Abstract
Cryptotanshinone is one of the major tanshinones isolated from the roots of the plant Salvia miltiorrhiza Bunge (Danshen). Danshen has been widely used in traditional Chinese medicine for treatment of a variety of diseases, including coronary artery disease, acute ischemic stroke, hyperlipidemia, chronic renal failure, chronic hepatitis, and Alzheimer’s disease, showing no serious adverse effects. Recent studies have shown that cryptotanshinone not only possesses the potential for treatment and prevention of the above-mentioned diseases, but also is a potent anticancer agent. Here we briefly summarize the physical and chemical properties and the pharmacokinetic profiles of cryptotanshinone, and then comprehensively review its anticancer activities as well as the underlying mechanisms.
Keywords: Cryptotanshinone, tanshinones, cancer, cell proliferation, cell death, mTOR
1. Introduction
Phytochemicals, naturally occurring substances in plants, have attracted considerable public and scientific interest to find their activities to relieve human diseases, especially the two commonest killers in the industrialized world, cardiovascular disease and cancer [1]. Salvia miltiorrhiza Bunge, also known as red sage or Chinese sage, is a deciduous perennial plant in the genus Salvia, highly valued for its roots, called Danshen, in traditional Chinese medicine [2, 3]. It typically grows to 30-60 cm high, with branching stems, oval leaves and purple flowers, and mainly distributes in northeastern China, Korea and Japan [4], at 90-1200 m elevation, preferring grassy places in forests, hillsides, and along stream banks [5]. Danshen is one of most widely used herbs in China and now largely exported to other countries [6, 7].
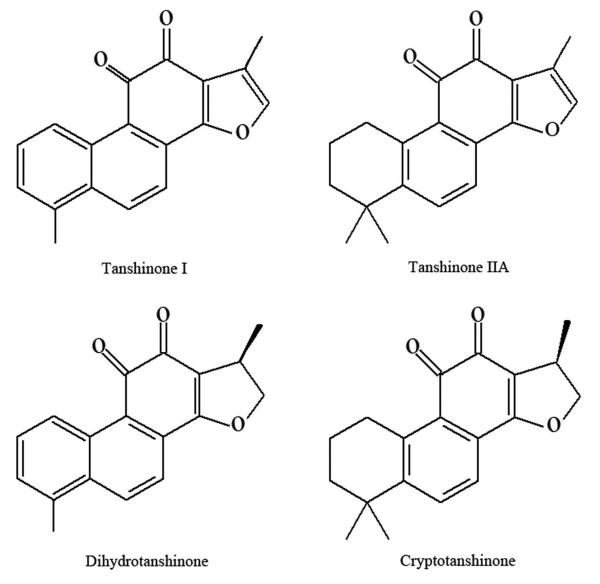
Danshen is typically characterized by dilating blood vessels and removing blood stasis, and thus has been clinically used to treat ischemic diseases including angina pectoris (chest pain) and ischemic stroke in China [4, 8]. Danshen has also been frequently used to treat hyperlipidemia, hepatic fibrosis, chronic renal failure, and gynecological diseases, such as dysmenorrhea, amenorrhea, and lochioschesis [9]. Modern studies have revealed that Danshen is an effective platelet aggregation inhibitor decreasing blood hypercoagulability and increasing coronary blood flow, and scavenges free radicals in ischemic diseases, thus reducing cellular damage from ischemia [3]. Furthermore, clinic trials have also indicated that Danshen is an effective agent for prevention and treatment of Alzheimer’s disease [3]. Danshen contains fat-soluble diterpenoids and water-soluble phenolic acids, as well as flavonoids and triterpenoids. Up to date, it has been identified that salvianolic acid B is one of the main hydrophilic extracts, whereas tanshinone I, tanshinone IIA, dihydrotanshinone, and cryptotanshinone (Fig.1) are the major lipophilic ingredients in Danshen. Most of Danshen extracts exhibit certain medicinal effects in vitro and in vivo [3]. In particular, recent studies have revealed that cryptotanshinone not only has potential to prevent ischemia, atherosclerosis, and Alzheimer’s disease, but also possesses diverse properties, such as antibacterial, anti-inflammatory, antioxidative [10], antidiabetic, anti-obesic, and anticancer activities (Table 1) [11]. Here, we briefly summarize the physical and chemical properties, as well as the pharmacokinetic profiles of cryptotanshinone, and then comprehensively review its anticancer activities and the underlying mechanisms.
Fig.1.
Structures of tanshinone I, tanshinone IIA, dihydrotanshinone and cryptotanshinone
Table 1.
