Abstract
Background
Hydroxyurea (HU) is highly effective treatment for Sickle Cell Disease (SCD). While pediatric use of HU is accepted clinical practice, barriers to use may impede its potential benefit.
Procedure
A survey of parents of children ages 5–17 years with SCD was performed across five institutions to assess factors associated with HU use.
Results
Of the 173 parent responses, 65 (38%) had children currently taking HU. Among parents of children not taking HU, the most commonly cited reasons were that their hematology provider had not offered it, their child was not sufficiently symptomatic and concerns about potential side effects. Even parents of HU users reported widespread concern about effectiveness, long-term safety and off-label use. In bivariate analyses, children’s ages, parental demographics such as education level, or travel time to their hematology provider were not correlated with HU use. Bivariate analysis and multivariate logistic regression revealed three significant factors associated with current HU use: better parental knowledge about its major therapeutic effects (p<0.001), sickle genotype (p=0.005) and institution of clinical care (p=0.04).
Conclusions
Pervasive concerns about HU safety exist, even among parents of current users. Varying knowledge among parents appears to be independent of their demographics, and is associated with HU use. Inter-institutional variability in parental knowledge and drug uptake highlights potentially potent site-specific influences on likelihood of HU use. Overall, these survey data underscore the need for strategies to bolster parental understanding about benefits of HU and address concerns about its safety.
Keywords: Hydroxyurea, sickle cell disease, knowledge, and barriers, outcomes research
Introduction
Sickle cell disease (SCD) is an inherited blood disease characterized by anemia, painful crises and organ damage beginning during childhood. [1] Hydroxyurea (HU) is currently the sole approved drug for SCD therapy. [2–3] It dramatically improves quality of life and decrease morbidity and health care utilization for children, and decreases mortality in adult users.[3–7] Its use for children is an accepted clinical practice despite lack of approval for pediatric use by the US Food and Drug Administration (FDA).[8–11] Efficacy of HU is firmly established among patients with homozygous sickle hemoglobin (HbSS) and HbS-beta zero thalassemia with recurrent serious episodes of pain or acute chest syndrome. More recently, HU use has expanded to additional clinical indications, to use in patients with other sickle cell genotypes such as HbSC and young children. [5,7,12] [13]
Despite HU’s clinical potential, many children with SCD do not use HU therapy, even if recommended by their SCD provider.[6,14] Need for research on specific barriers to HU use has been highlighted.[15] Insights from prior research in other health conditions suggest barriers to medication use among chronically ill under-served populations generally involve provider-patient relationship dynamics [16–18]. Additional barriers to medication use include incomplete knowledge of drug benefit and logistical factors that impede access to care [19–21]. As a chemotherapeutic agent, barriers to off-label HU therapy likely also include concerns about the safety of short- and long-term use [21]. While providers have been surveyed about their perceived barriers to HU therapy [15,22–25], no studies on barriers to HU among parents of children with SCD have been reported. Prescribing patterns for HU vary among individual providers and thus is anticipated to differ across institutions.[25] Prior studies have shown institutional variation in treatment of acute complications for sickle cell disease.[26–27]
This cross-sectional multi-site study of parents of children with SCD sought to evaluate parental perceptions about HU use in children receiving pediatric hematology clinical care. We hypothesized that several barriers limit uptake of HU for pediatric SCD, including child and parental demographics, awareness and knowledge about its use, impact on health, magnitude of concern for drug toxicity, relevance of enhancing fetal hemoglobin (HbF) levels and site of out-patient pediatric hematology care. An anonymous survey was administered to assess associations between HU use and these multiple potential variables.
Methods
This study was approved by and performed under policies of the Institutional Review Board of each of the five participating institutions. The study population consisted primarily of parents of children with SCD aged 5–17 years, with a small number of other primary caretakers such as grandparents or other adult family members (collectively referred to as “parents”) who were surveyed by a cross-sectional, written, self-administered anonymous questionnaire from May to August 2010. Subjects were recruited from five collaborating pediatric hematology centers as members of the NYCON Clinical and Translational Science Awards consortium: Albert Einstein College of Medicine, Columbia University, Yale University, Cornell University and University of Rochester. Parents of patients with an established diagnosis of any sickle hemoglobinopathy (Hb SS, SC, S-Beta0 Thalassemia, or other S-variant combination), receiving the majority of their pediatric sickle cell care at one of these pediatric hematology programs, irrespective of their HU status (currently using HU, past HU use or had never used HU) were approached for participation in the study. Parents were excluded from participating in the study if unable to complete paper surveys in English or Spanish with little or no assistance, if their children were on chronic transfusion therapy or had a known contraindication to HU, such as pregnancy or significant renal or hepatic dysfunction. Of the 133 parents approached for participation at Albert Einstein College of Medicine, 32 (24%) declined. Refusal rates are not available from the other sites. Of returned surveys, two were incomplete and were not included in the analysis.
