Abstract
Glutamine (GLN) can inhibit NF-kB activation and cytokine expression following sepsis. NF-kB activation and inflammatory cytokine expression, depend on neddylation of Cullin-1 (Cul-1) to proceed. Our aim was to evaluate whether GLN inhibits Cul-1 neddylation, and further determine if GLN-mediated Cul-1 deneddylation attenuates NF-kB activation and subsequent cytokine expression following experimental sepsis in the mouse. Sepsis induced via cecal-liagtion and puncture (CLP) led to a significant increase in lung Cul-1 neddylation. GLN administration post-sepsis led to enhanced lung Cul-1 deneddylation and attenuated NEDD8 expression (p< 0.01 vs. saline). Cul-1 deneddylation was associated with decreased NF-kB activation and IKBα degradation in GLN treated mice (*p < 0.01 vs. saline). Lastly, GLN treatment led to a significant decrease in lung TNF-α and IL-6 post-sepsis. These are the first data describing a direct effect of GLN on Cul-1 deneddylation and provide a possible mechanistic explanation for GLN’s anti-inflammatory effects.
Keywords: Lung Injury, NEDD8, IKB-α, Cecal-Ligation and Puncture, Sepsis
INTRODUCTION
Glutamine (GLN) has previously been shown to exert an anti-inflammatory effect following experimental sepsis[1]. This effect has been associated with attenuated NF-kB activation. Further, GLN administration can decrease lung injury and improve survival in models of experimental sepsis[2]. The mechanism responsible for GLN’s ability to regulate the inflammatory response and attenuate NF-kB expression is currently unclear.
One possible pathway underlying GLN’s inhibitory effect on the NF-kB pathway is the E3-SCFbTrCP ubiquitin ligase pathway. The E3 SCF ubiquitin ligase, which is vital for the polyubiquitination of IKBα[3], is comprised of SKP1, Cullin-1 (Cul-1), and the F-box domain of β-TrCP. Activity of the E3 ligase requires the regulatory subunit Cul-1 to be posttranslationally modified by the ubiquitin-like protein NEDD8. Loss of the NEDD8 modification is mediated by the COP9 signalosome and results in loss of SCF activity[4]. In detail, NEDD8 must be conjugated to the cullin subunit of the E3-SCG complex on Lys720 for optimal ubiquitin ligase activity[5; 6]. More importantly, NEDD8 modification of Cul-1 has been demonstrated to be required for the ubiquitination of IKB α and subsequent activation of NF-kB in mammalian cells[5]. In short, Deneddylated Cul-1 is incapable of ubiquitination of IkB α; hence NF-kB cannot become active and initiate the inflammatory response[4; 5].
To date, few physiologic regulators of Cul-1 neddylation have been described [5; 7]. One known physiologic effecter of Cul-1 deneddylation is the interaction of non-pathogenic commensal intestinal bacteria and gut epithelial cells [5]. Recent data reveals that this interaction results in the rapid loss neddylated Cul-1 and consequent repression of the NF-kB pathway[5; 8]. As commensal intestinal bacteria are vital for optimal cellular function and nutrient delivery in the intestinal epithelia, we hypothesized that it was also possible key epithelial cell nutrients[9], such as GLN, may play a role in modifying Cul-1 deneddylation.
Therefore, we examined the effect of GLN on Cul-1 neddylation, IkBα degradation, NF-kB activation, and inflammatory cytokine expression in lung tissue following experimental sepsis. The lung was chosen for evaluation in this model as is it is an organ that undergoes significant inflammatory injury following experimental sepsis induced via peritonitis[10]. Further, the ultimate cause of death in animals following this model of experimental sepsis is often severe lung injury[11].
MATERIALS AND METHODS
Animal Preparation
The experiments described in this paper were performed in adherence to the National Institute of Health guidelines for the use of experimental animals. Protocols were approved by the Animal Care and Use Committee of The University of Colorado Health Sciences Center. All experiments were also conducted and the animals cared for in accordance with the Guiding Principles for Research Involving Animals and Human Beings of the American Physiological Society.
