Abstract
Nephrolithiasis is a result of formation and retention of crystals within the kidneys. The driving force behind crystal formation is urinary supersaturation with respect to the stone forming salts, which means that crystals form when the concentrations of participating ions are higher than the thermodynamic solubility for that salt. Levels of supersaturation are kept low and under control by proper functioning of a variety of cells including those that line the renal tubules. It is our hypothesis that crystal deposition i.e. formation and retention in the kidneys, is a result of impaired cellular function, which may be intrinsic and inherent or triggered by external stimuli and challenges. Cellular impairment or dysfunction affects the supersaturation, by influencing the excretion of participating ions such as calcium, oxalate and citrate and causing hypercalciuria, hyperoxaluria or hypocitraturia. The production and excretion of macromolecular promoters and inhibitors of crystallization is also dependent upon proper functioning of the renal epithelial cells. Insufficient or ineffective crystallization modulators such as osteopontin (OPN), Tamm Horsfall protein (THP), bikunin (BK) etc are most likely produced by the impaired cells.
Keywords: Calcium Oxalate, Oxalate, Hyperoxaluria, Hypercalciuria, Hypocitraturia, Genetics
INTRODUCTION
Kidney stone formation is a common urological disorder with a lifetime risk in the U.S. of nearly 13% in men and 7% in women [1]. Recurrence of stone formation is common. Probability of recurrence in idiopathic stone formers is 40 to 50% within five years of the initial episode and 50% to 60% by 10 years. Thus approximately 40% of the stone formers do not produce another stone. Stone formers with systemic diseases such as cystinuria, primary hyperoxaluria, and primary hyperparathyroidism have a higher rate of recurrence. Calcium oxalate (CaOx) is the major constituent of most stones and is generally found mixed with calcium phosphate. Hypercalciuria, hyperoxaluria and hypocitraturia, alone or in combination are main abnormalities in most idiopathic calcium oxalate stone formers. Obviously stone formation is episodic and stone formers have specific abnormalities, some innate resulting in regular and frequent stone recurrences and others that require triggering and activation leading to long intervals between stone events. It is our hypothesis that abnormalities are the result of cellular dysfunctions, some inherited and endogenous and others extrinsic and environmental.
The stones can form anywhere in the urinary system, from kidneys to the bladder but in the industrialized and affluent societies, they are generally restricted to the kidneys. By and large the kidney stones form attached to the renal papillary tips. Randall first emphasized the importance of renal papilla when he described minute tubular calculi and subepithelial calcium plaques within and on human renal papillae and suggested that these could serve as focal points for stone development [2]. He suggested that interstitial subepithelial deposits of calcium phosphate or calcium carbonate arising from pathological conditions of the renal papilla erode through to the papillary surface forming a lesion, which he called type 1. He further suggested that excessive urinary supersaturation in association with tubular cell death results in crystal deposition in the collecting ducts producing a type 2 lesion. Both types of lesions acted as foci for further stone growth in the pelvis or papillary ducts. Thus Randall proposed a theory in which both urinary supersaturation and renal tubular damage/dysfunction play a part in stone formation. In this article we will review clinical and experimental data and examine the role of genetic basis of renal cellular dysfunction in the development of urinary supersaturation and the formation of CaOx kidney stones.
SUPERSATURATION AND CRYSTALLIZATION OF CALCIUM OXALATE
The formation of kidney stones or nephrolithiasis is a result of crystal formation in the kidneys. Crystal formation particularly that of calcium phosphate (CaP) and CaOx, is widespread within the urinary tract. Humans excrete millions of urinary crystals every day, indicating at least transient development of supersaturation. However few develop kidney stones, probably, because either the crystals do not form in the kidneys or the crystals that form do not stay there. It has been suggested that with a transit time across the kidney of 5-10 minutes, residence time for the crystals to nucleate and grow large enough to be trapped [3] is not enough. The inner diameter of various segments of renal tubules ranges from 15 - 60 μm [4]. The individual crystals of CaOx, growing at the rate of 1-2 μm/minute can not grow bigger than a few microns and are therefore excreted with urine without causing any stone episode. Thus crystal aggregation and means for crystal retention are essential for stone formation.
The driving force for crystallization is the development of supersaturation with respect to the precipitating salt [5]. However, supersaturation alone can not explain stone formation because people who have never formed stones can also pass highly supersaturated urine [6]. Human urine is a complex solution containing not only calcium (Ca) and oxalate (Ox) but also other ions and macromolecules that can interact with Ca and/or Ox and modulate crystallization. As a result urinary CaOx supersaturation depends not only on the concentration of Ca and Ox but also the presence of ions such as citrate and magnesium. As a result hypercalciuria, hyperoxaluria and hypocitraturia are major risk factors for calcific stone formation.
Superstauration and crystallization in the urine also depend upon the presence of macromolecules such as many proteins and lipids [7], which can bind or form complexes with Ca and/or Ox. These molecules can determine whether a crystal will nucleate and grow into a stone or excreted as a crystalluria particle. Normal mammalian urine contains many macromolecules, which inhibit crystal formation, growth and aggregation in the kidneys. On the other hand stone formers may produce structurally and functionally defective macromolecules leading to reduced inhibition or even promotion of crystallization and aggregation. Any cellular defect or dysfunction that can affect the quantity of participating urinary ions and production of macromolecules can influence CaOx supersaturation and crystallization in the kidneys. Some cellular dysfunctions are innate and the result of genetic modifications, which is the topic of current discussion. The fact that stone disease has a genetic basis has long been appreciated. There is however no agreement as to which genes are involved and to what extent.
Calciuria
Hypercalciuria is one of the major risk factors for the formation of idiopathic kidney stones. Genes encoding for soluble adenylate cyclase (sAC), vitamin D receptor (VDR), calcium sensing receptor (CaSR), sodium phosphate co-transporter-2( NPT-2), chloride channel-5 (CLC-5), transient receptor potential cations channel V (TRPV5) and claudin-16 have been implicated in hypercalciuria and idiopathic nephrolithiasis (Table 1), many divergent results notwithstanding [8]. sAC is expressed in kidneys, intestine and bone [9]. In the kidneys the expression is seen in epithelia lining distal tubules, thick ascending limbs and collecting ducts [10]. sAC is suggested to be a bicarbonate exchanger [9,10]. Individuals with sAC mutations are hypercalciuric osteopenic stone formers [8, 11, 12].
Table 1.
Genes involved in hypercalciuria, gene products and their renal expression and phenotype. CD, collecting duct; DCT, distal convoluted tubule; IC, intercalated cell PT, proximal tubule; MCD, medullary collecting duct; TAL, thick ascending limb
| Gene | Gene Product/Function | Renal Tubular Expression | Renal Phenotype |
|---|---|---|---|
| VDR | Vitamin D Receptor | DCT, CD | Decreased calcium reabsorption leading to Hypercalciuria and Nephrocalcinosis |
| CLCN5 | Cl/H antiporter | PT, TAL, αIC | Inactivating mutation causes Hypercalciuria, Hyperphosphaturia, Low molecular weight proteinuria, Nephrocalcinopsis, Stones |
| CASR | Calcium Sensing Receptor | PT (apical), MCD (principal cell, apical), TAL (basal), DCT (basal) |
Gain of Function mutation produces Hypercalciuria, Nephrocalcinosis, Stones |
| CLDN16 | Tight Junction protein | TAL, DCT | Hypercalciuria, Magnesium wasting, Nephrocalcinosis, Stones |
| NPT2a/c | Sodium phosphate co-transporter |
PT | Hypercalciuria, Hypophosphatemia, Phosphate wasting, Nephrocalcinosis, Stones |
| TRPV5 | Calcium selective transient receptor potential channel |
DCT, Connecting Tubule | Hypercalciuria, Hyperphosphaturia |
| sAC | Soluble adenylate cyclase/bicarbonate exchanger |
DCT, TAL, CD | Hypercalciuria, Stones |
| KLOTHO | Aging suppression protein/ Regulator of calcium homeostasis |
DCT | Hypercalciuria |
VDR is expressed in vitamin D sensitive tissues and VDR genes and their polymorphisms are suggested to play significant role in hypercalciuria and stone formation [8, 13-18]. In the kidneys, VDR is mainly expressed in the distal tubules and collectinmg ducts. Vitamin D-dependent reabsorption mainly occurs in the distal tubules. The concept of VDR involvement in hypercalciuria and stone formation is strengthened by investigations of hypercalciuric rats produced by selective breeding of normal Sprague-Dawley rats over 60 generations. These rats have high intestinal expression of VDR, increased calcium absorption in the intestine, increased bone resorption, decreased calcium reabsorption in the kidney, and produce calcium phosphate stones in the urinary space [13]. The hypercalciuric rats produce CaOx stones when made hyperoxaluric through dietary manipulation [19]. Human investigations of VDR gene and polymorphism have however led to conflicting data. Increased numbers of vitamin D receptors were found on peripheral blood lymphocytes of some hypercalciuric patients but no abnormality of the VDR gene was detected [20]. In a study of French-Canadian sibling pairs a susceptibility locus associated with stones and hypercalciuria was identified on chromosome 12q12-14 near the VDR gene [21]. Somewhat similar results were obtained investigating Indian families with hypercalciuric stone forming members [14]. In another study, this time with European hypercalciuric stone forming families, no linkage was found between chromosome 12q12-14 and hypercalciuria [22].
