Abstract
Although high-density lipoprotein-cholesterol (HDL-C) levels in large epidemiological studies are inversely related to the risk of coronary heart disease (CHD), increasing the level of circulating HDL-C does not necessarily decrease the risk of CHD events, CHD deaths, or mortality, HDL can act as an anti- or a proinflammatory molecule, depending on the context and environment. Based on a number of recent studies, it appears that the anti- or proinflammatory nature of HDL may be a more sensitive indicator of the presence or absence of atherosclerosis than HDL-C levels. The HDL proteome has been suggested to be a marker, and perhaps a mediator, of CHD. Apolipoprotein A-1 (apoA-I), the major protein in HDL is a selective target for oxidation by myeloperoxidase, which results in impaired HDL function. Improving HDL function through modification of its lipid and/or protein content maybe a therapeutic target for the treatment of CHD and many inflammatory disorders. HDL/apoA-I mimetic peptides may have the ability to modify the lipid and protein content of HDL and convert dysfunctional HDL to functional HDL. This review focuses on recent studies of dysfunctional HDL in animal models and human disease, and the potential of apoA-I mimetic peptides to normalize the composition and (function of lipoproteins.
Keywords: ApoA-I mimetic peptides, High-density lipoprotein, Inflammation, Oxidative stress
Developing Apolipoprotein A-1 (ApoA-I) Mimetic Peptides
Improvement of atherosclerosis in animal models1,2 and in preliminary human studies makes apoA-I an interesting therapeutic candidate; however, the complexity of large-scale synthesis of apoA-I, with its 243 amino acid residues, resulting in high cost and need for it to be given intravenously, makes it less attractive. In the initial studies, weekly intravenous doses for 5–6 weeks pointed to potential therapeutic benefit,3 hut subsequent larger clinical trials did not fully confirm these promising results.4 This seems to be an unlikely therapy for the millions of patients with atherosclerosis if longer periods of intravenous administration are required.
In 1985 an 18-amino-acid (AA) peptide that did not have sequence homology with apoA-I, but mimicked the class A amphipathic helixes contained in apoA-I, was designed and synthesized by Segrest, Anantharamaiah and colleagues.5–7 There were 2 phenylalanine residues on the hydrophobic face and hence the peptide was called 2F. Although many of the lipid-binding properties of apoA-I were mimicked by 2F, it was not able to alter lesions in a mouse model of atherosclerosis.8 A series of 88-AA peptides were then tested for their ability to inhibit low-density lipoprotein (LDL) oxidation by artery wall cells in culture and for production of monocyte chemotactic protein-1 (MCP-1) in response to LDL-derived oxidized lipids.9,10 Two peptides with 4 or 5 phenylalanine residues, namely 4F and 5F, were chosen for testing in animal models.
The peptide 5F synthesized from L-amino acids (L-5F) and injected into mice improved the anti-inflammatory properties of high-density lipoprotein (HDL) in mice and significantly protected the mice from diet induced atherosclerosis.11 When the peptide 4F was synthesized from all D-amino acids 11 (D-4F) bland administered orally in the drinking water the anti-inflammatory properties of HDL were significantly improved and atherosclerotic lesions were significantly reduced in both apoE-null mice on a chow diet and in LDL receptor-null mice on a Western diet.12 Interestingly, this occurred without any alteration in the plasma cholesterol or HDL-cholesterol level.12
In animal models, administration of the 4F peptide improved a number of model pathological processes, including type I diabetes,13,14 type II diabetes and obesity,15,16 influenza A pneumonia,17 scleroderma,18 hyperlipidemia and sickle cell-induced vascular dysfunction,19,20 hepatic fibrosis,21 Alzheimer disease.22 vascular dementia.23 and hyperlipidemia-induced renal inflammation.24 Buga et al reported that, in hyperlipidemic mice. L-4F decreases platelet aggregation by binding plasma-oxidized lipids that cause platelet hyperreactivity.25 The 4F peptide was found to synergize with statins and cause regression of atherosclerotic lesions in old apoE-null mice.26 It additionally inhibited accelerated vein graft atherosclerosis in a mouse model27 and markedly reduced chronic rejection in a heart transplant mouse model.28 In humans with coronary heart disease (CHD), a single oral dose of D-4F significantly improved the anti-inflammatory properties of HDL.29