The overview of biological activity of cryptotanshinone
| Biological activity | Targets | Possible application in diseases |
|---|---|---|
| Cardiovascular effect | ||
| Vasorelaxation | Ca2+ Endothelin-1 |
Coronary artery disease Acute ischemic stroke Chronic renal failure |
| Cerebrovascular effect | ||
| Neuroprotection | ACHE* BuCHE* |
Alzheimer’s disease |
| Antibacterial activity | Superoxide radicals | G+ & G− bacterial infection |
| Anti-inflammatory activity | NF-κB, COX-1, IL-1, IL-2 TLR4*, TAK1* |
Inflammation |
| Antioxidant activity | Superoxide radicals SOD, CAT, GPx |
Aging; Cancer; Obesity Alzheimer’s disease |
| Antidiabetes | AMPK, PPARγ | Diabete; Obesity Hyperlipidemia |
| Anti-fibrosis | MMPs, JNK | Liver, lung, heart, kidney fibrosis |
| Anticancer | Detailedly discussed below |
ACHE: Acetylcholinesterase; BuCHE: Butyrylcholinesterase; TLR4: Toll-like receptor 4; TAK1: TGF-β-activated kinase 1;
2. Physical and chemical properties of cryptotanshinone
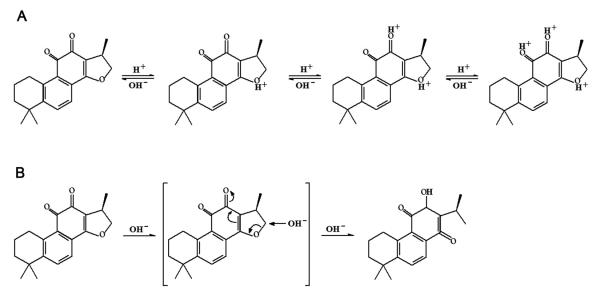
Cryptotanshinone, also named cryptotanshinon or tanshinone C, is a cell-permeable diterpene quinone, one of the major tanshinones (including tanshinone I, tanshinone IIA, dihydrotanshinone and cryptotanshinone) (Fig.1) derived from the roots of Salvia miltiorrhiza Bunge [12, 13]. Cryptotanshinone is an orange-brown powder, which is now commercially available. Its chemical name is 1,2,6,7,8,9-hexahydro-1,6,6-trimethyl-(R)-phenanthro(1,2-b)furan-10,11-dione (CAS registry number: 35825-57-1), with a molecular formula of C19H20O3, a molecular weight of 296.36 and a melting point of 184°C. Cryptotanshinone is not water-soluble, but soluble in dimethyl sulfoxide, methanol, ethanol, chloroform and ether, and turns red when interacting with concentrated sulfuric acid [12, 13]. Cryptotanshinone belongs to phenanthraquinone derivatives, and photochemical reaction would occur, when exposed to light [14]. This is evidenced by the findings that the content of cryptotanshinone was found to be obviously different in the methanol extract of Danshen or in cryptotanshinone methanol solution before and after illumination [14]. Additionally, the solubility of cryptotanshinone varies slightly between pH 2 to pH 8, but largely between pH 10 to pH 12 [15], implying that cryptotanshinone is highly sensitive to alkaline conditions as well. The fluorescence property and the molecular structure of cryptotanshinone are undergoing considerable change in different media [15]. When in strong acidic or in strong basic medium, the fluorescence is quenched completely and shifted to a higher frequency [15]. If the strong acidity is adjusted to neutrality, the fluorescence intensity can be retrieved [15]. The reaction process [15] is illustrated in Fig.2. Therefore, it is suggested that cryptotanshinone be stored and protected from light and under dry and cool (2-8°C) condition.
Fig.2.
Tautomerism of cryptotanshinone under acidic (A) and strong alkaloid (B) conditions.
Synthetic cryptotanshinone was firstly achieved by Hiroshi Kakisawa and Yoshinobu Inouye, two Japanese chemists, in 1969 [16]. Synthesis of cryptotanshinone took multiple steps, involving Diels-Alder adduct reaction between 3-methylbenzofuran-4,7-quinone and 6,6-dimethyl-1-vinylcyclohexene in ethanol at room temperature, followed by air oxidation and further adduction with concentrated sulfuric acid in ethanol, catalyzed by 5% Pd-C (Palladium on carbon) (Fig.3) [16].
Fig.3.
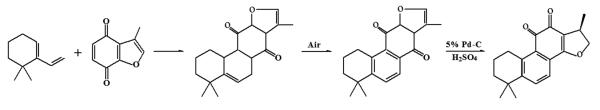
The route of synthesis of cryptotanshinone.
