Abstract
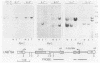
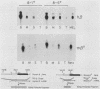
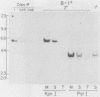
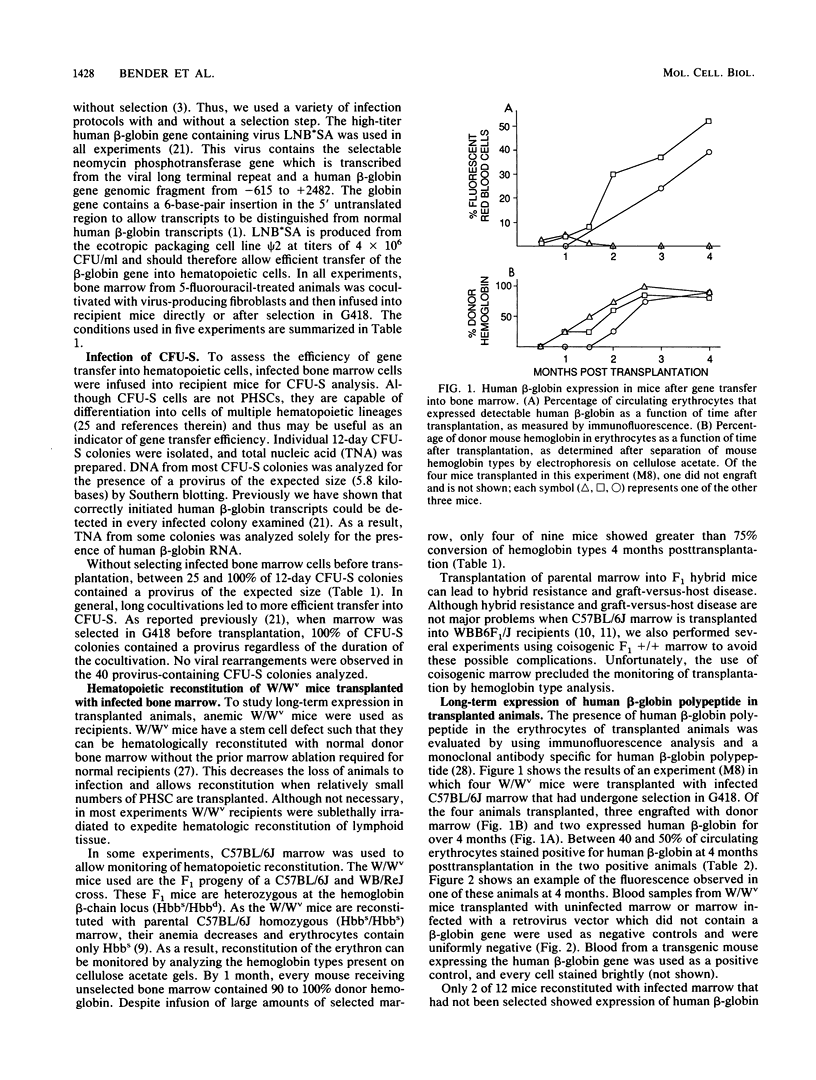
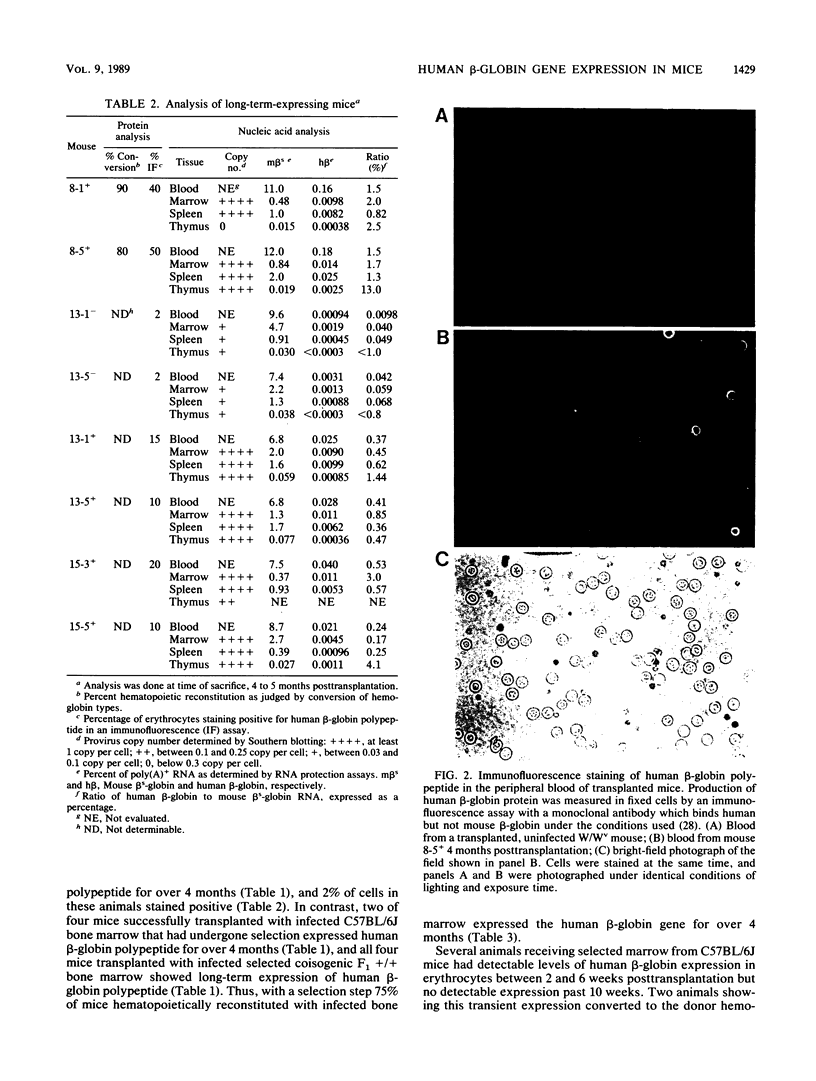
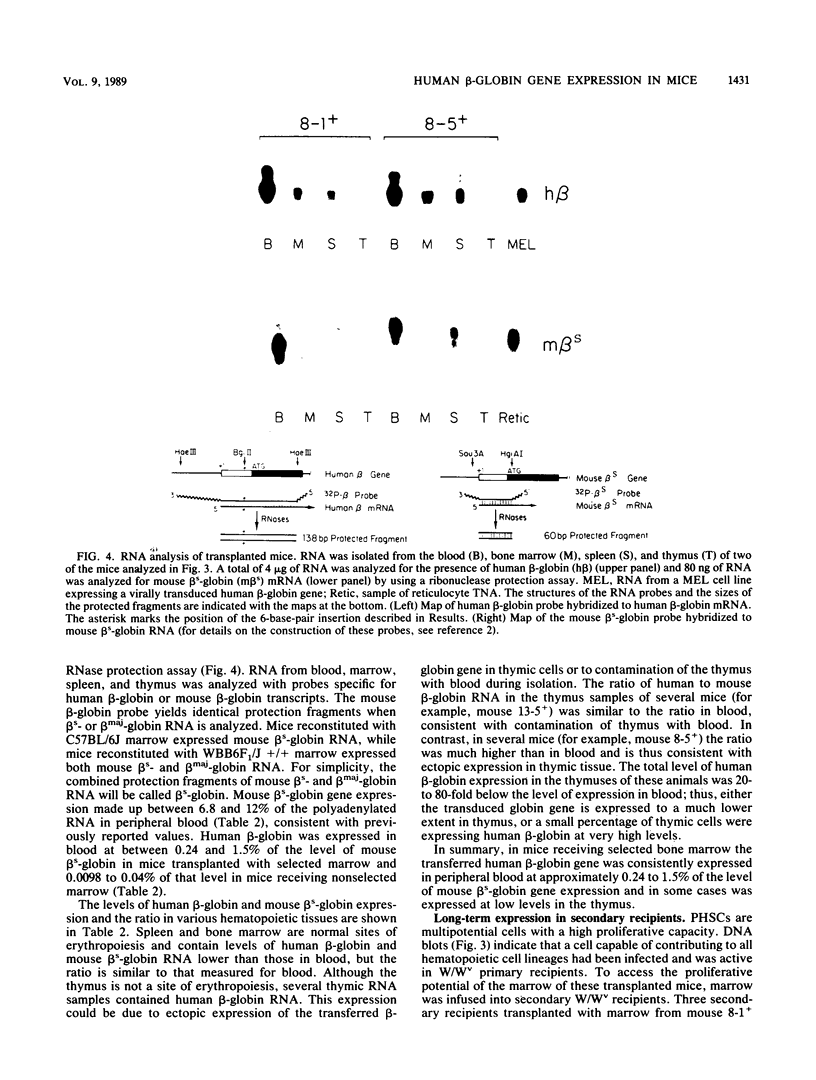
Murine bone marrow was infected with a high-titer retrovirus vector containing the human beta-globin and neomycin phosphotransferase genes. Anemic W/Wv mice were transplanted with infected marrow which in some cases had been exposed to the selective agent G418. Human beta-globin expression was monitored in transplanted animals by using a monoclonal antibody specific for human beta-globin polypeptide, and hematopoietic reconstitution was monitored by using donor and recipient mice which differed in hemoglobin type. In some experiments all transplanted mice expressed the human beta-globin polypeptide for over 4 months, and up to 50% of peripheral erythrocytes contained detectable levels of polypeptide. DNA analysis of transplanted animals revealed that virtually every myeloid cell contained a provirus. Integration site analysis and reconstitution of secondary marrow recipients suggested that every mouse was reconstituted with at least one infected stem cell which had extensive repopulation capability. The ability to consistently transfer an active beta-globin gene into mouse hematopoietic cells improves the feasibility of using these techniques for somatic cell gene therapy in humans.