Abstract
Background
Accurate assessment of the pregnant woman’s thyroid status is critical, for both the initiation of thyroid hormone therapy and for the adjustment of thyroid hormone dose in those already receiving thyroid hormone. Trimester-specific intervals are especially important during pregnancy when thyroid insufficiency may be associated with adverse obstetric outcome and fetal neurodevelopmental deficits. We defined pregnancy-specific reference intervals for thyroxine (T4) and 3,5,3′-triiodothyronine (T3). We used a novel isotope dilution tandem mass spectrometry (LC/MS/MS) method, and compare these to reference intervals obtained by immunoassays (IAs) performed on the same samples.
Methods
Concentrations of circulating T4 and T3 were measured simultaneously during first, second and third trimesters and postpartum in iodine-sufficient, healthy, singleton pregnancies using API-3000 LC/MS/MS with deuterium-labeled internal standard (l-thyroxine-d2). Immunoassays were conducted on the same samples (T4 Dade Behring RxL, T3 DPC-Immunolite).
Results
Linear regression is reported for method comparisons; for T4, the slope decreased from r=0.900 in nonpregnant women to 0.802–0.820 during pregnancy. For T3, correlations between LC/MS/MS and immunoassays were weaker in all cases (r=0.407–0.574).
Conclusion
In this longitudinal study, we established trimester-specific reference intervals for T4 and T3 by LC/MS/MS and compare these to intervals obtained by immunoassays.
Keywords: Thyroxine T4, Triiodothyronine T3, Reference intervals, Pregnancy, Thyroid hormones, Isotope dilution tandem mass spectrometry LC/MS/MS
1. Introduction
Early in pregnancy, even mild thyroid insufficiency has been implicated to be associated with neurodevelopmental deficits and pregnancy-related complications [1,2]. Commonly, serum TSH measurement was considered the most sensitive detector of thyroid dysfunction both in pregnant and nonpregnant individuals [3]. However, T4 and T3 are the hormones that are responsible for thyroid hormone effects, and during pregnancy, thyroid function is altered. In order to evaluate women in pregnancy, it is essential to establish trimester-specific thyroid function reference intervals in healthy, iodine-sufficient women.
Circulating T4 and a small percentage of circulating T3 (20%) are synthesized within the thyroid gland. The bulk of circulating T3 is produced via monodeiodination of T4 by intracellular enzymes present in the thyroid follicular cells and in the cells of target tissues [4]. Most circulating thyroid hormones are bound to plasmabinding proteins (99.98% of T4 and of 99.7% T3), thus increasing their biological half-life and enabling their transport. The plasma-binding proteins include thyroxine-binding globulin (TBG), transthyretin and albumin [5,6]. In serum drawn from healthy adults, total serum T4 (TT4) (free and protein-bound hormone) is present at about 60-fold higher concentration than total T3 (TT3).
Pregnancy induces complex changes in circulating maternal steroid hormones and in TBG concentrations. In addition to the stimulatory effects of estrogen on TBG synthesis, a major contribution to the increased TBG concentration during pregnancy is the reduced plasma clearance of the protein due to changes in TBG glycosylation induced by estrogen. It is commonly thought that TT4 and TT3 increase in the setting of pregnancy-induced increases in serum TBG concentrations. The concentrations of TBG double by the 16th to 20th week of gestation [7]. In addition to the two- to threefold increase in serum TBG, modest decreases in both serum transthyretin and albumin are commonly found in pregnancy [8]. Delivery leads to a rapid reversal of this process, resulting in a decrease in TBG concentrations causing T4 and T3 concentrations to return to pre-gestational serum levels within 4–6 weeks. In order to determine abnormalities in thyroid hormone concentrations during the progression of pregnancy, it is necessary to determine their reference intervals for each of the trimesters. The reference intervals will be different when the population is iodine deficient.
Immunoassays (IAs) are notoriously unreliable with increasing evidence in the published literature supporting their lack of specificity [9-11]. The presence of circulating iodothyronine-binding auto-antibodies that interfere with total T4 and T3 IAs is a known phenomenon [12-14]. These autoantibodies may give falsely high, or falsely low values of thyroid hormone measurements depending on the assay separation method used and are often in discordance with the clinical features [12-14]. Direct serum free T4 (FT4) and free T3 (FT3) measurements are technically difficult to perform since they are measured in the picomole range and, to be valid, must be free from interference by the much higher total hormone concentrations. It is therefore easier to measure the total thyroid hormone concentrations that are measured at nanomolar levels.
