Abstract
Background
The aim of this study was to provide a contemporary picture of the presentation, etiology and outcome of infective endocarditis (IE) in a large patient cohort from multiple locations worldwide.
Methods
Prospective cohort study of 2781 adults with definite IE admitted to 58 hospitals in 25 countries between June 2000 and September 2005.
Results
The median age of the cohort was 57.9 (IQR 43.2–71.8) years and 72% had native valve IE. Most (77%) patients presented early in the disease (<30 days) with few of the classic clinical hallmarks of IE. Recent health-care exposure was found in one quarter of patients. Staphylococcus aureus was the most common pathogen (31%). Mitral (41%) and aortic (38%) valves were infected most commonly. Complications were common: stroke (17%); embolization other than stroke (23%); heart failure (32%) and intracardiac abscess (14%). Surgical therapy was common (48%) and in-hospital mortality remained high (18%). Prosthetic valve involvement (OR 1.47, 95%CI 1.13–1.90), increasing age (OR 1.30, 95%CI 1.17–1.46 per 10-year interval), pulmonary edema (OR 1.79, 95%CI 1.39–2.30), S. aureus infection (OR 1.54, 95%CI 1.14–2.08), coagulase-negative staphylococcal infection (OR 1.50, 95%CI 1.07–2.10), mitral valve vegetation (OR 1.34, 95%CI 1.06–1.68), and paravalvular complications (OR 2.25, 95%CI 1.64–3.09) were associated with increased risk of in-hospital death, while viridans streptococcal infection (OR 0.52, 95%CI 0.33–0.81) and surgery (OR 0.61, 95%CI 0.44–0.83) were associated with decreased risk.
Conclusions
In the early 21st century, IE is more often an acute disease, characterized by a high rate of S. aureus infection. Mortality remains relatively high.
Infective endocarditis (IE) is a disease of high morbidity and mortality. Although first described in the mid-sixteenth century, it was Osler's Gulstonian Lectures1–3 to the Royal College of Physicians in 1885 that created the impetus for systematic study of IE. Beginning in the early 1900s, investigators have frequently reported on the manifestations of this disease.4–11 Yet, despite advances over the last century in diagnosis,12 medical therapy,13 and surgical treatment,14, 15 mortality rates have not changed substantially in the past 25 years.5, 9, 16–18 The current in-hospital mortality rate for patients with IE is 15–20%,5, 16 with one-year mortality approaching 40%.16, 18, 19 This is in stark contrast to sustained and ongoing improvements observed in other cardiovascular diseases such as myocardial infarction.20
Unfortunately, definitive studies of IE have been limited by its relative infrequency - a problem compounded by the wide range of causative organisms, at-risk populations, and underlying risk factors for infection. Most studies have consisted of case reports or single-center studies which limit the scope and statistical power necessary for definitive conclusions. Moreover, the lack of multinational studies has prevented an understanding of how geographic differences in patient characteristics and management affect outcome in patients with IE.
A prospective multicenter approach is essential for addressing the limitations associated with prior investigations of IE and, importantly, for examining therapeutic choices in a definitive way. Therefore, the International Collaboration on Endocarditis (ICE) was established to facilitate a multinational, multicenter approach to the study of IE. From this collaboration, the ICE–Prospective Cohort Study (ICE–PCS) was designed to assess the current characteristics of patients with IE. In this study, we describe this large cohort of patients, with particular emphasis on the current clinical presentation, microbial etiology and outcome of patients with IE.
METHODS
International Collaboration on Endocarditis–Prospective Cohort Study
The International Collaboration on Endocarditis (ICE) began in June 1999. The ICE investigators later developed the ICE–PCS.21 Enrollment in ICE–PCS began on June 2000, and for the purposes of this study was closed on September 2005; the present study includes data from 58 sites in 25 countries.
All patients aged 18 years or older with IE from sites that met criteria for participation were included in the study. Sites had to meet the following criteria: 1) minimum enrollment of 12 cases per year in a center with access to cardiac surgery; 2) patient identification procedures in place to ensure consecutive enrollment and minimize ascertainment bias;21 3) high-quality data, including query resolution; and 4) Institutional Review Board (IRB)/Ethics Committee approval or waiver based upon local standards.
The ICE-PCS database is maintained at the Duke Clinical Research Institute, which serves as the coordinating center for the ICE studies, with IRB approval from Duke University School of Medicine.
Patient Selection
Patients were prospectively identified at each site to ensure consecutive enrollment.21 A total of 3284 patients were enrolled into ICE-PCS, of which 2781 had definite IE by the modified Duke criteria (Table 1).22 The 2781 patients with definite IE were included in this analysis.
Table 1.
Definition of infective endocarditis according to the modified Duke criteria22
| Definite infective endocarditis |
| Pathologic criteria |
|
| Clinical criteria (see below for definitions) |
|
| Possible infective endocarditis |
|
| Rejected |
|
| Definition of terms used in the modified Duke criteria for the diagnosis of infective endocarditis (IE): |
| Major criteria |
| Blood culture positive for IE |
|
| Evidence of endocardial involvement |
| Echocardiogram positive for IE (TEE recommended in patients with prosthetic valves, rated at least “possible IE” by clinical criteria, or complicated IE [paravalvular abscess]; TTE as first test in other patients), defined as follows: |
|
| New valvular regurgitation (worsening or changing of pre-existing murmur not sufficient) |
| Minor criteria |
|
Abbreviations: TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Excludes single positive cultures for coagulase-negative staphylococci and organisms that do not cause endocarditis.
Data Collection
A case report form of 275 variables was developed by the ICE group according to standard definitions.21, 23, 24 Data were collected prospectively by site investigators during the index hospitalization and were then sent either to the coordinating center for data entry or were entered directly by the site investigators through a secure internet data entry system. Queries were developed on critical variables and were distributed to the sites for reconciliation. Once complete, the reconciled queries were returned to the coordinating center for final data entry.
Definitions
Definitions of the variables included in the ICE-PCS case report form have been reported in detail elsewhere.23 Community-acquired IE was defined as IE diagnosed at the time of admission (or within 48 hours of admission) in a patient not fulfilling the criteria for health care-associated infection. Health care-associated IE was defined as either nosocomial IE or non-nosocomial health care-associated IE. Nosocomial IE was defined as IE developing in a patient hospitalized for more than 48 hours prior to onset of signs/symptoms consistent with IE. Non-nosocomial health care-associated IE was defined as IE diagnosed within 48 hours of admission in an outpatient with extensive health care contact as reflected by any of the following criteria: (1) receipt of intravenous therapy, wound care, or specialized nursing care at home within the 30 days prior to the onset of IE; (2) attendance at a hospital or hemodialysis clinic or receipt of intravenous chemotherapy within the 30 days prior to the onset of IE; (3) hospitalization in an acute care hospital for 2 or more days in the 90 days prior to the onset of IE; or (4) residence in a nursing home or long-term care facility. In an effort to group centers based on geographic similarities, regions were defined as follows: North America (10 sites from USA), South America (8 sites from Brazil, Argentina, and Chile), Europe (22 sites from Croatia, France, Germany, Italy, Netherlands, Spain, Sweden, Ireland, Romania, Russia, Slovenia and United Kingdom) and Other (18 sites from Australia, Israel, India, Lebanon, Malaysia, New Zealand, Singapore, Thailand and South Africa).
