Abstract
Objectives
Plasma tryptophan levels are associated with delirium in critically ill patients. Although tryptophan has been linked to the pathogenesis of other neurocognitive diseases through metabolism to neurotoxins via the kynurenine pathway, a role for kynurenine pathway activity in intensive care unit brain dysfunction (delirium and coma) remains unknown. This study examined the association between kynurenine pathway activity as determined by plasma kynurenine concentrations and kynurenine/tryptophan ratios and presence or absence of acute brain dysfunction (defined as delirium/coma-free days) in intensive care unit patients.
Design, Setting, and Patients
This was a prospective cohort study that utilized patient data and blood samples from the Maximizing Efficacy of Targeted Sedation and Reducing Neurologic Dysfunction trial, which compared sedation with dexmedetomidine vs. lorazepam in mechanically ventilated patients.
Measurements and Main Results
Baseline plasma kynurenine and tryptophan concentrations were measured using high-performance liquid chromatography with or without tandem mass spectrometry. Delirium was assessed daily using the Confusion Assessment Method for the Intensive Care Unit. Linear regression examined associations between kynurenine pathway activity and delirium/coma-free days after adjusting for sedative exposure, age, and severity of illness. Among 84 patients studied, median age was 60 yrs and Acute Physiology and Chronic Health Evaluation II score was 28.5. Elevated plasma kynurenine and kynurenine/tryptophan ratio were both independently associated with significantly fewer delirium/coma-free days (i.e., fewer days without acute brain dysfunction). Specifically, patients with plasma kynurenine or kynurenine/tryptophan ratios at the 75th percentile of our population had an average of 1.8 (95% confidence interval 0.6–3.1) and 2.1 (95% confidence interval 1.0–3.2) fewer delirium/coma-free days than those patients with values at the 25th percentile (p = .006 and p < .001, respectively).
Conclusions
Increased kynurenine pathway activation, assessed by plasma kynurenine and kynurenine/tryptophan ratio, was associated with fewer days alive and without acute brain dysfunction in intensive care unit patients. Future studies are warranted to clarify this relationship and investigate potential therapeutic interventions.
Keywords: brain dysfunction, delirium, kynurenine, mechanically ventilated
Delirium in intensive care unit (ICU) patients is an independent predictor of increased costs (1), length of hospital stay (2–4), and poor outcomes, such as long-term cognitive impairment (5, 6) and death (7–11). Despite its high prevalence among mechanically ventilated patients (12–14), the pathophysiology of delirium remains hypothetical and unproven (15, 16). Abnormalities in neurotransmission (17–19), inflammation (20–22), and cerebral blood flow (23, 24) are leading hypotheses implicated as directly or indirectly contributing to acute brain dysfunction, which clinically manifests as delirium and coma. Furthermore, markers of central nervous system damage such as S100B-protein have been associated with acute brain dysfunction (23, 25, 26). We have previously shown that the ratio of tryptophan to other large neutral amino acids was significantly associated with delirium (27), implicating tryptophan—a precursor for the neurotransmitter serotonin—in the pathogenesis of delirium. It was unclear, however, whether perturbations in tryptophan levels were associated with delirium because of alterations in serotonin concentrations in the brain or because of the production of downstream metabolites of tryptophan that are potentially neurotoxic.
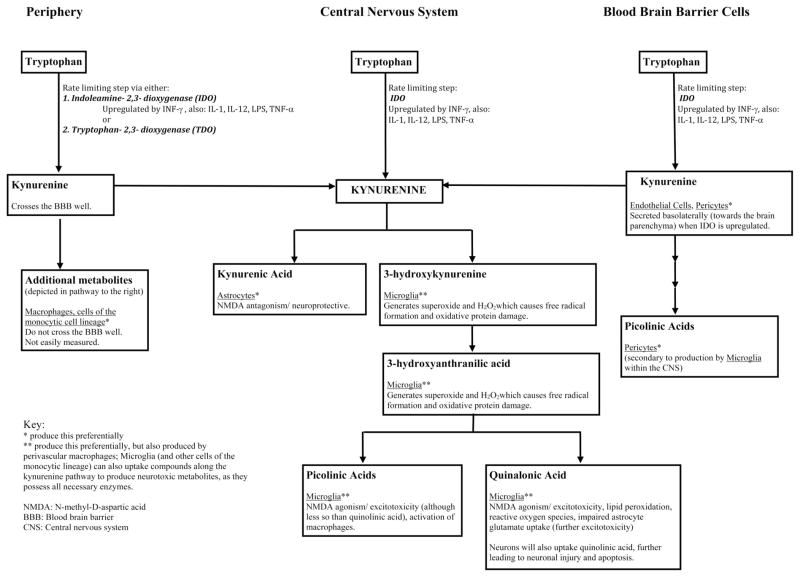
Although previous hypotheses implicating tryptophan in the pathogenesis of delirium have focused on the serotonin/methoxyindole pathway of tryptophan metabolism, which may lead to disturbances in serotonin and melatonin neurotransmission (27–29), it has been estimated that close to 90%–95% of tryptophan metabolism occurs via the kynurenine pathway (30, 31) (Fig. 1). This less commonly recognized pathway of tryptophan metabolism has been implicated in the pathogenesis of numerous central nervous system disorders, including Alzheimer disease (32–34), Huntington’s disease (35), amyotrophic lateral sclerosis (36), human immunodeficiency virus-induced cerebral dysfunction (34, 37), depression (30), psychosis (38), seizure disorders (39), and traumatic brain injury (40). These neurologic injuries are hypothesized to be secondary to metabolites of the kynurenine pathway such as quinolinic acid, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, and picolinic acids (41, 42) (Fig. 1), which can lead to neuronal and microglial cell injury, excitotoxicity, and apoptosis (32, 43–45). There is evidence now that microglia and astrocyte activation can itself promote neuronal and glial apoptosis and that this may play an important role in delirium and subsequent cognitive impairment (46, 47). Thus metabolites of the kynurenine pathway may be associated with delirium via both microglial activation and subsequent neuronal injury.