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. A., Miller A. D., Gelinas R. E. Expression of the human beta-globin gene after retroviral transfer into murine erythroleukemia cells and human BFU-E cells. Mol Cell Biol. 1988 Apr;8(4):1725–1735. doi: 10.1128/mcb.8.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Johnson G. R., Kelso A., Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med. 1987 Aug;4(4):229–250. [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P. W., Jolly J. D., Jones J. B., Anderson W. F., Lothrop C. D., Jr Gene transfer into hematopoietic progenitor cells from normal and cyclic hematopoietic dogs using retroviral vectors. Blood. 1988 Mar;71(3):717–722. [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Harrison D. E. A semiquantitative measure of immune responses against erythropoietic stem cell antigens. Immunogenetics. 1987;26(3):123–129. doi: 10.1007/BF00365900. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., DeLaittre J. A. Processing by the thymus is not required for cells that cure and populate W/WV recipients. Blood. 1979 Nov;54(5):1152–1157. [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M. Population of lymphoid tissues in cured W-anemic mice by donor cells. Transplantation. 1976 Jul;22(1):42–46. doi: 10.1097/00007890-197607000-00007. [DOI] [PubMed] [Google Scholar]
- Harrison D. E. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980 Jan;55(1):77–81. [PubMed] [Google Scholar]
