Abstract
Objectives
To describe the interrelationships of thyroid functions based on trimester-specific concentrations in healthy, iodine-sufficient pregnant women across trimesters, and postpartum.
Methods
Circulating total 3,5,3′-triidothyronine (T3) and thyroxine (T4) concentrations were determined simultaneously using liquid chromatography tandem mass-spectrometry (LC/MS/MS). Free thyroxine (FT4), thyroid-stimulating hormone (TSH), and thyroglobulin (Tg) were measured using immunoassay techniques. Linear mixed effects models and correlations were calculated to determine trends and associations, respectively, in concentrations.
Results and conclusions
Trimester-specific T3, FT4, TSH, and Tg concentrations were significantly different between the first and third trimesters (all p < 0.05); second and third trimester values were not significantly different for FT4, TSH, and Tg (all p > 0.25) although T3 was significantly higher in the third, relative to the second trimester. T4 was not significantly different at any trimester (all p > 0.80). With two exceptions, analyte concentrations tended not to be correlated at each trimester and at 1-year postpartum. One exception was that T3 and T4 tended to be associated (all p < 0.05) at all time points except the third trimester (ρ = 0.239, p > 0.05). T4 and FT4 concentrations tended to correlate positively during pregnancy (ρ 0.361–0.382, all p < 0.05) but not postpartum (ρ = 0.179, p > 0.05). Trends suggest that trimester-specific measurements of T3, FT4, Tg, and possibly TSH are warranted.
Introduction
Maternal thyroid dysfunction can have untoward impact on the progress of pregnancy and on fetal outcomes. As a result of increased concentrations of estrogen, human chorionic gonadotrophin (hCG), and other factors during pregnancy, there are complex changes in the concentrations of 3,5,3′-triiodothyronine (T3), thyroxine (T4), and thyrotropin (TSH). As a result of the stimulatory effect of estrogen, thyroid-binding globulin (TBG) synthesis increases, and its plasma clearance decreases (1–3). The concentrations of TBG more than doubles by the 16th to 20th week of gestation (4), and there are moderate decreases in both serum transthyretin and albumin (5). This requires an increase in T4 and T3 production in order to maintain adequate pools of free thyroxine (FT4) and free triiodothyronine (FT3) for the metabolic needs of the mother and the developmental needs of the fetus. These changes reflect physiologic adaptations to optimize maternal thyroid status for fetal development. After delivery there is a rapid reversal of this process, serum TBG concentrations return to normal within 4–6 weeks and serum T4 and T3 return to pregestational serum levels.
The interpretation of the changes in thyroid hormone concentrations during normal pregnancy should be based on an understanding of the normal physiologic changes during pregnancy, the iodine adequacy and ambient goitrogens in different geographical regions, medications and medical considerations (6–10). Goiter frequently accompanies pregnancy in geographic areas with iodine deficiency, but not in iodine-replete areas (11). In areas with adequate iodine, autoimmune thyroid disease is the more common cause of thyroid insufficiency. Our results suggest that there is a need for trimester-specific reference intervals for thyroid-associated analytes, because their monitoring is important for the welfare of the mother as well as the fetus (12). In this study we sought to describe the interrelationships between key thyroid hormones during normal pregnancy and approximately 1-year postpartum in healthy, iodine-sufficient women.
Since the 1990s, tandem mass spectrometry has increasingly become an integral part of clinical laboratory testing and newborn screening programs because of its ability to detect more then 30 disorders in a single blood spot and a single analysis. We recently developed novel methodology using isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) to assay T3 and T4 in a single sample. The method has been described and validated (13). This method has advantages over traditional immunoassays which lack specificity, cross-react with other analytes and are also affected by heterophilic, nonspecific antibodies (14,15).
Good accuracy with low susceptibility to interferences of MS/MS measurements is important in the case of thyroid hormones because 99.98% of T4 and 99.7% of T3 in serum drawn from healthy adults are normally bound to plasma binding proteins. In addition, FT4, TSH, Tg, and antithyroid peroxidase antibody (TPOAb) concentrations were determined on the same samples by current immunoassay techniques. We describe the interrelationships between concentrations of key thyroid hormones, reflecting thyroid function throughout pregnancy and postpartum, in a group of healthy, iodine-sufficient women, to assist clinicians who care for pregnant women and other women of reproductive age in identifying those at risk for thyroid dysfunction, and to diagnose thyroid disorders during pregnancy.