Figure 1.
Tryptophan metabolism by the kynurenine pathway. The first and rate-limiting step in the kynurenine pathway is the formation of kynurenine from tryptophan via indoleamine-2,3-dioxygenase (IDO), found ubiquitously, or tryptophan-2,3-dioxygenase (TDO). Kynurenine concentrations within the central nervous system (CNS) are related to peripheral production (42) and transportation into the brain via the large amino acid transporter in the blood brain barrier (50), basolateral secretion of kynurenine by blood brain barrier (BBB) cells (endothelial cells, pericytes) (45), or from local CNS synthesis of kynurenine pathway metabolites (i.e. astrocytes, microglia, perivascular macrophages) (45, 51). These mechanisms are upregulated by cytokines and inflammatory/immune signals, including interferon gamma (INF-γ) (48, 49), interleukins 1 and 12 (IL-1, IL-12) (63, 64), lipopolysaccharide (LPS), and tumor necrosis factor alpha (TNF-α) (65, 66), which are often increased in patients with critical illness. Increased central kynurenine concentrations lead to production of beneficial (kynurenic acid) and neurotoxic kynurenine pathway metabolites. At times of stress and with inflammation, the pathway preferentially produces neurotoxic metabolites including quinolinic acid, 3-hydroxy-kynurenine, anthranilic acid, and picolinic acids (41, 42, 45, 51). An imbalance favoring the production of neurotoxic metabolites leads to neuronal and glial cell injury, excitotoxicity, and apoptosis (30, 32, 37, 43–47), which may be clinically manifested as delirium or coma.
The first and rate-limiting step in the kynurenine pathway is the formation of kynurenine from tryptophan via ubiquitously expressed indoleamine-2,3-dioxygenase or hepatic tryptophan-2,3-dioxygenase (Fig. 1). Because indoleamine-2,3-dioxygenase and tryptophan-2,3-dioxygenase are rate-limiting steps of the kynurenine pathway, the plasma kynurenine concentration and the kynurenine/tryptophan ratio are both excellent surrogate measures of kynurenine pathway activity (48, 49). Kynurenine concentration within the central nervous system is controlled by three main mechanisms: kynurenine movement from the peripheral circulation (42) across the blood brain barrier through the large neutral amino acids transporter (50); basolateral secretion of kynurenine from blood–brain barrier cells such as endothelial cells and pericytes (45); and kynurenine synthesis from tryptophan within the central nervous system by astrocytes and microglia (45, 51). Each of these mechanisms are upregulated in inflammatory states or in response to immune system activation, resulting in increased kynurenine in the central nervous system (45, 51).
Increased kynurenine in the brain can lead to the production of both neuroprotective and neurotoxic metabolites. An imbalance in the production of beneficial and neurotoxic kynurenine metabolites might explain the development of acute brain dysfunction in ICU patients. Kynurenic acid, for example, is a metabolite with neuroprotective properties (30, 44) that is produced by astrocytes in response to increased kynurenine levels (Fig. 1). Quinolinic acid, alternatively, is a neurotoxic metabolites of tryptophan and kynurenine produced by microglia (41, 42), which also produce 3-hy-droxykynurenine, 3-hydroxyanthranilic acid (51) and picolinic acid. In conditions of stress and with inflammation, the kynurenine pathway preferentially produces quinolinic acid and the downstream neurotoxic metabolites (45, 51). As mentioned, peripherally produced kynurenine contributes significantly to the central kynurenine concentration and production of metabolites, and thus serves as an excellent and easily measured surrogate marker of kynurenine pathway activity in the brain (51, 52). However, metabolites of kynurenine, such as kynurenic acid and quinolinic acid, cannot be easily measured in the periphery, their central nervous system level measurements are not practical, and they do not readily cross the blood barrier (42).