Animals and Experimental Protocol
Experiments were performed on male C57BL/6 mice (25g body weight). The mice were purchased from Charles River Laboratories, Inc. and were maintained on a standard diet and water ad libitum. Animals were housed at constant temperature with 10 and 14 hours of light and dark exposure, respectively. Animals underwent an acclimatization period of at least 7 days before use in experiments. Sepsis was induced by cecal ligation and puncture (CLP). Following anesthesia with intraperitoneal injection of Ketamine (40 mg/kg) and Xylazine (6mg/kg), a 1 cm incision was made in the abdominal wall and the cecum was carefully extruded. Approximately 25% of the cecum was then ligated just below the ileocecal valve to avoid bowel obstruction. The cecum was punctured twice using a sterile 22-gauge needle and was squeezed to extrude fecal material into the peritoneal cavity. The muscle and skin layers of the abdomen were then closed. All of the above manipulations were performed by the same surgeon to ensure consistency. This length of cecum ligated was chosen after evaluation of multiple cecal length ligation distances as preveiously described [12]. In this set of studies, this specific distance was chosen as it consistently yielded approximately 70% mortality in control animals. Immediately after the procedure, the animals were given 5% body weight of normal saline for fluid resuscitation and allowed to recover. Thirty minutes following CLP, mice were given either 0.75 g/kg GLN or normal saline intravenously. A sham group of animals for each of the treatment groups was also performed in which the abdomen was opened and the cecum manipulated, however no cecal ligation or cecal puncture was performed, and the abdomen was closed.
Tissue Collection
Male C57BL/6 mice underwent CLP, and were given GLN or saline control 30 minutes following CLP (n=5/timpoint). Lung tissue was collected at 1 h, 2 h, 6 h, and 24 h following cecal ligation and puncture. Sham animal tissue for each group was also collected following the sham procedure. All tissues were removed and immediately frozen in liquid nitrogen and stored in −80 ° C.
TNF-αand IL-6 Detection
TNF-α and IL-6 concentrations from lung tissue were measured using an enzyme-linked immunosorbent assay (ELISA). Lung tissue samples were homogenized in homogenization buffer containing a protease inhibitor cocktail tablet (Roche Molecular, Indianapolis, Indiana). Lung homogenate was then centrifuged for 15 minutes at 10,000 revolutions/min, and supernatant was removed and frozen at −80°C. The supernatant was then analyzed utilizing an ELISA kit for TNF- α from Endogen (Woburn, Massachusetts) and an ELISA kit for IL-6 from Roche (Mannheim, Germany). Results were determined spectrophotometrically using a microplate reader. (Thermo Lab Systems, Opsys MR, Chantily, Virginia).
Nuclear and Cytoplasmic Protein Extraction
All nuclear and cytoplasmic protein extractions were performed on ice with ice-cold reagents. Protease inhibitors were added to reagents prior to use, and the NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce, Rockford IL) was utilized in order to obtain nuclear fractions. The fractions were then stored at −80° C and used for Western blot analysis.
Western Blot Analysis
Lung tissue was removed and immediately frozen in liquid nitrogen and stored at −80° C until analysis. Western blotting was performed as previously described [13], and cytoplasmic extracts were prepared as stated above. For Cullin-1 detection, the membranes were incubated with rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, Cat. #sc-11384). For IKBα detection, membranes were incubated with rabbit polyclonal antibody specific to IkB α (Santa Cruz Biotechnology, Santa Cruz, CA, Cat. # sc-847). For NEDD8 detection, membranes were incubated with rabbit polyclonal antibody (Affinity Bioreagents, Cat. #OPA1–07001). All membranes were then washed and incubated with secondary donkey anti-rabbit antibody HRP (Santa Cruz Biotechnology, Santa Cruz, CA, Cat. # sc-2305). Densitometry was determined using UVP Chemilumenscent Darkroom System (UVP Inc., Upland, California). All western blot densitometry was normalized using HSC-73 (heat shock constitutive protein) to control for protein loading.
NF-κB Detection
A kit detecting activation of NF-kB and binding of the p65 subunit was utilized (Pierce Biotechnology, Rockford, IL). Nuclear extracts were prepared as described above. Samples were assayed in duplicate in a 96-well plate coated with the consensus NF-kB binding element. After incubation with anti-p65 antibody, followed by a secondary antibody linked to HRP, plates were developed with a chemiluminescent substrate and read in the UVP Chemiluminescent Darkroom System (UVP Inc., Upland, California). An internal positive control of TNF-α activated nuclear extract was used as a reference point for maximal signal. “Cold competition” for this assay was performed by adding wild type NF-kB competitor consensus binding element to wells. This reduced the signal level to near zero. A mutated NF-kB binding sequence had no effect on signal, ensuring signal specificity.