Statistical analyses
Continuous variables were represented as medians with 25th and 75th percentiles. Categorical variables were represented as frequencies and percentages of the specified group. Univariable comparisons were made with the chi-square test or Kruskal Wallis test as appropriate. In order to account for the possibility that patients referred to study hospitals from other health care facilities may represent a different population than those who were admitted directly, data from the latter group only were analyzed separately where indicated.
A generalized estimating equation (GEE) method was used to determine factors associated with in-hospital mortality. Age, gender, whether transferred from another healthcare facility and variables found to have a univariable association with in-hospital mortality (P <0.10) were entered into the final exploratory model. The GEE method produces consistent parameter estimates that measure association between in-hospital death and the baseline covariates while accounting for the correlation in outcomes of patients from the same hospital. Likelihood ratio tests were used to compare models with and without interaction terms. Final parameter estimates were converted to odds ratios (ORs) with corresponding 95% Wald confidence intervals (CIs). The model was validated by the bootstrap procedure. Some 200 estimates were obtained by fitting the GEE model to 200 datasets obtained by randomly selecting 2781 observations with replacement from the actual data. Bootstrap estimates were computed by averaging the 200 parameter estimates and bootstrap confidence intervals were computed sorting the parameter estimates in ascending order and selecting the 5th estimate for the lower confidence limit and the 195th estimate for the upper confidence limit.
Statistical analyses were performed using STATA version 8.2 (StataCorp, College Station, TX, USA).
RESULTS
Patients were enrolled in ICE–PCS from the following regions: North America (n = 597, 21%); South America (n = 254, 9%); Europe (n = 1213, 44%); and Other (n = 717, 26%). Baseline characteristics and predisposing factors are shown in Table 2. The median age of the cohort was 57.9 years (mean 56.5 years; IQR 43.2–71.8 years). The majority of patients in the cohort (72%) had native valve IE, and most patients (77%) were admitted within one month of the initial signs of illness. The most common underlying condition was diabetes mellitus (16%), but 10% of South American patients were diabetics, compared to over one quarter of North American patients. Similarly, less than 5% of patients from outside North America were on hemodialysis, compared with over 20% of North American patients.
Table 2.
Baseline characteristics and predisposing conditions in 2781 patients with definite endocarditis. Data are number (%) unless otherwise stated.
| Total Cohort | Patients admitted directly to study sites onlya | Region |
P value for the difference in regions | ||||
|---|---|---|---|---|---|---|---|
| North America | South America | Europe | Other | ||||
| A. Baseline Characteristics | |||||||
| Age, years (median (IQR)) | 57.9 (43.2–71.8) | 59.8 (44.2–73.1) | 52.9 (44.1–66.4) | 56.8 (40.3–70.4) | 61.4 (45.1–72.7) | 58.0 (40.5–72.9) | <0.001 |
| Male gender | 1889/2777 (68) | 1045/1556 (67) | 388/596 (65) | 179/254 (70) | 873/1212 (72) | 449/715 (63) | <0.001 |
| First sign to admission < 1 month | 2088/2711 (77) | 1201/1529 (79) | 496/582 (85) | 166/244 (68) | 896/1174 (76) | 530/711 (75) | <0.001 |
| Hemodialysis | 220/2777 (8) | 130/1556 (8) | 124/596 (21) | 20/254 (8) | 49/1210 (4) | 27/717 (4) | <0.001 |
| Diabetes | 447/2764 (16) | 261/1550 (17) | 158/592 (27) | 25/253 (10) | 169/1207 (14) | 95/712 (13) | <0.001 |
| HIV positive | 58/2748 (2) | 41/1540 (3) | 16/594 (3) | 4/236 (2) | 33/1211 (3) | 5/707 (1) | 0.02 |
| Cancer | 230/2772 (8) | 160/1553 (10) | 52/596 (9) | 15/251 (6) | 101/1210 (8) | 62/715 (9) | 0.56 |
| IE type | 0.05 | ||||||
| Native valve | 1901/2636 (72) | 1048/1471 (71) | 411/573 (72) | 167/246 (68) | 860/1166 (74) | 463/651 (71) | |
| Prosthetic valve | 563/2636 (21) | 321/1471 (22) | 116/573 (20) | 66/246 (27) | 227/1166 (20) | 154/651 (24) | |
| Pacemaker/ICD | 172/2636 (7) | 102/1471 (7) | 46/573 (8) | 13/246 (5) | 79/1166 (7) | 34/651 (5) | |
| B. Predisposing Conditions | |||||||
| Current IV drug use | 268/2746 (10) | 157/1540 (10) | 93/587 (16) | 1/249 (0.4) | 113/1203 (9) | 61/707 (9) | <0.001 |
| Previous IE | 222/2780 (8) | 138/1557 (9) | 66/596 (11) | 26/254 (10) | 84/1213 (7) | 46/717 (6) | 0.003 |
| Invasive procedure within 60 days | 690/2581 (27) | 392/1463 (27) | 162/508 (32) | 64/247 (26) | 289/1145 (25) | 175/681 (26) | 0.03 |
| Chronic IV access | 244/2763 (9) | 142/1548 (9) | 148/595 (25) | 12/251 (5) | 56/1205 (5) | 28/712 (4) | <0.001 |
| Endocavitary device | |||||||
| Pacemaker | 262/2752 (10) | 146/1540 (9) | 55/595 (9) | 23/252 (9) | 137/1191 (12) | 47/714 (7) | 0.005 |
| ICD | 27/2720 (1) | 15/1521 (1) | 16/593 (3) | 0/249 (0) | 8/1172 (1) | 3/706 (0.4) | <0.001 |
| Congenital heart disease | 311/2656 (12) | 167/1481 (11) | 62/582 (11) | 53/244 (22) | 111/1156 (10) | 85/674 (13) | <0.001 |
| Native valve predisposition | 884/2761 (32) | 538/1547 (35) | 147/596 (25) | 93/252 (37) | 370/1201 (31) | 274/712 (38) | <0.001 |
Abbreviations: HIV, human immunodeficiency virus; IE = infective endocarditis, IV = intravenous, ICD = implantable cardioverter defibrillator
Excludes patients transferred to study hospitals from other health care facilities
Predisposing conditions were common in patients with definite IE (Table 2). Although intravenous drug use remains important (10%), the most common predisposing conditions were related to valvular heart disease. Degenerative valve disease, e.g. significant mitral (43%) and/or aortic (26%) valve regurgitation, was the most frequent native valve predisposing factor. In contrast, rheumatic heart disease was uncommon; only 92 patients (3%) had rheumatic mitral valve disease. Valvular predisposing conditions also included the presence of a prosthetic valve in 618 (23%) patients.
Chronic intravenous access was as common as intravenous drug use in the overall cohort; 61% of patients in this study with chronic intravenous access were from North America (Table 2).
Clinical and laboratory findings on admission are presented in Table 3. The classical signs that are often considered diagnostic for IE were infrequent.
Table 3.