Woo et al30 recently demonstrated that HDL is pro-inflammatory in apoE−/−Fas−/− mice that spontaneously develop IgG autoantibodies, glomerulonephritis, osteopenia, and atherosclerotic lesions on a normal chow diet. They observed that the level of dysfunctional HDL was decreased following an injected dose of L-4F, but not alter scrambled L-4F administration. The authors reported that L-4F treatment, alone or with pravastatin, significantly reduced IgG anti-dsDNA and IgC anti-oxidized phospholipids (anti-oxPLs), proteinuria, glomerulonephritis, and osteopenia in a murine lupus model of accelerated atherosclerosis. The authors further elaborated that despite the enlarged aortic lesions, the presence of increased smooth muscle content, decreased macrophage infiltration, and decreased pro-atherogenic chemokines in mice treated with L-4F plus pravastatin suggest protective mechanisms on the lupus-like disease. Charles-Schoeman et al reported that combination D-4F/pravastatin reduced disease activity in rat collagen-induced arthritis, an animal model of rheumatoid arthritis.31 Favorable changes in cytokines were observed with treatment, and D-4F/pravastatin therapy was associated with improvement in HDL anti-inflammatory properties.31
Watanabe et al reported that administration of 4F converted pro-inflammatory HDL to anti-inflammatory HDL by lowering the levels of hemoglobin (Hb)/haptoglobin (Hp) associated with HDL in hyperlipidemic mice.32 After treatment of ApoE null mice with 4F. some of the Hb/Hp associated with HDL appeared to return to the non-lipoprotein fractions of the serum. They hypothesized that the decrease in HDL-associated Hb/Hp by 4F treatment may be related to a 4F mediated reduction of tissue inflammation.
Anti-Inflammatory Properties of the Mimetic Peptides
In the aforementioned studies it was difficult to explain why it was that while the concentration of apoA-I in most of the animal models and in the humans was 35 μmol/L, 4F concentrations of 4 nmol/L in humans and 130 mol/L in animal models could be biologically active.
In cultures of human aortic endothelial cells, adding only 4 nmol/L of L-4F together with 35 μmol/L of apoA-I significantly reduced the LDL-induced MCP-1, whereas this was not achieved following the addition of human apoA-I at a concentration of 35 μmol/L.33
The 4F peptides and apoA-I bind non-oxidized lipids such as 1-palmiloyl-2-arachidonoyl-sn-glycero-3-phosphotyleholine (PAPC) with similar affinities. Oxidation of PAPC produces a series of oxidation products, including 1-palmitoyl-2-(5,6-epoxyisoproslane E2)-sn-glycero-3-phosphorylcholinc (PEIPC), which potently stimulate human aortic endothelial cells to produce MCP-1.33 It was found that the binding affinity of PEIPC for L-4F was approximately 5 million-fold greater than it was for apoA-I33
A series of apoA-I mimetic peptides were designed and tested and it was hypothesized that apoA-I mimetic peptides that bind pro-inflammatory oxidized lipids similarly to apoA-I will not be anti-inflammatory and those that bind oxidized lipids with much higher affinity than is the case for apoA-I will be.33
HDL and apoA-I reduce inflammatory responses to lipo-polysaccharide (LPS). Dai et al34 reported that the apoA-I mimetic peptide, 4F. prevents LPS-induced changes in blood pressure and vascular reactivity. The authors proposed that 4F results in endotoxin neutralization by promoting the localization of LPS to the HDL fraction. They also suggested that 4F may therefore prevent hemodynamic changes associated with the nitric oxide synthase 2 (NOS2) induction that results from LPS administration.34
HDL and its major protein component, apoA-I, and apoA-I mimetic peptide 4F exert anti-inflammatory effects, inhibit monocyte chemotaxis/adhesion, and reduce the vascular macrophage content in inflammatory conditions (Figure 1). Smythies et al35 showed that by regulating the expression of key cell surface receptors on monocyte-derived macrophages (MDMs), 4F modulates the function of MDMs. They provided evidence that 4F. similar to apoA-I. induces profound functional changes in MDMs, possibly because of differentiation to an anti-inflammatory phenotype.