Statistical Analysis
Results are presented as an average +/− S.D. Lung tissue cytokines, western blotting, and NF-kB activation were compared using the Student’s T Test or ANOVA followed by Student-Newman Keul’s test where applicable. Results were considered significant at a p value < 0.05.
RESULTS
Glutamine treatment results in inhibition of pulmonary Cullin-1 neddylation
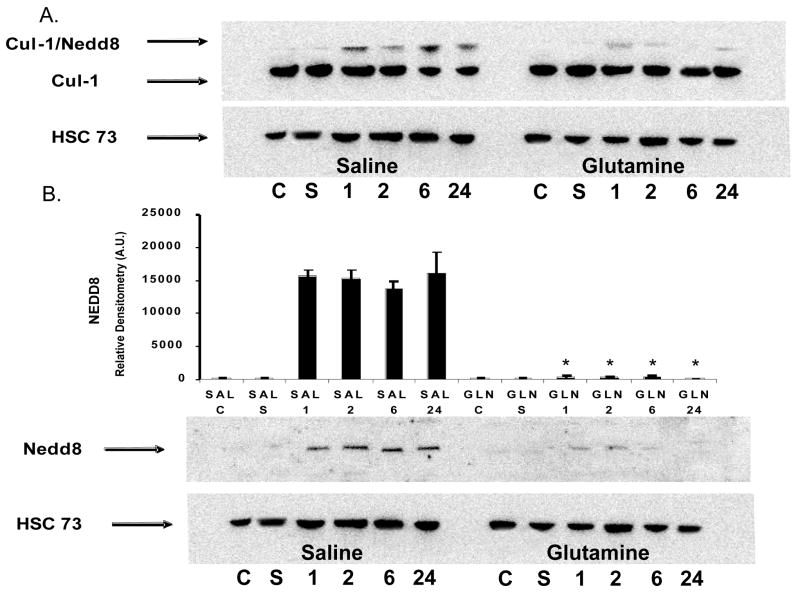
The effect of GLN treatment on Cul-1 neddylation was analyzed in lung tissue cytosolic extracts from mice given GLN or saline (n=5/group) 30 min post CLP were assessed for the Cul-1/NEDD8 complex as well as for NEDD8 independently. Following sepsis via CLP, mice treated with saline expressed an increase in Cul-1 neddylation as well as an increase in independent NEDD8 expression over time (Figure 1A and 1B). GLN treatment led to an inhibition of lung Cul-1 neddylation at 1,2,6 and 24 hrs following CLP (*-p- < 0.01 vs. saline)(Figure 1A). GLN treatment also led to the inhibition of independent NEDD8 expression (*-p- < 0.01 vs. saline)((Figure 1B). As shown in figure 1A and 1B, healthy control animal (C) and Sham animal (S) tissues (n=5/group) were also assessed and no Cul-1 neddylation or independent NEDD8 expression was observed in these groups.
Figure 1.
Expression of Cul-1/NEDD8 and NEDD8 in GLN and saline treated wild-type mice following sepsis. A). Saline treated animals expressed enhanced lung Cul-1 neddylation over time. GLN treatment led to an inhibition of lung Cul-1 neddylation at 1,2,6 and 24 hrs following CLP. No Cul-1 neddylation was observed in healthy control animal (C) and SHAM animal (S) lung tissues. B). Independent western blots of NEDD8 alone depict enhanced NEDD8 expression following sepsis in saline treated animals. GLN treatment significantly decreased NEDD8 expression at all time points following sepsis (*-p- < 0.01 vs. saline). Heat Shock Constitutive protein (HSC 73) was utilized as a loading control for all western blots and graphical data is normalized to HSC73. Representative western blot shown of n=5.