Clinical and LAboratory findings on admission in 2781 patients with definite endocarditis and historical comparisons
| Findings | Number (%) |
|---|---|
| Fever >38°C | 2322/2428 (96) |
| Splinter hemorrhages | 213/2655 (8) |
| Osler's nodes | 77/2648 (3) |
| Janeway lesions | 123/2650 (5) |
| Roth spots | 50/2649 (2) |
| Vascular embolic event | 456/2665 (17) |
| Conjunctival hemorrhage | 122/2655 (5) |
| Splenomegaly | 284/2662 (11) |
| New murmur | 1068/2232 (48) |
| Worsening of old murmur | 359/1787 (20) |
| Elevated erythrocyte sedimentation rate | 1611/2645 (61) |
| Elevated C-reactive protein | 1632/2650 (62) |
| Elevated rheumatoid factor | 138/2549 (5) |
| Hematuria | 666/2587 (26) |
In 2756 (99%) of the 2781 patients, blood cultures were taken to determine the causative microorganism. Of the 310 patients (11%) with negative blood cultures, 192 (62%) had received antibiotics within seven days of the blood culture. In addition to blood culture information, serologic tests and valve cultures were performed in a minority of cases. Of the 2781 patients, 277 (10%) were determined to have culture/serology-negative IE.
The causative microorganisms isolated from blood cultures are shown in Table 4. Gram-positive organisms were predominant (83%), with S. aureus accounting for 31% of all infections. S. aureus was also the most common organism in each major risk group, including intravenous drug users and intracardiac device IE (Table 5). Positive serological tests for Coxiella burnetii were reported from 27 patients (17 from Europe, two from North America, one from South America and seven from other regions), but only nine were reported to have reciprocal antibody titers >800. Similarly, 22 patients had positive serological tests for Bartonella spp. (18 from Europe, one from South America and three from other regions), but only three were reported to have reciprocal antibody titers >800. There was one case of infection due to Tropheryma whipplei.
Table 4.
Microbiologic etiology by region in 2781 patients with definite endocarditis.
| Total Cohort n = 2781 n (%) | Patients admitted directly to study sites onlya n= 1558 n (%) | Region |
P value for the difference between regions | ||||
|---|---|---|---|---|---|---|---|
| North America n = 597 n (%) | South America n = 254 n (%) | Europe n = 1213 n (%) | Other n = 717 n (%) | ||||
| S. aureus | 869 (31) | 487 (31) | 256 (43) | 43 (17) | 339 (28) | 231 (32) | <0.001 |
| Coag Neg staph. | 304 (11) | 161 (10) | 69 (12) | 18 (7) | 156 (13) | 61 (9) | 0.005 |
| Viridans group strep | 483 (17) | 288 (19) | 54 (9) | 66 (26) | 198 (16) | 165 (23) | <0.001 |
| S. bovis | 165 (6) | 101 (7) | 9 (2) | 17 (7) | 116 (10) | 23 (3) | <0.001 |
| Other strep | 162 (6) | 101 (7) | 38 (6) | 16 (6) | 66 (5) | 42 (6) | 0.86 |
| Enterococci | 283 (10) | 158 (10) | 78 (13) | 21 (8) | 111 (9) | 73 (10) | 0.05 |
| HACEK | 44 (2) | 26 (2) | 2 (0.3) | 6 (2) | 19 (2) | 17 (2) | 0.02 |
| Fungi / yeast | 45 (2) | 25 (2) | 20 (3) | 3 (1) | 13 (1) | 9 (1) | 0.002 |
| Polymicrobial | 28 (1) | 23 (2) | 8 (1) | 1 (0.4) | 13 (1) | 6 (1) | 0.60 |
| Culture negative | 277 (10) | 122 (8) | 41 (7) | 51 (20) | 123 (10) | 62 (9) | <0.001 |
| Other | 121 (4) | 66 (4) | 22 (4) | 12 (5) | 59 (5) | 28 (4) | 0.61 |
Abbreviations : strep = streptococci; HACEK = Haemophilus spp., Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; PVIE = prosthetic valve infective endocarditis.
Excludes patients transferred to study hospitals from other health care facilities
Table 5.
Microbiologic etiology by IE type in 2781 patients with definite endocarditis.
| Native Valve IE |
Intracardiac-Device IE |
|||
|---|---|---|---|---|
| Drug Abusers (n=237) n (%) | Non-Drug Abusers (n=1644) n (%) | PVIE (n=563) n (%) | Other devices (n=172)a n (%) | |
| S. aureus | 160 (68) | 457 (28) | 129 (23) | 60 (35) |
| Coag Neg staph. | 7 (3) | 148 (9) | 95 (17) | 45 (26) |
| Viridans group strep | 24 (10) | 345 (21) | 70 (12) | 14 (8) |
| S. bovis | 3 (1) | 119 (7) | 29 (5) | 5 (3) |
| Other strep | 5 (2) | 118 (7) | 26 (5) | 7 (4) |
| Enterococci | 11 (5) | 179 (11) | 70 (12) | 10 (6) |
| HACEK | 0 (0) | 30 (2) | 13 (2) | 1 (1) |
| Fungi / Yeast | 3 (1) | 16 (1) | 23 (4) | 2 (1) |
| Polymicrobial | 6 (3) | 16 (1) | 5 (1) | 0 (0) |
| Culture negative | 12 (5) | 154 (9) | 65 (12) | 18 (11) |
| Other | 6 (3) | 62 (4) | 38 (7) | 10 (6) |
| Surgical therapy | 89/234 (38)b | 784/1639 (48) | 274/561 (49) | 104/172 (61) |
| In-hospital mortality | 23/236 (10)b | 281/1643 (17) | 131/561 (23) | 17/172 (10) |
Abbreviations : IE = infective endocarditis; strep = streptococci; HACEK = Haemophilus spp., Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; PVIE = prosthetic valve infective endocarditis.
Including pacemakers and implantable cardioverter defibrillators.
For pure right-sided IE only: 23/107 (22%) had surgical therapy and 6/108 (6%) died in hospital.
S. aureus was the most common organism in three of four regions, whereas viridans group streptococci were the most common organisms isolated from patients in South America. The frequency of Streptococcus bovis IE was much higher in Europe and South America compared to the other regions, and IE due to HACEK bacteria was relatively uncommon in North America. The majority of C. burnetii and Bartonella infections were from Europe.
The location of acquisition was determined in 94% of patients; community acquisition (71%) was more common than nosocomial (14%) or non-nosocomial health care–associated IE (9%) in the total cohort (Figure 1). North America had a much higher proportion of health care-associated infections (37%) compared with other regions, mainly due to a larger proportion with non-nosocomial health care–associated IE. The microbial etiology of IE varied with location of acquisition, with a higher proportion with staphylococcal IE and a lower proportion with viridans streptococcal IE among those with health care-associated IE. Among patients with community-acquired infection, 34% had staphylococcal IE and 23% had virdans streptococcal IE, while the corresponding figures for nosocomial infection were 70% and 1% respectively, and for non-nosocomial health care-associated infection were 68% and 4% respectively.
Figure 1.
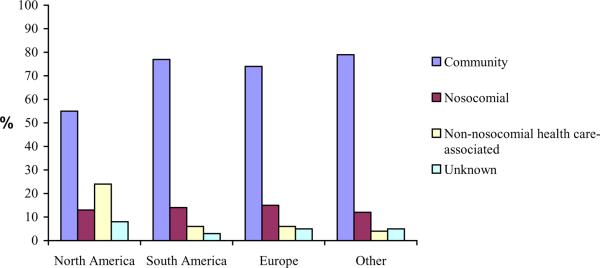
Geographic comparison of location of acquisition in 2781 patients with definite endocarditis.