We hypothesized that elevations of both plasma kynurenine and kynurenine/tryptophan ratio would be associated with neurologic injury, and thus fewer days free from acute brain dysfunction in critically ill patients.
MATERIALS AND METHODS
Our cohort comprised patients from the Maximizing Efficacy of Targeted Sedation and Reducing Neurologic Dysfunction (MENDS) double-blind, randomized, controlled trial (53). Briefly, the MENDS trial included 103 adult mechanically ventilated medical and surgical ICU patients from two tertiary care centers enrolled between August 2004 and April 2006. Exclusion criteria included neurologic disease that would confound the diagnosis of delirium (e.g., previous large stroke, severe dementia, cerebral palsy), active seizure disorders, Child-Pugh class B or C cirrhosis, moribund state with planned withdrawal of life support, family or physician refusal, alcohol abuse, active myocardial ischemia, second-degree or third-degree heart block, pregnancy or lactation, severe hearing disabilities, and inability to understand English (53). The Institutional Review Boards at Vanderbilt University approved the study.
Demographic data and severity of illness, measured with the Acute Physiology and Chronic Health Evaluation II score (54) and the Sequential Organ Failure Assessment score (55), were computed from data recorded in the computerized medical record. Mental status was assessed daily from enrollment until hospital discharge or for up to 12 days using the Confusion Assessment Method for the ICU (12, 13). The level of sedation was measured according to the Richmond Agitation-Sedation scale (56, 57). Utilizing these criteria, delirium and coma were defined as follows: delirium, Richmond Agitation-Sedation scale score of −3 to + 4 with a positive Confusion Assessment Method ICU score; and coma, Richmond Agitation-Sedation scale score of −5 or −4 (unresponsive or responsive to physical stimuli alone, respectively).
As a measure of the burden of acute brain dysfunction, we chose delirium/coma-free days (DCFDs) instead of duration of delirium or delirium-free days. In this study, DCFDs represented the number of days out of the 12-day period after enrollment during which patients were alive without delirium or coma (53). This event-free outcome takes into account the contribution of delirium, coma, and death, thus providing an accurate measure of the duration of normal cognitive status. Alternatively, delirium days do not account for death, such that a patient who dies early in the course of the assessment period could have only a few delirium days despite having worse outcomes. Similarly, the delirium-free day outcome could be erroneously increased because of longer periods of coma, because a patient who is comatose cannot have delirium diagnosed during their coma, a period which thus would be considered delirium-free.
Baseline blood samples were collected from patients within 48 hrs of enrollment, and plasma was separated and stored at −80°C. Plasma tryptophan concentrations were measured using high-performance liquid chromatography (58), and plasma kynurenine was measured with high-performance liquid chromatography plus tandem mass spectrometry using a variation of a previously described method (59). The kynurenine and kynurenine/tryptophan ratio were both used as indicators of kynurenine pathway activity, because tryptophan metabolism to kynurenine is the rate-limiting step in the kynurenine pathway (60). Specifically, the kynurenine/tryptophan ratio reflects tryptophan metabolism to kynurenine.
Statistical Analysis
Baseline demographic data are presented using medians and interquartile ranges for continuous variables and proportions for categorical variables. We used linear regression to study the associations between plasma kynurenine concentrations and kynurenine/tryptophan ratios (the two primary exposure variables that were assessed in independent regression models) and DCFDs (the outcome in each model), after adjusting for sedative group (i.e., dexmedetomidine or lorazepam), age, and severity of illness as indicated by the acute physiology component of Acute Physiology and Chronic Health Evaluation II. Both kynurenine concentrations and kynurenine/tryptophan ratios were transformed using a natural logarithm to improve model fit. Nonlinearity of the associations examined were evaluated using restricted cubic splines in the regression analyses. All analyses were performed using R version 2.10 (R Development Core Team 2009, Vienna, Austria; http://www.R-project.org).