Serum TT4 and TT3 concentrations are most commonly measured by immunoassay methods. In proficiency testing of samples for different methods of measurement of both T4 and T3, the College of American Pathologists Proficiency Testing Program (CAPPT) reported that the difference in specificity of the various antibodies used in thyroid hormone IAs can vary by a factor of 2. In addition to method differences, under certain conditions, such as pregnancy, estrogen therapy or genetic abnormalities in protein binding have also reportedly made IA methods for T4 and T3 diagnostically unreliable [15,16].
The use of tandem mass spectrometry has been shown to be accurate, specific, reliable, simple and fast. Using this technique, the proteins are precipitated, and both T4 and T3 are measured in the samples simultaneously. We report here new reference intervals for healthy, iodine-sufficient women using an isotope dilution tandem mass spectrometry method [17] for the simultaneous measurements of T4 and T3 during pregnancy and postpartum and compared the new established reference intervals with those obtained by the traditional IA.
2. Materials and methods
The research was conducted at the Georgetown University Medical Center’s General Clinical Research Center.
2.1. Chemicals and reagents
Standards of thyroxine (T4) and 3,5,3′-triidothyronine (T3) were from Sigma (St. Louis, MO). A stable deuterium-labeled internal standard, l-thyroxine-d2 was synthesized as previously described [18]. HPLC grade methanol was from VWR Scientific. All other chemicals were of analytical grade and from Sigma.
2.2. Sample preparation
Serum samples were obtained from the Karolinska Institute in Sweden. Informed consents were obtained, and the Karolinska IRB and Georgetown University Medical Center (GUMC) IRB approved the study. The samples were obtained from healthy women with no history of thyroid disease undergoing their first pregnancy, during gestation week 12 (first trimester), gestation week 22 (second trimester), gestation week 32 (third trimester) and approximately 1 year postpartum. All women were of Caucasian inheritance/non-Hispanic, with the mean age of 30 years (25–38 years). Gestational dates were confirmed by ultra-sound. All pregnancies were viable, singleton pregnancies. All the women were on normal diets and did not suffer from severe hyperemesis nor any known thyroid disease. Sweden is an iodine-sufficient country, and although urinary iodine (UI) concentrations were not specifically measured in the women participating in our study, in another study conducted in Sweden in 1998 [19], mean values (95% confidence interval) for 24-h urine during gestation week (GW) 11–13 were 1.40 (1.19–1.61) μmol/day, GW 24 UI 1.33 (1.14–1.51) μmol/day, GW 32, UI 1.45 (1.06–1.84) μmol/day and GW 38 UI 1.14 (0.88–1.39) μmol/day. Serum samples were stored frozen at −70 °C and thawed at room temperature, and all samples were analyzed at the same time.
2.3. LC/MS/MS and Immunoassay procedures
An API 3000 tandem mass spectrometer (SCIEX, Toronto, Canada) equipped with TurboIonSpray and Shimadzu HPLC system was used to perform the analysis. Negative ion multiple reaction-monitoring (MRM) mode was used. LC/MS/MS for T4 and T3 was performed as previously described [17].
2.4. Immunoassays for T4, T3, TSH and TPOAb
Serum T4 concentrations were measured by immunoassay using the Dade Behring. RxL Dimension Clinical Chemistry Analyzer (Newark, DE) with between-day precision giving CVs of <6% at all concentrations tested, while concentrations of serum T3 were measured using the DPC-Immulite Automated Analyzer (Diagnostics Product, CA) with between-day precision giving CVs of <11% at all concentrations tested [20]. Serum TT4 values are method dependent; the typical reference intervals approximate 58–160 nmol/l (4.5–12.6 μg/dl). Likewise, serum TT3 reference intervals approximate 1.2–2.7 nmol/l (80–180 ng/dl) [21]. TSH analysis was performed by immunoassay using the Dade Behring RxL Dimension analyzer (Newark). Between-day precision gave CVs <10% at all concentrations tested. The manufacturer’s reference range for TSH was 0.34–4.82 μIU/ml. TPOAb analysis was conducted on all samples using the DPC-Immulite analyzer (DPC) employing DPC reagents and according to the manufacturer’s specifications. TPOAb test was considered positive at levels >35 IU/ml.
All of the women in the sample were TPOAb negative during pregnancy. However, three of the women (5.8%) tested TPOAb-positive in the postpartum period. In addition, two women had a slightly higher than normal TSH concentration (above 5 μIU/ml) during the second trimester.