- Harrison D. E. F1 hybrid resistance: long-term systemic effects sensitive to irradiation and age. Immunogenetics. 1981;13(3):177–187. doi: 10.1007/BF00350784. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Bodine D. M., Perry L., Papayannopoulou T., Nienhuis A. W. Expression of the human beta-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Bender M. A., Harris E. A., Kaleko M., Gelinas R. E. Design of retrovirus vectors for transfer and expression of the human beta-globin gene. J Virol. 1988 Nov;62(11):4337–4345. doi: 10.1128/jvi.62.11.4337-4345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Waki N., Asai H., Kitamura Y. Long-term monoclonal reconstitution of erythropoiesis in genetically anemic W/Wv mice by injection of 5-fluorouracil-treated bone marrow cells of Pgk-1b/Pgk-1a mice. Blood. 1987 Dec;70(6):1758–1763. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. In vivo proliferative and differential properties of murine bone marrow cells separated on the basis of rhodamine-123 retention. Exp Hematol. 1988 Dec;16(11):903–907. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Farquhar M., Lindsley D., Brice M., Papayannopoulou T., Nute P. E. Monoclonal antibodies specific for globin chains. Blood. 1983 Mar;61(3):530–539. [PubMed] [Google Scholar]
- Stead R. B., Kwok W. W., Storb R., Miller A. D. Canine model for gene therapy: inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988 Mar;71(3):742–747. [PubMed] [Google Scholar]
- Whitney J. B., 3rd Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem Genet. 1978 Aug;16(7-8):667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]