Several of the restriction fragment length polymorphisms (RFLPs) of the VDR gene have been implicated in hypercalciuria and stone formation. Links have been established in some cases and not confirmed in others. An association between FokI polymorphism and calcium oxalate stone disease [16, 23, 24] and TaqI polymorphism and severe recurrent stone disease [12] has been suggested. No association between stone formation and FokI or TaqI polymorphism has been reported [25-27]. In one study ApaI and BsmI polymorphisms coincided only with fasting hypercalciuria [27].
CaSR is expressed in kidneys, intestine, parathyroid and bone [28, 29] and among other functions is involved in renal handling of calcium and water. In the kidneys it is expressed in the apical membranes of proximal tubular epithelial cells and principal cells of the medullary collecting ducts and basolateral membranes of epithelial cells lining the thick ascending limb of the loop of Henle as well as the distal tubules. Polymorphism of CaSR gene has been shown to be associated with calcific stone formation. The relative risk of hypercalciuria is increased in individuals with gain of function mutation [30, 31]. Activating CaSR mutation in a mice model leads to ectopic calcification [32].
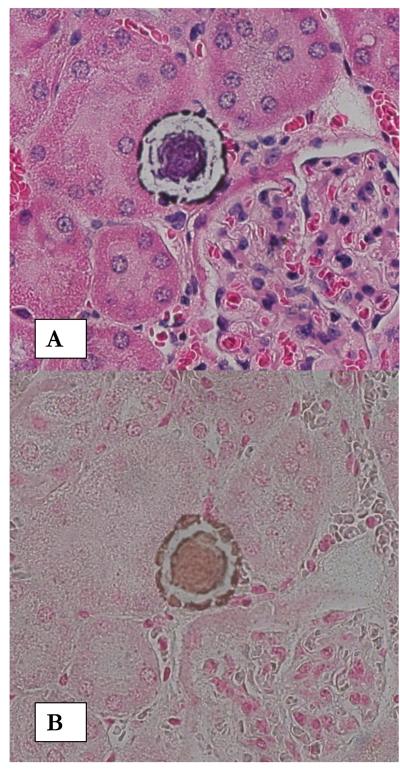
Renal phosphate wasting with nephrolithiasis is reported in hereditary hypophosphatemic rickets with hypercalciuria as well as Dent’s disease. Since NPT2a encodes for proximal tubular sodium phosphate cotransporter, which is involved in reabsorption of filtered phosphate, this gene is considered a candidate gene for hereditary renal phosphaturia. Mutation in NPT2 gene encoding sodium phosphate co-transporter in proximal tubule has been implicated in hypophosphatemia and kidney stone [33]. Variant NPT2a were found in two of 20 study subjects with osteoporosis or recurrent stone disease with renal phosphate leak. A later study of a cohort of 98 families of hypercalciuric stone formers found a number of genetic variations in the gene. But the variations were not associated with significant abnormalities of phosphate or calcium handling [34]. Recently mutations in genes encoding NPT2c transporter have been identified in consanguineous kindreds and additional families with hereditary hypophosphatemic rickets and hypercalciuria indicating that NPT2c may play significant role in the kidneys [35]. Mice with disrupted NPT2a co-transporter gene created by targeted mutagenesis exhibit increased urinary excretion of Pi, ~80% decrease in renal brush border membrane Na/Pi cotransport, and hypophosphatemia, which leads to increased serum 1,25 (OH) 2D levels, overexpression of intestinal calcium channels, intestinal calcium hyperabsorption and development of hypercalciuria [36]. NPT2a -/- mice develop renal deposits of apatitic calcium phosphate in their kidneys [37], present in newborn, weanling as well as adult mice (Figure 1).
Figure1.
Calcium phosphate deposition in kidneys of NPT2a -/- mice. A. H&E stained paraffin section of a kidney from a 13 month old mouse showing what appear to be interstitial deposits in the renal cortex. Renal tubules are completely devoid of any crystals. Deposits show ring like substructure. Original magnification X 40. B. Von Kossa staining of a similar crystal deposit illustrates that it is calcium phosphate crystals.
Chloride/proton antiporter CLC-5 is encoded by CLCN5 gene and is expressed in the renal epithelia lining proximal tubule and thick ascending limb of the loop of Henle and alpha intercalated cells of the collecting ducts. Inactivating mutations of CLCN5 cause Dent’s disease, an X-linked recessive tubulopathy characterized by low molecular weight proteinuria, hypercalciuria, nephrocalcinosis, nephrolithiasis and progressive renal failure [38]. CLC-5 is critical in endosome acidification and involved in membrane trafficking via receptor-mediated endocytic pathway. Loss of chloride channel CLC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules [39]. Mice lacking CLC-5 gene show endocytic disruption [40, 41] and develop renal tubular defects with low molecular weight proteinuria, hypercalciuria and nephrocalcinosis [41, 42].
One cause of hypercalciuria is decreased calcium reabsorption by renal tubules which occurs by passive entry of Ca++ through apical Ca++ channels (TRPV5 and TRPV6) followed by diffusion through the cytosol facilitated by calbindin-D28K with eventual extrusion across the basolateral membrane. Currently, it is thought that the primary rate limiting step for transepithelial Ca++ reabsorption is the apical entry step. TRPV5 is the main channel responsible for apical Ca++ entry.TRPV5 is a calcium selective channel expressed in distal convoluted tubule and connecting tubule and a member of transient receptor potential (TRP) channel family, which includes 28 ion channels that act as cellular sensors and regulate a variety of cell functions [43, 44]. TRPV5 channels mediate calcium reabsorption in the kidneys and their expression is regulated by parathyroid harmone, 1,25 di-hydroxyvitamin D3, estrogen and dietary calcium. TRPV5 -/- mice have impaired Ca reabsorption and high plasma 1,25 di-hydroxyvitamin D3 with compensatory hyperabsorption of dietary calcium and severe calcium wasting [45]. Despite elevated urinary calcium excretion there is no renal calcification because of significant polyuria. Human mutations of TRPV5 have so far not been reported. Thus the importance of this gene in idiopathic hypercalciuria is unknown. TRPV5-mediated calcium reabsorption could however be regulated by other molecules such as “with no lysine kinase 4 (WNK4) [46]. Mutation of WNK4 causes autosomal dominant pseudohypoaldosteronism type II, which is characterized by hypercalciuria associated with hyperkalemic hypertension.