Echocardiography was used commonly (99% of patients). More than one half (59%) of the patients had both transthoracic and transesophageal echocardiography. Of the 2781 patients, 87% had echocardiographic evidence of vegetation, whereas new, significant, valvular regurgitation was discovered in 64% of patients. Abscess was the most common paravalvular complication (15% of patients), while 92% of patients with prosthetic valve IE had evidence of a prosthetic valve complication such as dehiscence or new paravalvular regurgitation.
Congestive heart failure was the most common complication in all regions (Table 6). In general, the highest complication rates occurred in North America and Europe.
Table 6.
vegetation findings, complications, treatment, and outcome in 2781 patients with definite endocarditis
| Total Cohort n (%) | Patients admitted directly to study sites onlya n (%) | Region |
P value for the difference between regions | ||||
|---|---|---|---|---|---|---|---|
| North America n (%) | South America n (%) | Europe n (%) | Other n (%) | ||||
| A. Vegetation Findings | |||||||
| Vegetation present | 2406/2764 (87) | 1325/1545 (86) | 530/594 (89) | 223/254 (88) | 1041/1201 (87) | 612/715 (86) | 0.257 |
| AV | 1031/2741 (38) | 524/1535 (34) | 198/593 (33) | 117/252 (46) | 460/1189 (39) | 256/707 (36) | 0.003 |
| MV | 1125/2740 (41) | 640/1534 (42) | 253/593 (43) | 103/252 (41) | 474/1188 (40) | 295/707 (42) | 0.70 |
| TV | 323/2741 (12) | 177/1534 (12) | 107/593 (18) | 18/252 (7) | 129/1189 (11) | 69/707 (10) | <0.001 |
| PV | 29/2739 (1) | 11/1534 (1) | 8/593 (1) | 5/252 (2) | 7/1187 (1) | 9/707 (1) | 0.15 |
| B. Complications | |||||||
| Stroke | 462/2727 (17) | 225/1528 (15) | 118/595 (20) | 37252 (15) | 199/1169 (17) | 108/711 (15) | 0.11 |
| Embolization, non-stroke | 611/2709 (23) | 324/1524 (21) | 139/587 (24) | 46/251 (18) | 295/1163 (25) | 131/708 (19) | 0.002 |
| CHF | 876/2713 (32) | 414/1527 (27) | 207/591 (35) | 97/249 (39) | 383/1162 (33) | 189/711 (27) | <0.001 |
| Intracardiac abscess | 389/2707 (14) | 176/1522 (12) | 101/590 (17) | 48/250 (19) | 156/1157 (13) | 84/710 (12) | 0.005 |
| Persistent positive blood culture | 251/2699 (9) | 131/1515 (9) | 124/586 (21) | 7/250 (3) | 82/1153 (7) | 38/710 (5) | <0.001 |
| New conduction abnormality | 217/2695 (8) | 100/1511 (7) | 70/591 (12) | 25/250 (10) | 72/1152 (6) | 50/702 (7) | <0.001 |
| C. Treatment/Outcome | |||||||
| Surgical therapy | 1335/2769 (48) | 574/1549 (37) | 268/595 (45) | 141/252 (56) | 613/1210 (51) | 313/712 (44) | 0.001 |
| In-hospital mortality | 490/2774 (18) | 274/1555 (18) | 108/596 (18) | 43/254 (17) | 231/1210 (19) | 108/714 (15) | 0.17 |
Abbreviaitons: AV, aortic valve; MV, mitral valve; TV, tricuspid valve; PV, pulmonic valve; CHF, congestive heart failure.
Excludes patients transferred to study hospitals from other health care facilities
There were also geographic differences in both treatment and outcome, although the magnitude of this variation was not large (Table 6). Surgical treatment was common for the entire cohort, (48%), and in-hospital mortality was 18%. Table 7 shows the results of the regression modeling for in-hospital mortality, together with the estimates from bootstrap validation. The following variables were independently associated with an increased risk of in-hospital death: involvement of a prosthetic valve, increasing age, radiographic pulmonary edema, S. aureus infection, coagulase-negative staphylococcus infection, presence of a mitral valve vegetation, and paravalvular complications. Variables independently associated with a decreased risk of in-hospital death were: elevated ESR, infection with a viridans group streptococcus, and surgery during the current IE episode. The estimates obtained by bootstrap validation were similar to those of the original model and support the validity of this model. Differences between models were noted for four variables: diabetes, health care-associated acquisition, coagulase-negative staphylococcus infection, and presence of a mitral valve vegetation.
Table 7.
Results of multivariable regression modelling of associations with In-hospital death in 2781 patients with definite endocarditis.
| Variablea | Original Model |
Bootstrap Modelc |
|||
|---|---|---|---|---|---|
| ORb | 95% CI | p-value | ORb | 95% CI | |
| Age in ten year intervals | 1.30 | 1.17–1.46 | <0.001 | 1.23 | 1.14–1.31 |
| Male gender | 0.99 | 0.74–1.34 | 0.97 | 1.02 | 0.79–1.25 |
| Transferred from another health care facility | 0.97 | 0.74–1.29 | 0.85 | 1.17 | 0.92–1.42 |
| Prosthetic valve endocarditis | 1.47 | 1.13–1.90 | 0.004 | 1.34 | 1.05–1.70 |
| Hemodialysis | 1.06 | 0.73–1.53 | 0.76 | 1.01 | 0.65–1.42 |
| Diabetes | 1.28 | 0.88–1.86 | 0.20 | 1.45 | 1.08–1.85 |
| Intravenous drug use | 0.93 | 0.51–1.70 | 0.82 | 0.81 | 0.47–1.24 |
| Cancer | 1.04 | 0.65–1.67 | 0.86 | 1.23 | 0.80–1.70 |
| Other chronic illness | 1.36 | 0.95–1.95 | 0.10 | 1.28 | 0.99–1.61 |
| Invasive procedure | 0.96 | 0.66–1.39 | 0.82 | 0.94 | 0.73–1.18 |
| Congenital heart disease | 1.22 | 0.74–2.02 | 0.44 | 1.18 | 0.75–1.61 |
| Elevated erythrocyte sedimentation rate | 0.57 | 0.44–0.73 | <0.001 | 0.59 | 0.47–0.72 |
| Radiographic pulmonary edema | 1.79 | 1.39–2.30 | <0.001 | 2.03 | 1.56–2.53 |
| Health care-associated acquisition | 1.30 | 0.85–1.98 | 0.23 | 1.32 | 1.02–1.69 |
| S. aureus IE | 1.54 | 1.14–2.08 | 0.005 | 1.72 | 1.31–2.18 |
| Coagulase-negative staphylococci IE | 1.50 | 1.07–2.10 | 0.02 | 1.36 | 0.93–1.87 |
| Viridans group streptococci IE | 0.52 | 0.33–0.81 | 0.004 | 0.52 | 0.35–0.71 |
| Mitral valve vegetation | 1.34 | 1.06–1.68 | 0.01 | 1.20 | 0.93–1.45 |
| Paravalvular complications | 2.25 | 1.64–3.09 | <0.001 | 2.00 | 1.57–2.49 |
| Surgery during this episode | 0.61 | 0.44–0.83 | 0.002 | 0.56 | 0.44–0.69 |
All dichotomous variables except for age.
Adjusted for all other variables in the model.