2.5. Statistical analysis
Statistical analysis, including the linear regression correlations, was conducted using Prism software. In addition paired t-test were calculated to determine if measurements by LC/MS/MS were different from IA by trimesters.
3. Results
New reference intervals for TT4 and TT3 during the first, second and third trimesters of pregnancy and approximately 1 year postpartum in normal iodine-sufficient women have been determined employing the percentile approach and using tandem mass spectrometry. The reference intervals established by tandem mass spectrometry are compared here with those established by IA on the same samples. The reference intervals (2.5th and 97.5th percentiles) are shown in Table 1 for the women during their first, second and third trimesters of pregnancy and for the same women approximately 1 year after delivery. The mean TSH concentrations were slightly suppressed during the first trimester (geometric mean 0.89 mIU/l vs. 1.06 mIU/l in the same women while not pregnant). All TSH values were <3.0 μIU/ml. In two women, the TSH concentrations reached 6.75 and 5.31 μIU/ml on week 22 but were back within range by third trimester. We excluded these women from the data analysis. Comparisons between tandem mass spectrometry and IA results are shown below for nonpregnant, first, second and third trimester pregnancy (Figs. 1-8). In all figures, the units are in nanograms per milliliter; to derive T3 values of nanograms per deciliter, multiply nanograms per milliliter values by 100; for T4 micrograms per deciliter values, divide nanograms per milliliter values by 10.
Table 1.
The reference intervals (2.5th and 97.5th percentiles) for TT4 and TT3 using isotope dilution tandem mass spectrometry and using immunoassays
| Triiodothyronine TT3a | Reference intervals by IA, ng/dl (nmol/l) |
Reference intervals by LC/MS/MS ng/dl (nmol/l) |
|---|---|---|
| First trimester |
n=50, 92–218 (1.42–3.36) |
n=52, 96–267 (1.48–4.12) |
| Second trimester |
n=49, 112–278 (1.73–4.29) |
n=51, 107–274 (1.64–4.22) |
| Third trimester |
n=49, 111–265 (1.71–4.08) |
n=51, 105–297 (1.62–4.57) |
| 1 year postpartum |
n=48, 74–146 (1.48–2.24) |
n=52, 80–214 (1.23–3.29) |
| Thyroxine TT4b | Reference intervals by IA, μg/dl (nmol/l) |
Reference intervals by LC/MS/MS μg/dl (nmol/l) |
|---|---|---|
| First trimester |
n=50, 6.3–14.6 (80.8–187.0) |
n=52, 5.8–14.2 (74.6–181.9) |
| Second trimester |
n=49, 6.4–14.8 (82.2–190.1) |
n=51, 6.3–14.4 (80.3–184.7) |
| Third trimester |
n=49, 6.3–16.7 (80.3–207.0) |
n=51, 5.9–14.2 (75.1–181.5) |
| 1 year postpartum |
n=48, 4.4–14.9 (56.9–191.3) |
n=52, 4.8–11.7 (60.9–150.4) |
To derive T3 ng/dl×0.0154=nmol/l.
T4 μg/dl×12.8=nmol/l.
Fig. 1.
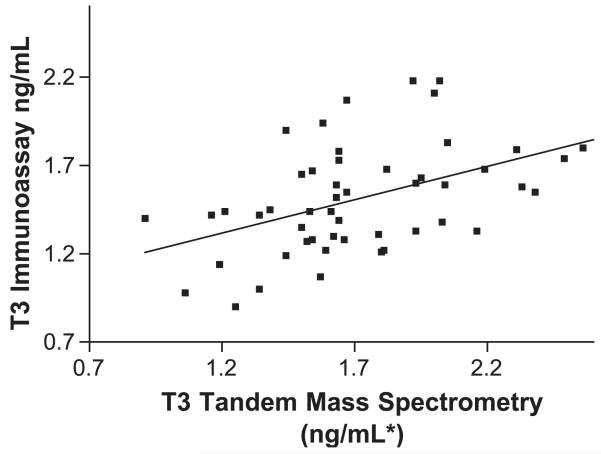
T3 first trimester mass spectrometry vs. DPC Immulite. IA=0.38MS+0.86, r=0.486, Sy.x=0.2688, p<0.001. *To derive T3 values of ng/dl, multiply ng/ml values by 100.
Fig. 8.