RESULTS
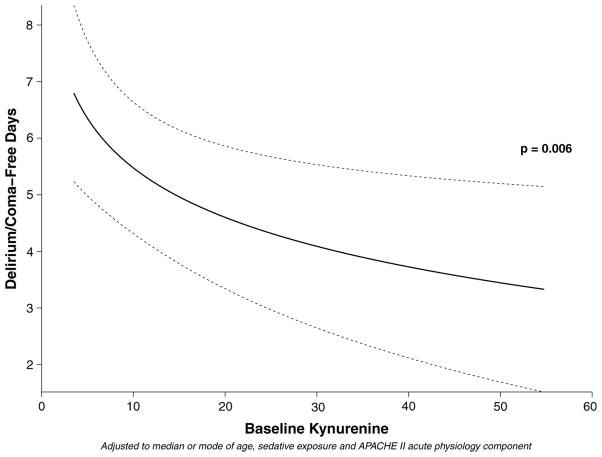
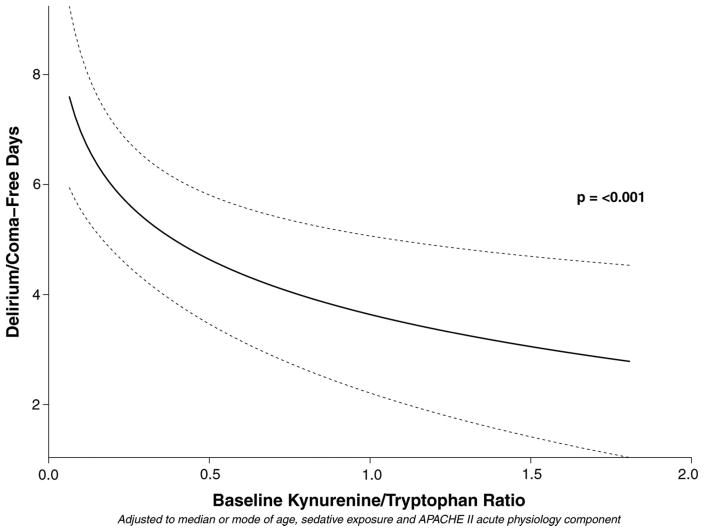
Eighty-four of the 103 MENDS participants had plasma available for analysis. Demographic data for the cohort are shown in Table 1. After adjusting for age, sedation regimen, and severity of illness, increasing plasma kynurenine concentrations at baseline were independently associated with fewer DCFDs, i.e., worse neurologic outcome (Table 2 and Fig. 2). Other covariates being equal, patients with a kynurenine concentration at the 75th percentile kynurenine in this population had an average of 1.8 (95% confidence interval, 0.6–3.1; p = .006) fewer days without acute brain dysfunction than did patients with a kynurenine concentration at the 25th percentile (Table 2 and Fig. 2). Similarly, after adjusting for confounders, an increased kynurenine/tryptophan ratio was independently associated with fewer days without acute brain dysfunction (Table 3 and Fig. 3), such that patients with kynurenine/tryptophan ratios at the 75th percentile had 2.1 fewer DCFDs (95% CI, 1.0–3.2; p < .001) than did patients with kynurenine/tryptophan ratios at the 25th percentile. In keeping with results from our previous studies (61, 62), sedation with dexmedetomidine was associated with a greater number of DCFDs than sedation with lorazepam in both the kynurenine and the kynurenine/tryptophan ratio models (p = .007 for both; Tables 2 and 3, respectively).
Table 1.
Demographic and baseline characteristicsa
| Variable | n = 84 |
|---|---|
| Age, yrs | 60 (46–66) |
| Male, No. (%) | 42 (50%) |
| White, No. (%) | 73 (87%) |
| Sepsis on admission, No. (%) | 36 (43%) |
| Acute Physiology and Chronic Health Evaluation II | 28.5 (24–32) |
| Sequential Organ Failure Assessment Score | 9.0 (8–12) |
| Mental status at enrollment | |
| Comatose | 45 (54%) |
| Delirious | 26 (31%) |
| Normal | 13 (15%) |
| Intensive care unit length of stay (days) | 9.3 (5.3–17.4) |
| Hospital length of stay (days) | 16 (8–23) |
| Delirium at least once | 72 (86%) |
| Delirium, days | 3.0 (1.0–6.0) |
| Coma, days | 3.0 (1.0–5.0) |
| Delirium-free, days | 7.3 (4.5–10.0) |
| Delirium/coma-free, days | 4.0 (0.56–8.0) |
| Died within 28 days of enrollment | 23 (27%) |
Median (interquartile range) unless otherwise noted.
Table 2.
