Abstract
BACKGROUND
Multiple observational studies have suggested that BRCA-associated ovarian cancers have improved survival compared to BRCA-negative ovarian cancers. Most of these studies, however, have combined BRCA1 and BRCA2 patients or evaluated only BRCA1 patients. We sought to examine if BRCA1− and BRCA2-associated ovarian cancers were associated with different outcomes.
METHODS
A single-institution retrospective analysis of patients seen between January 1, 1996 and February 1st, 2011 for a new diagnosis of histologically confirmed Stage III or IV serous ovarian, fallopian tube, or primary peritoneal cancer and who underwent BRCA mutation testing on one of two IRB approved follow-up studies. Patients tested for BRCA mutations beyond 24 months of diagnosis were excluded from analysis to minimize selection bias from including patients referred for genetic testing because of long survival.
RESULTS
Data from 190 patients (143 BRCA−, 30 BRCA1+, 17 BRCA2+) were analyzed. During the study period, 73 deaths were observed (60 BRCA−, 10 BRCA1+, 3 BRCA2+). Median follow-up time for the remaining 117 survivors was 2.5 years. At 3 years, 69.4%, 90·7%, and 100% of BRCA−, BRCA1+, and BRCA2+ patients were alive, respectively. On univariate analysis, age, BRCA2, debulking status, and type of first-line therapy (intravenous or intraperitoneal) were significant predictors of overall survival (OS). On multivariate analysis, BRCA2 status (HR .20; 95% CI, .06–.65; P=.007) but not BRCA1 status (HR .70; 95% CI, .36–1.38; P=.31) predicted for improved OS compared to BRCA-patients. When carriers of BRCA2 mutations were directly compared to carriers of BRCA1 mutations, BRCA2 mutation status appeared to confer an improved OS (HR .29; 95% CI, 0.08–1.05; P=.060), although this finding did not reach significance.
CONCLUSION
Our data suggest that BRCA2 status confers an overall survival advantage compared to both BRCA− and BRCA1 status in high-grade serous ovarian cancer. This finding may have important implications for clinic trial design.
Keywords: Ovarian cancer, BRCA1, BRCA2, PARP inhibitors
Introduction
Ovarian cancer is the leading cause of death from gynecologic malignancies in the United States and is the fourth most common cause of cancer death in women.1 An estimated 21,000 cases are diagnosed in the United States each year, resulting in 15,000 deaths. It is now recognized that approximately 10% of unselected cases, and 16-21% of the high-grade serous subtype, are due to a hereditary susceptibility, of which BRCA1 and BRCA2 mutations account for the majority.2-4
Although BRCA1 and BRCA2 mutations are both implicated in the development of hereditary ovarian cancers, each represents a clinically distinct entity in several important ways. The penetrance of ovarian cancer differs in these two populations with a lifetime risk of 36-60% and 16-27% in BRCA1 and BRCA2 carriers, respectively.5-7 BRCA1 carriers also develop ovarian cancers approximately 10 years earlier then BRCA2 carriers with median age at diagnosis of 53 and 62 years, respectively.8, 9 The distinct underlying biology of BRCA1 and BRCA2 ovarian cancers has also been characterized in comparative gene array profiles that have demonstrated more then 100 non-redundant genes with significantly different expression levels.10
Despite these clear biological and clinical differences, investigators typically have grouped together BRCA1 and BRCA2 carriers when investigating the prognosis and survival of this subset of ovarian cancer patients. In several observational studies, BRCA+ ovarian cancers have been shown to be associated with improved overall survival (OS).9, 11-18 The majority of these studies, including the three largest to date, have combined BRCA1 and BRCA2 patients in their survival analyses.9, 14, 19 Only one report has independently evaluated survival of BRCA1 and BRCA2 patients but the power of this analysis was limited due to the inclusion of only six BRCA2+ serous ovarian cancers.16
We investigated the survival of BRCA1 and BRCA2 associated ovarian cancers independently in patients seen at this institution. Our goal was to determine if these two ovarian cancer syndromes were associated with different prognoses.