Materials and Methods
Sample preparation
The study was approved by the Georgetown University Medical Center (GUMC) and Karolinska IRB and informed consents were obtained. The samples were obtained from healthy, iodine-sufficient women on normal diets undergoing their first pregnancy. All of the women were of Caucasian inheritance/non-Hispanic, with the mean age of 30 years (25–38 years) attending a pregnancy clinic at the Karolinska Institute, Sweden. The samples were obtained during gestation week 12 (first trimester), gestation week 22 (second trimester), gestation week 32 (third trimester), and approximately 1-year postpartum. Gestational dates were confirmed by ultrasound. All pregnancies were viable, non-multiple pregnancies. This study was part of a larger study assessing steroid hormone levels throughout pregnancy.
Chemicals and reagents
Standards of T4 and T3 were purchased from Sigma (St. Louis, MO). A stable deuterium-labeled internal standard, levo-thyroxine-d2 was synthesized according to procedures described in the literature (16,17) by Dr. T. Class, Chemistry Department, Georgetown University. High-performance liquid chromatography (HPLC) grade methanol was purchased from VWR Scientific (West Chester, PA). All other chemicals were of analytical grade and purchased from Sigma.
Serum samples were stored frozen at −70°C, thawed at room temperature, and all samples were analyzed at the same time. All the women were on normal diets, did not suffer from severe hyperemesis or any known thyroid disease, and had normal thyroid volume. Sweden is an iodine-sufficient country, and although urinary iodine (UI) concentrations were not specifically measured in the women participating in our study, in a different study also conducted in Sweden in 1998 (18) (which did not include the same cohort of women) the mean values (95% confidence interval) for 24-hour urine during gestation week (GW) 11–13 were 1.40 (1.19–1.61) μmol/d, GW 24 UI = 1.33 (1.14–1.51) μmol/d, GW 32 UI = 1.45 (1.06–1.84) μmol/d and GW 38 UI = 1.14 (0.88–1.39) μmol/d.
Total T4 and total T3 LC/MS/MS procedures
An API 3000 liquid chromatography tandem mass-spectrometer (SCIEX, Toronto, Canada) equipped with TurboIonSpray and Shimadzu HPLC system was used to perform the analysis. Negative ion multiple reaction-monitoring (MRM) mode was used. LC/MS/MS for T4 and T3 was performed as previously described (13). Serum total T4 values are method-dependent; the typical reference intervals, determined for nonpregnant women using immunoassay, approximate 58–160 nmol/L (4.5–12.6 μg/dL). Likewise, serum total T3 reference intervals, measured by immunoassay for nonpregnant women, approximate 1.2–2.7 nmol/L (80–180 ng/dL).
TSH analysis was performed by immunoassay using the Dade Behring Inc. RxL Dimension Clinical Chemistry Analyzer (Newark, DE). Between-day precision gave coefficients of variation (CVs) less than 10% at all concentrations tested. The manufacturer’s reference range for TSH was 0.34–4.82 μIU/mL.
FT4 concentrations were measured by immunoassay using the Dade Behring Inc. RxL Dimension Clinical Chemistry Analyzer (Newark, DE). FT4 measurements are notorious for being method-dependent (19).
Tg concentrations of serum Tg were measured using the DPC-Immulite Automated Analyzer (Diagnostics Product Corporation, Los Angeles, CA) using DPC reagents and according to the manufacturer’s specifications.
TPOAb analysis was conducted on all samples using the DPC-Immulite Automated Analyzer (Diagnostics Product Corporation) using DPC reagents and according to the manufacturer’s specifications. TPOAb test was considered positive at levels greater than 35 IU/mL.
Statistical analysis
SPSS version 11.5 (SPSS Inc., Chicago, IL) was used to carry out all of the analyses. Descriptive statistics were calculated for each analyte separately by trimester. Medians, along with means and standard errors are presented in Table 1; TSH values were logarithmically transformed to facilitate the use of normal-theory analytic methods and geometric means (and associated standard errors) were calculated for this analyte. To assess trends in analytes during pregnancy, mixed effects linear regression models were run to determine the effect of trimester on hormone level (with subject a random effect). Nonparametric correlations (Spearman’s ρ) were calculated to determine associations between analytes at each time point.