T4 nonpregnant 1 year post-delivery mass spectrometry vs. Dade. IA=1.10MS-3.2, r=0.900, Sy.x=8.743, p<0.001.
During the first trimester, T3 values using LC/MS/MS ranged between 96 and 267 ng/dl, whereas using IA, the values ranged between 72 and 218 ng/dl. Some of the values that are out of range for LC/MS/MS fall within the range for IA and would therefore not be detected, and several values that are within range for LC/MS/MS are out of the reference interval for IA. The mean value for T3 determined by IA is smaller (Table 2), with a smaller range (standard deviation) than those derived by LC/MS/MS. The observed values for T3 derived by IA were significantly correlated with those derived by LC/MS/MS (r=486 pb0.001). The measurements of T3 by IA were significantly lower than those by LC/MS/MS (t(49)=4.1, p<0.001).
Table 2.
The means and medians for TT4 and TT3 using isotope dilution tandem mass spectrometry and using immunoassays
| Triiodothyronine TT3a | IA |
LC/MS/MS |
||
|---|---|---|---|---|
| Mean ng/dl (±S.D.) (nmol/l) |
Median ng/dl (nmol/l) |
Mean ng/dl (± S.D.) (nmol/l) |
Median ng/dl (nmol/l) |
|
| First trimester |
n=50, 152.1 (±30) (2.34) |
148.5 (2.29) |
n=52, 174.5 (±40) (2.70) |
164 (2.53) |
| Second trimester |
n=49, 169.2 (±35) (2.60) |
171 (2.63) |
n=51, 183.2 (±42) (2.82) |
181 (2.79) |
| Third trimester |
n=49, 175.0 (±37) (2.69) |
167 (2.57) |
n=51, 208 (±47) (3.20) |
207 (3.19) |
| 1 year postpartum |
n=48, 102 (±19) (1.57) |
97.5 (1.49) |
n=52, 125 (±28) (1.93) |
121 (1.86) |
| Thyroxine TT4b | IA |
LC/MS/MS |
||
|---|---|---|---|---|
| Mean μg/dl (±S.D.) (nmol/l) |
Median μg/dl (nmol/l) |
Mean μg/dl (±S.D.) (nmol/l) |
Median μg/dl (nmol/l) |
|
| First trimester |
n=50, 10.9 (±1.7) (139.2) |
10.6 (136.3) |
n=52, 10.1 (±1.7) (129.1) |
9.97 (127.7) |
| Second trimester |
n=49, 11.1 (±1.9) (142.4) |
10.9 (139.5) |
n=51, 10.1 (±1.7) (129.3) |
10.2 (130.6) |
| Third trimester |
n=49, 11.3 (±2.1) (144.5) |
11.2 (143.4) |
n=51, 10.2 (±2) (130.5) |
10.2 (130.6) |
| 1 year postpartum |
n=48, 7.4 (±2.1) (94.7) |
6.9 (88.3) |
n=52, 6.9 (±1.6) (89.1) |
6.6 (85.1) |
To derive T3 ng/dl×0.0154=nmol/l.
T4 μg/dl×12.8=nmol/l.
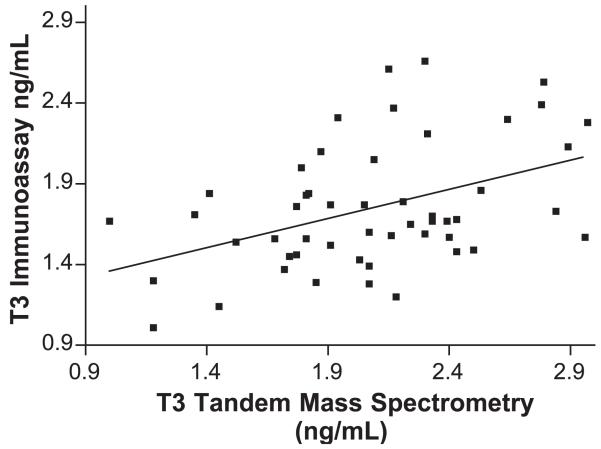
Similarly, during the second trimester, using LC/MS/MS T3 concentrations ranged between 107 and 274 ng/dl, whereas using IA, the values were similar. The mean values for T3 determined by IA were lower at all times (Table 2), with a smaller range (standard deviation) than those derived by LC/MS/MS. The observed values for T3 derived by IA were significantly correlated with those derived by LC/MS/MS at all times (p<0.001). The measurements of T3 by IA are significantly lower than those by LC/MS/MS (t(49)=2.4, p<0.05) for the second trimester.