Associations between kynurenine plasma level and delirium/coma-free days
| Variable | 25th Percentile | 75th Percentile | Difference in Mean Delirium/Coma-Free Day (95% Confidence Interval) | p |
|---|---|---|---|---|
| Age at enrollment | 47.8 | 66.0 | −0.8 (−1.8 to 0.1) | .10 |
| Acute Physiology and Chronic Health Evaluation II acute physiology score component | 11.0 | 20.0 | −0.7 (−1.9 to 0.5) | .23 |
| Dexmedetomidine vs. lorazepama | Lorazepam | Dexmedetomidine | 2.2 (0.7–3.8) | .007 |
| Baseline kynurenine plasma level (μM) | 5.0 | 21.4 | − 1.8 (− 3.1 to − 0.6) | .006 |
Linear regression was used to study the role of kynurenine in delirium/coma-free days after adjusting for age, severity of illness, and study drug. The difference in means represents the difference in delirium/coma-free days between patients at the 75th percentile of kynurenine levels of our population vs. the 25th percentile. Thus, a patient at the 75th percentile for kynurenine levels had 1.8 fewer days without acute brain dysfunction than a patient at the 25th percentile. This comparison between percentiles is more clinically relevant than the traditionally used one-unit change.
Patients in this cohort received sedation with either dexmedetomidine or lorazepam. Patients using dexmedetomidine (as compared to lorazepam) had 2.2 more days alive and free from delirium and coma.
Figure 2.
Increasing kynurenine levels (μM, micromolar units) are associated with fewer delirium/coma-free days. Interpretative example: Other covariates being equal, patients with a kynurenine concentration at the 75th percentile (kynurenine 21.4 μM in this population) had an average of 1.8 fewer delirium/coma-free days than patients with kynurenine levels at the 25th percentile (5 μM).
Table 3.
Associations between kynurenine-to-tryptophan ratios and delirium-to-coma-free days
| Variable | 25th Percentile | 75th Percentile | Difference in Mean Delirium/Coma-Free Days (95% Confidence Interval) | p |
|---|---|---|---|---|
| Age at enrollment | 47.8 | 66.0 | −0.6 (−1.6 to 0.3) | .19 |
| Acute Physiology and Chronic Health Evaluation II acute physiology score component | 11.0 | 20.0 | −0.4 (−1.6 to 0.8) | .48 |
| Dexmedetomidine vs. lorazepama | Lorazepam | Dexmedetomidine | 2.1 (0.6 to 3.6) | .007 |
| Baseline kynurenine/tryptophan ratio | 0.1 | 0.6 | − 2.1 (− 3.2 to − 1.0) | −.001 |
Linear regression was used to study the role of kynurenine/tryptophan in delirium/coma-free days after adjusting for age, severity of illness, and study drug. The difference in means represents the difference in delirium/coma-free days between patients at the 75th percentile of kynurenine-to-tryptophan ratios of our population vs. the 25th percentile. Thus, a patient at the 75th percentile for kynurenine/tryptophan levels had 2.1 fewer days without acute brain dysfunction than a patient at the 25th percentile. This comparison between percentiles is more clinically relevant than the traditionally used one-unit change.
Patients in this cohort received sedation with either dexmedetomidine or lorazepam. Patients using dexmedetomidine (as compared to lorazepam) had 2.1 more days alive and free from delirium and coma.
Figure 3.
Increasing kynurenine/tryptophan ratios are associated with fewer delirium/coma-free days. Interpretative example: Other covariates being equal, patients with a kynurenine/tryptophan ratio at the 75th percentile (0.6 in this population) had an average of 2.1 fewer delirium/coma-free days than patients with a kynurenine/tryptophan ratio at the 25th percentile (0.1).
DISCUSSION
This is the first published study to quantify and analyze kynurenine pathway activation and its association with brain dysfunction (delirium and coma) in mechanically ventilated ICU patients. Increasing baseline plasma kynurenine concentrations and kynurenine/tryptophan ratios were found to be independently associated with fewer days alive and free from delirium and coma (DCFDs).
There are biologically plausible mechanisms to explain the association of higher kynurenine and kynurenine/tryptophan ratios with brain dysfunction during the acute phase of a critical illness. First, although the kynurenine compound itself is not attributed with neurotoxicity, there are >20 different metabolites known to have neurotoxic effects (44). Of these, quinolinic acid is the most notable and has been shown to lead to neurotoxicity via multiple mechanisms that include direct neuronal excitotoxicity (via its activity as a N-methyl-D-aspartic acid receptor agonist), induction of lipid peroxidation, production of reactive oxygen species, and prevention of glutamate uptake by astrocytes (44). Although neurons do not express enzymes of the kynurenine pathway, they internalize quinolinic acid through a “scavenging” mechanism when the extracellular concentration of quinolinic acid is high, ultimately leading to neuronal injury and apoptosis (32). Other important kynurenine metabolites with known neurotoxic and/or neuroexcitatory properties are shown in Figure 1. Second, the kynurenine pathway and, therefore, the potential production of neurotoxic metabolites are upregulated by cytokines and inflammatory/immune signals, including interferon-γ (48, 49), interleukins 1 and 12 (63, 64), lipopolysaccharide, and tumor necrosis factor-α (65, 66), which are often increased in patients with critical illness. Finally, a compelling mechanism explaining the development of delirium and coma may involve an imbalance between concentrations of kynurenine neuroprotective and neurotoxic metabolites. Kynurenic acid, produced by astrocytes, has neuroprotective properties because of its activity as an N-methyl-D-aspartic acid antagonist that leads to decreased glutamate output (and thus decreased excitotoxicity). Microglia and macrophages, however, may produce even greater amounts of neurotoxic metabolites such as quinolinic acid (44), which could counteract the neuroprotective effects of kynurenic acid and therefore could be responsible for acute brain dysfunction in the form of delirium or coma. Despite these plausible mechanisms, however, the precise molecular mechanism among critically ill patients remains unclear without detailed in vivo data.