Claudins are present in membranes of tight junctions in a number of epithelia [47]. Claudin-16 also called paracellin-1 (PCLN-1) is exclusively expressed in thick ascending limb of the loop of Henle and plays a role in paracellular transport of calcium and magnesium [48]. Loss of function mutations in CLDN16 gene encoding for claudin-16 have been identified in patients with familial hypomagnesemia with hypercalciuria and nephrocalcinosis [49]. Results of a study showed that many heterozygous relatives of patients with familial hypomagnesemia with hypercalciuria and nephrocalcinosis produced stones but the prevalence of nephrolithiasis or hypercalciuria was not significantly different from that in the general population [49]. A missense mutation of CLDN16 found in two families was associated with self limiting childhood hypercalciuria, which decreased with age and was not associated with progressive renal decline [50]. Klotho is an aging suppression protein predominantly expressed in renal distal convoluted tubules, parathyroid gland and epithelial cells of the choroid plexus [51, 52, 53]. Over-expression of klotho increases life span in mice [52]. In humans, a correlation between polymorphisms of klotho gene and life span, osteoporosis and coronary artery disease has been shown [53]. Klotho-deficient mice are hypercalcemic and hypercalciuric, show reduced renal absorption of calcium and reduced TRPV5 expression and have higher circulating levels of 1.25-D. Klotho inhibits the expression of 25-hydroxyvitamin D 1alpha hydroxylase, a key enzyme for synthesis of 1,25dihydroxyvitamin D (1,25-D) [54]. The treatment of cells in culture with klotho increases expression of TRPV5 channels [55]. Based on these and other observations klotho is considered a critical regulator of calcium homeostasis. Klotho is also known to serve as a coreceptor for bone-derived FGF23 [56], which promotes renal phosphate-wasting and vitamin D activation. The Klotho-FGFR complex binds to FGF23 with a higher affinity than FGFR or Klotho alone.
Oxaluria
Urinary oxalate, the critical risk factor in CaOx nephrolithiasis, is derived from dietary as well as endogenous sources and its concentration is controlled by production in the liver, by the erythrocytes, conversion of ascorbate and absorption and secretion in the gut and kidneys, involving many enzymes, transporters and exchangers [57]. Glyoxylate is the immediate precursor of endogenous oxalate in the liver as well as erythrocytes. In the liver, glycolate is oxidized by peroxisomal glycolate oxidase to glyoxylate, which is further oxidized to oxalate by lactate dehydrogenase. Under normal conditions glyoxylate is catalyzed by peroxysomal enzyme alanine glyoxylate aminotransferase (AGT) into glycine, which is metabolized to serine. Alternatively glycoxylate is reduced to glycolate by the widely distributed cytosolic enzyme D-glyoxylate reductase/d-glycerate dehydrogenase / hydroxypyruvate reductase. Deficient AGT activity due to mutation or mistargeting, results in failure to detoxify glyoxylate, which is either oxidized to oxalate or reduced to glycolate, leading to significantly increased urinary excretion of oxalate a condition called primary hyperoxaluria type 1 [58]. Deficiency of the enzyme d-glycerate dehydrogenase, leads to increased oxidation of glyoxylate to oxalate and its significantly increased urinary excretion. This condition is called primary hyperoxaluria type 2 [59].
A null mutant mouse was generated by targeted mutagenesis of the homologous alanine-glyoxylate amino transferase gene, Agxt, in embryonic stem cells. Mutant mice though developed normally, exhibited hyperoxaluria and CaOx crystalluria [60]. Urinary oxalate was normalized and CaOx crystalluria stopped by hepatic expression of human AGT1, the protein encoded by AGXT, by adenoviral vector-mediated gene transfer in Agxt(-/-) mice. The expression of wild-type human AGT1 was predominantly localized in peroxisomes of the mouse liver, while that of the most common mutant form of AGT1 (G170R) was primarily localized in the mitochondria.
Glyoxylate is also suggested to be the precursor of oxalate in the erythrocytes, where oxalate is transported via Band3 (AE1) anion transport protein Slc4a1. In the kidneys, Slc4a1 is expressed on the basolateral membrane of type A acid secretory intercalated cells of the collecting duct epithelium and is responsible for chloride/bicarbonate exchange [61]. Mice lacking Slc4a1 develop nephrocalcinosis, hypercalciuria, hyperphosphaturia and hypocitraturia [62]. Mutations in human gene Slc4a1 that encodes for band 3 protein causes distal renal tubular acidosis, which is associated with hypercalciuria and considered one of the risk factors of recurrent nephrolithiasis.
A significant amount of urinary oxalate is derived from diet and some stone formers may be hyperabsorbers of oxalate [63, 64]. Results of a study showed that idiopathic calcium oxalate stone formers with hyperoxaluria had significantly increased oxalate excretion after a 5 mmol oral oxalate load than those with normal urinary oxalate excretion [64]. Three members of solute-linked carrier 26 (Slc26) family of anion exchangers with role in oxalate absorption are expressed along the intestinal tract [65-67]. Slc26a3 is localized to the apical membrane of the epithelial lining of the colon. Slc26a6 is localized in the apical membrane of the small intestine and stomach epithelial cells. Slc26a7 is localized to the basolateral membrane of the stomach’s parietal cells.
Several members of Slc26 family of anion exchangers are also expressed in the kidneys [68]. In the proximal tubules Slc26a1 (Sat-1) mediates the transport of sulfate and oxalate across the basolateral membrane [69]. Slc26a6 (CFEX, Pat-1) is located in the apical membrane of the proximal tubular epithelial cells and primarily mediates chloride - oxalate exchange [70]. Targeted deletion of oxalate/anion exchanger gene Slc26a6 in two separate lines of mice led to hyperoxaluria attributable to defective intestinal excretion [71, 72]. In one model of Slc26a6 -/- mice hyperoxaluria is attributed to loss of secretion in the distal ileum accompanied by increased oxalate absorption [71]. In the other model loss of most duodenal oxalate secretion without change in the oxalate absorption [72] leads to hyperoxaluria, hyperoxalemia and calcium oxalate nephrolithiasis. Results of the studies with Slc26a6 -/- mice indicate that factors regulating these genes may affect oxalate homeostasis and possibly promote CaOx nephrolithiasis.
So far no polymorphism of the gene or its variants, have however, been identified among stone formers. But species-specific differences between mouse and human genes exist. Mouse Slc26a6 and human SLC26A6 share only 78% amino acid identity and exhibit significant differences in anion selectivity. The Slc26a6 in the mouse mediates bidirectional electrogenic chloride/oxalate transport while the same mediated by SLC26A6 in humans is electroneutral.
Citraturia
Urinary citrate is another significant determinant of CaOx supersaturation and an important risk factor for CaOx nephrolithiasis. Citrate in the urine binds to calcium forming a soluble compound thereby lowering free ionic calcium, reducing urinary supersaturation with respect to CaOx and CaP and inhibiting their precipitation [5]. In addition urinary citrate is shown to inhibit crystal nucleation, as well as growth and aggregation [73]. Results of several studies indicate that stone formers excrete less citrate in their urine than the non-stone formers [74-76]. The incidence of hypocitratuiria in stone forming population is reported to range from 19% to 63% [73]. Any cellular abnormality which leads to altered urinary excretion of citrate impacts CaOx supersaturation and nephrolithiasis. Low urinary citrate levels are found in many conditions such as potassium depletion and renal tubular acidosis [77].