Table 1.
Biochemical Parameters of Thyroid Function (Mean ± SE) During Gestation and at One-Year Postpartum in Healthy Iodine-Sufficient Women
|
Trimester
|
||||
|---|---|---|---|---|
| Analyte * | First | Second | Third |
1-Year post
partum |
| Total T3 LC/MS/MS | 174.0 ± 6.0 ng/dLa,b | 182.6 ± 6.3 | 208.2 ± 7.18 | 126.0 ± 4.23 |
| (2.68 ± 0.09) nmol/L | (2.81 ± 0.1) | (3.21 ± 0.11) | (1.94 ± 0.06) | |
| Total T4 LC/MS/MS | 10.0 ± 0.25 μg/dL | 10.0 ± 0.26 | 10.1 ± 0.29 | 7.0 ± 0.23 |
| (127.7 ± 3.6) nmol/L | (127.7 ± 3.6) | (129.3 ± 3.6) | (89.2 ± 2.9) | |
| FT4 | 0.96 ± 0.03 ng/dL | 0.82 ± 0.02 | 0.82 ± 0.02 | 1.07 ± 0.04 |
| (12.3 ± 0.4) pmol/L | (10.5 ± 0.3) | (10.5 ± 0.2) | (13.6 ± 0.5) | |
| TSHc | 0.89 ± 0.58 mIU/L | 1.17 ± 0.08 | 1.16 ± 0.08 | 1.06 ± 0.07 |
| Tg | 15.48 ± 1.96 μg/L | 14.92 ± 2.05 | 18.55 ± 2.8 | 13.95 ± 1.6 |
Values are given as mean ± SE in the respective units.
For conversion: T3 ng/dL × 0.0154 = nmol/L; T4 μg/dL × 12.8 = nmol/L.
TSH values are geometric means and their SEs.
T3 triiodothyronine; T4, thyroxine; LC/MS/MS, liquid chromatography tandem mass spectrometry; FT4, free thyroxine; TSH, thyrotropin; Tg, thyroglobulin; SE, standard error.
Results
In our attempt to delineate data for serum TSH during the trimesters of pregnancy from pregnant women whom we believe to be without evidence for thyroid disease, we measured TPOAb on all of the women. All of the women in the sample were anti-TPOAb negative during pregnancy. However, three of the women (5.8%) tested TPOAb-positive in the postpartum period. In addition, 2 women had a slightly higher than normal TSH concentration (above 5 mIU/L) during the second trimester. These 5 women (9.6% of sample) were excluded from the analyses. Table 1 presents the descriptive statistics for total T3, total T4, FT4, TSH, and Tg at each trimester and 1-year postpartum for the 47 women whose thyroid function was within normal limits.
T3 and T4 concentrations are both higher during pregnancy than in the postpartum period (see also Table 3 described below). T4 levels remained stable throughout pregnancy. In contrast, T3 values increased during each successive trimester, with the third trimester mean being approximately 20% higher than the first trimester mean. FT4 concentrations were lower during pregnancy than in the postpartum period. The mean FT4 decreased by about 15% from the first trimester to the second and then remained stable during the remainder of pregnancy. TSH concentrations were slightly lower than postpartum values (about 16%) during the first trimester, but remained above the postpartum baseline during the second and third trimester. Tg concentrations during the third trimester were substantially higher (by approximately 25%) than postpartum, first, and second trimester concentrations.
Table 3.
Ratios of Analyte Value During Pregnancy to Postpartum Levela
| Analyte * | First Trimester | Second Trimester | Third Trimester |
|---|---|---|---|
| T3b (LC/MS/MS) | 1.20 ± (0.06) | 1.49 ± (0.06) | 1.69 ± (0.06) |
| T4b (LC/MS/MS) | 1.46 ± (0.04) | 1.46 ± (0.04) | 1.46 ± (0.04) |
| FT4 (IA) | 0.90 ± (0.03) | 0.78 ± (0.01) | 0.78 ± (0.02) |
| TSHc (IA) | 0.80 ± (0.05) | 1.11 ± (0.06) | 1.11 ± (0.06) |
| Tg (IA) | 1.11 ± (0.08) | 1.07 ± (0.08) | 1.33 ± (0.12) |
Values are dimensionless—the ratios are constant across unit type and mean SEs are given in parenthesis.