There are many potential implications of our findings of fewer days without acute brain dysfunction associated with increased kynurenine concentrations and kynurenine/tryptophan ratios. Recent data have shown that each additional day of ICU delirium is associated with a 10% increased risk of death at both 6 months and 1 yr after discharge; the cumulative effect of multiple days spent in delirium on mortality risk, therefore, is multiplicative rather than additive (9–11). If the results of this report are corroborated, then measurement of kynurenine pathway activity could allow for early identification of patients at risk for development of prolonged periods of delirium and/or coma. Additionally, genetic polymorphisms associated with increased kynurenine pathway activity could be identified that may provide guidance in identifying ICU patients at risk for worse neurologic outcomes. For example, a polymorphism of the interferon-γ gene associated with elevated kynurenine levels has been shown to decrease longevity (30). Although the associations shown in this study do not prove causation, the known roles of neurotoxic kynurenine metabolites in the pathogenesis of numerous forms of cognitive dysfunction (24, 26–34) lend credence to the hypothesis that increased kynurenine pathway activity plays a role in the pathogenesis of delirium and/or coma during critical illness. Modulation of kynurenine pathway activity has been proposed as a way of treating a variety of diseases (43, 67), and it is possible that inhibition of kynurenine pathway activity could be used to prevent the development or mitigate the duration of delirium and/or coma. Work using animal models of cognitive dysfunction is needed to understand better the role of kynurenine pathway activity in the pathogenesis of delirium before interventional trials can be designed and executed.
Strengths of our study include the prospective collection of baseline data and delirium outcomes by experienced research personnel using a validated delirium monitoring instrument and the measurement of kynurenine concentration and kynurenine/tryptophan ratios using state-of-the-art high-performance liquid chromatography and tandem mass spectroscopy instrumentation in a laboratory that specializes in kynurenine assays (Proteomics and Mass Spectrometry Laboratory at Vanderbilt). Limitations included our inability to measure patients’ dietary tryptophan intake, which may have been helpful because tryptophan administration is known to increase kynurenine pathway activity (68). The measurement of tryptophan intake, however, is difficult in a clinical setting because of variability of dietary need, uncertainty regarding individual absorption of tryptophan, and release of tryptophan from endogenous stores via protein breakdown and/or decreased albumin binding. Whereas it is assumed that kynurenine pathway compounds within the central nervous system are responsible for producing neurobehavioral effects, we were unable to directly measure central nervous system levels given that lumbar punctures or other invasive procedures to obtain central nervous system samples are risky in critically ill patients. Plasma kynurenine levels, however, serve as an excellent surrogate marker until future methods become available, and direct central nervous system measurements may prove to be unnecessary (37, 69). In this study, we were only able to measure kynurenine pathway activity at baseline and, hence, could not study the acute effects of changes in kynurenine pathway activity over time during critical illness. A study among acute traumatic brain injury patients, however, noted that the average peak of quinolinic acid in cerebrospinal fluid after injury was 72–83 hrs, suggesting that elevations in downstream metabolites and potential neurotoxic effects may also correlate with the timing of activation and migration of microglia and macrophages in vivo (40). Furthermore, we did not measure kynurenine activity after hospital discharge, which would have allowed study of the role of the kynurenine pathway in long-term cognitive impairment. This may be important given data from traumatic brain injury patients who continue to have elevated kynurenine/tryptophan ratios many years after the initial injury (70). Finally, we were not able to separate our analysis by more specific categories of cognition (i.e., disorganized thinking vs. delirium vs. coma) because of the patient sample size. This would significantly limit the power of our study after adjusting for covariates in the analysis. In the future, with larger sample sizes, this would help to determine whether variations of the kynurenine pathway exist among these groups.
In conclusion, this prospective cohort study of mechanically ventilated ICU patients showed that increased baseline plasma levels of kynurenine or increased kynurenine pathway activity were independent predictors of greater duration of acute brain dysfunction. These findings suggest a role for tryptophan metabolites via the kynurenine pathway in the pathogenesis of delirium. Future studies are warranted to characterize further the association between kynurenine pathway activity and neurologic outcomes in critically ill patients.