For the third trimester, LC/MS/MS values ranged between 105 and 297 ng/dl, whereas using IA, thevalues were 111 and 265 ng/dl. The measurements of T3 by IA are significantly lower than those by LC/MS/MS (t(48)=5.1, p<0.001).
One-year postpartum T3 values determined by LC/MS/MS ranged between 80 and 214 ng/dl, whereas using IA, the values were 74 and 146 ng/dl. The measurements of T3 by IA were significantly lower than those by LC/MS/MS (t(47)=5.6, p<0.001).
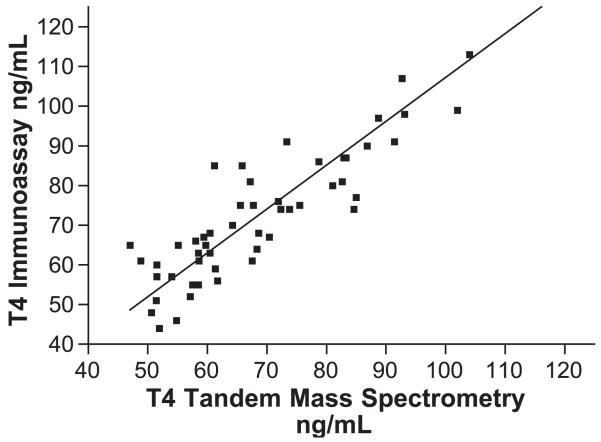
Using LC/MS/MS, T4 the values ranged between 5.8 and 14.2 μg/dl, whereas using IA, the values are 6.3 and 14.6 μg/dl. The observed values for T4 derived by IA were significantly correlated with those derived by LC/MS/MS (r=0.805, p<0.0001). The mean value for T4 by IA is slightly higher, with nearly identical range, than that derived by LC/MS/MS. All the measurements of T4 by IA were significantly higher than those by LC/MS/MS (t(49)=−5.3, p<0.001) for the first trimester, (t(48)=−6.4, p<0.001) for the second trimester, (t(48)=−6.0, p<0.001) for the third trimester and (t(47)=−3.0, p<0.005) for T4 1 year postpartum.
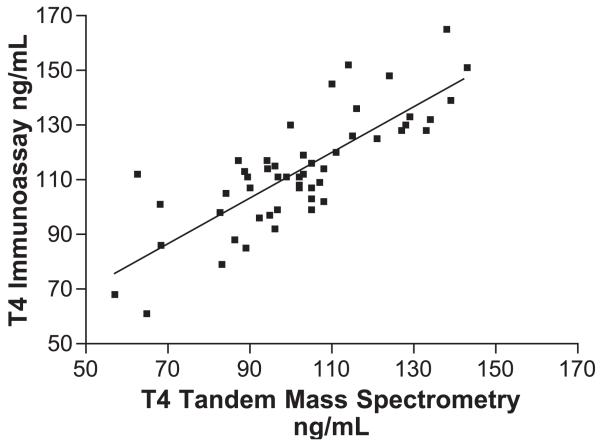
Using LC/MS/MS second trimester values ranged between 6.3 and 14.4 μg/dl, whereas using IA, the values are 6.4 and 14.8 μg/dl. T4 third trimester values using LC/MS/MS ranged between 8.0 and 17.0 μg/dl, whereas using IA, the values were 6.0 and 14.0 μg/dl.
Over three trimesters of pregnancy, correlation coefficients between T3 measured by MS and IA were near 0.45 (range: 0.436–0.574, p≤0.001); postpartum measurements were only weakly correlated (r=0.407, p=0.05). During pregnancy the association between T4 measurements by each method was much greater, near 0.80 (range: 0.802–0.819, p<0.001), and postpartum, the association was very high (r=0.900, p<0.001).
In spite of these measures of association, paired t-tests were conducted to compare the measurements by LC/MS/MS and IA at each time point. T3 measured by LC/MS/MS was significantly higher than T3 measured by IA in every case (all p<0.05) by average amounts ranging from 13.7 ng/dl (second trimester) to 32.9 ng/dl (third trimester). T4 measured by LC/MS/MS was significantly lower than T4 measured by IA in every case (all p<0.005) by average amounts ranging from 0.4 μg/dl (postpartum) to 1.1 μg/dl (third trimester).