Glutamine treatment results in educed activation of pulmonary NF-kB and degradation of IKBα following sepsis
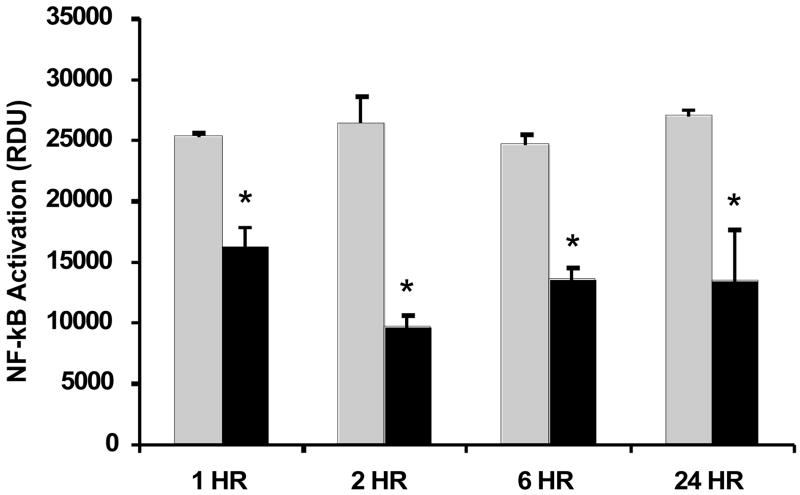
To determine if the GLN-mediated loss of Cul-1 neddylation also resulted in a reduction of NF-kB activation, lung tissue nuclear extracts from mice given GLN or saline 30 min post CLP (n=5/group) lung tissues were assayed for specific nuclear p65 binding activity at 1h, 2h, 6h and 24h following sepsis. Figure 2 shows that sepsis-induced activation of NF-kB signaling was decreased significantly at all time points following sepsis in the GLN treated mice (p < 0.01 versus saline treated mice). Similar to EMSA (electro-mobility shift assay), addition of wild-type (WT) NF-kB competitor duplex highly suppressed detection of signal. Incubation with a mutated NF-kB p65 probe showed no effect, validating the signal specificity of these experiments. An internal positive control of TNF-α activated nuclear extract was used as a reference point for maximal signal.
Figure 2.
Effect of GLN treatment on expression on NF-kB activation in lung tissue nuclear extracts using a specific nuclear p65 binding activity transcription factor assay kit (Pierce, Rockford, IL). Sepsis-induced activation of NF-kB signaling was inhibited significantly in mice given GLN treatment at all time points post sepsis (*-p < 0.01 versus saline treated). (Black bars= GLN treated mice; Grey Bars= saline treated mice). The results shown are representative of an experiment that was conducted in triplicate
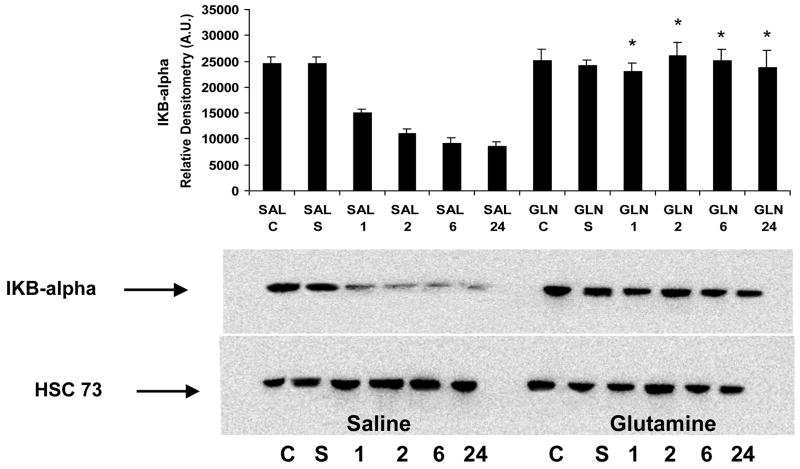
Activation of IkB kinase (IKK) leads to phosphorylation, ubiquitination, and degradation of IkBα which allows NF -kB to translate to the nucleus and induce transcription [14]. Western blot analysis shows that saline treated mouse lung cytosolic extracts exhibited a significant degradation of IkBα over time, while the GLN treated mouse lung tissue did not exhibit this degradation. (Figure 3). As shown in Figure 3, Healthy control animal (C) and Sham animal (S) tissues were also assessed, and found to exhibit no degradation of IkBα.
Figure 3.
Effect of GLN treatment on cytosolic IKBα expression. Representative western blot of degradation of IkBα in mice given GLN or saline following sepsis. Mice given glutamine did not show degradation of IKB-α in lung tissue following CLP (*- p <0.01 versus saline). IkBα protein levels were assessed from cytosolic extracts prepared 1,2,6 and 24 hrs after the induction of sepsis. Healthy control animal (C) and Sham animal (S) tissues were also assessed. Heat Shock Constitutive protein (HSC 73) was utilized as a loading control for all western blots and graphical data is normalized to HSC73. Representative western blot shown of n=5.