Both T4 and T3 were measured by LC/MS/MS.
TSH values reflect geometric means and their SEs.
T3 triiodothyronine; T4, thyroxine; FT4, free thyroxine; TSH, thyrotropin; Tg, thyroglobulin; LC/MS/MS, liquid chromatography tandem mass spectrometry; SE, standard error.
The concentrations of the analytes at each trimester and at 1-year postpartum tended not to correlate with each other, with two exceptions (Table 2). One exception was that T3 and T4 (both measured by LC/MS/MS) tended to be associated (Spearman’s ρ range 0.407–0.445, all p < 0.05) at all time points except the second trimester (ρ = 0.159, p > 0.05); the second exception was that levels of T4 and FT4 tended to correlate positively during pregnancy (ρ range 0.361–0.382, all p < 0.05), but not postpartum (ρ = 0.179 p > 0.05).
Table 2.
Correlation Coefficients (Spearman’S ρ) for Thyroid Analytes by Trimester
| T 3 | T 4 | FT 4 | Tg | TSH | |
|---|---|---|---|---|---|
| Trimester 1 | |||||
| T3 | 1 | ||||
| T4 | 0.445a | 1 | |||
| FT4 | −0.078 | 0.361a | 1 | ||
| Tg | 0.036 | 0.256 | 0.034 | 1 | |
| TSH | 0.257 | 0.057 | −0.05 | 0.072 | 1 |
| Trimester 2 | |||||
| T3 | 1 | ||||
| T4 | 0.427a | 1 | |||
| FT4 | 0.076 | 0.382a | 1 | ||
| Tg | 0.064 | 0.076 | 0.092 | 1 | |
| TSH | 0.243 | 0.084 | −0.239 | 0.105 | 1 |
| Trimester 3 | |||||
| T3 | 1 | ||||
| T4 | 0.239 | 1 | |||
| FT4 | 0.159 | 0.376b | 1 | ||
| Tg | 0.270 | 0.202 | 0.057 | 1 | |
| TSH | −0.001 | −0.061 | −0.234 | −0.010 | 1 |
| Postpartum | |||||
| T3 | 1 | ||||
| T4 | 0.407a | 1 | |||
| FT4 | 0.007 | 0.179 | 1 | ||
| Tg | 0.101 | 0.185 | 0.014 | 1 | |
| TSH | 0.204 | −0.164 | −0.236 | 0.057 | 1 |
p < 0.01.
p < 0.05.
T3 triiodothyronine; T4, thyroxine; FT4, free thyroxine; Tg, thyroglobulin; TSH, thyrotropin.
Table 3 and Figure 1 show the ratio of mean analyte values for each woman relative to her postpartum baseline, thus showing how each analyte changed over the course of the study. The arithmetic mean for untransformed TSH values is depicted for consistency of scale with the other analytes. In order to reflect the individualized representation in the mixed effects models and to display all analytes on common axes, we divided the trimester values by the postpartum value of each analyte at each trimester. Thus, a mean ratio value of 1.4 indicates that the average value for the ratio of each woman’s measured analyte for that trimester relative to her postpartum value is 1.4.
FIG. 1.
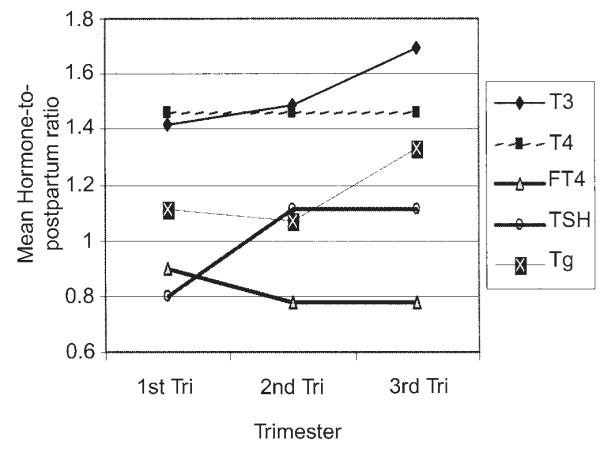
Thyroid hormones, thyrotropin (TSH), and thyroglobulin mean ratios by trimester (values relative to 1-year postpartum). Ratios were calculated for each woman relative to her own baseline (postpartum). The means of the ratios are shown.