Methods
Patients
Institutional Review Board approval was obtained for this retrospective analysis. Eligible patients were seen at Memorial Sloan-Kettering Cancer Center (MSKCC) between January 1, 1996 and February 1st, 2011 for a new diagnosis of histologically confirmed Stage III or IV serous ovarian, fallopian tube, or primary peritoneal cancer and underwent BRCA mutation testing on one of two IRB approved follow-up studies being conducted by the Clinical Genetics Service investigating the clinical significance of germline BRCA mutations. Details of these two follow-up studies have been published previously.20 For the present study, patients with non-high grade (low malignant potential or FIGO grade 1) histology and/or recurrent disease were excluded. Diagnoses were confirmed by a fulltime gynecologic pathologist at a high volume comprehensive cancer center. In the event there was uncertainty regarding the diagnosis, the case was reviewed at a gynecologic pathology consensus conference. Patients tested for BRCA mutations beyond 24 months of diagnosis were also excluded from analysis to minimize the selection bias that could result from including patients referred for genetic testing because of long survival. All BRCA1 and BRCA2 mutations were predicted to be deleterious. Patients with variants of unknown significance were considered to be BRCA negative. All patients received cytotoxic chemotherapy as per appropriate institutional protocol at time of diagnosis. This therapy could be intravenous alone (IV) or combined intravenous and intraperitoneal (IP). Because the standard regimen for IP chemotherapy changed over the time studied, patients were considered to have received IP chemotherapy if they received any IP cisplatin as part of first-line therapy.
Statistical Methods
This was a single institutional retrospective analysis with the primary objective of determining OS of patients by BRCA status (BRCA1+, BRCA2+, BRCA−). The associations between clinical factors and BRCA status were tested by either using the Wilcoxon-rank sum test or Kruskal-Wallis test for the continuous variables or the Fisher-exact test for categorical variables. OS time is calculated from diagnosis to death or last follow-up date. Univariate OS analyses for BRCA status, age, and stage were performed using the log-rank test for categorical variables or Wald test based on Cox proportional hazards model for continuous variable. A landmark survival analysis was used in order to evaluate time-dependent covariates such as timing of initiation of chemotherapy or attempt at surgical debulking.21 This analysis is conditional on being at risk at the landmark time so patients with follow-up time shorter than the landmark time were excluded. Variables are regarded significant at a significance level of 0.05. Forward selection technique was used to build multivariate model using a significance level of 0.05 for the variable to remain in the model.22 Analyses were conducted using SAS version 9.2 (SAS Institute, Carey, North Carolina).
BRCA Testing
During the study period, patients presenting for treatment of newly diagnosed pelvic serous cancer were not required to undergo genetic counseling or testing. Between 1996 and 2008 patients were typically referred based on at least one of the following: 1) family history of breast cancer prior to age 50 or ovarian cancer at any age in a first or second degree relative, 2) Eastern European (Ashkenazi) Jewish heritage 3) patient request, or 4) physician request. Since July 2008, genetic counseling has been offered to (but not required of) all patients diagnosed with high-grade serous ovarian cancer irrespective of family history. For patients whose four grandparents were all of Ashkenazi Jewish heritage, germline DNA was screened for the three Ashkenazi founder mutations (BRCA1*185delAG, BRCA1*5382insC and BRCA2*6174delT). If these were wild-type and there was a strong family history of ovarian or early onset breast cancer, sequencing of the coding and flanking intronic regions was performed by Myriad Genetics (Salt Lake City, UT). For participants who were not of exclusive Ashkenazi ancestry, sequencing of BRCA1 and BRCA2 was performed. Testing for structural rearrangements was obtained based on individual patient characteristics and testing coverage.
Results
A total of 190 patients (143 BRCA−, 30 BRCA1+, 17 BRCA2+) were eligible for analysis. No patient had mutations in both BRCA1 and BRCA2. Eighty-six (45%) of patients reported at least one Ashkenazi Jewish grandparent, while the remaining 105 (55%) were of entirely non-Ashkenazi Jewish decent. Baseline demographic and treatment data are listed in Table 1. There were no significant differences between the BRCA1 and BRCA2 patients in the distribution of age, stage, debulking outcomes (optimal versus suboptimal), or first-line therapy (IV versus IP). Comparing across all three groups the BRCA+ patients were more likely to have been optimally debulked and receive IP based first-line chemotherapy.