Acknowledgments
Dr. Girard is supported by the National Institutes of Health (AG034257). Dr. Ely is supported by the VA Clinical Science Research and Development Service (VA Merit Review Award) and the National Institutes of Health (AG027472). Drs. Girard and Ely are both supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). Dr. Boomershine is supported by the National Institutes of Health (U01GM092691, K08DK080219-02S1, and K08DK080219) and Pfizer (GA9002FP). The tryptophan metabolites analysis was supported by the Vanderbilt CTSA grant UL1 RR024975 from NCRR/NIH. Dr. Boomershine has received honoraria from Amgen, Astra-Zeneca, Pfizer, Eli Lilly, Forest Pharmaceuticals, Takeda Pharmaceuticals North America, and URL Pharma, and a research grant from Pfizer Inc. Dr. Pandharipande was supported by the VA Clinical Science Research and Development Service (VA Career Development Award). The ASCCA-FAER-Abbot Physician Scientist Award funded the Maximizing Efficacy of Targeted Sedation and Reducing Neurologic Dysfunction study. Dr. Girard has received honoraria from Hospira. Dr. Ely has received a research grant and honoraria from Hospira, Pfizer, Eli Lilly, GSK, and a research grant from Aspect Medical Systems. Dr. Pandharipande has received a research grant from Hospira and honoraria from Hospira, GSK, and Orion Pharma.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
References
- 1.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 2.Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: A prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson JC, Gordon SM, Hart RP, et al. The association between delirium and cognitive decline: A review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 6.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51:591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 8.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 10.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care unit patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: Evaluation of a new screening tool. Intensive Care Med. 2001;27:859– 864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 15.Trzepacz PT. The neuropathogenesis of delirium. A need to focus our research. Psychosomatics. 1994;35:374–391. doi: 10.1016/S0033-3182(94)71759-X. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado JR. Pathoetiological model of delirium: A comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24:789–856. ix. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol. 1998;11:138–145. doi: 10.1177/089198879801100304. [DOI] [PubMed] [Google Scholar]
- 18.Inouye SK, Ferrucci L. Elucidating the pathophysiology of delirium and the interrelationship of delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1277–1280. doi: 10.1093/gerona/61.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin. 2008;24:45–65. viii. doi: 10.1016/j.ccc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald A, Adamis D, Treloar A, et al. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing. 2007;36:222–225. doi: 10.1093/ageing/afl121. [DOI] [PubMed] [Google Scholar]
- 21.Kudoh A, Takase H, Katagai H, et al. Postoperative interleukin-6 and cortisol concentrations in elderly patients with postoperative confusion. Neuroimmunomodulation. 2005;12:60– 66. doi: 10.1159/000082365. [DOI] [PubMed] [Google Scholar]
- 22.Pfister D, Siegemund M, ll-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong TG, Bogardus ST, Jr, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci. 2006;61:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 24.van Munster BC, Bisschop PH, Zwinderman AH, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010;74:18–23. doi: 10.1016/j.bandc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 25.van Munster BC, Korevaar JC, Korse CM, et al. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. 2010;25:234–239. doi: 10.1002/gps.2326. [DOI] [PubMed] [Google Scholar]
- 26.Pandharipande PP, Morandi A, Adams JR, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009;35:1886–1892. doi: 10.1007/s00134-009-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Mast RC, Fekkes D. Serotonin and amino acids: partners in delirium pathophysiology? Semin Clin Neuropsychiatry. 2000;5:125–131. doi: 10.153/SCNP00500125. [DOI] [PubMed] [Google Scholar]
- 28.Lewis MC, Barnett SR. Postoperative delirium: the tryptophan dyregulation model. Med Hypotheses. 2004;63:402–406. doi: 10.1016/j.mehy.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Uchida K, Aoki T, Ishizuka B. Postoperative delirium and plasma melatonin. Med Hypotheses. 1999;53:103–106. doi: 10.1054/mehy.1998.0724. [DOI] [PubMed] [Google Scholar]
- 30.Oxenkrug GF. Genetic and hormonal regulation of tryptophan kynurenine metabolism: Implications for vascular cognitive impairment, major depressive disorder, and aging. Ann N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- 31.Allegri G, Bertazzo A, Biasiolo M, et al. Kynurenine pathway enzymes in different species of animals. Adv Exp Med Biol. 2003;527:455–463. doi: 10.1007/978-1-4615-0135-0_53. [DOI] [PubMed] [Google Scholar]