T3 levels increased, relative to postpartum levels, at each successive trimester, beginning at an average of 1.20 times the postpartum level in the first trimester to 1.69 by the third trimester. By contrast T4 beginning at 1.46 in the first trimester did not change during the course of pregnancy. On the other hand, the mean ratio of FT4 was 0.90 in the first trimester (but dropped to 0.78 in both second and third trimesters. The TSH ratio decreased to 0.8 for the first trimester, rose to 1.11 for the second and third trimester. Tg levels were slightly higher than the postpartum level in the first (1.11) and second (1.07) trimesters and increased to the highest level in the third trimester (1.33).
The changes in some of the analytes during the course of pregnancy are relatively small, compared with the 95% confidence interval (CI). However, the significant progressive increase in T3 levels throughout pregnancy can be appreciated, as well as a significant early decrease in TSH during the first trimester, increasing during the second trimester. Early decrease in FT4 with nonoverlapping CIs can also be seen occurring between the first and second trimesters. Differences in these analytes across the trimesters of pregnancy were examined using mixed-effects linear models built with subject as a random effect and trimester as a fixed effect. These models were limited to analyte measurements during pregnancy because of the artifactual effect that inclusion of the postpartum baseline would impart. The linear regression models tested the overall hypothesis that the analyte values were different across trimesters. These were followed by comparisons of measurements of subsequent trimester values (i.e., first to third, second to third). Table 4 presents the results of these models.
Table 4.
Results of Mixed Effects Models: Did Analyte Values Differ Over Time?
| Analyte/comparison * | Test statistic a | p Value |
|---|---|---|
| T3b (LC/MS/MS) | F(2, 5.1) = 14.02 | p < 0.01 |
| First trimester versus third | t(13.6) = −5.2 | p < 0.001 |
| Second trimester versus third | t(13.9) = −3.9 | p < 0.002 |
| T4b (LC/MS/MS) | F(2, 3.9) = 0.33 | p > 0.90 |
| First trimester versus third | t(9.8) = −0.08 | p > 0.80 |
| Second trimester versus third | t(0.8) = −0.25 | p > 0.80 |
| FT4 (IA) | F(2, 3.9) = 16.05 | p = 0.013 |
| First trimester versus third | t(25.0) = 5.2 | p < 0.001 |
| Second trimester versus third | t(7.4) = 0.41 | p > 0.60 |
| TSH (by IA)c | F(2, 2.6) = 7.74 | p = 0.080 |
| First trimester versus third | t(4.48) = −3.3 | p = 0.020 |
| Second trimester versus third | t(2.0) = 0.1 | p > 0.90 |
| Tg (by IA) | F(2, 3.9) = 9.15 | p = 0.034 |
| First trimester versus third | t(5.2) = −1.9 | p = 0.119 |
| Second trimester versus third | t(0.7) = −3.1 | p = 0.260 |
F statistic reflects overall effect of trimester; t statistic reflects comparison of that trimester’s mean observation with 3rd trimester’s mean observation.
T4 and T3 were measured by LC/MS/MS.
TSH values were log-transformed prior to this analysis.
T3 triiodothyronine; T4, thyroxine; FT4, free thyroxine; TSH, thyrotropin; Tg, thyroglobulin; LC/MS/MS, liquid chromatography tandem mass spectrometry.
T3, FT4, and Tg were significantly different across trimesters (all F tests for trimester p < 0.05); T3 levels significantly increased at each successive trimester (both t tests comparing first and second to third p < 0.01). FT4 decreased between the first and third trimester (p < 0.001), but there was no difference between the second and third trimester values (p > 0.60), suggesting that the decrease occurred between the first and second trimesters. TSH increased between the first and third trimester (p < 0.05), but not between the second and third trimester (p > 0.90), again implying that the increase took place between the first and second trimesters. Tg was significantly associated with trimester (F(2,3.9) = 9.15, p < 0.05), but changes in Tg levels were not significantly different when trimester-specific concentrations were compared to the third trimester value (both p > 0.10). The only analyte that was not significantly different at any trimester was T4 (both p > 0.8).