Table 1. Patient Demographics.
| Variable | All | BRCA (−) | BRCA1 (+) | BRCA2 (+) | Test for Group | Test for BRCA1 vs. BRCA2 |
|---|---|---|---|---|---|---|
| All | 190 | 143 | 30 | 17 | ||
| Age at Diagnosis | ||||||
| Median (Mean) | 59(58.45) | 61(59.72) | 55(53.7) | 56(56.12) | 0.008 | 0.41 |
| Range | 32-78 | 37-78 | 32-73 | 40-74 | ||
| Stage | ||||||
| III | 148(77.9%) | 110(76.9%) | 23(76.7%) | 15(88.2%) | 0.63 | 0.46 |
| IV | 42(22.1%) | 33(23.1%) | 7(23.3%) | 2(11.8%) | ||
| Optimal Debulking | ||||||
| No | 45(23.7%) | 41(28.7%) | 2(6.7%) | 2(11.8%) | 0.012 | 0.61 |
| Yes | 145(76.3%) | 102(71.3%) | 28(93.3%) | 15(88.2%) | ||
| IP/IV (2 missing) | ||||||
| IV | 95(50.5%) | 82(57.7%) | 10(33.3%) | 3(18.8%) | 0.001 | 0.49 |
| IP | 93(49.5%) | 60(42.3%) | 20(66.7%) | 13(81.3%) |
Overall Survival
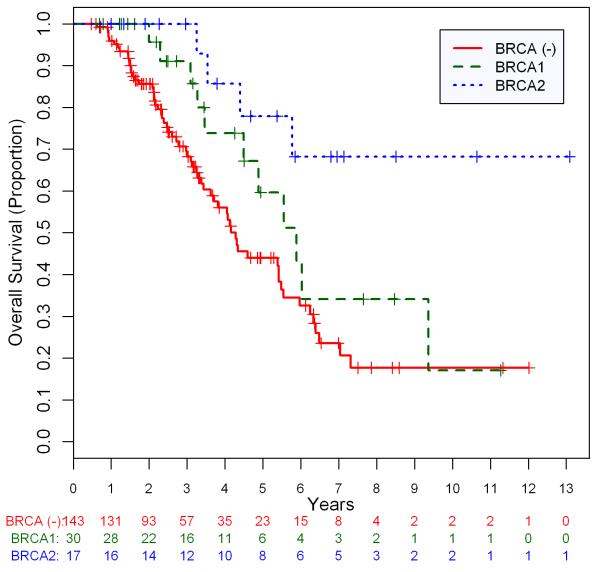
Survival data and univariate OS analysis are presented in Table 2. During the period of follow-up there were a total of 73 deaths (10 BRCA1+, 3 BRCA2+, 60 BRCA−). OS rate at three years for the BRCA−, BRCA1+, and BRCA2+ patients was 69·4%, 90·7%, and 100%, respectively. The median survival for BRCA− and BRCA1+ patients was 4.3 years and 5.6 years, respectively. With a median follow-up of 5.3 years for the surviving BRCA2+ patients, median survival had not yet been reached. Median follow-up time for the remaining 117 surviving patients was 2·5 years (range: .47–13.1).
Table 2. Univariate Overall Survival Analyses.
| Variable | N | Death | 3-year OS rate (95%CI) | Median Survival Year (95%CI) | HR (95%CI) | log-rank P |
|---|---|---|---|---|---|---|
| All | 190 | 73 | 75.8%(67.7-82.1%) | 5.4(4.2-5.9) | ||
|
| ||||||
| BRCA Status | ||||||
| BRCA (−) | 143 | 60 | 69.4%(59.4-77.3%) | 4.3(3.6-5.5) | Ref. Level | 0.005* |
| BRCA1 (+) | 30 | 10 | 90.7%(67.6-97.6%) | 5.6(3.5-Not Estimable) | 0.61(0.31-1.2) | |
| BRCA2 (+) | 17 | 3 | 100% | Not Reached | 0.19(0.06-0.62) | |
|
| ||||||
| Age at diagnosis (increase by 10) | 1.38(1.08-1.77) | 0.01 | ||||
|
| ||||||
| Stage | ||||||
| III | 148 | 58 | 79.7%(70.9-86.1%) | 5.4(4.3-6.0) | Ref. Level | 0.38 |
| IV | 42 | 15 | 60.3%(39.4-76.1%) | 3.5(2.7-Not Estimable) | 1.29(0.73-2.29) | |
|
| ||||||
| Optimal Debulking (2 excluded in the landmark analysis) | ||||||
| No | 44 | 23 | 45.6%(27.2-62.3%) | 3.0(1.7-4.0) | Ref. Level | 0.005 |
| Yes | 144 | 50 | 70.6%(60-78.9%) | 4.9(3.7-5.8) | 0.5(0.31-0.82) | |
|
| ||||||
| IP/IV (2 missing, 1 excluded in landmark analysis used) | ||||||
| IV | 94 | 47 | 58.1%(45.3-69%) | 3.7(2.7-5.0) | Ref. Level | 0.005 |
| IP | 93 | 26 | 74.2%(60.3-83.9%) | 5.0(3.8-Not Estimable) | 0.51(0.32-0.83) | |
The log-rank p value for BRCA1 vs. BRCA− is 0.150; BRCA2 vs. BRCA− is 0.003; BRCA1 vs. BRCA2 is 0.050.