4. Discussion
Thyroid insufficiency during early pregnancy may be associated with pregnancy-related complications and neurodevelopmental deficits in the conceptus. During normal pregnancy, there is an overall increase in thyroid-related activity including a two- to three-fold increase in TBG concentrations, sharp increases in TT4 and TT3 concentrations early in pregnancy, an increase in serum thyroglobulin and an increase in renal iodide clearance [22]. The normal increases in TT4 plateau early in the second trimester at approximately 50% greater concentrations than pre-pregnancy values, often outside the normal nonpregnant reference interval. Current thyroid function tests do not provide trimester-specific intervals.
Large variations exist in serum concentrations of thyroid hormones. One source of variation is biological variation, comprised of within-person variation (circadian and seasonal) and the variation between individuals [23]. Since the ratio of within- to between-individual variation is low for serum T4 and T3, it is important to recognize that the laboratory reference intervals may be relatively insensitive to aberrations from normality in the individual, and TSH measurements should be included to diagnose abnormalities. The other sources of variation can result from the methods of analysis.
Technically, it has been easier to develop methods to measure the total thyroid hormone concentrations, in comparison to tests that estimate the very low free hormone concentrations. This is because TT4 and TT3 are measured at nanomolar levels, whereas free hormone concentrations (FT4 and FT3) are measured in the picomole range. To be valid, FT4 and FT3 must be free from interference by the much higher total hormone concentrations. When TBG concentrations are abnormal, FT4 estimate tests are normally preferred over TT4 immunoassay measurements. However, current non-dialysis FT4 assays are binding-protein dependent and cannot be compared in absolute terms across methods [24,25].
Serum T4 and T3 detection methods have evolved through a variety of technologies since the 1950s. Radioimmunoassay (RIA) methods to detect thyroid hormone were developed in the 1970s. Serum T4 and T3 concentrations are currently measured by competitive immunoassay methods that are mostly nonisotopic and use enzymes, fluorescence or chemiluminescence molecules as signals [26]. Most necessitate the inclusion of a displacing agent to release the hormone from binding proteins [27] together with the large sample dilution to facilitate the binding of hormone to the antibody reagent. In these cases, abnormal serum TT4 and TT3 concentrations are more commonly encountered as a result of binding protein abnormalities and not thyroid dysfunction [21,28].
Since the concentration of circulating T3 is 10-fold lower than the concentration of circulating T4, there is both a technical sensitivity and precision challenge, even despite the use of a higher specimen volume. A reliable normal-range measurement and high-range T3 measurement is critical for diagnosing hyperthyroidism. In our samples, the lower values for T3 and T4 are higher by IA than MS especially during the second and third trimesters. The clinical manifestations of this may mean that some women who have subnormal levels are not detected by using IA.
Numerous reports of interferences in thyroid hormone immunoassays have been published in the last 20 years. In highly sensitive single- or double-antibody immunoassays, the presence of circulating endogenous antibodies directed against different antigens may cause either falsely depressed or falsely increased values of thyroid hormones, depending on the nature of the interfering antibody or the assay design. Because the abnormal values may influence the clinical decisions, these measurements and the reference intervals have important clinical consequence and may lead to unnecessary clinical investigations as well as inappropriate treatments [15]. The three major possible sources of antibody interference in thyroid hormone immunoassays are autoantibodies, heterophilic antibodies, including human anti mouse antibodies (HAMA) [14], and rheumatoid factors. Autoantibodies can cause an analyte-specific interference in thyroid assays [15], in contrast to heterophilic antibodies and rheumatoid factors, which may be responsible for method-specific disturbances in a wide range of immunoassays, including thyroid hormone measurement techniques. None of these interfere with TH measurements by isotope dilution tandem mass spectrometry, which is a candidate reference method and the current gold standard for T4/T3 measurement.
The correlation coefficients (r) for the T4 comparisons (LC/MS/MS vs. IA) are significantly better than for the T3 comparisons. Nevertheless, the slope of the T4-regression equations decreased significantly when comparing the nonpregnant group with the first, second or third trimester pregnancy groups (from 0.900 in nonpregnant women to 0.798–0.82 during pregnancy). This effect, is probably due to changes in circulating plasma proteins and thyroid antibodies during pregnancy. Our preliminary data for nonpregnant and pregnant women indicate a weaker correlation for T3 in both groups (r varies between 0.442 and 0.461). The solid line in Figs. 1-8 represent the best fit linear regression.