Reduced pro-inflammatory cytokine release following sepsis in mice treated with Glutamine
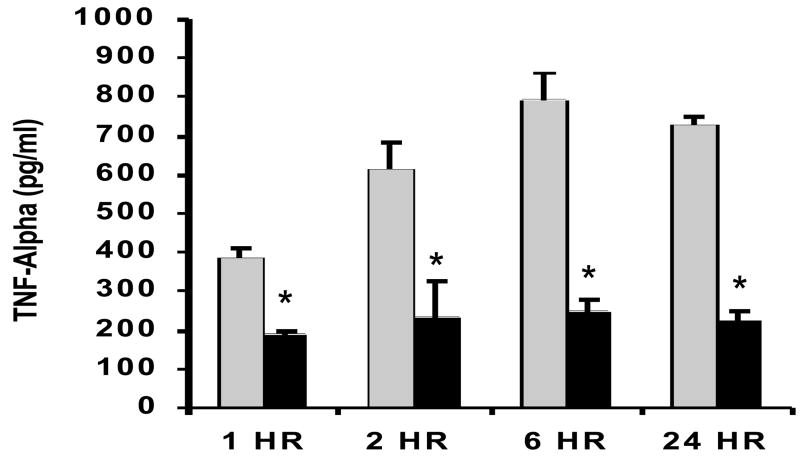
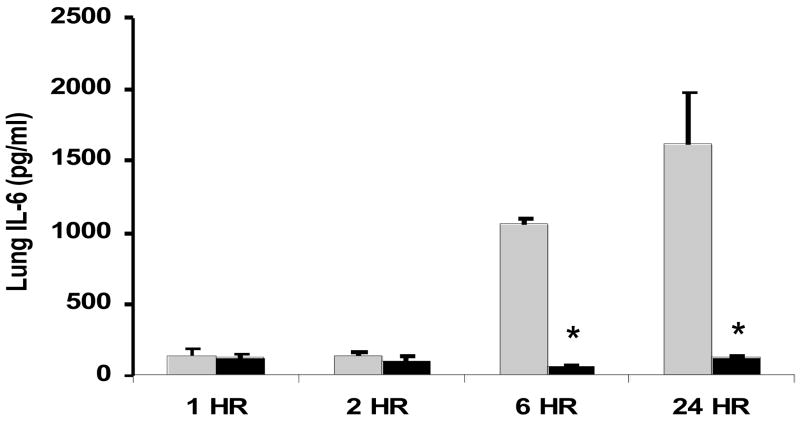
To examine whether GLN treatment could modulate pro-inflammatory cytokine release following CLP-induced polymicrobial sepsis, we studied septic animals given GLN or saline (n=5/group) at 1h, 2 h and 6 h and 24 h post-CLP procedure. GLN treatment significantly decreased release of lung TNF-α at all time points following sepsis and IL-6 at 6h and 24h post-CLP (p < 0.01 versus saline treated mice) (Fig 4A and 4B).
Figure 4.
Figure 4A and 4B. Effect GLN treatment lung concentrations of pro-inflammatory cytokines following cecal ligation and puncture (CLP). Mice given GLN or saline (n=5/group) underwent CLP, and lung tissue was collected at 1,2, and 6 and 24 hrs. Lung tissue was homogenized and TNF-α (4A) and IL-6 4B) (were assessed via ELISA as described in methods. GLN treatment led to a significant decrease in TNF-α at all time points and a significant decrease in IL-6 at 6h and 24 h post sepsis. (*-p < 0.01 versus saline treated mice). (Black bars= GLN treated mice; Grey Bars= saline treated mice)
DISCUSSION
Sepsis leads to the activation of the inflammatory response which is key to the survival of the organism following an infectious insult[15]. This response, when not appropriately regulated, can lead to significant tissue injury. This is particularly true in sepsis, where lung injury due to unchecked inflammation occurs commonly[16]. Previous data has shown that GLN treatment can attenuate lung injury following a septic insult and this may relate to GLN’s ability to regulate the inflammatory response[2; 17]. However, there has been no mechanistic link to explain GLN’s regulation of key transcription factors responsible for the inflammatory response.