Impact of age, stage, debulking status, and type of first line therapy (IV or IP) on overall survival were analyzed using univariate analyses. Of these, only age (10-year HR 1.38; 95% CI, 1.08-1.77), optimal debulking status (HR .50; 95% CI, .31-.82), and IP therapy (HR .51; 95% CI, .32-.83) were statistically significant predictors of survival. Surgical stage (III versus IV) was not significantly associated with survival, although the majority of patients had stage III disease (78%, 148/190). When BRCA status (BRCA1+, BRCA2+ and BRCA−) was examined in univariate fashion, BRCA2 (HR .19; 95% CI, .06 – .62), but not BRCA1 (HR .61; 95% CI, .31-1.20), status was a significant predictor of OS.
The effect of BRCA status on OS was also evaluated in a multivariate analysis. The results are presented in Table 3. Using forward-selection technique, the covariates age, optimal vs. sub-optimal debulking, and BRCA mutation status were selected for inclusion in the final multivariate model. Type of first-line therapy was not included as it did not reach significance in the final model. This was likely due to substantial correlation between debulking status and receipt of IP chemotherapy. In the final multivariate model, only BRCA2 status (HR .20; 95% CI, .06 – .65; P=.007) and age (10-year HR 1.32; 95% CI, 1.02 – 1.71; P=.035) remained significant predictors of OS. Optimal debulking status was of borderline significance and BRCA1 status was not a significant predictor of survival (HR 0·70, 95% CI 0·36 – 1·38; p=0·31). When carriers of BRCA2 mutations were directly compared to carriers of BRCA1 mutations in our multivariate model setting, BRCA2 mutation status appears to predict for substantially improved survival (HR .29; 95% CI, .08 – 1.05; P=.06) although this finding did not quite reach significance. The overall survival of the BRCA·, BRCA1 and BRCA2 patients are shown in Figure 1.
Table 3. Multivariate Overall Survival*.
| Variable | HR (95%CI) | P |
|---|---|---|
| Age at diagnosis (in 10) | 1·32 (1·02 - 1·71) | 0·035 |
| BRCA 1 vs. BRCA (−) | 0·70 (0·36 - 1·38) | 0·31 |
| BRCA 2 vs. BRCA (−) | 0·20 (0·06 - 0·65) | 0·007 |
| Optimal vs. Suboptimal Debulking | 0·60 (0·36 - 1·00) | 0·050 |
Landmark analysis was used to build this multivariate model.
Figure 1.
Kaplan-Meier curves illustrating overall survival for patients with breast cancer gene (BRCA)-negative, BRCA1-positive, and BRCA2-positive high-grade serous ovarian cancer.
Discussion
Our data suggest that BRCA2-associated serous ovarian cancer may have meaningfully different outcomes from not only BRCA-negative, but also BRCA1-associated, high-grade serous ovarian cancer. This finding, if confirmed, will have an important impact on clinical trial design given the 16-21% prevalence of germline BRCA mutations in unselected patients with serous ovarian cancer. The ongoing and upcoming trials of poly (ADP-ribose) polymerase (PARP) inhibitors in high-grade epithelial ovarian cancer, many of which will specifically enrich for BRCA1 and BRCA2 carriers, may be at particular risk for confounded results if stratification of these two groups is not considered.
