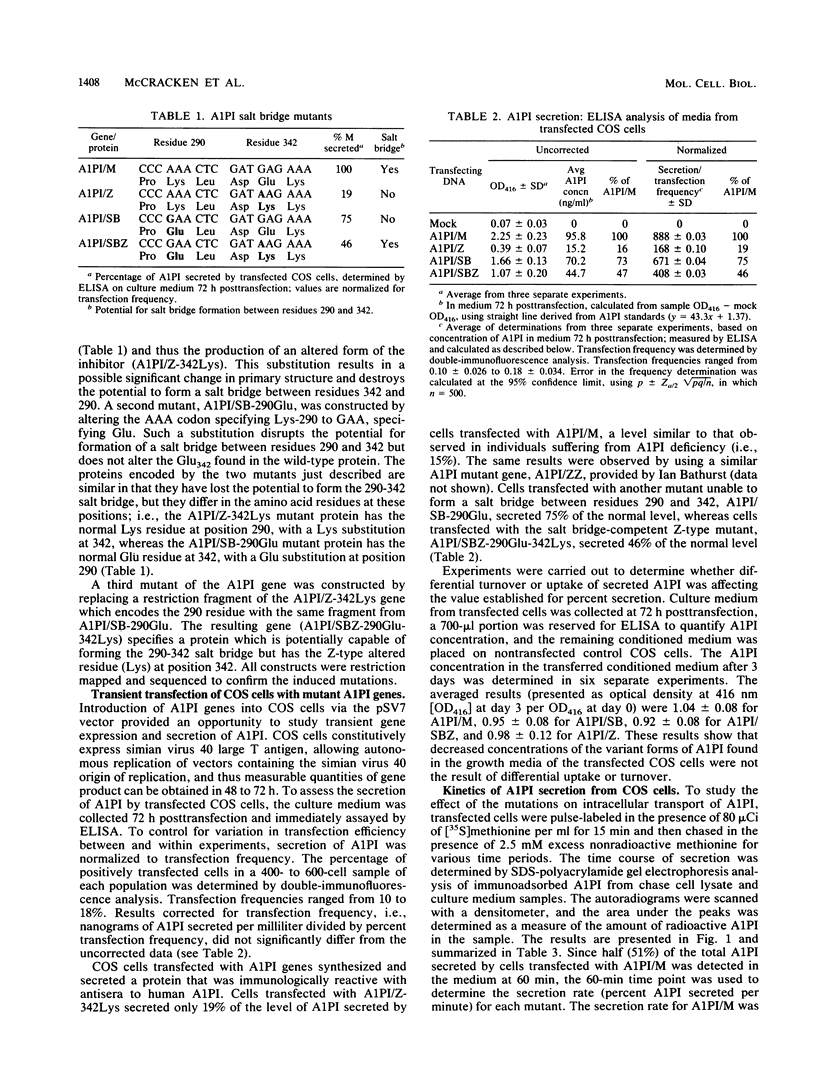
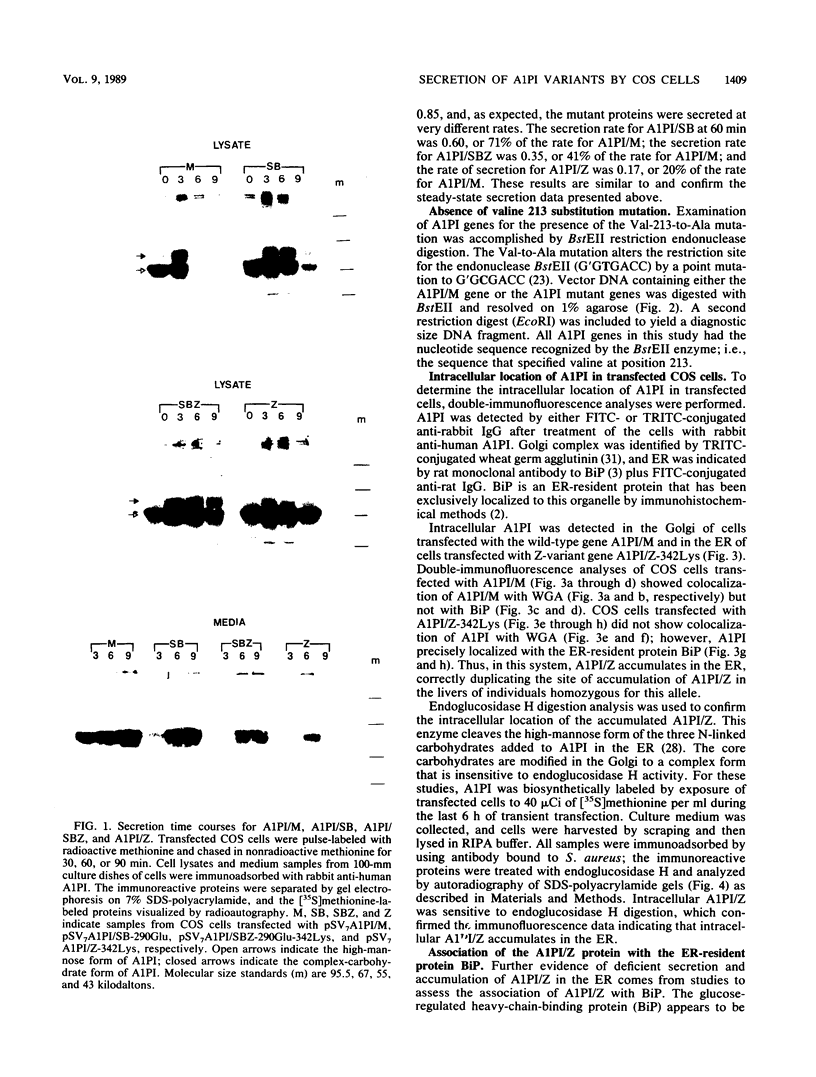
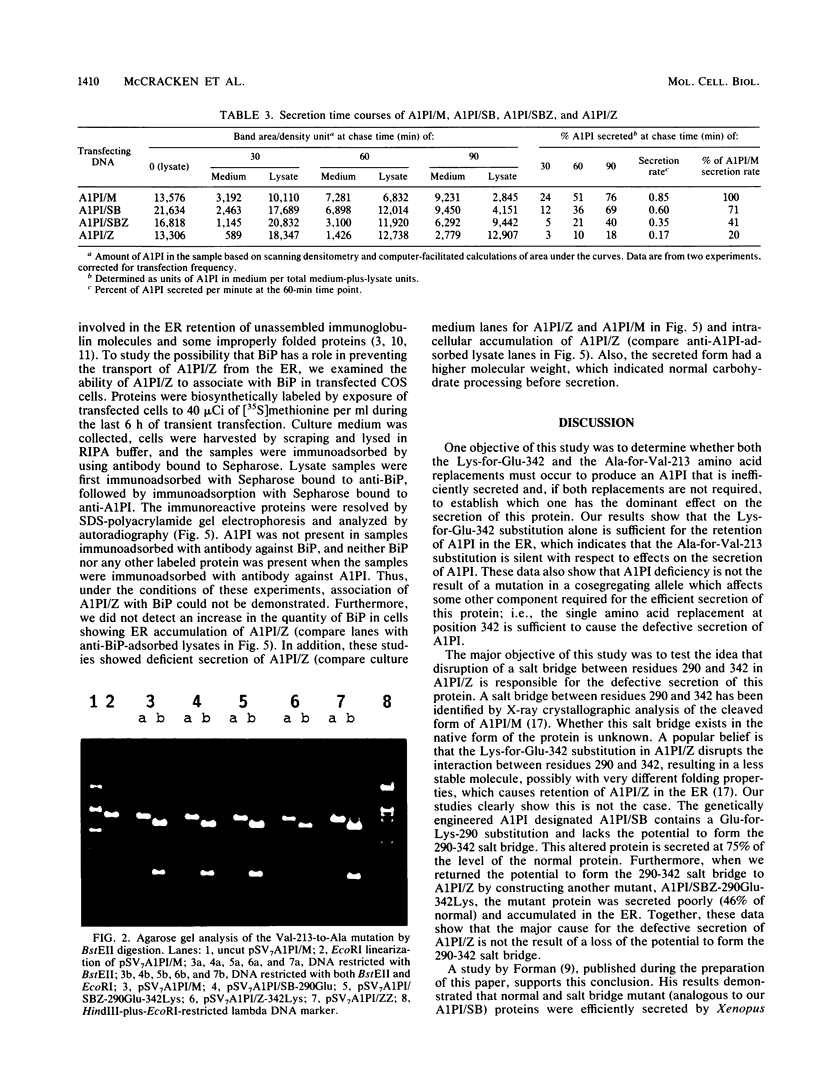
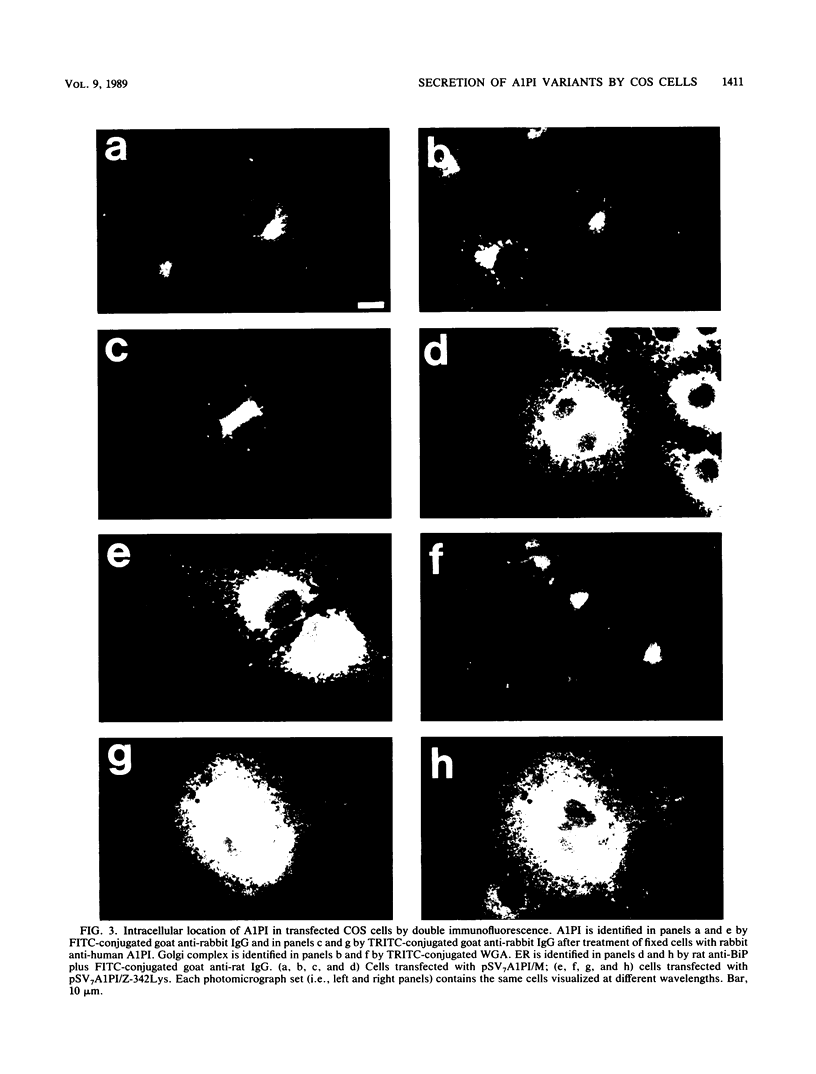
Human alpha-1-proteinase inhibitor (A1PI) deficiency, associated with the Z-variant A1PI (A1PI/Z) gene, results from defective secretion of the inhibitor from the liver. The A1PI/Z gene exhibits two point mutations which specify amino acid substitutions, Val-213 to Ala and Glu-342 to Lys. The functional importance of these substitutions in A1PI deficiency was investigated by studying the secretion of A1PI synthesized in COS cells transfected with A1PI genes altered by site-directed mutagenesis. This model system correctly duplicates the secretion defect seen in individuals homozygous for the A1PI/Z allele and shows that the substitution of Lys for Glu-342 alone causes defective secretion of A1PI. The substitution of Lys for Glu-342 eliminates the possibility for a salt bridge between residues 342 and 290, which may decrease the conformational stability of the molecule and thus account for the secretion defect. However, when we removed the potential to form a salt bridge from the wild-type inhibitor by changing Lys-290 to Glu (A1PI/SB-290Glu), secretion was not reduced to the 19% of normal level seen for A1PI/Z-342Lys; in fact, 75% of