The multiple choice survey included questions regarding parent/child demographics (without personal identifiers), disease complications, awareness and knowledge of HU, perceived barriers to HU, and recommendations related to HU use offered by their child’s sickle cell provider. Survey questions were developed based on existing provider surveys and postulated barriers to HU outlined by the NIH Consensus Development Panel on Hydroxyurea Use. [15,23–24] The survey instrument was pilot tested with a sample of potential respondents to ensure its comprehensibility. Knowledge was assessed by four questions about the effects of HU. Respondents were categorized by the number of questions about HU effects they had correctly answered: those answering 1–2 questions correctly were categorized as having some knowledge of HU, while those answering 3 or more questions correctly were categorized as having significant knowledge. Parents were asked about their specific concerns relating to HU use. Concerns were rated on a four point scale; 1=not important to 4=very important. We combined the two response categories somewhat important and very important for our analyses. (Survey is available by request to Dr. Oyeku.)
Parents were approached to complete English or Spanish versions of the survey in waiting rooms or clinic rooms during regularly scheduled appointments at times when not otherwise occupied. Completed self-administered, close-ended surveys were returned to study staff in self-sealed envelopes that recorded only study site. Parents requesting additional information about HU were provided with information sheets after completing the survey.
Descriptive statistics were calculated for independent variables including parent/child demographics, institution, parental knowledge of HU, and concerns about HU. The dependent variable was HU use trichotomized into three categories: current HU use, past HU use and never used HU. Univariate analyses were conducted to determine the frequency of perceived barriers to HU use within the participant sample. Bivariate analyses were performed on all three groups (current HU use; past HU use and never used HU). Mann-Whitney U tests and Kruskal Wallis tests were used to test the association between HU use and the number of medications taken on a daily basis and the number of annual clinic visits across the three larger study sites with at least 30 responses each. Chi-square tests and Spearman correlations tested associations between HU use and demographic variables, institution, parental knowledge, and concerns about HU. Variables found to be significant in bivariate analyses were introduced into a multivariable logistic regression model to determine factors independently associated with current HU use among children of surveyed parents. Parental demographic factors of age, education, income, primary language or travel time to clinic were not significantly associated with HU use in bivariate analyses, and thus were not included in multivariate analysis. Although child’s age was not significant in bivariate analyses, this variable was included in the regression model since age is an important factor associated with HU use identified in previous studies [7,12]. Although the multivariable model adjusted for institution of clinical care, we conducted additional multivariable analyses stratified by institution to assess for potential effect modification by this variable. These stratified analyses were conducted in the two sites with the largest sample sizes. The response for each child was the unit of analysis. Additional sensitivity analyses were performed using only one data point for each parent. Analyses were conducted with SPSS Version 19.
Results
Demographics
A total of 173 responses for children with SCD were obtained from 157 parents (Table 1). Of these, 12 parents had two children with SCD and two parents had three affected children. Most parents (83%) were female and 39% were 30–39 years old. Parents self-identified primarily as African American (64%) or Hispanic/Latino (26%). Most parents spoke English (85%), earned a salary of $40,000 or less (52%) and a minority (37%) were married. Parental education level was mainly divided between those with high school degree or equivalent (38%) or post-high school education (45%). Half (51%) of children of parent respondents were aged 5–10 years and in elementary school (49%), while 19% of children were in middle school, and 32% in high school or college. Most parents (71%) identified their child as having HbSS disease.
Table I.