- 32.Rahman A, Ting K, Cullen KM, et al. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009;4:e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 34.Guillemin GJ, Brew BJ, Noonan CE, et al. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 35.Beal MF, Matson WR, Storey E, et al. Kynurenic acid concentrations are reduced in Huntington’s disease cerebral cortex. J Neurol Sci. 1992;108:80– 87. doi: 10.1016/0022-510x(92)90191-m. [DOI] [PubMed] [Google Scholar]
- 36.Guillemin GJ, Meininger V, Brew BJ. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:166–176. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 37.Heyes MP, Brew BJ, Martin A, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 38.Barry S, Clarke G, Scully P, et al. Kynurenine pathway in psychosis: Evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–294. doi: 10.1177/0269881108089583. [DOI] [PubMed] [Google Scholar]
- 39.Heyes MP, Wyler AR, Devinsky O, et al. Quinolinic acid concentrations in brain and cerebrospinal fluid of patients with intractable complex partial seizures. Epilepsia. 1990;31:172–177. doi: 10.1111/j.1528-1167.1990.tb06302.x. [DOI] [PubMed] [Google Scholar]
- 40.Sinz EH, Kochanek PM, Heyes MP, et al. Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J Cerebral Blood Flow Metab. 1998;18:610– 615. doi: 10.1097/00004647-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Heyes MP, Saito K, Crowley JS, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 42.Kita T, Morrison PF, Heyes MP, et al. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem. 2002;82:258–268. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 43.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 44.Sas K, Robotka H, Toldi J, et al. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Owe-Young R, Webster NL, Mukhtar M, et al. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J Neurochem. 2008;105:1346–1357. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- 46.Cerejeira J, Firmino H, Vaz-Serra A, et al. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 47.Sharshar T, Polito A, Checinski A, et al. Septic-associated encephalopathy–Everything starts at a microlevel. Crit Care. 2010;14:199. doi: 10.1186/cc9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Guillemin GJ. Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. Int J Tryptophan Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrocksnadel K, Wirleitner B, Winkler C, et al. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- 51.Fukui S, Schwarcz R, Rapoport SI, et al. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 52.Gal EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- 53.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 54.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818– 829. [PubMed] [Google Scholar]
- 55.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 57.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 58.Fekkes D, van Dalen A, Edelman M, et al. Validation of the determination of amino acids in plasma by high-performance liquid chromatography using automated pre-column derivatization with o-phthaldialdehyde. J Chromatogr B Biomed Appl. 1995;669:177–186. doi: 10.1016/0378-4347(95)00111-u. [DOI] [PubMed] [Google Scholar]
- 59.Wade Calcutt M, Lee W, Puzanov I, et al. Determination of chemically reduced pyrrolobenzodiazepine SJG-136 in human plasma by HPLC-MS/MS: Application to an anticancer phase I dose escalation study. J Mass Spectrom. 2008;43:42–52. doi: 10.1002/jms.1268. [DOI] [PubMed] [Google Scholar]
- 60.Matthews HR, Matthews KS, Opella SJ. Selectively deuterated amino acid analogues. Synthesis, incorporation into proteins and NMR properties. Biochim Biophys Acta. 1977;497:1–13. doi: 10.1016/0304-4165(77)90134-9. [DOI] [PubMed] [Google Scholar]
- 61.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34– 41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liebau C, Baltzer AW, Schmidt S, et al. Interleukin-12 and interleukin-18 induce in-doleamine 2,3-dioxygenase (IDO) activity in human osteosarcoma cell lines independently from interferon-gamma. Anticancer Res. 2002;22:931–936. [PubMed] [Google Scholar]
- 64.Hu S, Sheng WS, Peterson PK, et al. Cytokine modulation of murine microglial cell superoxide production. Glia. 1995;13:45–50. doi: 10.1002/glia.440130106. [DOI] [PubMed] [Google Scholar]
- 65.Smith DG, Guillemin GJ, Pemberton L, et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol. 2001;7:56– 60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- 66.Fujigaki S, Saito K, Sekikawa K, et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol. 2001;31:2313–2318. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 67.Costantino G. New promises for manipulation of kynurenine pathway in cancer and neurological diseases. Expert Opin Ther Targets. 2009;13:247–258. doi: 10.1517/14728220802665734. [DOI] [PubMed] [Google Scholar]
- 68.Forrest CM, Mackay GM, Stoy N, et al. Tryptophan loading induces oxidative stress. Free Radic Res. 2004;38:1167–1171. doi: 10.1080/10715760400011437. [DOI] [PubMed] [Google Scholar]
- 69.Heyes MP, Lackner A. Increased cerebrospinal fluid quinolinic acid, kynurenic acid, and L-kynurenine in acute septicemia. J Neurochem. 1990;55:338–341. doi: 10.1111/j.1471-4159.1990.tb08857.x. [DOI] [PubMed] [Google Scholar]
- 70.Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2006;13:30– 42. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]