Italicised values indicate differences between the original and bootstrap models
Of the total cohort of patients with definite IE, 1174 (42%) had been transferred to a study hospital from another health care facility. Analysis of the data after excluding these patients revealed few differences from analysis of the whole cohort (Tables 2, 4, and 6). Notable differences were that transferred patients were more likely to undergo surgery (63% of transferred patients cf 37% of non-transferred patients; p <0.001), and were more likely to have congestive heart failure as a complication (39% cf 27%; p <0.001). In-hospital mortality (18%) and microbial etiology were similar for both groups of patients.
COMMENT
Despite more than a century of study and recent advances in diagnosis and treatment, IE remains an incompletely understood disease with high morbidity and mortality. Textbook descriptions of the clinical features and epidemiology of IE are still largely based on data obtained from several decades ago. Lack of progress is partly related to the fundamental difficulty in studying this type of disease. By necessity, most studies are derived from case reports or small case series from single sites, with few large cohort studies or randomized trials. A shift in approach is necessary to further the understanding of endocarditis and to definitively study therapeutic choices. The ICE-PCS represents a new effort in broadening our understanding of endocarditis. This study is by far the largest prospective cohort study of IE to date. The size of the cohort coupled with the multinational perspective has enabled several important observations to be made.
changes in patient characteristics of IE
Our findings reveal that, in much of the world, IE is no longer a subacute or chronic disease occurring primarily in younger patients with rheumatic valvular abnormalities. In contrast, most patients in this investigation presented early and demonstrated few of the classic clinical findings traditionally associated with IE. For example, in the 1960s and 1970s, Osler's nodes were recorded in 11–23% and splenomegaly in 20–44% of patients with IE.9, 10, 25, 26 In our study, predisposing valvular conditions were common, but were primarily due to the presence of degenerative valve disease or a prosthetic valve, rather than rheumatic heart disease. Forty years ago, approximately 50% of cases of IE in the United States were superimposed on preexisting rheumatic lesions,27 compared with <5% in the present study. Prosthetic valve endocarditis was present in one fifth of our patients, as discussed in detail elsewhere.24
An emerging population at risk for IE consists of patients with healthcare–associated infections. Overall, IE was attributed to a health care exposure in nearly 25% of the patients. These findings confirm those of recent reports from small single-center studies16,28 and provide evidence that these population changes are occurring in many regions of the world. The health care setting will continue to gain importance in relation to complications such as IE, mainly due to aging societies that rely upon increasingly invasive medical care.29, 30
Our analysis has provided evidence of geographic differences for several important characteristics in patients with IE. For example, although the overall IE population characteristics were influenced by contact with health care services and medical interventions, this specific finding was not observed in the centers from South America. In addition, the association between health care-associated IE was most striking in North America.
changes in microbiologic characteristics of IE
Another observation arising from this investigation is the shift in the microbiology of IE. S. aureus is now the most common cause of IE in much of the world, confirming several recent investigations5, 16, 31 and the earlier findings of the ICE-PCS.23 This shift is due in part to the global presence of risk factors for S. aureus–associated IE (for example, injection drug use, health care contact, and invasive procedures). Given the growing antimicrobial resistance in S. aureus,32 including vancomycin,33–35 the importance of this pathogen as a potentially lethal infection is cause for concern.
We also noted a substantially higher prevalence of S. bovis IE in Europe, that HACEK IE was relatively uncommon in North America, and that most cases of Q fever and bartonella IE came from Europe. Whether these findings reflect differences in patient characteristics, regional health care access, diagnostic bias or other factors remains to be determined. For IE due to micro-organisms that are difficult to culture, geographic differences may, at least partially, reflect variation in the threshold for performing additional diagnostic tests. This may be the case for Q fever and bartonella IE which often rely on serological and/or nucleic acid amplification tests for diagnosis.36 However, it is also clear that there are geographic differences in the incidence of these two infections.37
These changes in the patients and pathogens have important implications for the diagnosis and management of IE. For example, new risk groups have been identified that necessitate careful diagnostic attention in the presence of fever and bacteremia. In addition, the acute nature of IE in the modern era may require an accelerated evaluation strategy that provides the opportunity for early diagnosis and treatment decisions in patients at high risk for complications and death.
In-Hospital Mortality
We have found several factors that were independently associated with in-hospital mortality. Some of these factors, such as increasing age, presence of pulmonary edema, and paravalvular complications, were not surprising. In addition, prosthetic valve IE and staphylococcal IE were also associated with an increased risk of in-hospital death, while there was a decreased risk associated with viridans streptococcal IE. Interestingly, an elevated ESR was associated with a decreased risk of death, although the reason for this is unclear. Elevated ESR may be associated with more chronic infection, thereby signifying a more chronic clinical course. Importantly, we have found that early surgery may be critical in improving survival in patients with definite IE. This finding adds detail to recent reports supporting early surgical intervention38, 39 and adds credence to the practice of a combined medical and surgical approach from admission for patients with IE, specifically in those with congestive heart failure and prosthetic valve infections. Our finding that nearly 50% of patients had surgery indicates that the threshold for early surgical treatment has lowered.
Study Limitations
This is an observational study of patients from centers with a particular interest in IE. These hospitals are typically referral centers with cardiac surgical programs. Consequently, the study population is unlikely to be a true population-based sample, thereby limiting epidemiologic inferences. This potential selection bias may be less evident in some sites (e.g. New Zealand) where most cases of IE within the catchment area would be eligible for enrollment in the study. It might be expected that patients transferred from other health care facilities would represent a different population than those who presented directly to study hospitals. In particular, the former group may have more complicated disease and greater indications for surgery. However, when the two groups were compared, patients transferred from other facilities had similar characteristics to those presenting directly to study hospitals, with notable exceptions being that a larger proportion of the former group underwent surgery during their initial hospitalization and had congestive heart failure as a complication. Consequently, we believe it is important to present data from both groups of patients and that exclusion of referred patients may create a greater selection bias.
While study sites spanned all non-Antarctic continents, there was a heavy weighting towards wealthy countries in Europe, North America and Australasia, with few sites in Asia and Africa. There would undoubtedly be greater geographic differences in patient and microbiologic characteristics of IE if sampling was able to more closely resemble the global population distribution. The study lacked long-term follow-up of patients, thereby limiting the ability to analyze outcome beyond initial hospitalization. The precise timing of all complications was not recorded and may affect the ability to determine the clinical significance of some findings.
Conclusions
IE remains a serious and deadly disease despite recent advances in diagnosis and treatment. Notably, IE has shifted to a disease in which the presentation is more acute than previously described and, throughout much of the world, is characterized by a high rate of S. aureus infection in patients with previous health care exposure. More care must be taken to effectively treat all patients with S. aureus bacteremia and to identify patients with high potential for complications.40 We have documented geographic differences in the presentation, microbial etiology, treatment, and outcome of patients with IE. In addition, we have found initial evidence that early surgery may be important in improving patient outcomes. Since nearly 50% of patients with IE undergo surgery, early identification of surgical indications may improve mortality. More research also needs to focus on stroke prevention (e.g. when to operate on vegetations), the identification of most effective therapy (e.g. role of new antibiotics and combination treatment), and understanding reasons for the high prevalence of S. bovis IE in Europe and the near absence of HACEK IE in North America.