Citrate is derived from intestinal absorption as well as endogenous metabolism [73, 77-79]. It is filtered freely by the glomerulus and its urinary excretion is regulated primarily by rate of its re-absorption in the proximal tubules. 65% to 90% of the filtered citrate is reabsorbed in the proximal tubules with the assistance of sodium-citrate co-transporter present in the apical membrane [80]. The transporter is encoded by 3Na-citrate2- cotransporter-1(NaDc-1) which has been isolated from human as well as rat and rabbit kidneys. Examination of an association between citrate excretion and single nucleotide polymorphism (SNP) in exon 12 of hNaDC-1 in recurrent stone formers, suggest that the B allele of 1550V polymorphism of hNaDC-1 may be associated with reduction in urinary excretion of citrate and hypocitraturia [81]. Similar polymorphism was also found in hypocitraturic non stone formers indicating that factors other than hypocitraturia are also involved in the formation of kidney stones. Involvement of NaDC-1 in citrate excretion was also investigated in an animal model of CaOx nerphrolithiasis [82]. Hyperoxaluria was induced in male Wistar rats by the administration of ethylene glycol, which is known to lead to hypocitraturia and CaOx nephrolithiasis. NaDC-1 mRNA levels in the kidneys were determined by northern blot analyses and its protein expression was examined by immunohistochemistry. Both mRNA and protein levels increased significantly in hyperoxaluric rats. Administration of potassium citrate significantly elevated urinary citrate and downregulated NaDC-1 expression in the kidneys.
Pyrophosphaturia
Pyrophosphate is present in urine at concentrations of 15-100μM. In a seeded crystal growth system, it inhibits COM crystal growth by 50% at 16-20 μM [83-86]. It can also inhibit COM crystal growth inside a gel matrix [87] and effectively inhibits the growth of CaPs [88, 89]. If it is equally efficient in urine it can contribute 50% crystal COM growth inhibition in the collecting ducts (5 times dilution) and up to 80% in the urine. Hypopyrophosphaturia is postulated to be a metabolic risk factor for recurrent kidney stone formers [90]. Mutations in pyrophosphate transporter, ANKH, are associated with defects of calcification such as craniometaphyseal dysplasia and chodrocalcinosis. ANKH polymorphism has been associated with changes in bone mineral density and with ankolysing spondylitis (AS), the most common form of spondyloarthropathy. A family-based association analyses of 201 multiplex AS families with nine ANKH intragenetic and two flanking microsatellite markers showed that two variants located in two different regions of the ANKH gene were associated with AS [91]. Association also revealed gender-genotype specificity. Lifetime kidney stone incidence for patients with AS and spondyloarthropathy has been demonstrate to be twice as high as rheumatoid controls and almost three times higher than in the normal population [92]. Likelihood of renal stone formation increases with extension of disease duration [93].
Macromoleculuria
In addition to small molecules such as citrate and pyrophosphate, crystallization in the kidneys is also modulated by a number of macromolecules [94]. Osteopontin (OPN), Tamm-Horsfall protein (THP), bikunin, and urinary prothrombin fragment-1 are four of the more extensively examined crystallization modulator (Table 2). OPN is synthesized in the kidneys and excreted in the urine at levels sufficient to inhibit CaOx crystallization [95]. Stone formers have been reported to excrete less OPN in their urine than the normal healthy non stone forming individuals [96]. OPN’s role as an inhibitor of stone formation was further strengthened by observations that experimental induction of hyperoxaluria in OPN knock out mice leads to significant deposition of CaOx crystals in the kidneys, whereas OPN wild type mice showed upregulation of OPN production and were not affected [97]. Mutations in the genes regulating the synthesis of OPN could be a predisposing genetic factor for stone formation.
Table 2.
Crystallization Modulating Macromolecules, Their Expression and Production in Renal Epithelium
| Name | Role in CaOx Crystallization and Nephrolithiasis |
Other Features and Functions |
|---|---|---|
| Tamm-Horsfall Protein |
Inhibitor of Aggregation | Inflammation, Renoprotective |
| Osteopontin | Free OPN Inhibits Crystal Nucleation, Growth, Aggregation and Attachment, Immobilized OPN promotes crystal Attachment |
Calcium Binding, Renoprotective, Tissue repair and inflammation, Chemoattractant for Monocytes/Macrophages |
| Prothrombin Fragment-1 |
Inhibitor of Growth, and Aggregation |
Calcium Binding, Coagulation |
| Bikunin and Inter-α-Inhibitor |
Inhibitor of Nucleation, Growth, Aggregation and Attachment |
Metastasis, Tissue Reapir and Remodeling |
| α-1-microglobulin | Inhibitor of Crystallization | Immunosuppressive, Mitogenic |
| CD-44 | Promoter of Crystal Attachment | Tissue repair and Remodeling |
| Calgranulin | Inhibitor of Crystal Growth and Aggregation |
Calcium Binding, Tissue Remodeling and Inflammation |
| Heparan Sulfate | Inhibitor of Crystal Aggregation and Attachment |
Tissue Remodeling |
| Osteonectin | Calcium Binding, Tissue Remodelling |
|
| Fibronectin | Inhibitor of Crystal Aggregation , Attachment and Endocytosis |
Morphogenesis, Wound Healing and Metastasis |
| Matrix Gla Protein | Inhibitor of Crystal Deposition | Inhibitor of Mineralization |
Recently an association between kidney stone risk and a single nucleotide polymorphism (SNP) of the human OPN gene has been reported. The entire human OPN gene of Asian Japanese stone patients and matching controls was sequenced, haplotype-tagging SNPs (htSNP) searched and association between haplotypes and nephrolithiasis determined [98]. Six novel polymorphisms were identified and a significant association was found between relative probability of stone formation and two haplotypes located in the OPN promoter. Interestingly OPN appeared to be dually associated with nephrolithiasis risk. One haplotype (T-G-T-G) was associated with reduced risk while another (G-T-T-G) one with the increased risk. Role of polymorphism in protein production, molecular structure and crystallization inhibition and nephrolithiasis risk is not known. The post-translational modifications of OPN including phosphorylation, glycosylation and sulfation [99, 100] appear directly pertinent to stone formation. Inhibition of hydroxyapatite crystal growth by OPN was markedly reduced after dephosphorylation [101] and phosphorylation of OPN peptides markedly enhanced the inhibition of CaOx crystal growth [102].
THP is a kidney specific protein, synthesized in cells of the thick ascending limbs of the loop of Henle. It coats luminal side of the epithelium and is most abundant in the human urine (50-100mg/day). It is consistently present in the stone matrix and has high affinity with calcium phosphate crystals. An analysis of proteins associated with CaOx and CaP crystals experimentally induced in vitro in the human urine showed THP as the most abundant protein in the matrix of the CaP crystals [103]. Normal THP is a potent inhibitor of CaOx crystal aggregation [104] and reduced urinary excretion of THP by stone formers has been reported [105]. In addition stone formers are shown to produce THP with abnormal molecular structure which promotes crystal aggregation [106]. First direct evidence for THP’s involvement in stone formation was provided by ablating the murine THP gene [107, 108]. Kidneys of 16% of mice lacking THP gene and protein production contained intratubular as well as interstitial CaP deposits in the medullary and papillary collecting ducts. Induction of hypercalciuria and hyperoxaluria by the administration of vitamin D3 and ethylene glycol respectively, lead to copious crystal deposition in the kidneys of 76% of the THP knockout mice. There was no crystal deposition in kidneys of the wild type mice, with or without the excessive intake of calcium and oxalate. Ablation of both the THP and OPN genes showed spontaneous deposition of CaP crystal deposition in 39% of the THP/OPN double knockout mouse. Induction of hypercalciuria and hyperoxaluria resulted in 95% of the mice lacking both OPN and THP to suffer from deposition of CaOx crystals in their kidneys [108]. These results indicate that defects in both THP and OPN may contribute to crystallization in the kidneys and stone formation.
A number of naturally occurring THP mutations have been reported and linked to autosomal dominant medullary cystic disease and familial juvenile hyperurecaemic nephropathy. Mutations lead to intracellular trafficking defects, retention within the endoplasmic reticulum and reduction in THP secretion and excretion [109, 110]. Renal stone disease has so far not been described in patients with any of these mutations.