3. Pharmacokinetic profiles of cryptotanshinone
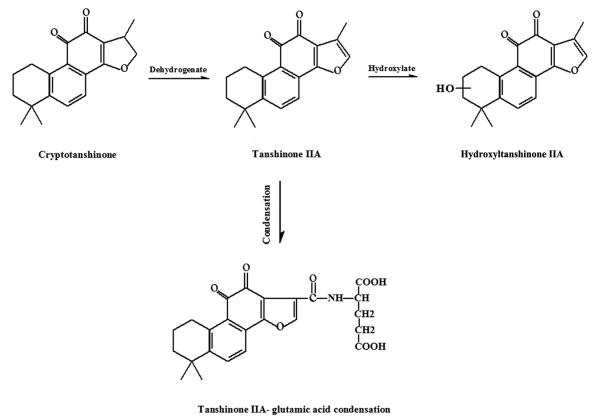
Pharmacokinetic studies of cryptotanshinone have been carried out in rats, mice and pigs [17-20]. In rats, cryptotanshinone was found to mainly distribute in liver, lung, brain and heart, while little was detected in spleen and kidney [17]. Only 0.34% of the dose was recovered in the 48-h urine as unchanged form after a single oral administration, indicating that a great part of cryptotanshinone was metabolized in the body [17]. In addition to cryptotanshinone, the following metabolites, including tanshinone IIA, hydroxylated forms of tanshinone IIA, and a glutamic acid-tanshinone IIA conjugate, were isolated and identified from urine and bile samples (Fig.4) [17].
Fig.4.
Chemical structures of major metabolites of cryptotanshinone in rodents.
In pigs, cryptotanshinone was given intravenously at a single dose of 10 mg/kg, and cryptotanshinone concentration in serum was measured by high performance liquid chromatography. Half time (t1/2) of cryptotanshinone distribution in the body was 2.36 min, and t1/2 of its elimination was 64.78 min [18, 19], also suggesting that cryptotanshinone distributed and was metabolized quickly. In addition, tanshinone IIA was detected after 4.6 min [18, 19], further highlighting that cryptotanshinone could be converted to tanshinone IIA by the metabolism transformation very rapidly in the body. Oral administration of 20 mg/kg cryptotanshinone in pigs resulted in non-detectable cryptotanshinone and tanshinone IIA in serum. However, when the dose increased to 40 mg/kg, 0.043 μg/ml of cryptotanshinone in serum was detected at 1 h of post-administration [18, 19]. These data indicate that the bioavailability of cryptotanshinone is very low when orally administered. The biliary and urinary excretion of both cryptotanshinone and its metabolite tanshinone IIA was minimal (0.3% of total dose over 48 h) after oral, intramuscular, or intravenous dosing, but the fecal recovery was 12% [19]. It remains elusive why such low bioavailability of cryptotanshinone occurs. Possibly, this is related to first-pass metabolism and poor intestinal absorption [20]. Using Caco2 cells, P-glycoprotein-overexpressed MDCKII cells and single-pass rat intestinal perfusion, Zhang et al found that cryptotanshinone was a substrate of P-glycoprotein, an ATP-dependent efflux pump, that pumped cryptotanshinone into the luminal side, resulting in low bioavailability [20].
Due to the low bioavailability of cryptotanshinone, a lot of efforts have been tried to increase serum level of the compound. For example, hydroxylpropyl-β-cyclodextrin-included complex of cryptotanshinone was prepared for oral administration [21]. After a single oral dose, cryptotanshinone in the inclusion complex was absorbed slowly and the Cmax and AUC0–t were dose-dependent, as detected by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) [21]. The oral bioavailability of cryptotanshinone in rats was increased by 2.5-fold (2.05% for parent cryptotanshinone versus 6.90% for cryptotanshinone inclusion complex) at 60 mg/kg [21]. After a single oral dose of cryptotanshinone inclusion complex at 53.4 mg/kg, the bioavailability of cryptotanshinone was 11.1% ± 1.8% in dogs [21]. The t1/2 values of cryptotanshinone in rats and dogs were 5.3-7.4 and 6.0-10.0 h, respectively [21]. Compared with the parent cryptotanshinone, the oral inclusion complex has a higher bioavailability and longer half life. However, excretion data in rats did not show significant change between the two preparations [21, 22]. Of interest, oral administration of liposoluble ethanol extract of Danshen, in comparison with the equivalent dose of single cryptotanshinone or tanshinone IIA administration, increased the plasma concentrations of cryptotanshinone and tanshinone IIA by about 8 and 10 folds, respectively [23], suggesting that a drug-drug interaction may occur between the coexisting tanshinones, which affects their absorption, transformation and metabolism.