Our analysis has several strengths. Unlike several other survival analyses of BRCA ovarian cancers, we restricted our analysis to only advanced stage (III/IV), high-grade serous tumors, the ovarian cancer subtype that accounts for the majority of the morbidity and mortality related to ovarian cancer.23 We also chose to limit or cohort to patients seen at our institution at the time of diagnosis because the likelihood of recurrence following initial therapy could be linked to BRCA status. The patients presented here were also treated almost exclusively during a 15-year period at a single institution with common surgical and medical standards over this period of time, minimizing the possibility that our analysis was confounded by subtle differences in surgical or chemotherapeutic management styles across institutions or time. Our analysis was also limited to patients tested for BRCA mutations within 2-years of initial diagnosis of ovarian cancer. This restriction, which caused us to remove 43 patients from analysis, eliminated patients who may have been BRCA tested due to unexpected longevity or persistent sensitivity to chemotherapy. Finally, the control (BRCA−) patients in our analysis were all confirmed non-carriers rather then untested matched controls as had been the case in some previously published reports.11, 17
This analysis does have several limitations. Our findings are based on a convenience sample of patients tested for BRCA mutations. During the time period included in this report, our institution treated approximately 1200 unique cases of newly diagnosed and recurrent Stage III/IV high-grade serous ovarian cancers, meaning only approximately 15% of potential cases were captured for our analysis. This relatively low capture rate reflects several factors including the lack of universal BRCA mutation testing and the use of strict inclusion and exclusion criteria designed to minimize bias. In addition, only patients tested for BRCA mutations on one of two IRB approved follow-up protocols were included due to unique human subjects issues and privacy concerns related to germline genetic testing. It is not possible to speculate how a higher rate of capture would have affected our results.
Differences in the BRCA1, BRCA2 and BRCA− cohorts are also worth addressing. The BRCA2 cohort had only 11.8% of Stage IV patients compared to 23.3% and 23.1% in the BRCA1 and BRCA− cohorts. Although neither difference was statistically significant, repeat analysis limited to Stage III patients did not change the conclusions presented here (data not shown). Patients with BRCA1 or BRCA2 mutations were also more likely to be optimally debulked and receive IP-based first-line chemotherapy. Because IP chemotherapy has traditionally been restricted to patients who have been optimally debulked, it is likely that these two findings are correlated.
Our analysis found a trend towards improved survival in BRCA1 patients compared to BRCA− patients, although this finding did not reach significance (HR .70; 95% CI, .36-1.38; P=.31). This finding is consistent with other published data. In the large multivariate analysis reported by Chetrit, BRCA1 status demonstrated a similar impact on survival (HR .82; 95% CI, .63-1.05; P=.10).14 It is unclear whether this result would reach statistical significance in an even larger cohort.
Finally, the data presented here do not provide insight into the reason for improved survival in BRCA2 compared to BRCA1 and BRCA− patients. The relative contributions of improved chemotherapy sensitivity versus underlying differences in tumor biology are unclear. A case-controlled study by Tan and colleagues found higher rates of platinum sensitivity across first, second and third-line therapy for BRCA1/2 mutation positive ovarian cancer patients compared to matched controls.18 However, their report included only 22 BRCA carriers (17 BRCA1, 5 BRCA2) and did not attempt to compare BRCA1 and BRCA2 outcomes. More recently our group examined predictors of survival in a subset of the patients included in the present report.15 In this prior work, both the presence of a BRCA mutation and platinum sensitivity were independent predictors of survival, suggesting that both tumor biology and chemotherapy sensitivity may contribute to prolonged survival. This prior study, however, was also not powered to look at differences between carriers of BRCA1 and BRCA2 mutations. Ultimately, the mechanisms through which BRCA1 and BRCA2 might result in different tumor biology are poorly understood and require further research.