In summary, we define here trimester-specific reference intervals for T4 and T3 in pregnancy and in the same women 1 year postpartum. The selection criteria for establishing these pregnancy-related intervals for T4 and T3 were iodine sufficiency, normal singleton pregnancy, no thyroidal or other known disease nor severe hyperemesis. T4 and T3 reference intervals were determined simultaneously by using isotope dilution LC/MS/MS. In addition, we used IA to measure and define trimester-specific thyroid hormone reference values on the same samples (Immulite for T4 and DPC for T3). The reference intervals obtained for both T4 and T3 during the first, second and third trimesters of pregnancy in iodine-sufficient women are higher than the concentrations in the same women a year postpartum. T3 concentrations increase with the progression of pregnancy. T3 values are almost double those measured in nonpregnant women, and T4, 50% higher. These intervals, to our knowledge, are the first to be reported in the literature in a longitudinal study, as well as measured by LC/MS/MS. It is clear that T3 values obtained by IA (Immulite) do not correlate well with those obtained by a definitive isotope dilution tandem mass spectrometry procedure, throwing into question IAs for T3. While T4 IA results in the nonpregnant group correlate well with those obtained by isotope dilution tandem mass spectrometry, comparisons during pregnancy are less favorable.
We conducted and are in the process of data analysis of other analyte assays on the same samples including eight steroid hormones and other diabetes makers using LC/MS/MS and other thyroid function tests using IA.
Fig. 2.
T3 second trimester mass spectrometry vs. DPC Immulite. IA=0.41MS+0.91, r=0.574, Sy.x=0.2523, p<0.001. *To derive T3 values of ng/dl, multiply ng/ml values by 100.
Fig. 3.
T3 third trimester mass spectrometry vs. DPC Immulite. IA=0.36MS+1.00, r=0.436, Sy.x=0.3505, p<0.001.
Fig. 4.
T3 nonpregnant 1 year post-delivery mass spectrometry vs. DPC Immulite. IA=0.29MS+0.65, r=0.407, Sy.x=0.1732, p<0.05.
Fig. 5.
T4 first trimester mass spectrometry vs. Dade. IA=0.83 MS+25.4, r=0.805, Sy.x=10.34, p<0.001. *For T4 μg/dl values, divide ng/ml values by 10.
Fig. 6.
T4 second trimester mass spectrometry vs. Dade. IA=0.90MS+20.5, r=0.819, Sy.x=11.12, p<0.001.
Fig. 7.
T4 third trimester mass spectrometry vs. Dade. IA=0.84MS+27.6, r=0.802, Sy.x=12.59, p<0.001.
Acknowledgements
Presented in part at the CDC “Scientific Workshop on Maternal Thyroid Disease: The impact of Maternal Thyroid Disease on the Developing Fetus: Implications for Diagnosis, Treatment and Screening,” Atlanta, GA, January 12–13, 2004. This study was supported by NCI grant 5RO1 CA-89950-03 and NIH GCRC grant number 5-MO1-RR-13297-S1. We are grateful to Dr. R. Tractenberg, GCRC Georgetown University, for assistance with some of the statistical analysis and to Dr. N. Soukhova, Bioanalytical Core Laboratory, Georgetown Clinical Research Center for technical assistance.
References
- [1].Glinoer D, Riahi M, Grun JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab. 1994;79:197–204. doi: 10.1210/jcem.79.1.8027226. [DOI] [PubMed] [Google Scholar]
- [2].Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- [3].Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- [4].Lum SM, Nicoloff JT, Spencer CA, Kaptein E. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest. 1984;73:570–5. doi: 10.1172/JCI111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Robbins J. Thyroid hormone transport proteins and physiology of hormone binding in Werner and Ingbar’s “The Thyroid”. In: Braverman LE, Utiger RD, editors. A fundamental and clinical text. Lippincott-Raven; Philadelphia: 2000. pp. 103–20. [Google Scholar]
- [6].Schussler GC. The thyroxine-binding proteins. Thyroid. 1999;10:141–9. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- [7].Glinoer D. What happens to the normal thyroid during pregnancy? Thyroid. 1999;9:631–5. doi: 10.1089/thy.1999.9.631. [DOI] [PubMed] [Google Scholar]