Demographic characteristics of survey respondents.
| Demographic Characteristics (N= 173) | N (%)* |
|---|---|
|
| |
| Institution | |
| A | 98 (57%) |
| B | 30 (17%) |
| C | 8 (5%) |
| D | 6 (4%) |
| E | 31 (18%) |
|
| |
| Hydroxyurea Use by Child | |
| Current Use | 65 (38%) |
| Never Used | 91 (53%) |
| Past Use | 7 (4%) |
| Did not answer | 10 (6%) |
|
| |
| Parent Age* | |
| 20–29 | 19 (11%) |
| 30–39 | 68 (39%) |
| 40–49 | 49 (28%) |
| >=50 | 24 (14%) |
|
| |
| Parent Gender* | |
| Male | 20 (12%) |
| Female | 143 (83%) |
|
| |
| Child Age | |
| 5–10 | 88 (51%) |
| 11–17 | 85 (49%) |
|
| |
| Child Gender* | |
| Male | 86 (50%) |
| Female | 83 (48%) |
|
| |
| Parent Education* | |
| Grade School | 18 (10%) |
| HS diploma/GED | 65 (38%) |
| Associates/Bachelor’s degree | 46 (27%) |
| Masters’/Doctoral degree/Other | 31 (18%) |
|
| |
| Child’s Sickle Cell Genotype* | |
| Hgb SS | 122 (71%) |
| Hgb SC | 31 (18%) |
| Hgb S-Beta0 Thalassemia/S-X Variant | 11 (6%) |
|
| |
| Parent’s Race/Ethnicity* | |
| Black | 110 (64%) |
| Hispanic/Latino | 45 (26%) |
| Other (Asian, etc.) | 9 (5%) |
|
| |
| Parent’s Language(s) spoken** | |
| English | 147 (85%) |
| Spanish | 43 (25%) |
| Other (French, etc.) | 28 (16%) |
|
| |
| Travel Time to Hematology program* | |
| 30 minutes | 106 (61%) |
| 45 minutes | 33 (19%) |
| ≥ 60 minutes | 24 (14%) |
|
| |
| Parent’s Marital Status* | |
| Single | 78 (43%) |
| Married | 67 (37%) |
| Other (Divorced, separated, etc.) | 26 (15%) |
|
| |
| Parent’s Income* | |
| $20,000 or less | 56 (32%) |
| $20,000–40,000 | 34 (20%) |
| More than $40,000 | 40 (23%) |
| Don’t Know | 28 (16%) |
N can vary due to missing data, and may not add up to 100%;
percentages over 100% reflect more than one language spoken.
Medication Use and Annual Clinic Visits
Among 173 responses, 65 (38%) indicated that their child is currently taking HU, seven (4%) responded that their child was a previous HU user, and 91 (53%) stated that their child had never been a HU user (10 (6%) did not answer). The most common medications taken by all the children were reported to be folic acid (83%) and penicillin (28%). Other commonly used medications included medications for pain management, asthma, allergic rhinitis and other conditions. The frequency of children currently on HU varied by institution (p=0.001), as did the number of medications regularly taken (p=0.001).
Children currently taking HU took significantly more daily medications compared to those not taking HU (p<0.001) (Table 2). Among those currently on HU, the number of daily medications varied significantly by institution (p=0.02). Among children not currently on HU, the number of daily medications varied, but not significantly by institution (p=0.08). Current HU users had significantly more SCD outpatient clinic visits during the preceding 12 months than non-users (p<0.001) (Table 2), while annual clinic visits did not vary significantly by institution for either HU users or non-users (p=0.72; p=0.59 respectively).
Table II.
Distribution of Medication Use and Annual Clinic Visits Between Current Hydroxyurea (HU) Users and Non-users, by parental report (N =173).
| Daily Medications*1 | Current HU Users (N=61) % of total users |
HU Non-users (N=67) % of total non-users |
|---|---|---|
| 1–2 | 41 | 84 |
| 3 | 34 | 12 |
| 4 or more | 25 | 4.5 |
| Annual Clinic Visits*2 |
Current HU Users (N=63) % of total users |
HU Non-users (N=86) % of total non-users |
| 1–2 | 29 | 55 |
| 3–4 | 11 | 23 |
| 5 or more | 60 | 22 |
Statistically significant difference between Current HU Users and Non-users. (p<0.001)
Missing: N=45
Missing: N=24
Awareness about HU
Overall, 63% of parents had heard about HU. Among those whose children were currently using HU, 97% had heard of it, compared to 39% of HU non-users who had not heard of it (13% were uncertain or did not respond). Awareness of HU was significantly associated with its current use (p<0.001) and varied by institution (p=0.002).