A limited number of transcription factors regulate inflammatory pathways, the most important transcriptional regulator is NF-kB[18]. Following activation by a wide array of mediators, including cytokines, bacterial toxins, or oxidative stress, the signal transduction cascade is initiated, which causes the phosphorylation of IkBα on Ser32 and -36 by IKK complex [19]. Phosphorylation of IkBα is followed by ubiquitination via the E3 ligase SCFβTRCP and is targeted for proteasomal degradation by the 26S proteasome[20]. Following IkBα degradation, NF-kB can translocate to the nucleus and bind to the promoters of proinflammatory genes, leading to enhanced gene expression and amplification of the inflammatory response. The extent of NF-kB activation depends on multiple factors, one key factor is the variable E3 activity of the SCFβTRCP complex. This complex is regulated by a reversible modification with the ubiquitin-like protein NEDD8[21].
The data presented here demonstrate for the first time that GLN given following a septic insult can inhibit Cul-1 neddylation and total NEDD8 expression in the lung. As previously described Cul-1 neddylation is a vital regulator of NF-kB activation, and ultimately the progression of the inflammatory response to sepsis and other injury. Our data confirms GLN is also able to suppress NF-kB transcriptional activation and translocation to the nucleus. This is associated with significant inhibition of IkBα phosphorylation, ubiquitination and degradation in lung tissue. Finally, GLN’s effect on Cul-1 deneddylation led to concomitant attenuation of inflammatory cytokine expression in the lung following experimental sepsis in the mouse. These results may provide the first indication of a mechanistic explanation for GLN’s anti-inflammatory effects in sepsis.
These results reveal that the attenuation of Cul-1 neddylation by GLN may provide a therapeutic intervention for disease processes mediated by dysregulated or unchecked inflammation. The effect of GLN on regulation of systemic inflammation could be applied therapeutically not only for the treatment of sepsis and lung injury, but potentially to other inflammatory diseases as well.
References
- 1.Singleton KD, Beckey VE, Wischmeyer PE. Glutamine prevents activation of NF-kappaB and stress kinase pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock. 2005;24:583–9. doi: 10.1097/01.shk.0000185795.96964.71. [DOI] [PubMed] [Google Scholar]
- 2.Singleton KD, Wischmeyer PE. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1839–45. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 3.Cope GA, Deshaies RJ. COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell. 2003;114:663–71. doi: 10.1016/s0092-8674(03)00722-0. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–51. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 5.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol. 2005;175:4194–8. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 6.Ohh M, Kim WY, Moslehi JJ, Chen Y, Chau V, Read MA, Kaelin WG., Jr An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep. 2002;3:177–82. doi: 10.1093/embo-reports/kvf028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–11. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. Embo J. 2007;26:4457–66. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preiser JC, Wernerman J. Glutamine, a life-saving nutrient, but why? Crit Care Med. 2003;31:2555–6. doi: 10.1097/01.CCM.0000084863.47943.6F. [DOI] [PubMed] [Google Scholar]
- 10.Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914–21. [PubMed] [Google Scholar]
- 11.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Singleton KD, Wischmeyer PE. Distance of cecum ligated influences mortality, tumor necrosis factor-alpha and interleukin-6 expression following cecal ligation and puncture in the rat. Eur Surg Res. 2003;35:486–91. doi: 10.1159/000073387. [DOI] [PubMed] [Google Scholar]
- 13.Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol. 2001;90:2403–10. doi: 10.1152/jappl.2001.90.6.2403. [DOI] [PubMed] [Google Scholar]
- 14.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–11. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 16.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 17.Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med. 2005;33:1206–13. doi: 10.1097/01.ccm.0000166357.10996.8a. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 19.Wojcik C, Di Napoli M. Ubiquitin-proteasome system and proteasome inhibition: new strategies in stroke therapy. Stroke. 2004;35:1506–18. doi: 10.1161/01.STR.0000126891.93919.4e. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–97. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 21.Amir RE, Iwai K, Ciechanover A. The NEDD8 pathway is essential for SCF(beta -TrCP)-mediated ubiquitination and processing of the NF-kappa B precursor p105. J Biol Chem. 2002;277:23253–9. doi: 10.1074/jbc.M200967200. [DOI] [PubMed] [Google Scholar]