Acknowledgments
In addition to all of the named ICE investigators at each site, we would like to acknowledge the support given to this project from all of the personnel at each site and at the coordinating center that have allowed this project to move forward. This study was supported in part by the following: National Institutes of Health grants AI-068804 (VGF) and K23 HL70861-01 (CHC), AHA BGIA 0265405U (CHC), the “Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Madrid (Spain) - Red Española de Investigación en Patología Infecciosa” ((REIPI RD06/0008) and FIS 05/0170) (JMM), the Fundación Privada Máximo Soriano Jiménez (Barcelona, Spain) (JMM), the Institut d'Investigacions Biomèdiques August Pi i Sunyer and the “Conselleria de Salut de la Generalitat de Catalunya, Barcelona (Spain) (IDIBAPS, Barcelona Spain) (JMM).
Role of the Sponsors The sponsors played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Appendix
Study Investigators David Gordon MBBS, PhD, FRACP, FRCPA, Uma Devi MD (Flinders Medical Centre, Adelaide, Australia); Denis Spelman MD (Alfred Hospital, Amiens, France); Jan T.M. van der Meer MD, PhD (University of Amsterdam, Amsterdam, Netherlands); Carol Kauffman MD, Suzanne Bradley MD, William Armstrong MD (Ann Arbor VA Medical Center, Ann Arbor, USA); Efthymia Giannitsioti MD, Helen Giamarellou MD, PhD (Attikon University General Hospital, Athens, Greece); Stamatios Lerakis MD, FAHA, FACC, FASE, FCCP (Emory University, Atlanta, USA); Ana del Rio MD PhD, Asuncion Moreno MD PhD, Carlos A. Mestres MD, PhD, FETCS, Salvador Ninot, MD, Carlos Paré MD PhD, Cristina Garcia de la Maria PhD, Yolanda Armero, Elisa de Lazzari BSc, Francesc Marco MD PhD, Jose M Gatell MD PhD, Manel Almela MD, Manuel Azqueta MD, Marta Sitges, MD PhD, Xavier Claramonte MD, Maria Jesús Jiménez-Expósito MD PhD, Natividad de Benito MD PhD, Jose Ramirez MS, PhD, Noel Perez MD, José M. Miró MD, PhD (Hosp. Clinic - IDIBAPS: University of Barcelona, Barcelona, Spain); Benito Almirante MD, Nuria Fernandez-Hidalgo MD, Pablo Rodriguez de Vera MD, Pilar Tornos MD, Vicente Falcó MD Xavier Claramonte MD, (Hospital Universitari Vall d'Hebron, Barcelona, Spain); Nisreen Sidani RN, MSN, Souha Kanj-Sharara MD, FACP, Zeina Kanafani MD, MS (American University of Beirut Medical Center, Beirut, Lebanon); Annibale Raglio MD, DTM&H, Antonio Goglio MD, Fabrizio Gnecchi MD, Fredy Suter MD, Grazia Valsecchi MD, Marco Rizzi MD, Veronica Ravasio MD (Ospedali Riuniti di Bergamo, Bergamo, Italy); Bruno Hoen MD, PhD, Catherine Chirouze MD, Efthymia Giannitsioti MD, Joel Leroy MD, Patrick Plesiat MD, Yvette Bernard MD (University Medical Center of Besançon, Besançon, France); Anna Casey, Peter Lambert BSc, PhD, DSc, Richard Watkin MRCP, Tom Elliott BM, BS, BMedSci, PhD, DSc, FRCPath (Queen Elizabeth Hospital, Birmingham, UK); Mukesh Patel MD, William Dismukes MD (University of Alabama at Birmingham, Birmingham, USA); Angelo Pan MD, Giampiero Caros MD (Spedali Civili - Università di Brescia, Brescia, Italy); Amel Brahim Mathiron Christophe Tribouilloy MD, PhD, Thomas Goissen MD (South Hospital Amiens, Bron Cedex, France); Armelle Delahaye, Francois Delahaye MD, MPH, FESC, Francois Vandenesch MD, PhD (Hopital Louis Pradel, Bron Cedex, France); Carla Vizzotti MD, Francisco M. Nacinovich MD, Marcelo Marin MD, Marcelo Trivi MD, Martin Lombardero MD (Instituto Cardiovascular, Buenos Aires, Argentina); Claudia Cortes MD, José Horacio Casabé MD (Instituto de Cardiología y Cirugía Cardiovascular, Buenos Aires, Argentina); Javier Altclas MD, Silvia Kogan MD (Sanatorio Mitre, Buenos Aires, Argentina); Liliana Clara MD, Marisa Sanchez MD (Hospital Italiano, Buenos Aires, Argentina); Anita Commerford MD, Cass Hansa MD, Eduan Deetlefs MD, Mpiko Ntsekhe MD, Patrick Commerford MD (Groote Schuur Hospital, Cape Town, South Africa); Dannah Wray MD, MHS, Lisa L. Steed PhD, Preston Church MD, Robert Cantey MD (Medical University of South Carolina, Charleston, USA); Arthur Morris MD, FRCPA (Diagnostic Medlab, Auckland, New Zealand); David Holland MB, ChB, PhD, FRACP, FRCPA (Auckland City Hospital, Auckland, New Zealand); David Murdoch MD, MSc, FRACP, FRCPA, Stephen Chambers MD, MSc, FRACP (University of Otago, Christchurch and Christchurch Hospital, Christchurch, New Zealand); Kerry Read MB, ChB, FRACP (North Shore Hospital, Auckland, New Zealand); Nigel Raymond MB, ChB, FRACP (Wellington Hospital, Wellington, New Zealand); Selwyn Lang MB, ChB, FRACP, FRCPA (Middlemore Hospital, Auckland, New Zealand); Despina Kotsanas BSc (Hons), Tony M. Korman MD (Southern Health, Clayton, Australia); Gail Peterson MD, Jon Purcell BS, Paul M. Southern, Jr. MD (UT-Southwestern Medical Center, Dallas, USA); Manisha Shah MD, Roger Bedimo MD, MS (Dallas VA Medical Center, Dallas, USA); Arjun Reddy, Donald Levine MD, Gaurav Dhar MD (Wayne State University, Detroit, USA); Alanna Hanlon-Feeney, Margaret Hannan MD, BCh, BAO, MSc, MRCPath, FRCPI, Sinead Kelly MD (Mater Hospitals, Dublin, Ireland); Andrew Wang MD, Christopher H. Cabell MD, MHS, Christopher W. Woods MD, MPH, Daniel J Sexton MD, Danny Benjamin, Jr MD, MPH, PhD, G Ralph Corey MD, Jay R McDonald MD, Jeff Federspiel, John J Engemann MD, L. Barth Reller MD, Laura Drew RN, BSN, L.B. Caram MD, Martin Stryjewski MD, MHS, Susan Morpeth MB, ChB, Tahaniyat Lalani MD, Vance Fowler, Jr MD, MHS, Vivian Chu MD (Duke University Medical Center, Durham, USA); Bahram Mazaheri PhD, Carl Neuerburg, Christoph Naber MD (University Essen, Essen, Germany); Eugene Athan MD, Margaret Henry BSc (Hons), PhD, Owen Harris MD (Barwon Health, Geelong, Australia); Eric Alestig MD, Lars