Figure 1.
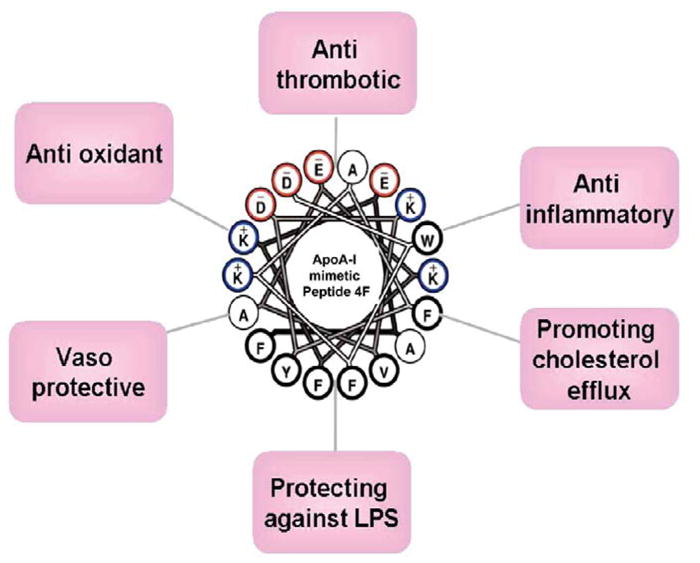
Pleiotropic effects of the apoA-I mimetic peptide 4F.
Effect of 4F on Serum Paraoxonase 1 (PON1) and Oxidized Lipids
PON1 is a HDL-associated enzyme and responsible for the antioxidativc properties of HDL. Forte el al showed that ApoE−/− mice had elevated levels of lysophosphatidylcholine and bioactive oxidized phospholipids compared with controls on a chow diet.36 Elevated oxidized phospholipids may have, in part, contributed to Spontaneous lesions in these mice on a chow diet. A Western diet decreased PON1 activity in these mice by 38%.36 It has been suggested that the removal of oxidized fatty acids from HDL might cause the return of PON1 activity.
Although PON1 has the ability to prevent lipid oxidation and may even inactivate oxidized lipids once formed under conditions of excess inflammatory pressure,37,38 the ability of HDL to protect itself and other lipid containing molecules and structures can be reduced. Reduction of lipid and protein oxidation by agents such as HDL mimetic peptides may prove to be an effective way of supporting the protective role of HDL.39
Recently Imaizumi et al40 reported that injecting L-4F into apoE null mice resulted in a significant reduction in plasma levels of 15 hydroxyeicosatetraenoic acid (HETF), 5-HETE, 13-hydroxyoctadecadienoic acid (HODE) and 9-HODE. Plasma levels of 14,15-epoxyeicosatrienoic acid, which are derived from the cytochrome P450 pathway, were elevated and plasma levels of 20-HETE were unchanged following injection of L-4F. An increase in the plasma levels of 13-HODE and 9-HODE was observed following injection of 13(S)-hydroperoxyoctadecadienoic acid (HPODE) into wild-type C57BL/6J mice. This was accompanied by a significant reduction in the anti-inflammatory properties of HDL. Injection of 13(S)-HPODE into atherosclerosis-resistant C3H/HeJ mice resulted in a similar but much more blunted response. When L-4F was injected at a site different from that at which the l3(S)-HPODE was administered, it resulted in significantly lower plasma levels of 13-HODE and 9-HODE and significantly less loss of HDL’s anti-inflammatory properties in both atherosclerosis-susceptible or-resistant mouse strains. The authors concluded that L-4F administration differentially alters mouse plasma levels of oxidized fatty acids. The additionally suggested that the reduced reaction of the C3H/HeJ mouse to these potent lipid oxidants may play a role in the resistance of this line of mice to atherosclerosis.40
The liver is the organ with the highest accumulation of oxidized lipids. Previous studies have shown that there is a selective hepatic uptake of oxidized LDL and HDL cholesterol esters mediated by scavenger receptors on the liver.41,42 Morgantini et al reported that the livers of diabetic mice contained high levels of oxidized fatty acids, which were significantly reduced by treatment with the 4F peptide.43 Concomitant with the decrease in hepatic levels of oxidized fatty acid, there was also a significant decrease in aortic atherosclerosis in the 4F-treated diabetic mice.43
Morgantini et al showed ex-vivo incubation of plasma samples from diabetic patients with L-4F induced a significant improvement in HDL’s anti-inflammatory properties, as well as in samples from healthy volunteers.44 Together with the fact that administration of the 4F peptide improved HDL function in various animal models, these results may indicate that oxidized lipids in HDL are strongly related to HDL function because the effect of the apoA-I mimetic is related to its ability to avidly bind oxidized lipids.33
Effect of ApoA-l Mimetic Peptides on Cancer
Kozak et al reported that apoA-I, transthyretin, and transferrin, can better predict early-stage ovarian cancer than serum CA-125.45 However, it was not clear whether the reduced level of these markers in ovarian cancer patients was the result of cancer development or was causal in disease progression.