Bikunin is the so-called light chain of Inter alpha inhibitor (IαI) and related molecules collectively referred to as the IαI family [111]. These molecules are normally synthesized in the liver and are common in plasma. They are composed of a combination of heavy chains, H1 (60 kDa), H2 (70 kDa), H3 (90 kDa) covalently linked via a chondroitin sulfate bridge to bikunin (35-45 kDa). Separate genes located on three different chromosomes encode these chains. Bikunin originates from a precursor that also codes for α1-microglobulin (α1-m). The heavy and light chains also exist independently as single molecules. IαI (180-240 kDa) is a heterotrimer consisting of bikunin linked to heavy chains H1and H2. Pre-α-inhibitor (PαI, 125 kDa) is composed of bikunin and heavy chain H3. Both heavy and light chains have been identified in the urine. Bikunin isolated from the stone patients, contained less sialic acid and exhibited less crystallization inhibitory activity than that purified from the urine of healthy subject [112]. A significantly higher proportion of stone patients had a 25kDa bikunin in their urine in addition to the normal 40kDa species [113]. 25kDa bikunin was similar to the deglycosylated bikunin and was less inhibitory. Yet another study found decreased urinary excretion of bikunin by stone forming patients [114].
IαI proteins have been shown to inhibit CaOx crystallization in vitro [112, 114-117]. The inhibitory activity is confined to the carboxy terminal of the bikunin fragment of IαI. Both rat and human urinary bikunin inhibited nucleation and growth of CaOx crystals. Treatment with chondroitinase AC had no effect on this inhibitory activity, which was destroyed by pronase treatment indicating that the activity lies not with the chondroitin chain but with the peptide. Bikunin has also been implicated in modulating adhesion of CaOx crystals to the renal epithelial cells [118]. MDCK cells were exposed in culture to CaOx monohydrate crystals in the presence or absence of various protein fractions isolated from normal human urine. A single fraction with a molecular weight of 35 kDa was found to be most inhibitory of crystal adhesion. This protein inhibited crystal adhesion at the minimum concentration of 10ng/ml and completely blocked it at 200ng/ml. Amino acid sequence of the first 20 amino acids of the N-terminal was structurally homologous with bikunin.
α1-microglobulin (α1-m) is also an inhibitor of CaOx crystallization in vitro [119]. α1-m was isolated from human urine. Two species of 30 and 60 kDa, recognized by the antibody against α1-m, were isolated. Both inhibited CaOx crystallization in a dose dependent manner. Using an ELISA assay, urinary concentration of α1-m was found to be significantly lower in 31 CaOx stone formers than in 18 healthy subjects (2.95 + 0.29 vs. 5.34 + 1.08 mg/l respectively, P = 0.01). As mentioned above genes at three different chromosomes are involved in encoding for various IαI related proteins. Alpha-1-microglobulin / bikunin precursor gene that encodes for both bikunin and α1-m is regulated by a number of transcription factors. Mutations in any of the genes may produce structural and secretory variations seen in the kidney stone formers.
CONCLUDING REMARKS
Intrinsic cellular dysfunctions which lead to hyperoxaluria, hypercalciuria, and hypocitraturia, individually or in combination, can lead to increased urinary CaP and/or CaOx supersaturation. Mild supersaturation by itself, can however, only produce small particle crystalluria. The crystals do not grow and aggregate, do not come in contact with the epithelial cells for long durations, are not retained inside the kidneys, and are excreted in the urine without causing any pathological changes and urolithiasis. Mild hyperoxaluria and hypercalciuria provoke protective responses. The exposed cells respond by producing macromolecular crystallization inhibitors leading to reduced crystal nucleation, growth and aggregation. Any crystals formed are excreted in the urine. If crystals come in contact with the cells, they are endocytosed and moved to lysosomes for removal. Other crystals are sent to the renal interstitium where macrophages become involved in crystal elimination [120].
Additional cellular dysfunctions which produce reduced or inefficient macromolecular inhibitors can however, cause crystals to grow and aggregate or attach to renal epithelial cells and thus be retained inside the kidneys. In addition CaP may crystallize in the renal interstitium and later evolve into a platform on papillary surface for the deposition of CaOx. In case of excessive urinary excretion of oxalate or calcium such as in the primary or enteric hyperoxaluria secondary to bariatric surgery and distal renal tubular acidosis, supersaturation can reach very high levels. A large number of crystals sufficient to slow down their movement through the tubular lumens, are formed. Crystals may eventually plug the renal tubules. Slowing of the crystal movement and the blockage of renal tubules would result in prolonged interaction between crystals and renal epithelium leading to cellular dysfunction and degradation. Renal cells respond according to severity of the challenge. Response may be physiological leading to the production of active crystallization inhibitors or pathological producing defective inhibitors promoting crystal aggregation and adherence. In addition damage to the cells can lead to both crystal nucleation and adherence. Crystallization modulators, both ionic and macromolecular can affect supersaturation by binding calcium and/or oxalate. The most critical aspect of stone formation is the migration of interstitial crystal deposits to the papillary surfaces, which is most likely directed by inflammatory cells and the production of metalloproteinases.
Urinary supersaturation with respect to the stone salts is controlled by urinary concentration of various participating ions as well as urinary conditions such as pH. All of these parameters are regulated by various genes, whose products are not only participants in the crystallization but may have additional functions. For example alterations in VDR gene would not only affect calcium metabolism but also cell proliferation and various immune responses etc. In addition cellular responses to stone promoting conditions such as hyperoxaluria, hypercalciuria, and exposure to a variety of crystals are also a part of the process of stone formation. Various genes involved in how cells respond are thus indirectly involved and any alteration in these genes may also lead to the pathogenesis of nephrolithiasis.
ACKNOWLEDGEMENTS
Research supported by NIH grants # RO1DK065658 and RO1DK59765, and The University of Florida Center for the Study of Lithiasis and Pathological Calcification.