Knowledge about HU
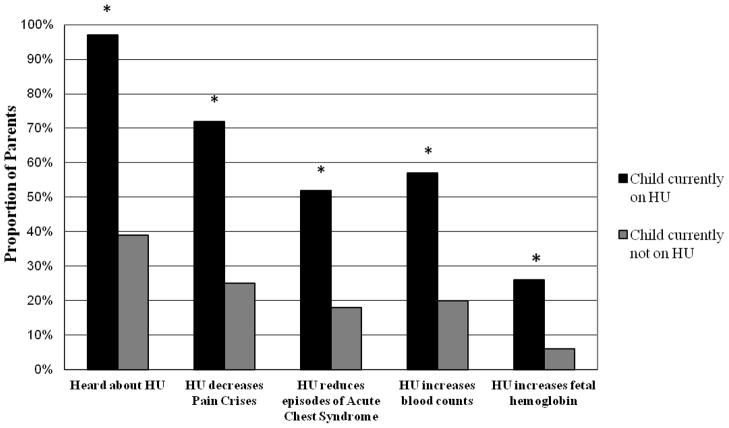
Parents were asked four specific questions to assess the extent of their knowledge about the effects of HU on SCD. Among all respondents, 45% of parents correctly indicated that its use decreased the frequency of pain crises, 30% indicated that it decreased episodes of acute chest syndrome, 33% indicated that it increased blood counts, while only 15% indicated that HU increased fetal hemoglobin levels. Parents of current HU users significantly more often correctly cited specific clinical benefits of HU than parents of non-users. (p<0.001, Figure 1) The proportion of parents having significant parental knowledge (answering 3 or more questions correctly about HU) varied by institution (p=0.004). In contrast, parents having some knowledge (answering 1 or 2 questions correctly about HU) did not vary significantly by institution (p=0.61). Level of parental knowledge about HU was not significantly associated with parental demographics such as parental age, gender, education, race/ethnicity, language or income.
Figure 1. Incomplete Awareness or Knowledge about Hydroxyurea (HU) among Parents.
While substantial proportions of parents do not know about HU ameliorative effect, parents whose children are non-users (N= 91) are significantly less cognizant about HU than parents of users (N= 65);*= p<0.001.
Perceived Effectiveness among Parents of Current HU Users
Parents of children currently taking HU (N=65) were asked to estimate the drug’s effectiveness by assessing the frequency of events such as pain crises, acute chest syndrome, emergency department (ED) visits, overnight hospital stays, and days missed from school since starting HU. Overall, parents (72–78%) noted one or more of these complications had improved, while some (12–18%) indicated the frequency of one or more of these events were unchanged, and a minority (9–15%) noted that one or more of these events had worsened since onset of HU use. By parental report, the number of episodes of acute chest syndrome, pain crises, emergency room visits, hospital visits or missed days from school did not vary significantly by institution (all p > 0.70). As an anonymous survey, overall duration and intensity of HU treatment were not verified.
Parental Concerns about HU among Current HU and Past HU users
Among current HU users (N=65), 89% of parents ranked their most common serious concerns as the unknown carcinogenic potential of HU and its possible unknown adverse effects (Table 3). Other common concerns among current users were: uncertainty about the risk of adverse effects (as high as 89%); that HU would not improve their child’s health (83%); and lack of FDA approval for pediatric use (81%). The small number of parents of past users (N=7) precluded statistically meaningful comparison with parents of current users (N=65). These findings were similar when data on parents of previous and current users were combined. Among past users, parents did not cite any consistent reasons for stopping HU. Concerns among non-users of HU are described separately below.
Table III.
Concerns about Hydroxyurea (HU) Among Parents whose Children Currently Use HU.