Discussion
Using the recently developed and standardized LC/MC/MC method to measure total T4 and total T3 simultaneously (13), we studied a group of pregnant women, who are believed to be sufficient in iodine and without evidence of thyroid disease. We found changes in total T4, TSH, FT4, and that were consistent with the findings of others (7,11,20). In this study T3 increased through pregnancy and was highest in the third trimester in contrast to other reports of a T3 plateau at 20 weeks (7). Thus the T3/T4 molar ratio actually increased in late pregnancy in these women, although it remained within the normal range (21). We also detected no increase in Tg until the third trimester. Increased Tg has been shown by others in relatively iodine deficient women (22,23).
Tandem mass spectrometry LC/MS/MS has advantages over traditional immunoassays that lack specificity, cross-react with other analytes, and are also affected by heterophilic nonspecific antibodies (14,15). The LC/MS/MS assay for simultaneous measurement of T3 and T4 includes stable-isotope internal standards in each sample that allow accuracy and reproducibility. It appears to be largely free of interference or cross-reactivity, a problem that affects immunoassays, as demonstrated in our method-comparison study (24). In comparison to immunoassays that may achieve comparable or better sensitivities and require substantial manual labor and prolonged incubation times, our LC/MS/MS assay is fast, with little hands-on time and no need for sample extraction and derivatization. In our case, because of greater specificity and only measuring the analyte of interest, LC/MS/MS consistently provides somewhat lower results than immunoassays for T4 (15).
Establishing normal reference intervals for these analytes in women without thyroid dysfunction has important implications for detecting women with thyroid insufficiency in pregnancy who would need to be treated and monitored throughout pregnancy. Specific reference intervals for FT4, T4, T3 in addition to TSH during pregnancy may be particularly important for several reasons. First, it would be important to know what the FT4 levels are in the first trimester as they are higher than in other trimesters in a euthyroid pregnancy, and this is time when the fetus is wholly dependent on T4 from the mother. Accurate reference intervals for T4 and FT4 would then provide the ability to detect a deficiency at this critical time and provide a subtle indication of maternal hypothyroidism. Such hypothyroxinemia may be masked during the first trimester if that determination relied solely on an elevated TSH because of the stimulatory effect of high hCG levels on the thyroid gland. It is possible that high, sustained estrogen levels during this time are associated with transient lowering of serum TSH (25,26) thus curtailing its rising above the normal nonpregnant range.
Second, maintenance of normal maternal thyroid hormone levels is known to be a determinant of adequate fetal thyroid hormone levels early in pregnancy (20) and adequate mental and psychomotor development in infants (27). Better and easier measurements of T4 and FT4 during early pregnancy would contribute to better insights into the more appropriate method of detecting thyroid deficiency at this critical time fir the fetus. Mild maternal hypothyroidism (subclinical hypothyroidism) has been implicated as the cause of neuro-psycho-intellectual deficit in offspring (28,29). Accurate reference intervals in early pregnancy would make it possible to better define this condition. In cases of overt hypothyroidism the fetal consequences can be extreme (30). However, it appears that the maternal level and delivery of FT4 and not T3 to the fetus is critical for the neuropsychological development of the fetus (31). A deficiency may not be reflected by the degree of TSH elevation (32). It is imperative for normal intervals of thyroid hormones to be established for pregnant women especially if it should be determined necessary that T4 levels in early pregnancy can be used to screen for fetal and pregnancy risk.
Studies to date have relied on elevated TSH to determine the increment of thyroid hormone needed by hypothyroid patients during pregnancy. Some studies demonstrate that 85% of hypothyroid women needed an increase in LT4 supplementation during pregnancy (33,34). However, this increased requirement may only be experienced by a subset of hypothyroid patients (35). Once time-specific reference intervals are established, the LC/MS/MS can be used to structure these adjustments individually to support the rate of increase and not just absolute values.