REFERENCES
- 1.Stamatelou KK, Francis MF, Jones CA, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Internal. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Randall A. The etiology of primary renal calculus. Intl Abst Surg. 1940;71:209–240. [Google Scholar]
- 3.Finlayson B, Reid S. The expectation of free or fixed particles in urinary stone disease. Invest Urol. 1978;15:442–448. [PubMed] [Google Scholar]
- 4.Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kid Intl. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh JP. Calcium oxalate crystallization in vitro. In: Khan SR, editor. Calcium oxalate in biological systems. CRC Press; Boca Raton, Florida: 1995. p1. [Google Scholar]
- 6.Robertson WG, Peacock M, Nordin BEC. Activity products in stone forming and non-stone forming urine. Clin Sci. 1968;34:579–594. [PubMed] [Google Scholar]
- 7.Khan SR, Kok DJ. Modulators of urinary stone formation. Frontierts in Bioscience. 2004;9:1450–148. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 8.Vezzoli G, Soldati L, Gambaro G. Update on primary hypercalciuria from a genetic perspective. J Urol. 2008;179:1676–1682. doi: 10.1016/j.juro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Geng W, Wang Z, Zhang J, Reed BY, Pak CY, Moe OW. Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol. 2005;288:C1305–C1311. doi: 10.1152/ajpcell.00584.2004. [DOI] [PubMed] [Google Scholar]
- 10.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) Is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed BY, Heller HJ, Gitomer WL, Pak CY. Mapping a gene defect in absorptive hypercalciuria to chromososme 1q23.3-q24. J Clin Endocrinol Metab. 1999;84:3907–3911. doi: 10.1210/jcem.84.11.6155. [DOI] [PubMed] [Google Scholar]
- 12.Reed BY, Gitomer WL, Heller HJ, Hsu MC, Lemke M, Padalino P, et al. Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spine bone density. J Clin Endocrinol Metab. 2002;87:1476–1481. doi: 10.1210/jcem.87.4.8300. M. [DOI] [PubMed] [Google Scholar]
- 13.Bushinsky DA. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens. 1999;8:479–489. doi: 10.1097/00041552-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Khullar M, Relan V, Singh SK. VDR gene and urinary calcium excretion in nephrolithiasis. Kidney Int. 2006;69:943–951. doi: 10.1038/sj.ki.5000176. [DOI] [PubMed] [Google Scholar]
- 15.Recker F, Rubben H, Bex A, Constantinides C. Morphological changes following ESWL in the rat kidney. Urol Res. 1989;17:229–233. doi: 10.1007/BF00262598. [DOI] [PubMed] [Google Scholar]
- 16.Relan V, Khullar M, Singh SK, Sharma SK. Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res. 2004;32:236–241. doi: 10.1007/s00240-004-0414-x. [DOI] [PubMed] [Google Scholar]
- 17.Rendina D, Mossetti G, Viceconti R, Sorrentino M, Castaldo R, Manno G, et al. Association between vitamin D receptor gene polymorphisms and fasting idiopathic hypercalciuria in recurrent stone-forming patients. Urology. 2004;64:838–842. doi: 10.1016/j.urology.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Soylemezoglu O, Ozkaya O, Gonen S, Misirlioglu M, Kalman S, Buyan N. Vitamin D receptor gene polymorphism in hypercalciuric children. Pediatr Nephrol. 2004;19:724–730. doi: 10.1007/s00467-004-1490-4. [DOI] [PubMed] [Google Scholar]
- 19.Bushinsky DA, Asplin JR, Grynpass MD, et al. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Intl. 2002;61:975–987. doi: 10.1046/j.1523-1755.2002.00190.x. [DOI] [PubMed] [Google Scholar]
- 20.Zerwekh JE, Hughes MR, Reed BY, Breslau NA, Heller HJ, Lemke M, et al. Evidence for normal vitamin D receptor messenger ribonucleic acid and genotype in absorptive hypercalciuria. J Clin Endocrinol Metab. 1995;80:2960–2965. doi: 10.1210/jcem.80.10.7559881. [DOI] [PubMed] [Google Scholar]
- 21.Scott P, Ouimet D, Valiquette L, Guay G, Proulx Y, Trouve ML, et al. Suggestive evidence for a susceptibility gene near the vitamin D receptor locus in idiopathic calcium stone formation. J Am Soc Nephrol. 1999;10:1007–1013. doi: 10.1681/ASN.V1051007. [DOI] [PubMed] [Google Scholar]
- 22.Muller D, Hoenderop JG, Vennekens R, Eggert P, Harangi F, Mehes K, et al. Epithelial Ca(2_) channel (ECAC1) in autosomal dominant idiopathic hypercalciuria. Nephrol Dial Transplant. 2002;17:1614–1621. doi: 10.1093/ndt/17.9.1614. [DOI] [PubMed] [Google Scholar]
- 23.Bid HK, Kumar A, Kapoor R, Mittal RD. Association of vitamin D receptor-gene (FokI) polymorphism with calcium oxalate nephrolithiasis. J Endourol. 2005;19:111–115. doi: 10.1089/end.2005.19.111. [DOI] [PubMed] [Google Scholar]
- 24.Chen W-C, Chen H-Y, Lu H-F, Hsu C-D, Tsai F-J. Association of the vitamin D receptor gene start codon Fok I polymorphism with calcium oxalate stone disease. BJU International. 2001;87:168–174. doi: 10.1046/j.1464-410x.2001.02074.x. [DOI] [PubMed] [Google Scholar]
- 25.Nishijima S, Sugaya K, Naito A, Morozumi M, Hatano T, Ogawa Y. Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol. 2002;167:2188–2195. [PubMed] [Google Scholar]
- 26.Ozkaya O, Soylemezoglu O, Misirlioglu M, Gonen S, Buyan N, Hasanoglu E. Polymorphisms in the vitamin D receptor gene and the risk of calcium nephrolithiasis in children. Eur Urology. 2003;44:150–154. doi: 10.1016/s0302-2838(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 27.Rendina D, Mossetti G, Viceconti R, Sorrentino M, Castaldo R, Manno G, et al. Association between vitamin D receptor gene polymorphisms and fasting idiopathic hypercalciuria in recurrent stone-forming patients. Urology. 2004;64:838–842. doi: 10.1016/j.urology.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Devuyst O, Pirson Y. Genetics of hypercalciuric stoen forming diseases. Kid Intl. 2007;72:1065–1072. doi: 10.1038/sj.ki.5002441. [DOI] [PubMed] [Google Scholar]
- 29.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2004;42:35–48. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 30.Vezzoli G, Tanini A, Ferrucci L, Soldati L, Bianchin C, Franceschelli F, et al. Influence of calcium-sensing receptor gene on urinary calcium excretion in stone-forming patients. J Am Soc Nephrol. 2002;13:2517–2125. doi: 10.1097/01.asn.0000030077.72157.d2. [DOI] [PubMed] [Google Scholar]
- 31.Vezzoli G, Terranegra A, Arcidiacono T, Biasion R, Coviello D, Syren ML, et al. R990G polymorphism of calcium-sensing receptor does produce a gain-of-function and predispose to primary hypercalciuria. Kidney Int. 2007;71:1155–1163. doi: 10.1038/sj.ki.5002156. [DOI] [PubMed] [Google Scholar]
- 32.Hough TA, Bogani D, Cheeseman MT, Favor J, Nesbit MA, Thakker RV, Lyon MF. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci USA. 2004;101:13566–13571. doi: 10.1073/pnas.0405516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, et al. Nephrolithiasis and osteoporosis caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–988. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 34.Lapointe Y, Tessier J, Paquette Y, Wallendorff B, Coady MJ, Pichette V, et al. NPT2 gene variation in calcium nephrolithiasis with renal phosphate leak. Kidney Int. 2006;69:226–233. doi: 10.1038/sj.ki.5000437. [DOI] [PubMed] [Google Scholar]
- 35.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-Iic in maintaining phosphate homeostasis. Am J Human Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tennenhouse HS. Regulation of phosphorus homeostasis by the type !!a Na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 37.Khan SR, Glenton PA. Calcium oxalate crystal deposition in kidneys of hypercalciuric mice with disrupted type IIa sodium-phosphate cotransporter. Am J Physiol Renal Physiol. 2008;294:F1109–1115. doi: 10.1152/ajprenal.00620.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrong OM, Norden AG, Feest TG. Dent’s disease; a familial proximal renal tubular syndrome with low-molecularweight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. QJM. 1994;87:473–493. [PubMed] [Google Scholar]
- 39.Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, et al. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A. 2003;100:8472–8479. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devuyst O, Jouret F, Auzanneau C, Courtoy PJ. Chloride channels and endocytosis: new insights from Dent’s disease and CIC-5 knockout mice. Nephron Physiol. 2005;99:69–73. doi: 10.1159/000083210. [DOI] [PubMed] [Google Scholar]