Lipid transport, inflammation, and oxidative stress play a major role in the initiation and progression of cancer. Camuzcuoglu et al reported that decreased serum paraoxonase activity and increased serum lipid hydroperoxide levels correlated with stage, grade and the level of tumor marker CA-125 of ovarian cancer.46 As mentioned before, apoA-I mimetic peptides strongly bind oxidized lipids and mimic the anti-inflammatory and antioxidant functionalities of apoA-I. Su et al reported that overexpression of human apoA-I in transgenic mice significantly improved survival and decreased tumor size in a mouse model of ovarian cancer.47 In addition, treatment with the apoA-I mimetic peptide 4F or 5F reduced in vitro viability and proliferation of mouse and human ovarian cancer cells and inhibited in vivo tumor growth in a mouse model of ovarian cancer.47
It has been demonstrated that the pro-inflammatory lysophospholipid, lysophosphalidic acid (LPA), stimulates tumor development, increases angiogenesis and promotes metastasis of cancer.48 Su et al reported that although both apoA-I and 4F bind LPA, the binding affinity of 4F for LPA is more than 6 orders of magnitude greater than is the binding affinity for LPA by apoA-I.47 They reported from in vitro experiments that LPA-induced ovarian cancer cell growth was significantly reduced by 4F, and also showed that plasma levels of LPA were significantly reduced in an ovarian cancer mouse model that received 4F or 5F.47 Binding and removal of LPA may be one of the mechanisms for the inhibition of tumor development by apoA-I mimetic peptides. Gao et al reported that the apoA-I mimetic peptide, L-5F, reduced tumor angiogenesis in vivo and inhibited vascular endothelial growth factor (VEGF)/basic fibroblast growth factor (bFGF)-stimulated proliferation, migration, and invasion, as well as the tube formation of human umbilical vascular endothelial cells.49 They demonstrated that L-5F inhibits angiogenesis by mechanisms that may involve the removal of LPA-like bioactive lipids from the tumor environment. L-5F significantly suppressed LPA-stimulated proliferation of ovarian cancer cells and production of VEGF.49 They also showed that in vivo VEGF levels were reduced in both tumor tissue and the circulation after treatment with L-5F. and that L-5F inhibited LPA-stimulated cell viability and VEGF secretion from ovarian cancer cell lines. L-5F also inhibited VEGF-and bFGF-induced activation of their corresponding receptors, VEGFR2 and FGFR1, as well as downstream signaling pathways.49 Those authors hypothesized that apoA-I mimetic peptides may not only remove LPA-like bioactive lipids but also alter lipid composition/structure of cellular membranes and may thus lead to alteration in the function of membranes receptors such as VEGF2 and FGFR1.