Olaison MD,PhD, Lotta Wikstrom, Ulrika Snygg-Martin MD (Sahlgrenska Universitetssjukhuset/Östra, Goteborg, Sweden); Johnson Francis MD,DM, K Venugopal MD,DM, Lathi Nair MD, DM, Vinod Thomas MD, DM (Medical College Calicut, Kerla, India); Jaruwan Chaiworramukkun MD, Orathai Pachirat MD, Ploenchan Chetchotisakd MD, Tewan Suwanich MD (Khon Kaen University, Khon Kaen, Thailand); Adeeba Kamarulzaman MBBS, FRACP, Syahidah Syed Tamin MD (University of Malaya Medical Center, Kuala Lumpur, Malaysia); Manica Mueller Premru MD, PhD, Mateja Logar MD, PhD, Tatjana Lejko-Zupanc MD, PhD (Medical Center Ljublijana, Ljublijana, Slovenia); Christina Orezzi, John Klein MD (St. Thomas' Hospital, London, UK); Emilio Bouza MD, PhD, Mar Moreno MD, PhD, Marta Rodríguez-Créixems MD, PhD, Mercedes Marín MD, Miguel Fernández MD, Patricia Muñoz MD, PhD, Rocío Fernández, Victor Ramallo MD (Hospital General Universitario Gregorio Marañón, Madrid, Spain); Didier Raoult MD, PhD, Franck Thuny MD, Gilbert Habib MD, FACC, FESC, Jean-Paul Casalta MD, Pierre-Edouard Fournier MD (Faculté de Médecine de Marseille, Marseille, France); Natalia Chipigina PhD, Ozerecky Kirill MD, Tatiana Vinogradova MD, PhD, Vadim P. Kulichenko PhD (Russian Medical State University, Moscow, Russia); O.M. Butkevich PhD (Learning Medical Centre of Russian Presidential Affairs Government, Moscow, Russia); Christine Lion MD, Christine Selton-Suty MD, Francois Alla MD, PhD, Hélène Coyard, Thanh Doco-Lecompte MD (CHU Nancy-Brabois, Nancy, France); Diana Iarussi MD, Emanuele Durante-Mangoni MD, PhD, Enrico Ragone MD, PhD, Giovanni Dialetto MD, Marie Françoise Tripodi MD, Riccardo Utili MD, Roberta Casillo MD, PhD (II Università di Napoli, Naples, Italy); A. Sampath Kumar MD, Gautam Sharma MD (All India Institute of Medical Sciences, New Delhi, India ); Stuart A. Dickerman MD (New York University Medical Center, New York, USA );Alan Street, Damon Peter Eisen MBBS, MD, FRACP, Emma Sue McBryde MBBS, PhD, FRACP, Leeanne Grigg (Royal Melbourne Hospital, Parkville, Australia); Elias Abrutyn MD (Drexel University College of Medicine, Philadelphia, USA); Christian Michelet MD, PhD, Pierre Tattevin MD, Pierre Yves Donnio PhD (Pontchaillou University, Rennes, France); Claudio Querido Fortes MD (Hospital Universitario Clementino Fraga Filho/UFRJ, Rio de Janeiro, Brazil ); Jameela Edathodu MRCP, Mashael Al-Hegelan MD (King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia); Bernat Font MD, Ignasi Anguera MD, PhD, Joan Raimon Guma MD (Hospitál de Sabadell, Sabedell, Spain); M Cereceda MD, Miguel J. Oyonarte MD, Rodrigo Montagna Mella Md (Hospital Clinico Universidad de Chile, Santiago, Chile); Patricia Garcia MD, Sandra Braun Jones MD (Hosp. Clínico Pont. Universidad Católica de Chile, Santiago, Chile); Auristela Isabel de Oliveira Ramos MD (Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil); Marcelo Goulart Paiva MD, Regina Aparecida de Medeiros Tranchesi MD (Hospital 9 de Julho, São Paulo, Brazil); Lok Ley Woon BSN, Luh-Nah Lum BSN, Ru-San Tan MBBS, MRCP (National Heart Centre, Singapore, Singapore); David Rees MD, Pam Kornecny MD, Richard Lawrence MD, Robyn Dever MD (St. George Hospital, Sydney, Australia); Jeffrey Post MD, Phillip Jones MD, Suzanne Ryan MHSc, GCDM (The University of New South Wales, Sydney, Australia ); John Harkness MD, Michael Feneley MD (St. Vincent's, Sydney, Australia); Ethan Rubinstein MD, LLB, Jacob Strahilewitz MD (Tel Aviv University School of Medicine, Tel Aviv, Israel); Adina Ionac MD,PhD, Cristian Mornos MD, Stefan Dragulescu MD,PhD (Victor Babes University of Medicine and Pharmacy, Timisoar, Romania); Davide Forno MD, Enrico Cecchi MD, Francesco De Rosa MD, Massimo Imazio MD, FESC, Rita Trinchero MD (Maria Vittoria Hospital, Torino, Italy); Franz Wiesbauer MD, Rainer Gattringer MD (Vienna General Hospital, Vienna, Austria); Ethan Rubinstein MD,LLB, Greg Deans MD (University of Manitoba, Winnipeg, Canada); Arjana Tambic Andrasevic MD, PhD, Bruno Barsic MD, PhD, Igor Klinar MD, Josip Vincelj MD, PhD, FESC, Suzana Bukovski MD, Vladimir Krajinovic MD (Univ. Hospital for Infectious Diseases, Zagreb, Croatia).
ICE Coordinating Center, Durham, USA Christopher Cabell MD, MHS, Judy Stafford MS, Khaula Baloch BA, Paul Pappas MS, Thomas Redick MPH, Tina Harding RN, BSN
ICE Steering Committee A W Karchmer MD, Arnie Bayer MD, Bruno Hoen MD,PhD, Christopher H Cabell, MD, MHS, Daniel J Sexton, MD, David T. Durack MD, D Phil, FACP, FRCP, FRACP, Elias Abrutyn MD, Ethan Rubinstein MD,LLB, G Ralph Corey MD, José M. Miró, MD, PhD, Phillipe Moreillon, Susannah Eykyn, Vance Fowler, Jr, MD, MHS, Lars Olaison MD, PhD
ICE Publications Committee Arnie Bayer MD, Bruno Hoen MD,PhD, Christopher H Cabell, MD, MHS, David Murdoch MD, MSc, FRACP, FRCPA, Elias Abrutyn MD, Eugene Athan MD, José M. Miró, MD, PhD, G Ralph Corey MD, Paul Pappas, MS, Vance Fowler, Jr, MD, MHS, Vivian Chu, MD
Footnotes
Author Contributions Dr Murdoch had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: DRM, GRC, BH, JMM, VGF, ASB, AWK,CHC
Acquisition of data: DRM, GRC, BH, JMM, VGF, ASB, AWK, LO, PM, STC, VHC, VF, DJH, PJ, JLK, NJR, KMR, MFT, RU, AW, CWW, CHC
Analysis and interpretation of data: DRM, GRC, PAP, CHC
Drafting of the manuscript: DRM, GRC, CHC
Critical revision of the manuscript: DRM, GRC, BH, JMM, VGF, ASB, AWK, LO, PAP, PM, STC, VHC, VF, DJH, PJ, JLK, NJR, KMR, MFT, RU, AW, CWW, CHC
Statistical analysis: DRM, PAP, CHC
All authors have seen and approved the final version of the manuscript.
Conflict Of Interest No authors have any conflict of interest to disclose regarding the work presented in this manuscript.