- 41.Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ. ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 42.Gunther W, Piwon N, Jentsch TJ. The ClC-5 chloride channel knock-out mouse-an animal model for Dent’s disease. Pflugers Arch. 2003;445:456–462. doi: 10.1007/s00424-002-0950-6. [DOI] [PubMed] [Google Scholar]
- 43.Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, et al. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol. 2001;281:F1021–27. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Landowski CP, Hedinger MA. Mechanisms and regulation of epithelial Ca absorption in health and disease. Annu Rev Physiol. 2008;70:257–271. doi: 10.1146/annurev.physiol.69.031905.161003. [DOI] [PubMed] [Google Scholar]
- 45.Hoenderop JG, van Leeuwen JP, van der Eerden BC, et al. Renal Ca2þ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang Y, Ferguson WB, Peng JB. WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00187.2006. [DOI] [PubMed] [Google Scholar]
- 47.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 48.Konrad M, Schlingmann KP, Gudermann T. Insights into the molecular nature of magnesium homeostasis. Am J Physiol Renal Physiol. 2004;286:F599–F605. doi: 10.1152/ajprenal.00312.2003. [DOI] [PubMed] [Google Scholar]
- 49.Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 50.Muller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–1301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arking DE. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuro-o M, Matsumora Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kanaem T, Kume E, Iwasaki H, Iida A, Shiraki-iida T, Nishikawa S, Nagai R, Nabeshima Y. mutation of the mouse klotho gene leads to a syndrome resembling aging. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 53.Ogata N, Matsumura Y, Shiraki M, Kawano K, Koshizuka Y, Hosoi T, Nakamura K, Kuro-o M, Kawaguchi H. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–41. doi: 10.1016/s8756-3282(02)00786-x. [DOI] [PubMed] [Google Scholar]
- 54.Tsuruoka S, Nishi K, Ioka T, Ando H, Saito Y, Kurabayashi M, nagai R, Fujimora A. defect in parathyroid hormone induced luminal calcium absorption in connecting tubuless of klotho mice. Nephrol Dial Transplant. 2006;21:2762–2767. doi: 10.1093/ndt/gfl335. [DOI] [PubMed] [Google Scholar]
- 55.Chang Q, Hoefs S, ven der Kemp AW, Topala AW, Bindels RJ, Hoenderop JG. The beta glucoronidase klotho hydrolyzes and activates TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 56.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marengo SR, Romani AMP. Oxalate in renal stone disease: the terminal metabolite that just won’t go away. Nature Clinical Practice Nephrol. 2008 doi: 10.1038/ncpneph0845. [DOI] [PubMed] [Google Scholar]
- 58.Danpure CJ, Rumsby G. Enzymology and molecular genetics of primary hyperoxaluria type 1, consequences for clinical management. In: Khan Saeed R., editor. calcium Oxalate in Biological Systems. CRC Press Inc; Boca Raton, Florida, USA: 1995. pp. 189–205. [Google Scholar]
- 59.Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Human Mol Genetics. 1999;8:2063–2069. doi: 10.1093/hmg/8.11.2063. [DOI] [PubMed] [Google Scholar]
- 60.Salido* Eduardo C., Li† Xiao M., Lu‡ Yang, Wang‡ Xia, Santana* Alfredo, Roy-Chowdhury]‡ Namita, Torres* Armando, Shapiro§¶ Larry J., Roy-Chowdhury Jayanta. Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci USA. 2006;103:18249–18254. doi: 10.1073/pnas.0607218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alper SL. Diseases of mutations in the SLC4A1/AE1 (band 3) Cl−/HCO −3 exchanger. In: Broer S, Wagner CA, editors. Membrane Transporter Diseases. Kluwer Academic/Plenum Publishers; New York: 2003. pp. 39–63. [Google Scholar]
- 62.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl-/HCO3- exchanger (slc4a1) J Am Soc Nephrol. 2007;18:1408–1418. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- 63.Knight J, Holmes RP, Assimos DG. Intestinal and renal handling of oxalate loads in normal individuals and stone formers. Urol Res. 2007;35:111–117. doi: 10.1007/s00240-007-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krishnamurthy M, Hruska KA, Chandhoke PS. The urinary response to an oral oxalate load in recurrent calcium stone formers. J Urol. 2003;169:2030–2035. doi: 10.1097/01.ju.0000062527.37579.49. [DOI] [PubMed] [Google Scholar]
- 65.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res. 2005;33:1–16. doi: 10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 66.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 67.Soleimani M. Expression, regulation and the role of SLC26 Cl-/HCO3- exchangers in kidney and gastrointestinal tract. Novartis Found Symp. 2006;273:91–102. [PubMed] [Google Scholar]
- 68.Soleimani M, Xu J. SLC26 chloride/nase exchangers in the kidney in health and disease. Seminars in Nephrol. 2006;26:375–385. doi: 10.1016/j.semnephrol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol. 2005;288:C957–C965. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 71.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol. 2006;290:G719–G728. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 72.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 73.Parks JH, Ruml LA, Pak CYC. Hypocitraturia. In: Coe FL, Favus MJ, Pak CYC, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Lippincott-Raven; Philadelphia: 1996. pp. 905–920. [Google Scholar]
- 74.Meyer JL, Smith LH. Growth of calcium oxalate crystals II: Inhibition by natural urinary crystal growth inhibitors. Invest Urol. 1975;13:36–39. [PubMed] [Google Scholar]
- 75.Pak CY, Nicar M, Northcutt C. The definition of the mechanism of hypercalciuria is necessary for the treatment of recurrent stone formers. Contrib Nephrol. 1982;33:136–151. doi: 10.1159/000407071. [DOI] [PubMed] [Google Scholar]
- 76.Shah O, Assimos DG, Holmes RP. Genetic and Dietary Factors in Urinary Citrate Excretion. J Endourol. 2005;19:177–182. doi: 10.1089/end.2005.19.177. [DOI] [PubMed] [Google Scholar]
- 77.Pak CY. Citrate and renal calculi. Miner Electrolyte Metab. 1987;13:257–266. [PubMed] [Google Scholar]
- 78.Hamm LL, Alpern RJ. Regulation of acid–base balance, citrate, and urine pH. In: Coe FL, Favus MJ, Pak CYC, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Lippincott-Raven; Philadelphia: 1996. pp. 289–302. [Google Scholar]
- 79.Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:885–893. doi: 10.1016/s0889-8529(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 80.Pajor AM. Conformationally sensitive residues in transmembrane domain 9 of the Na+/dicarboxylate co-transporter. J Biol Chemistry. 2001;276:29961–29968. doi: 10.1074/jbc.M011387200. [DOI] [PubMed] [Google Scholar]
- 81.Okamoto N, Aruga S, Matsuzaki S, Takahashi S, Matsushita K, Kitamura T. Associations between renal sodium-citrate co-transporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. International J Urol. 2007;14:344–349. doi: 10.1111/j.1442-2042.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 82.He Y, Chen X, Yu Z, Wu D, Lv Y, Shi S, Zhu H. Sodium dicarboxylate cotransporter-1 expression in renal tissues and its role in rat experimental nephrolithiasis. J Nephrol. 2004;17:34–42. [PubMed] [Google Scholar]
- 83.Kok DJ, Papapoulos SE, Blomen LMJ, Bijvoet OLM. Modulation of Calcium-Oxalate Monohydrate crystallization kinetics in vitro. Kidney Int. 1988;34:346–350. doi: 10.1038/ki.1988.187. [DOI] [PubMed] [Google Scholar]
- 84.Ryall RL, Harnett RM, Marshall VR. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta. 1988;112:349–356. doi: 10.1016/0009-8981(81)90458-7. [DOI] [PubMed] [Google Scholar]
- 85.Schwille PO, Rumenapf G, Wolfel G, Kohler R. Urinary pyrophosphate in patients with recurrent urolithiasis and in healthy controls: a reevaluation. J Urol. 1988;140:239–245. doi: 10.1016/s0022-5347(17)41573-4. [DOI] [PubMed] [Google Scholar]