- [8].Bartalena L. Recent achievements in studies on thyroid hormone-binding proteins. Endocr Rev. 1990;11:47–64. doi: 10.1210/edrv-11-1-47. [DOI] [PubMed] [Google Scholar]
- [9].Soldin SJ, Papanastasiou-Diamandis A, Heyes J, Lingwood C, Olley P. Are immunoassays for digoxin reliable? Clin Biochem. 1984;17:317–20. doi: 10.1016/s0009-9120(84)90637-4. [DOI] [PubMed] [Google Scholar]
- [10].Ghoshal AK, Soldin SJ. IMx tacrolimus II assay: is it reliable at low blood concentrations? A comparison with tandem MS/MS. Clin Biochem. 2002;35:389–92. doi: 10.1016/s0009-9120(02)00338-7. [DOI] [PubMed] [Google Scholar]
- [11].Soldin SJ, Steele BW, Witte DL, Wang E, Elin RJ. Lack of specificity of cyclosporine immunoassays. Results of a College of American Pathologists study. Arch Pathol Lab Med. 2003;127:19–22. doi: 10.5858/2003-127-19-LOSOC. [DOI] [PubMed] [Google Scholar]
- [12].Beck-Pecoz P, Romelli PB, Cattaneo MG, Faglia G, White EL, Barlow JW, et al. Evaluation of free thyroxine methods in the presence of iodothyronine-binding autoantibodies. J Clin Endocrinol Metab. 1984;58:736–9. doi: 10.1210/jcem-58-4-736. [DOI] [PubMed] [Google Scholar]
- [13].Sakata S, Nakamura S, Miura K. Autoantibodies against thyroid hormones or iodothyronine. Implications in diagnosis, thyroid function, treatment, and pathogenesis. Ann Intern Med. 1985;103:579–89. doi: 10.7326/0003-4819-103-4-579. [DOI] [PubMed] [Google Scholar]
- [14].Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124:921–3. doi: 10.5858/2000-124-0921-HAMA. [DOI] [PubMed] [Google Scholar]
- [15].Despres N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44:440–54. [PubMed] [Google Scholar]
- [16].Sarne DH, Refetoff S, Nelson JC, Linarelli LG. A new inherited abnormality of thyroxine-binding globulin (TBG-San Diego) with decreased affinity for thyroxine and triiodothyronine. J Clin Endocrinol Metab. 1989;68:114–9. doi: 10.1210/jcem-68-1-114. [DOI] [PubMed] [Google Scholar]
- [17].Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometric method for T4/T3. Clin Chim Acta. 2004;343:185–90. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tai SS, Sniegoski LT, Welch MJ. Candidate reference method for total thyroxine in human serum: use of isotope-dilution liquid chromatography–mass spectrometry with electrospray ionization. Clin Chem. 2002;48:637–42. [PubMed] [Google Scholar]
- [19].Elnagar B, Eltom A, Wide L, Gebre-Medhin M, Karlsson FA. Iodine status, thyroid function and pregnancy: study of Swedish and Sudanese women. Eur J Clin Nutr. 1998;52:351–5. doi: 10.1038/sj.ejcn.1600563. [DOI] [PubMed] [Google Scholar]
- [20].Babson AL, Olson DR, Palmieri T, Ross AF, Becker DM, Mulqueen PJ. The IMMULITE assay tube: a new approach to heterogeneous ligand assay. Clin Chem. 1991;37:1521–2. [PubMed] [Google Scholar]
- [21].Demers LM, Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf) 2003;58:138–40. doi: 10.1046/j.1365-2265.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- [22].Skjoldebrand L, Brundin J, Carlstrom A, Pettersson T. Thyroid associated components in serum during normal pregnancy. Acta Endocrinol (Copenh) 1982;100:504–11. doi: 10.1530/acta.0.1000504. [DOI] [PubMed] [Google Scholar]
- [23].Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–72. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- [24].Sapin R, d’Herbomez M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin Chem. 2003;49:1531–5. doi: 10.1373/49.9.1531. [DOI] [PubMed] [Google Scholar]
- [25].Roti E, Gardini E, Minelli R, Bianconi L, Flisi M. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J Endocrinol Invest. 1991;14:1–9. doi: 10.1007/BF03350244. [DOI] [PubMed] [Google Scholar]
- [26].Nelson JC, Wilcox RB. Analytical performance of free and total thyroxine assays. Clin Chem. 1996;42:146–54. [PubMed] [Google Scholar]
- [27].Evans SE, Burr WA, Hogan TC. A reassessment of 8-anilino-1-naphthalene sulphonic acid as a thyroxine binding inhibitor in the radioimmunoassay of thyroxine. Ann Clin Biochem. 1977;14:330–4. doi: 10.1177/000456327701400186. [DOI] [PubMed] [Google Scholar]
- [28].Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–87. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]