Ganapathy et al reported that 4F reduced the viability and proliferation of ID8 cells, a mouse epithelial ovarian cancer cell line, With a concomitant improvement of the antioxidant status of ID8 cells, as measured by lipid peroxide, protein carbonyl, superoxide anion, and hydrogen peroxide levels.50 They also showed that inhibition of ID8 cell proliferation by 4F required the upregulation of MnSOD protein and activity, which is a tumor suppressor protein that increases the dismutation rate of superoxide anion to hydrogen peroxide and inhibits cancer cell growth.50 They postulated that apoA-I mimetic peptides may be utilized as novel therapies for the treatment of diseases that are regulated by pro-oxidant processes, including cardiovascular disease and cancer.
Human Clinical Trials of the 4F Peptides
Bloedon et al29 reported that oral doses of D-4F of 4.3 mg/kg and 7.14 mg/kg significantly improved the HDL-inflammatory index HII) in patients with CHD who were already taking statins, but doses of 0.43 mg/kg and l.43 mg/kg did not. The improvement in HII with the former doses of D-4F occurred with Cmax plasma peptide levels of only 15.9±6.53 ng/ml.29 Because Van Lenten et al51 demonstrated that when given by injection D-4F and L-4F were equally efficacious, Watson el al52 designed clinical trials using L-4F in patients with CHD on a stable dose of statins. When L-4F was administered intravenously at a dose ranging from 0.042 to 1.43 mg/kg for 7 consecutive days there was no significant improvement in HII, despite achieving peptide plasma levels of 3,255+640 ng/ml for the dose used for statistical analysis (0.43 mg/kg).52 Subsequently, Navab et al53 determined that the dose administered and not the plasma level of the 4F peptide determined efficacy. They concluded that the failure of the 4F peptide to improve HII in the studies by Watson et al52 was because the doses used were below the threshold of efficacy established in the studies of Bloedon et al.29 Moreover, Navab et al53 found that regardless of whether the peptide was administered by injection or given orally, the concentration of peptide in the feces and the amount of peptide excreted in the feces for each dose administered was similar for similar doses, despite differences in plasma peptide levels of 1,000-fold.53 Navab et al concluded that the intestine maybe a major site of action for the 4F peptide, regardless of the route of administration.53
Summary and Conclusion
The levels and function of lipoproteins (HDL and LDL) are important determinants of cardiovascular health.54,55 Lipid oxidation products can increase the susceptibility of LDL to oxidative modification and reduce the anti-inflammatory capacity of HDL. The apoA-I mimetic peptides, 4F and 5F, have a high affinity for oxidized phospholipids and oxidized tatty acids. Removal of seeding molecules in LDL and inhibitory factors in HDL can help normalize the composition and function of lipoproteins, which could have a beneficial effect on HDL function, on lipoprotein metabolism, on endothelial function, and on platelet activation (Figure 2). Overall, the usefulness of several apolipoprotein mimetic peptides as research tools to understand and perturb the basic mechanisms of HDL metabolism, HDL function, atherosclerosis and more than a dozen inflammatory disorders has been firmly established in multiple animal models. Determining the role of apolipoprotein mimetic peptides as therapeutic agents in humans, however, will take years and many studies in a variety of patient populations.
Figure 2.
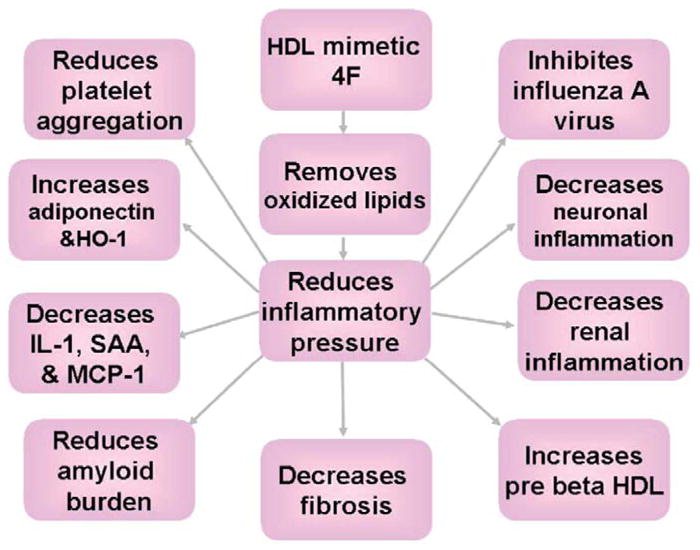
The apoA-I mimetic peptide, 4F, improves a number of pathological processes in animal models, probably by removing oxidized lipids.