- 86.Sidhu H, Gupta R, Thind SK, Nath R. Inhibition of calcium oxalate monohydrate crystal growth. by pyrophosphate, citrate and rat urine. Urol Res. 1986;14:299–303. doi: 10.1007/BF00262379. [DOI] [PubMed] [Google Scholar]
- 87.Achilles W, Coors D, Reifenberger B, Sallis JD, Schalk CH. Natural and artificial substances as inhibitors of crystal growth of calcium oxalates in gel matrices. In: Vahlensieck W, Gasser G, Hesse A, Schoeneich G, editors. Excerpta Medica Amsterdam; Urolithiasis, proceedings 1st European Symposium on urolithiasis; Bonn. 1989. pp. 65–67. ISBN 90 219 9865 3. [Google Scholar]
- 88.Grases F, Ramis M, Costa-Bauza A. Effects of phytate and pyrophosphate on brushite and hydroxyapatite crystallization - Comparison with the action of other polyphosphates. Urol Res. 2000;28:136–140. doi: 10.1007/s002400050152. [DOI] [PubMed] [Google Scholar]
- 89.Robertson WG. Factors affecting the precipitation of calcium phosphate in vitro. Calcif Tissu Res. 1973;11:311–322. doi: 10.1007/BF02547230. [DOI] [PubMed] [Google Scholar]
- 90.Laminski NA, Meyers AM, Sonnekus MI, Smyth AE. Prevalence of hypocitraturia and hypopyrophosphaturia in recurrent calcium stone formers: as isolated defects or associated with other metabolic abnormalities. Nephron. 1990;56:379–384. doi: 10.1159/000186179. [DOI] [PubMed] [Google Scholar]
- 91.Tsui HW, Inman RD, Paterson AD, Reveille JD, Tsui FW. ANKH varian is associated with ankylosing spondylitis: gender differences. Arthritis Res Ther. 2005;7:R513–525. doi: 10.1186/ar1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Canales BK, Leonard SM, Singh JS, Orzano IM, Zimmerman B, Weiland D, Monga M, Krug HS. Spondyloarthropathy: An Independent Risk Factorfor Kidney Stones. J Endourology. 2006;20:542–546. doi: 10.1089/end.2006.20.542. [DOI] [PubMed] [Google Scholar]
- 93.Korkmaz C, Ozcan A, Akcar N. Increased frequency of ultrasonographic findings suggestive of renal stone patients with ankolysing spondylitis. Clin Exp Rheumatol. 2005;23:389–392. [PubMed] [Google Scholar]
- 94.Khan SR, Kok DJ. Modulators of urinary stone formation. Frontierts in Bioscience. 2004;9:1450–148. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 95.Min W, Sgiraga H, Chalko C, Goldfarb S, Krishna GG, Hoyer JR. Quantitative studies of human urinary excretion of uropontin. Kidney Intl. 1998;53:189–193. doi: 10.1046/j.1523-1755.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 96.Nishio S, Hatanaka M, Takeda H, Iseda T, Iwata H, Yokoyama M. Analysis of urinary concentrations of calcium phosphate crystal-associated proteins: alpha-2-HS-glycoprotein, prothrombin F1, and osteopontin. J Am Soc Nephrol. 1999;10:S394–S396. [PubMed] [Google Scholar]
- 97.Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Steitz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J. Osteopontin is acritical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14:139–147. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- 98.Gao B, Yasui T, Itoh Y, Li Z, Okada A, Tozawa K, Hayashi Y, Kohri K. Association of osteopontin gene heplotypes with nephrolithiasis. Kidney Intl. 2007;72:592–598. doi: 10.1038/sj.ki.5002345. [DOI] [PubMed] [Google Scholar]
- 99.Fisher LW, Hawkins GR, Tuross N, Termine JD. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J.Biol.Chem. 1987;262:9702–9708. [PubMed] [Google Scholar]
- 100.Singh K, DeVouge MW, Mukherjee BB. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J.Biol.Chem. 1990;265:18696–18701. [PubMed] [Google Scholar]
- 101.Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins; structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J. 1994;300:723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoyer JR, Asplin JR, Otvos LJ. Phosphorylated osteopontin peptides suppress crystallization by inhibiting growth of calcium oxalate crystals. Kidney Int. 2001;60:77–82. doi: 10.1046/j.1523-1755.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 103.Atmani F, Glenton PA, Khan SR. Identification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in the urine of healthy and stone forming subjects. Urol Res. 1998;26:201–207. doi: 10.1007/s002400050047. [DOI] [PubMed] [Google Scholar]
- 104.Glauser A, Horhreiter W, Jaeger P, Hess B. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant. 2000;15:158–1587. doi: 10.1093/ndt/15.10.1580. [DOI] [PubMed] [Google Scholar]
- 105.Romero MC, Nocera S, Nesse AB. Decreased Tamm-Horsfall protein in lithiasis patients. Clin Biochem. 1997;30:63–67. doi: 10.1016/s0009-9120(96)00136-1. [DOI] [PubMed] [Google Scholar]
- 106.Schnierle P. A simple diagnostic method for the differentiation of Tamm-Horsfall glycoprotein from healthy probands and those from recurrent calcium oxalate renal stone formers. Experimentia. 1995;51:1068–1072. doi: 10.1007/BF01946918. [DOI] [PubMed] [Google Scholar]
- 107.Mo L, Huang H-Y, Zhu X-H, Shapiro E, Hasty DL, Wu X-Ru. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Intl. 2004;66:1159–1166. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 108.Mo L, Liaw L, Evan AP, Sommer AJ, Lieske JC, Wu X-R. Renal calcinosis and stone formation in mice lacking osteopontin,Tamm-Horsfall protein, or both. Am J Physiol renal Physiol. 2007;293:F1935–1943. doi: 10.1152/ajprenal.00383.2007. [DOI] [PubMed] [Google Scholar]
- 109.Bernascone I, Vavassori S, Di Pentima AD, Santambrogio S, Lamorte G, Amoroso A, Scolari F, Ghiggeri GM, Casari G, Polishchuk R, Rampoldi L. Defective Intracellular Trafficking of Uromodulin Mutant Isoforms. Traffic. 2006;7:1567–1579. doi: 10.1111/j.1600-0854.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 110.Serafini-Cessi F, Maligolini N, Cavallone D. Tamm-Horsfall protein glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 111.Biri H, Ozturk HS, Buyukkocak, Kacmaz M, Cimen MYB, Unal D, Birey M, Bozkirli I, Durak I. Antioxidant defense potential of rabbit renal tissues after ESWL: protective effects of antioxidant vitamins. Nephron. 1998;79:181–185. doi: 10.1159/000045022. [DOI] [PubMed] [Google Scholar]
- 112.Atmani F, Lacour B, Jungers P, Drüeke T, Daudon M. Reduced inhibitory activity of uronic-acid-rich protein in urine of stone formers. Urol Res. 1994;22:257–260. doi: 10.1007/BF00541903. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki S, Kobayashi H, Kageyama S, Shibata K, Fujie M, Terao T. Excretion of bikunin and its fragments in the urine of patients with renal stones. J Urol. 2001;166:268–274. [PubMed] [Google Scholar]
- 114.Medetognon-Benissan J, Tardivel S, Hennequin C, Daudon T, Drueke T, Lacour B. Inhibitory effect of bikunin on calcium oxalate crystallization in vitro and urinary bikunin decrease in renal stone formers. Urol Res. 1999;27:69–75. doi: 10.1007/s002400050091. [DOI] [PubMed] [Google Scholar]
- 115.Atmani F, Khan SR. Role of urinary bikunin in the inhibition of calcium oxalate crystallization. J Am Soc Nephrol. 1999;10:S385–S390. [PubMed] [Google Scholar]
- 116.Atmani F, Mizon J, Khan SR. Identification of uronic-acid-rich protein as urinary bikunin, the light chain of inter-α-inhibitor. Eur J Biochem. 1996;236:984–990. doi: 10.1111/j.1432-1033.1996.00984.x. [DOI] [PubMed] [Google Scholar]
- 117.Ryall RL, Harnett RM, Marshall VR. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin Chim Acta. 1988;112:349–356. doi: 10.1016/0009-8981(81)90458-7. [DOI] [PubMed] [Google Scholar]
- 118.DeWater R, Leenen PJM, Noordermeer C, Nigg AL, Houtsmiller AB, Kok DJ, Schroeder FH. Cytokine production induced by binding and processing of calcium oxalate crystals in cultured macrophages. Am J Kidney Dis. 2001;38:331. doi: 10.1053/ajkd.2001.26098. [DOI] [PubMed] [Google Scholar]
- 119.Tardivel S, Medetognon J, Randoux C, Kebede M, Drueke T, Daudon M, Hennequin C, Lacour B. Alpha-1-microglobulin: inhibitory effect on calcium oxalate crystallization in vitro and decreased urinary concentration in calcium oxalate stone formers. Urol Res. 1999;27:243–249. doi: 10.1007/s002400050117. [DOI] [PubMed] [Google Scholar]
- 120.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–57. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]