| Concerns1 (N=65) | Percent2 Concerned |
|---|---|
| Concerns about effectiveness of HU | |
| Risk of ineffectiveness | 83% |
| Uncertainty about risks and benefits of treatment | 83% |
| Not approved by Food and Drug Administration (FDA) for use in children with sickle cell disease | 81% |
| Concerns about personal/family impact | |
| Burden to self or to one’s family (i.e. lost work income, school absences due to appointments) | 45% |
| Frequency of clinic visits and blood tests for drug monitoring | 64% |
| Difficulty in obtaining prescription refills | 57% |
| Difficulty in taking HU | 53% |
| Remembering to give/take HU | 60% |
| Concerns about potential side effects | |
| Potential carcinogenic effect | 89% |
| Possible teratogenic effect from exposure | 77% |
| Future fertility | 69% |
| Additional side effects | 89% |
Concerns were rated on a 4 point scale. 1=Not Important to 4=Very Important;
Percent reflect concerns identified as “Somewhat or Very important” by respondents
Multivariate Logistic Regression Analysis of Factors Associated with Current HU Use
The multivariate model included those variables that were significantly associated with current HU use in our bivariate analyses: parental knowledge, site of hematology care and sickle cell genotype. As patient age has been reported as associated with pediatric use of HU, patient age was added to the multivariate model. [7–8,11–12]
Multivariate analyses revealed three factors independently and significantly associated with current HU use (Table 4): 1) Higher level of parental knowledge about HU, defined as the ability to identify 3–4 benefits (OR 21.3, 95% CI 6.6, 68.5), relative to identifying 1–2 benefits (OR 13.9, 95%CI 4.7, 40.8); 2) Children with HbSC had a 86% lower odds of currently being on HU relative to children with HbSS (OR 0.14, 95% CI 0.04, 0.6); 3) Patients receiving hematology care at institution E had an 80% lower odds of currently being on HU relative to institution A (OR 0.2, 95% CI 0.06, 0.9). The results were similar for sensitivity analyses limited to one child response per parent. Stratified analyses within institution showed a persistence of the association between parent knowledge and current HU use (Table 5). No significant differences were found by patient age or hemoglobinopathy. We did not observe any significant independent effect modification by institution on current HU use.
Table IV.
Multivariate Logistic Regression of Factors Associated with Current Hydroxyurea (HU) Use
| Characteristic | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
|
| |||
| Child’s Age | 0.99 | 0.9, 1.1 | 0.9 |
|
| |||
| Parental Knowledge of HU1 | |||
|
| |||
| No Knowledge | 1.0 | Ref | Ref |
|
|
|||
| Some knowledge | 13.9 | 4.7, 40.8 | <0.001 |
|
|
|||
| Significant knowledge | 21.3 | 6.6, 68.5 | <0.001 |
|
| |||
| Child’s Sickle Cell Genotype | |||
|
| |||
| Hemoglobin SS disease | 1.0 | Ref | Ref |
|
|
|||
| Hemoglobin SC disease | 0.14 | 0.04, 0.6 | 0.005 |
|
|
|||
| Hemoglobin S-Beta0 thalassemia disease | 0.18 | 0.02, 1.7 | 0.13 |
|
| |||
| Care at Institution | |||
|
| |||
| Institution A | 1.0 | Ref | Ref |
|
|
|||
| Institution B | 3.3 | 0.96, 11.1 | 0.06 |
|
|
|||
| Institution C | 0.4 | 0.06, 3.2 | 0.4 |
|
|
|||
| Institution D | 0.7 | 0.09, 5.7 | 0.8 |
|
|
|||
| Institution E | 0.2 | 0.06, 0.9 | 0.04 |
Regression model includes child’s age, parental knowledge, child’s sickle cell genotype, institution; Reference groups shown above with odds ratio of 1.0; Bold font indicates statistical significance;
Knowledge of HU: Some knowledge defined as: answered 1–2 knowledge questions correctly about HU, significant knowledge defined as: answered 3–4 knowledge questions correctly about HU.
Parental Reasons for Not Starting HU
Among parents whose children had never taken HU (N=91), twenty (22%) indicated that their child’s provider had recommended its use (three did not answer this question). The most common reasons parents cited for choosing not to start HU despite provider recommendation were: concerns about side effects (N=7); incomplete understanding about how HU would work for their child (N=5); and off-label use of HU for children (N=5).
Among never-users, 68 parents (75%) reported that their child’s provider had not recommended it. The most common reasons that would prompt them to ask the provider to prescribe HU were: more frequent acute events, such as emergency room visits for pain and other SCD complications (N=22; 32%); frequent hospitalizations for SCD complications (N=16; 25%); a better drug safety profile (N=16; 24%); and ease of administering to their child (N=8; 12%).