Acknowledgments
This work was supported in part by LIS Public Health Service Grunts HL-30568, HL-34343, HL-082823 and the Laubisch, Castera, and M.K. Grey Funds at UCLA, the Women’s Endowment, Carl and Roberta Deutsch Family Foundation, the Joan English Fund for Women’s Cancer Research; the VA Merit I Award (R.F.E.), the Ovarian Cancer Coalition, the Helen Beller Foundation, Wendy Stark Foundation and the Sue and Mel Geleibter Family Foundation.
Footnotes
Disclosure
Conflict of Interest: AMF, MN, and STR are Principals of Bruin Pharma and AMF is an officer of Bruin Pharma.
References
- 1.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant apoA-I milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 4.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 5.Anantharamaiah GM, Jones JL, Brouillette CG, Schmidt CF, Chung BH, Hughes TA, et al. Studies of synthetic peptide analogs of the amphipathic helix: Structure of complexes with dimyrisloyl phosphatidylcholine. J Biol Chem. 1985;260:10248–10255. [PubMed] [Google Scholar]
- 6.Yancey PG, Bielicki JK, Johnson WJ, Lund-Katz S, Palgunachari MN, Anantharamaiah GM, et al. Efflux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class a amphipathic peptides. Biochemistry. 1995;34:7955–7965. doi: 10.1021/bi00024a021. [DOI] [PubMed] [Google Scholar]
- 7.Venkatachalapathi YV, Phillips MC, Epand RM, Epand RF, Tytler EM, Segrest JP, et al. Effect of end group blockage on the properties of a class a amphipathic helical peptide. Proteins. 1993;15:349–359. doi: 10.1002/prot.340150403. [DOI] [PubMed] [Google Scholar]
- 8.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class a amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- 9.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 10.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Step 1. J Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 11.Garber DW, Dutta G, Chaddha M, Palgunachari MN, Hama SY, Navab M, et al. A new synthetic class a amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J Lipid Res. 2001;42:545–552. [PubMed] [Google Scholar]
- 12.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, et al. Oral administration of an apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 13.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, et al. D-4F Induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2015;111:3126–3134. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 14.Peterson SJ, Husney D, Kruger AL, Olszanecki R, Ricci F, Rodella LF, et al. Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type 1 diabetic rats. J Pharmacol Exp Ther. 2007;322:514–520. doi: 10.1124/jpet.107.119479. [DOI] [PubMed] [Google Scholar]
- 15.Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, et al. L-4f treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, et al. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation. 2002;106:1127–1132. doi: 10.1161/01.cir.0000030182.35880.3e. [DOI] [PubMed] [Google Scholar]
- 18.Weihrauch D, Xu H, Shi Y, Wang I, Brien J, Jones DW, et al. Effects of D-4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in light-skin mice. Am J Physiol Heart Circ Physiol. 2007;293:H1432–H1441. doi: 10.1152/ajpheart.00038.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ou J, Ou Z, Jones DW, Holzhauer S, Hatoum OA, Ackerman AW, et al. L-4F, an apolipoprolein A-I mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation. 2003;107:2337–2341. doi: 10.1161/01.CIR.0000070589.61860.A9. [DOI] [PubMed] [Google Scholar]