Limitations in this study include the lack of individual urinary iodine measurements of each of the women. Trimester-specific iodine data from a similar population, the knowledge that the population in Sweden is iodine sufficient and the normal thyroid volume were used to support a state of iodine sufficiency. It is possible that the rise of Tg and increased T3 to T4 ratio in the third trimester may speak to marginal iodine status becoming manifest at the end of pregnancy, but other thyroid function parameters do not suggest it. We have used the 1-year post pregnancy values to represent prepregnant values.
In conclusion, to our knowledge ours is the first longitudinal study examining T4 and T3 levels over time measured by LC/MS/MS in a pregnant population. These results show that, in iodine sufficient women T3, FT4, TSH, and Tg tend to change throughout the course of pregnancy, while T4, after the first trimester, does not. The concentrations of the analytes tended, in general, not to be correlated at each trimester and at 1-year postpartum, although T4 and FT4 concentrations tended to correlate positively during pregnancy but not postpartum.
Acknowledgments
This study was supported by National Cancer Institute (NCI) grant 5RO1 CA-89950-03 and supported in part by grant M01 RR-13297 from the National Center for Research Resources, National Institutes of Health. The research was conducted at the Georgetown University Medical Center’s General Clinical Research Center. The authors are grateful to Dr. L. Hilakivi-Clarke, Georgetown University, and Dr. E. Weiderpass of Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, and International Agency for Research on Cancer, Lyon, France for supplying the serum specimens, and to Dr. N. Soukhova Bioanalytical Core Laboratory, Georgetown Clinical Research Center for technical assistance. Special thanks to Dr. D. Glinoer for reading the manuscript and making helpful suggestions.
References
- 1.Lum SM, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest. 1984;73:570–575. doi: 10.1172/JCI111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins J. Thyroid Hormone Transport Proteins and Physiology of Hormone Binding. In: Braverman L, Utiger RD, editors. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. Lippincott-Raven; Philadelphia: 2000. pp. 103–120. [Google Scholar]
- 3.Schussler GC. The thyroxine-binding proteins. Thyroid. 2000;10:141–149. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- 4.Glinoer D. What happens to the normal thyroid during pregnancy? Thyroid. 1999;9:631–635. doi: 10.1089/thy.1999.9.631. [DOI] [PubMed] [Google Scholar]
- 5.Bartalena L. Recent achievements in studies on thyroid hormone-binding proteins. Endocr Rev. 1990;11:47–64. doi: 10.1210/edrv-11-1-47. [DOI] [PubMed] [Google Scholar]
- 6.De Leo V, la Marca A, Lanzetta D, Morgante G. Thyroid function in early pregnancy. I: Thyroid-stimulating hormone response to thyrotropin-releasing hormone. Gynecol Endocrinol. 1998;12:191–196. doi: 10.3109/09513599809015544. [DOI] [PubMed] [Google Scholar]
- 7.Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 8.Brent GA. Maternal thyroid function: Interpretation of thyroid function tests in pregnancy. Clin Obstet Gynecol. 1997;40:3–15. doi: 10.1097/00003081-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kol S, Karnieli E, Kraiem Z, Itskovitz-Eldor J, Lightman A, Ish-Shalom S. Thyroid function in early normal pregnancy: transient suppression of thyroid-stimulating hormone and stimulation of triiodothyronine. Gynecol Obstet Invest. 1996;42:227–229. doi: 10.1159/000291968. [DOI] [PubMed] [Google Scholar]
- 10.Glinoer D. Maternal thyroid function in pregnancy. J Endocrinol Invest. 1993;16:374–378. doi: 10.1007/BF03348861. [DOI] [PubMed] [Google Scholar]
- 11.Berghout A, Endert E, Ross A, Hogerzeil HV, Smits NJ, Wiersinga WM. Thyroid function and thyroid size in normal pregnant women living in an iodine replete area. Clin Endocrinol. 1994;41:375–379. doi: 10.1111/j.1365-2265.1994.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 12.Smallridge RC, Ladenson PW. Hypothyroidism in pregnancy: consequences to neonatal health. J Clin Endocrinol Metab. 2001;86:2349–2353. doi: 10.1210/jcem.86.6.7577. [DOI] [PubMed] [Google Scholar]