Discussion
A national consensus panel highlighted that barriers to HU use arise at multiple levels, including the patient and family level.[15] To our knowledge, this is the first report assessing factors associated with HU use from the perspective of parents of children with SCD. Our major findings are that parental awareness about HU and knowledge about its benefits are highly variable, and that even among users concerns about drug effectiveness and long-term safety were almost universal. Better parental knowledge of the drug’s therapeutic effects was significantly associated with more use, although directionality of this association is not possible from our cross-sectional data. Notably, parental demographics such as educational level, income, or primary language spoken did not independently predict HU use. Significant inter-institutional variation exists in parental knowledge about and the likelihood of HU use. As expected, children with HbSS were more likely to be taking HU than those with HbSC, and the majority of parents whose children were taking HU perceived a moderate to substantial improvement in disease manifestations. Our findings are consistent with a report of adults with SCD, in which limited knowledge about relative risks and benefits of HU was associated in its underuse. [21]
Our regional multi-site consortium of academic institutions with pediatric sickle cell programs identified significant differences between clinical sites in extent of HU uptake and parental knowledge about its impact on SCD. Many parents whose children were non-users stated that their hematology providers have not recommended HU, a perception that could not be verified. Nonetheless, considerable variation in HU use among pediatric hematology providers has been described, largely resulting from these same knowledge gaps. [25] Inter-institutional variation in treatment of acute complications and re-admission rates for SCD has been documented. [26–27] Inter-institutional differences in HU use and parental knowledge may be influenced by differences in patient-provider communication, care delivery systems, and/or case mix; these aspects were not assessed. All of these findings underscore the need for effective communication strategies to improve uptake of HU.
As an anonymous survey, confirmation of actual HU use or impact of HU on disease severity and health care utilization was not available. This survey did not specifically address intensity of HU prescription or adherence, or relative indications for specific disease complications. The relatively small proportion of those with HbSC precluded direct comparison to those with HbSS or Hb S-Beta0 Thalassemia. Specific concerns about HU among parents whose children have never used it were not directly compared to parents of current or previous users, as additional variables were assessed for the former group, such as reported provider recommendation. Due to the cross-sectional nature of the study design, inferences about the directionality of the association between parental knowledge and current HU use cannot be made, such as whether knowledge depended on site of care or actual HU use. Our findings might not be generalizable to children who do not routinely receive care from out-patient hospital-based SCD clinics. The proportion of respondents whose children take HU may be higher than that of the general sickle cell population, as more frequent treatment-related visits may have biased the overall clinic-based sampling. We focused on HU in children and adolescents ages 5 – 17 years, and did not address issues relevant to parents of younger or older patients.
In summary, our findings suggest that parental knowledge about HU is highly variable and is highly associated with HU use, and that concerns about long-term effectiveness and safety persist despite its use. Our data suggest that parental knowledge of HU and site of care are key factors associated with its use, and that use and knowledge were not associated with parental demographics. These findings suggest that increased acceptance of HU for children with SCD may require education targeted towards patients and families to increase knowledge gaps. Parents identified interest for both written and internet-based sources of information. However, the simple availability of these materials may not suffice to address these major issues. FDA approval for use in children with SCD and improved data on long-term impact on health and fertility may help reduce parental concerns. Updated National Heart Lung and Blood Institute’s recommendations on use of HU for SCD should help to clarify contemporary clinical indications and toxicities that providers may use to inform communication with patients and families about HU. Our survey findings also suggest that strategies to address institutional and provider level barriers to HU use may also be essential in overcoming challenges to pediatric uptake of this important therapeutic modality.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the parents who completed the survey and the study coordinators at our respective institutions who coordinated the data collection. This project was supported by the grants 3UL1RR024156-04S4 from National Center for Research Resources (NCRR) to Columbia University, as part of the Best Pharmaceuticals for Children Act program administered by NICHD. Support was also provided by grants UL1RR025750, KL2RR025749 and TL1RR025748 to Albert Einstein College of Medicine at Yeshiva University from NCRR
Footnotes
Conflict of Interest Statement
We have no financial conflicts of interest to disclose.