Until recently, all ovarian cancers have been managed similarly regardless of germline BRCA status. Although BRCA1/2+ ovarian cancers have been suggested to have a more favorable prognosis compared to BRCA− tumors for more than a decade, there have been no targeted therapies available to exploit the underlying biological differences between these tumors. Since the development of PARP inhibitors, which may be specifically active in BRCA deficient tumors, it has become increasingly importantly to molecularly sub-classify ovarian cancers.24 BRCA deficient tumors have alterations in homologous recombination (HR), the DNA repair pathway responsible for high-fidelity resolution of double-stranded DNA breaks and cross-links.25 PARP inhibitors block base excision repair, a lower fidelity salvage DNA repair pathway necessary to maintain genomic stability in tumors deficient in HR and are an extremely promising class of agents for treating BRCA deficient ovarian cancer.26 However, the mechanism of BRCA inactivation, and associated loss of HR function, may be important. Hennessy et al reported on a cohort of 44 ovarian cancer specimens tested for BRCA1/2 mutations.27 Thirty-three (75%) of the mutations were germline and the remaining 11 (25%) were somatic. In this analysis, patients with loss of BRCA function through either mechanism had improved progression free survival compared to patients with intact BRCA function. They did not, however, report the outcomes of germline and somatically mutated tumors separately. Finally, this analysis did not examine specimens for epigenetic BRCA inactivation. Data generated by The Cancer Genome Atlas (TCGA) ovarian project demonstrates that while approximately 50% of high-grade serous ovarian tumors have alterations in the HR pathway by BRCA1/2 germline mutation, somatic mutation, epigenetic silencing, or other putative HR defects, tumors with epigenetically silenced BRCA1 had significantly worse outcomes than tumors with germline and somatic BRCA1/2 mutations.19 These results strongly suggest, as does our data, that the method and type of BRCA inactivation may have different prognostic implications.
Although our analysis found BRCA2 to be an important predictor of survival compared to both BRCA− and BRCA1 status, it seems appropriate to consider this report hypothesis generating rather then definitive given the relatively small number of patients and events (only three deaths in the BRCA2 cohort). These conclusions will certainly need to be validated in prospective datasets. Our findings, however, indicate that in the emerging era of targeted therapies for molecularly characterized subtypes of ovarian cancer, the grouping of BRCA1 and BRCA2, two related but distinct cancer susceptibility syndromes, may not be appropriate, and strong consideration should be given to stratifying future studies in ovarian cancer according to BRCA1 and BRCA2 status.
Acknowledgments
Funding: Project Hope for Ovarian Cancer Research and Education; Kaleidoscope of Hope Foundation; Genet Fund
Footnotes
Financial Disclosures: NK has received consulting fees and has been an expert witness for Pfizer. No other authors have financial disclosures to report.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 3.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–10. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94(18):1365–72. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 6.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. The Breast Cancer Linkage Consortium Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62(3):676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT, Seynaeve C, Tilanus-Linthorst MM, Wagner A, et al. Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet. 2000;355(9220):2015–20. doi: 10.1016/s0140-6736(00)02347-3. [DOI] [PubMed] [Google Scholar]
- 9.Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283(17):2260–5. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 10.Jazaeri AA, Yee CJ, Sotiriou C, Brantley KR, Boyd J, Liu ET. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst. 2002;94(13):990–1000. doi: 10.1093/jnci/94.13.990. [DOI] [PubMed] [Google Scholar]
- 11.Aida H, Takakuwa K, Nagata H, Tsuneki I, Takano M, Tsuji S, et al. Clinical features of ovarian cancer in Japanese women with germ-line mutations of BRCA1. Clin Cancer Res. 1998;4(1):235–40. [PubMed] [Google Scholar]
- 12.Ben David Y, Chetrit A, Hirsh-Yechezkel G, Friedman E, Beck BD, Beller U, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20(2):463–6. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- 13.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97(9):2187–95. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 14.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. 2008;26(1):20–5. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher DJ, Konner JA, Bell-McGuinn KM, Bhatia J, Sabbatini P, Aghajanian CA, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2010 doi: 10.1093/annonc/mdq577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal T, Permuth-Wey J, Kapoor R, Cantor A, Sutphen R. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer. 2007;6(1):113–9. doi: 10.1007/s10689-006-9112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996;335(19):1413–6. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 18.Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26(34):5530–6. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 19.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, Satagopan J, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20(5):1260–8. doi: 10.1200/JCO.2002.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 22.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. 4th ed. McGraw-Hill/Irwin; Boston; New York: 2004. [Google Scholar]
- 23.Koonings PP, Campbell K, Mishell DR, Jr., Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989;74(6):921–6. [PubMed] [Google Scholar]
- 24.Sandhu SK, Wenham RM, Wilding G, McFadden M, Sun L, Toniatti C, et al. First-in-human trial of a poly(ADP-ribose) polymerase (PARP) inhibitor MK-4827 in advanced cancer patients (pts) with antitumor activity in BRCA-deficient and sporadic ovarian cancers. J Clin Oncol. 2010;28(7s) abstr 3001. [Google Scholar]
- 25.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 26.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28(22):3570–6. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]