- 13.Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometric method for T4/T3. Clin Chim Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124:921–923. doi: 10.5858/2000-124-0921-HAMA. [DOI] [PubMed] [Google Scholar]
- 15.Soldin OP, Hilakivi-Clarke L, Weiderpass E, Soldin SJ. Thyroxine and triiodothyronine concentrations in pregnancy: Reference intervals defined by isotope dilution tandem mass spectrometry and immunoassays. Clin Chim Acta. 2004;349:181–189. doi: 10.1016/j.cccn.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai SS, Sniegoski LT, Welch MJ. Candidate reference method for total thyroxine in human serum: use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem. 2002;48:637–642. [PubMed] [Google Scholar]
- 17.Burman KD, Bongiovanni R, Garis RK, Wartofsky L, Boehm TM. Measurement of serum T4 concentration by high performance liquid chromatography. J Clin Endocrinol Metab. 1981;53:909–912. doi: 10.1210/jcem-53-5-909. [DOI] [PubMed] [Google Scholar]
- 18.Elnagar B, Eltom A, Wide L, Gebre-Medhin M, Karlsson FA. Iodine status, thyroid function and pregnancy: Study of Swedish and Sudanese women. Eur J Clin Nutr. 1998;52:351–355. doi: 10.1038/sj.ejcn.1600563. [DOI] [PubMed] [Google Scholar]
- 19.d’Herbomez M, Forzy G, Gasser F, Massart C, Beaudonnet A, Sapin R. Clinical evaluation of nine free thyroxine assays: Persistent problems in particular populations. Clin Chem Lab Med. 2003;41:942–947. doi: 10.1515/CCLM.2003.143. [DOI] [PubMed] [Google Scholar]
- 20.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 21.Weeke J, Dybkjaer L, Granlie K, Eskjaer Jensen S, Kjaerulff E, Laurberg P, Magnusson B. A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinol. 1982;101:531–537. doi: 10.1530/acta.0.1010531. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen NG, Hornnes PJ, Hegedus L, Feldt-Rasmussen U. Serum thyroglobulin during the menstrual cycle, during pregnancy, and post partum. Acta Endocrinol. 1989;121:168–173. doi: 10.1530/acta.0.1210168. [DOI] [PubMed] [Google Scholar]
- 23.Glinoer D, deNayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, Kinthaert J, Lejeune B. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 24.Soldin OP, Tractenberg RE, Soldin SJ. Differences between measurements of T4 and T3 in pregnancy and in non-pregnant women using isotope dilution tandem mass spectrometry and immunoassays: Are there clinical implications? Clin Chim Acta. 2004;347:61–69. doi: 10.1016/j.cccn.2004.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklyn JA, Sheppard MC, Ramsden DB. Serum free thyroxine and free triiodothyronine concentrations in pregnancy. Br Med J. 1983;287:394. doi: 10.1136/bmj.287.6389.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrow GN. Thyroid function and hyperfunction during gestation. Endocr Rev. 1993;14:194–202. doi: 10.1210/edrv-14-2-194. [DOI] [PubMed] [Google Scholar]
- 27.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 28.Glinoer D, Delange F. The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid. 2000;10:871–887. doi: 10.1089/thy.2000.10.871. [DOI] [PubMed] [Google Scholar]
- 29.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 30.Delange F. The role of iodine in brain development. Proc Nutr Soc. 2000;59:75–79. doi: 10.1017/s0029665100000094. [DOI] [PubMed] [Google Scholar]
- 31.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 32.Connolly KJ, Pharoah POD. Iodine deficiency, maternal thyroxine levels in pregnancy and developmental disorders in children. In: Condliffe PG, editor. Iodine and the Brain. Plenum Press; New York: 1989. pp. 317–331. [Google Scholar]
- 33.Mandel SJ, Larsen PR, Seely EW, Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan MM. Monitoring thyroxine treatment during pregnancy. Thyroid. 1992;2:147–152. doi: 10.1089/thy.1992.2.147. [DOI] [PubMed] [Google Scholar]
- 35.Chopra IJ, Baber K. Treatment of primary hypothyroidism during pregnancy: Is there an increase in thyroxine dose requirement in pregnancy? Metabolism. 2003;52:122–128. doi: 10.1053/meta.2003.50019. [DOI] [PubMed] [Google Scholar]