normal secretion was observed. When the potential for salt bridge formation was returned to A1PI/Z-342Lys by changing Lys-290 to Glu, only 46% of normal secretion was seen. These data indicate that the amino acid substitution at position 342, rather than the potential to form the 290-342 salt bridge, is the critical alteration leading to the defect in A1PI secretion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bathurst I. C., Travis J., George P. M., Carrell R. W. Structural and functional characterization of the abnormal Z alpha 1-antitrypsin isolated from human liver. FEBS Lett. 1984 Nov 19;177(2):179–183. doi: 10.1016/0014-5793(84)81279-x. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callea F., Fevery J., Massi G., Lievens C., de Groote J., Desmet V. J. Alpha-1-antitrypsin (AAT) and its stimulation in the liver of PiMZ phenotype individuals. A "recruitment-secretory block" ("R-SB") phenomenon. Liver. 1984 Oct;4(5):325–337. [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Foreman R. C. Disruption of the Lys-290--Glu-342 salt bridge in human alpha 1-antitrypsin does not prevent its synthesis and secretion. FEBS Lett. 1987 May 25;216(1):79–82. doi: 10.1016/0014-5793(87)80760-3. [DOI] [PubMed] [Google Scholar]
- Fries E., Gustafsson L., Peterson P. A. Four secretory proteins synthesized by hepatocytes are transported from endoplasmic reticulum to Golgi complex at different rates. EMBO J. 1984 Jan;3(1):147–152. doi: 10.1002/j.1460-2075.1984.tb01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson J. O. Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBS Lett. 1976 Jun 1;65(2):195–197. doi: 10.1016/0014-5793(76)80478-4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Macintyre S. S., Kushner I., Samols D. Secretion of C-reactive protein becomes more efficient during the course of the acute phase response. J Biol Chem. 1985 Apr 10;260(7):4169–4173. [PubMed] [Google Scholar]
- McCracken A. A., Fishman N. F. Secretion of rat serum albumin by COS cells transfected with a spliced cDNA gene. A system to study protein sorting. J Biol Chem. 1986 Jan 15;261(2):508–511. [PubMed] [Google Scholar]
- Miller R. R., Kuhlenschmidt M. S., Coffee C. J., Kuo I., Glew R. H. Comparison of the chemical, physical, and survival properties of normal and Z-variant alpha-1-antitrypsins. J Biol Chem. 1976 Aug 10;251(15):4751–4757. [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Perlmutter D. H., Kay R. M., Cole F. S., Rossing T. H., Van Thiel D., Colten H. R. The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Tartakoff A. Exit of nonglycosylated secretory proteins from the rough endoplasmic reticulum is asynchronous in the exocrine pancreas. J Biol Chem. 1985 Jan 25;260(2):926–931. [PubMed] [Google Scholar]
- Sifers R. N., Carlson J. A., Clift S. M., DeMayo F. J., Bullock D. W., Woo S. L. Tissue specific expression of the human alpha-1-antitrypsin gene in transgenic mice. Nucleic Acids Res. 1987 Feb 25;15(4):1459–1475. doi: 10.1093/nar/15.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Truett M. A., Blacher R., Burke R. L., Caput D., Chu C., Dina D., Hartog K., Kuo C. H., Masiarz F. R., Merryweather J. P. Characterization of the polypeptide composition of human factor VIII:C and the nucleotide sequence and expression of the human kidney cDNA. DNA. 1985 Oct;4(5):333–349. doi: 10.1089/dna.1985.4.333. [DOI] [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Williams D. B., Swiedler S. J., Hart G. W. Intracellular transport of membrane glycoproteins: two closely related histocompatibility antigens differ in their rates of transit to the cell surface. J Cell Biol. 1985 Sep;101(3):725–734. doi: 10.1083/jcb.101.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Mega T. Carbohydrate composition of normal and variant human alpha 1-protease inhibitors. Arch Biochem Biophys. 1979 Jul;195(2):591–595. doi: 10.1016/0003-9861(79)90385-0. [DOI] [PubMed] [Google Scholar]