- 20.Ou J, Wang J, Xu H, On Z, Sorci-Thomas MG, Jones DW, et al. Effects of D-4F on vasodilation and vessel wall thickness in hyper-cholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on western diet. Circ Res. 2005;97:1190–1197. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLeve LD, Wang X, Kanel GC, Atkinson RD, MeCuskey RS. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handattu SP, Garber DW, Monroe CE, van Groen T, Radish I, Nayyar G, et al. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of alzheimer’s disease. Neurobiol Dis. 2009;34:525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buga G, Frank JS, Mottino GA, Hendizadeh M, Hakhamian A, Tillisch JH, et al. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a western diet. J Lipid Res. 2006;47:2148–2160. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Buga GM, Frank JS, Mottino GA, Hakhamian A, Narasimha A, Watson AD, et al. D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a western diet. J Lipid Res. 2008;49:192–205. doi: 10.1194/jlr.M700433-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Buga GM, Navab M, Imaizumi S, Reddy ST, Yekta B, Hough G, et al. L-4F alters hyperlipidemic (but not healthy) mouse plasma to reduce platelet aggregation. Arterioscler Thromb Vasc Biol. 2010;30:283–289. doi: 10.1161/ATVBAHA.109.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navab M, Anantharamaiah GM, Hama S, Hough G, Reddy ST, Frank JS, et al. D-4F and statins synergize to render HDL anti-inflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25:1426–1432. doi: 10.1161/01.ATV.0000167412.98221.1a. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Chyu KY, Faria Neto JR, Yano J, Nathwani N, Ferreira C, et al. Differential effects of apolipoprotein A-1-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;110:1701–1705. doi: 10.1161/01.CIR.0000142857.79401.69. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh GR, Schnickel GT, Garcia C, Shefizadeh A, Fishbein MC, Ardehali A. Inflammation/oxidation in chronic rejection: Apolipoprotein A-I mimetic peptide reduces chronic rejection of transplanted hearts. Transplantation. 2007;84:238–243. doi: 10.1097/01.tp.0000268509.60200.ea. [DOI] [PubMed] [Google Scholar]
- 29.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, et al. Safely, pharmacokinetics, and pharmacodynamics of oral apoA-1 mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo JM, Lin Z, Navab M, Van Dyck C, Trejo-Lopez Y, Woo KM, et al. Treatment with apolipoprotein A-1 mimetic peptide reduces lupus like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis Res Ther. 2010;12:R93. doi: 10.1186/ar3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charles-Schoeman C, Banquerigo ML, Hama S, Navab M, Park GS, Van Lenten BJ, et al. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin Immunol. 2008;127:234–244. doi: 10.1016/j.clim.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe J, Chou KJ, Liao JC, Miao Y, Meng HH, Ge H, et al. Differential association of hemoglobin with proinflammatory high density lipoproteins in atherogenic/hyperlipidemic mice: A novel biomarker of atherosclerosis. J Biol Chem. 2007;282:23698–23707. doi: 10.1074/jbc.M702163200. [DOI] [PubMed] [Google Scholar]
- 33.Van Lenten BJ, Wagner AC, Jung GL, Ruchala P, Waring AJ, Lehrer RI, et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai L, Datta G, Zhang Z, Gupta H, Patel R, Honavar J, et al. The apolipoprotein A-I mimetic peptide 4F prevents defects in vascular function in endotoxemic rats. J Lipid Res. 2010;51:2695–2705. doi: 10.1194/jlr.M008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, et al. Apolipoprotein A I mimetic 4F alters the function of human monocyte-derived macrophages. Am J Physiol Cell Physiol. 2010;298:C1538–1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forte TM, Subbanagounder O, Berliner JA, Blanche PJ, Clermont AO, Jia Z, et al. Altered activities of anti-atherogenic enzymes lcat, paraoxonase, and platelet-activating factor acetyl hydrolase in atherosclerosis-susceptible mice. J Lipid Res. 2002;43:477–485. [PubMed] [Google Scholar]
- 37.Mukamal KJ, Pai JK, Jensen MK, Rimm EB. Paraoxonase 1 polymorphisms and risk of myocardial infarction in women and men. Circ J. 2009;73:1302–1307. doi: 10.1253/circj.cj-08-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mashiba J, Koike G, Kamiunten H, Ikeda M, Sunagawa K. Vasospastic angina and microvascular angina are differentially influenced by PON1 A632G polymorphism in the Japanese. Circ J. 2005;69:1466–1471. doi: 10.1253/circj.69.1466. [DOI] [PubMed] [Google Scholar]