References
- 1.National Institutes of Health; National Heart Lung, and Blood Institute. The Management of Sickle Cell Disease. Bethesda, MD: 2002. [Google Scholar]
- 2.Charache S, Terrin ML, Moore RD, et al. Effect of Hydroxyurea on the Frequency of Painful Crises in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment.[see comment][erratum appears in JAMA2003 Aug 13;290(6):756] JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 4.Thornburg CD, Calatroni A, Panepinto JA. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J Pediatr Hematol Oncol. 2011;33(4):251–254. doi: 10.1097/MPH.0b013e3182114c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: what have we learned and what questions still remain? Curr Opin Hematol. 2011;18(3):158–165. doi: 10.1097/MOH.0b013e32834521dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candrilli SD, O’Brien SH, Ware RE, et al. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86(3):273–277. doi: 10.1002/ajh.21968. [DOI] [PubMed] [Google Scholar]
- 7.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) The Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinney TR, Helms RW, O’Branski EE, et al. Safety of Hydroxyurea in Children With Sickle Cell Anemia: Results of the HUG-KIDS Study, a Phase I/II Trial. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 9.Hoppe C, Vichinsky E, Quirolo K, et al. Use of hydroxyurea in children ages 2 to 5 years with sickle cell disease. J Pediatr Hematol Oncol. 2000;22(4):330–334. doi: 10.1097/00043426-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 11.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24(1):199–214. doi: 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115(26):5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health, National Heart, Lung and Blood Institute. Draft Recommendations on the Use of Hydroxyurea Therapy in Sickle Cell Disease. Nov 17, 2010. [Google Scholar]
- 14.Tripathi A, Jerrell JM, Stallworth JR. Clinical complications in severe pediatric sickle cell disease and the impact of hydroxyurea. Pediatr Blood Cancer. 2011;56(1):90–94. doi: 10.1002/pbc.22822. [DOI] [PubMed] [Google Scholar]
- 15.Brawley OW, Cornelius LJ, Edwards LR, et al. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008;148(12):932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowy DO, Hood S, LaJoie AS. Facilitators and barriers to self-management of type 2 diabetes among urban African American adults: focus group findings. Diabetes Educ. 36(6):897–905. doi: 10.1177/0145721710385579. [DOI] [PubMed] [Google Scholar]
- 17.Bogart LM, Wagner G, Galvan FH, et al. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among african american men with HIV. J Acquir Immune Defic Syndr. 53(5):648–655. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Jacobs EA, Moore RD, et al. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS. 24(7):415–420. doi: 10.1089/apc.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogedegbe G, Harrison M, Robbins L, et al. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis. 2004;14(1):3–12. [PubMed] [Google Scholar]
- 20.Champion VL, Springston J. Mammography adherence and beliefs in a sample of low-income African American women. Int J Behav Med. 1999;6(3):228–240. doi: 10.1207/s15327558ijbm0603_2. [DOI] [PubMed] [Google Scholar]
- 21.Haywood C, Jr, Beach MC, Bediako S, et al. Examining the characteristics and beliefs of hydroxyurea users and nonusers among adults with sickle cell disease. Am J Hematol. 2011;86(1):85–87. doi: 10.1002/ajh.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal JB, Strouse JJ, Beach MC, et al. Hydroxyurea for the treatment of sickle cell disease. Evid Rep Technol Assess (Full Rep) 2008;(165):1–95. [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzkron S, Haywood C, Jr, Hassell KL, et al. Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the Sickle Cell Disease Adult Provider Network. J Natl Med Assoc. 2008;100(8):968–973. [PubMed] [Google Scholar]
- 24.Zumberg MS, Reddy S, Boyette RL, et al. Hydroxyurea therapy for sickle cell disease in community-based practices: A survey of Florida and North Carolina hematologists/oncologists. Am J Hematol. 2005;79(2):107–113. doi: 10.1002/ajh.20353. [DOI] [PubMed] [Google Scholar]
- 25.Brandow AM, Jirovec DL, Panepinto JA. Hydroxyurea in children with sickle cell disease: practice patterns and barriers to utilization. Am J Hematol. 2010;85(8):611–613. doi: 10.1002/ajh.21749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobota A, Graham DA, Heeney MM, et al. Corticosteroids for acute chest syndrome in children with sickle cell disease: Variation in use and association with length of stay and readmission. Am J Hematol. 2010;85(1):24–28. doi: 10.1002/ajh.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobota A, Graham DA, Neufeld EJ, et al. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children’s hospitals: Risk factors and hospital variation. Pediatr Blood Cancer. 2012;58(1):61–65. doi: 10.1002/pbc.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.