References
- 1.Osler W. Gulstonian lectures on malignant endocarditis. Lecture I. Lancet. 1885;1(3210):415–418. doi: 10.1136/bmj.1.1263.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osler W. Gulstonian lectures on malignant endocarditis. Lecture II. Lancet. 1885;1(3211):459–464. doi: 10.1136/bmj.1.1263.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osler W. Gulstonian lectures on malignant endocarditis. Lecture III. Lancet. 1885;1(3212):505–508. [Google Scholar]
- 4.Cherubin CE, Neu HC. Infective endocarditis at the Presbyterian Hospital in New York City from 1938-1967. Am J Med. 1971;51(1):83–96. doi: 10.1016/0002-9343(71)90326-3. [DOI] [PubMed] [Google Scholar]
- 5.Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis. Results of a 1-year survey in France. JAMA. 2002;288(1):75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Horder TJ. Infective endocarditis: with an analysis of 150 cases and with special reference to the chronic form of the disease. Quart J Med. 1909;2:289–329. [Google Scholar]
- 7.Lamas CC, Eykyn SJ. Suggested modifications to the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: analysis of 118 pathologically proven cases. Clin Infect Dis. 1997;25(3):713–719. doi: 10.1086/513765. [DOI] [PubMed] [Google Scholar]
- 8.Osler W. Chronic infective endocarditis. Quart J Med. 1909;2:219–230. [Google Scholar]
- 9.Pelletier LL, Petersdorf RG. Infective endocarditis: a review of 125 cases from the University of Washington Hospitals, 1963-72. Medicine. 1977;56(4):287–313. [PubMed] [Google Scholar]
- 10.Rabinovich S, Evans J, Smith IM, January LE. A long-term view of bacterial endocarditis. 337 cases 1924 to 1963. Ann Intern Med. 1965;63(2):185–198. doi: 10.7326/0003-4819-63-2-185. [DOI] [PubMed] [Google Scholar]
- 11.Ribera E, Miró JM, Cortés E, et al. Influence of Human Immunodeficiency Virus 1 infection and degree of immunosuppression in the clinical characteristics and outcome of infective endocarditis in intravenous drug users. Arch Intern Med. 1998;158(18):2043–2050. doi: 10.1001/archinte.158.18.2043. [DOI] [PubMed] [Google Scholar]
- 12.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 13.Bayer AS, Bolger AF, Taubert KA, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98(25):2936–2948. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 14.Middlemost S, Wisenbaugh T, Meyerowitz C, et al. A case for early surgery in native left-sided endocarditis complicated by heart failure: results in 203 patients. J Am Coll Cardiol. 1991;18(3):663–667. doi: 10.1016/0735-1097(91)90785-8. [DOI] [PubMed] [Google Scholar]
- 15.Mullany CJ, Chua YL, Schaff HV, et al. Early and late survival after surgical treatment of culture-positive active endocarditis. Mayo Clin Proc. 1995;70(6):517–525. doi: 10.4065/70.6.517. [DOI] [PubMed] [Google Scholar]
- 16.Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162(1):90–94. doi: 10.1001/archinte.162.1.90. [DOI] [PubMed] [Google Scholar]
- 17.Delahaye F, Goulet V, Lacassin F, et al. Characteristics of infective endocarditis in France in 1991. a 1-year survey. Eur Heart J. 1995;16(3):394–401. doi: 10.1093/oxfordjournals.eurheartj.a060923. [DOI] [PubMed] [Google Scholar]
- 18.Nissen H, Nielsen PF, Frederiksen M, Helleberg C, Nielsen JS. Native valve infective endocarditis in the general population: a 10-year survey of the clinical picture during the 1980s. Eur Heart J. 1992;13(7):872–877. doi: 10.1093/oxfordjournals.eurheartj.a060285. [DOI] [PubMed] [Google Scholar]
- 19.Benn M, Hagelskjær LH, Tvede M. Infective endocarditis, 1984 through 1993: a clinical and microbiological survey. J Intern Med. 1997;242(1):15–22. doi: 10.1046/j.1365-2796.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- 20.American Heart Association . Heart disease and stroke statistics - 2004 update. American Heart Association; Dallas, TX: 2003. [Google Scholar]
- 21.Cabell CH, Abrutyn E. Progress toward a global understanding of infective endocarditis. Early lessons from the International Collaboration on Endocarditis investigation. Infect Dis Clin N Am. 2002;16(2):255–272. doi: 10.1016/s0891-5520(01)00007-1. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 23.Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis. A consequence of medical progress. JAMA. 2005;293(24):3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 24.Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297(12):1354–1361. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 25.Lerner PI, Weinstein L. Infective endocarditis in the antibiotic era (continued) N Engl J Med. 1966;274(5):259–266. doi: 10.1056/NEJM196602032740506. [DOI] [PubMed] [Google Scholar]
- 26.Venezio FR, Westenfelder GO, Cook FV, Emmerman J, Phair JP. Infective endocarditis in a community hospital. Arch Intern Med. 1982;142(4):789–792. [PubMed] [Google Scholar]
- 27.Weinstein L, Rubin RH. Infective endocarditis - 1973. Prog Cardiovasc Dis. 1973;16(3):239–274. doi: 10.1016/s0033-0620(73)80001-5. [DOI] [PubMed] [Google Scholar]
- 28.Spies C, Madison JR, Schatz IJ. Infective endocarditis in patients with end-stage renal disease: clinical presentation and outcome. Arch Intern Med. 2004;164(1):71–75. doi: 10.1001/archinte.164.1.71. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Renal Data System (USRDS) USRDS 1999 annual data report. National Institutes of Health; Bethesda, MD: 1999. [Google Scholar]
- 30.Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J. 2004;147(4):582–586. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Sanabria TJ, Alpert JS, Goldberg R, Pape LA, Cheeseman SH. Increasing frequency of staphylococcal infective endocarditis. Experience at a university hospital, 1981 through 1988. Arch Intern Med. 1990;150(6):1305–1309. [PubMed] [Google Scholar]
- 32.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290(22):2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control Vancomycin-resistant Staphylococcus aureus - New York, 2004. MMWR. 2004;2(3):322–323. [PubMed] [Google Scholar]
- 34.Tenover FC, Weigel LM, Appelbaum PC, et al. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob Agents Chemother. 2004;48(1):275–280. doi: 10.1128/AAC.48.1.275-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitener CJ, Park SY, Browne FA, et al. Vancomycin-resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis. 2004;38(8):1049–1055. doi: 10.1086/382357. [DOI] [PubMed] [Google Scholar]
- 36.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center. Etiologic diagnosis of 348 cases. Medicine. 2005;84(3):162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 37.Werner M, Fournier P-E, Andersson R, Hogevik H, Raoult D. Bartonella and coxiella antibodies in 334 prospectively studied episodes of infective endocarditis in Sweden. Scand J Infect Dis. 2003;35(10):724–727. doi: 10.1080/00365540310015980. [DOI] [PubMed] [Google Scholar]
- 38.Bishara J, Leibovici L, Gartman-Israel D, et al. Long-term outcome of infective endocarditis: the impact of early surgical intervention. Clin Infect Dis. 2001;33(10):1636–1643. doi: 10.1086/323785. [DOI] [PubMed] [Google Scholar]
- 39.Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA. 2003;290(24):3207–3214. doi: 10.1001/jama.290.24.3207. [DOI] [PubMed] [Google Scholar]
- 40.Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163(17):2066–2072. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]