- 39.Vakili L, Hama S, Kim JB, Tien D, Safarpoor S, Ly N, et al. The effect of HDL mimetic peptide 4F on PON1. Adv Exp Med Biol. 2010;660:167–172. doi: 10.1007/978-1-60761-350-3_15. [DOI] [PubMed] [Google Scholar]
- 40.Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamaiah GM, et al. L-4F deferentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett. 2010;4:139–148. doi: 10.2174/187231210791698438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fluiter K, Sattier W, De Beer MC, Connell PM, van der Westhuyzen DR, van Berkel TJ. Scavenger receptor B1 mediates the selective uptake of oxidized cholesterol esters by rat liver. J Biol Chem. 1999;274:8893–8899. doi: 10.1074/jbc.274.13.8893. [DOI] [PubMed] [Google Scholar]
- 42.Fluiter K, van der Westhuijzen DR, van Berkel TJ. In vivo regulation of scavenger receptor B1 and the selective uptake of high density lipoprotein cholesteryl esters in rat liver parenchymal and kupffer cells. J Biol Chem. 1998;273:8434–8438. doi: 10.1074/jbc.273.14.8434. [DOI] [PubMed] [Google Scholar]
- 43.Morgantini C, Imaizumi S, Grijalva V, Navab M, Fogelman AM, Reddy ST. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 2010;59:3223–3228. doi: 10.2337/db10-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgantini C, Natali A, Boldrini B, Navab M, Fogelman AM, Ferrannini L, et al. Anti-inflammatory and anti-oxidant properties of high density lipoproteins (HDL) are impaired in type 2 diabetes. Proceedings of American Diabetes Association’s 71st Scientific Sessions; June 2011; [Supplement to Diabetes] [Google Scholar]
- 45.Kozak KR, Amneus MW, Pusey SM, Su F, Luong MN, Luong SA, et al. Identification of biomarkers for ovarian cancer using strong anion-exchange proteinchips: Potential use in diagnosis and prognosis. Proc Natl Acad Sci USA. 2003;100:12343–12348. doi: 10.1073/pnas.2033602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camuzcuoglu H, Arioz DT, Toy H, Kurt S, Celik H, Erel O. Serum paraoxonase and arylesterase activities in patients with epithelial ovarian cancer. Gynecol Oncol. 2009;112:481–485. doi: 10.1016/j.ygyno.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Su F, Kozak KR, Imzumi S, Gao F, Amneus MW, Grijalva V, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci USA. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samadi N, Bekele R, Capatos D, Venkatraman G, Sariahmetoglu M, Brindley DN. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis and metastasis and chemo-resistance. Biochimie. 2011;93:61–70. doi: 10.1016/j.biochi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Gao F, Vasquez SX, Su F, Roberts S, Shah N, Grijalva V, et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Integr Biol (Camb) 2011;3:479–489. doi: 10.1039/c0ib00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganapathy E, Su F, Meriwether D, Devarajan A, Grijalva V, Gao F, et al. D-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the anti-oxidant enzyme MnSOD. Int J Cancer. 2011 doi: 10.1002/ijc.26079. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, et al. Lipoprotein inflammatory properties and serum amyloid a levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res. 2007;48:2344–2353. doi: 10.1194/jlr.M700138-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, et al. Treatment of patients with cardiovascular disease with L-4F, an apoA-I mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, et al. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether the peptide is administered subcutaneously or orally. J lipid Res. 2011;52:1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasuda T, Ishida T, Rader DJ. Update on the role of endothelial lipase in high-density lipoprotein metabolism, reverse cholesterol transport, and atherosclerosis. Cir J. 2010;74:2263–2270. doi: 10.1253/circj.cj-10-0934. [DOI] [PubMed] [Google Scholar]
- 55.Miyauchi K, Daida H. Clinical significance of intensive lipid-lowering therapy using statins in patients with coronary artery disease: LDL-cholesterol: The lower, the better; is it true for Asians? (Pro) Cir J. 2010;74:1718–1730. doi: 10.1253/circj.cj-10-0565. [DOI] [PubMed] [Google Scholar]