Besides, biotransformation also affects metabolism of cryptotanshinone. It has been described that cryptotanshinone was able to be biotransformed by Cunninghamella elegans to three new products, respectively identified as (3R,15R)-3-hydroxycryptotanshinone, (3S,15R)-3-hydroxycryptotanshinone, and (4S,15R)-18-hydroxycryptotanshinone, that are identical to three of the minor hydroxylated metabolites in vivo (Fig. 5) [24]. Although the activities of the hydroxylated forms of cryptotanshinone remain to be defined, the above finding suggests that microbial biotransformation be a feasible approach for large preparation of certain useful metabolites, if the amount of the metabolites is traceable in the body.
Fig.5.

Chemicals from biotransformation of cryptotanshinone by Cunninghamella elegans.
4. Therapeutic potential of cryptotanshinone for human cancer
Danshen has been used to treat coronary artery disease and ischemic stroke in China for centuries. Modern studies have demonstrated that cryptotanshinone from Danshen extracts has potent pharmacological activity against cardiovascular and cerebrovascular diseases [3]. Increasing evidence has implicated that cryptotanshinone possesses more activities, such as anti-Alzheimer’s disease, anti-bacteria, anti-inflammation, anti-oxidation, anti-diabetes, anti-obesity, anti-fibrosis, and anticancer as well [25]. Therefore, cryptotanshinone has received more and more attention. Here we focus on reviewing the current findings regarding the anticancer activities of cryptotanshinone and the underlying mechanisms. In particular, cryptotanshinone targeting the phosphatidylinositol 3′-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway will be discussed.
4.1. Inhibition of cell proliferation
In vitro studies have demonstrated that cryptotanshinone inhibits cell proliferation in a variety of cancer cell lines, including rhabdomyosarcoma, melanoma, cervical, colon, breast and prostate cancer cells [11, 26-28]. The inhibitory effect on cell proliferation is through inducing a cell cycle arrest either at G1/G0 or G2/M phase, depending on cell lines [11, 26-28]. It has been described that cryptotanshinone induced a G1/G0 cell cycle arrest in rhabdomyosarcoma (Rh30) and prostate cancer (DU145) cells by downregulating expression of cyclin D1 and phosphorylation of retinoblastoma protein (Rb) [11, 26], but induced a G2/M cell cycle arrest in melanoma (B16) cells via upregulating expression of Cdc25c, cyclin A1 and cyclin B1 [27]. Cryptotanshinone also upregulated expression of p21Cip1 and p27Kip1, two inhibitors for G1 or G1/S cyclin dependent kinases [11, 27]. In addition, Aurora A kinase, regulating mitosis and overexpressing in numerous cancer cells, was also proved to be an important anticancer target [29]. Cryptotanshinone inhibiting expression of Aurora A kinase led to significant suppression of prostate cancer cells in vitro and in mice [28].
4.2. Induction of cell death
Cryptotanshinone also induces cell death in cancer cells [27, 28, 30]. The underlying mechanisms remain to be elucidated. Studies have shown that cryptotanshinone induced apoptosis in prostate cancer (DU145) and rhabdomyosarcoma (Rh30) cells by downregulating expression of anti-apoptotic proteins including survivin and Mcl-1 [26, 30]. It has been speculated that this might be related to inhibition of the transcriptional activity of the signal transducer and activator of transcription 3 (Stat3) [26], as Mcl-1 and survivin are positively regulated by Stat3 at transcriptional level [31, 32]. Stat3, regulated by Janus kinases (JAKs), is constitutively activated in most human malignant tumors, and involved in the proliferation, angiogenesis, immune evasion and anti-apoptosis in cancer cells [33, 34]. Therefore, Stat3 has been regarded as a potential target for cancer therapy [33, 34]. However, cryptotanshinone inhibited Tyr705 phosphorylation of Stat3 independent of the JAKs [26]. Instead, by computational modeling, cryptotanshinone was found to bind to the SH2 domain of Stat3 and subsequently block Stat3 dimerization, nuclear translocation and Stat3-dependent transcription activity [26].
Mostly recently, we have demonstrated that cryptotanshinone induction of cell death was independent of activation of caspase cascade, as treatment with cryptotanshinone neither altered caspase3/7 enzyme activities, nor induced cleavage of caspase 3 or poly ADP-ribose polymerase (PARP) in Rh30 and DU145 cells [30]. It appears that cryptotanshinone induced caspase-independent cell death by activation of c-Jun N-terminal kinase (JNK) and p38 and inhibition of extracellular-signal-regulated kinases ½ (Erk1/2) in Rh30 and DU145 cells. This is strongly supported by the findings that: i) Inhibition of p38 with SB202190 or JNK with SP600125 attenuated cryptotanshinone-induced cell death. ii) Silencing p38 or c-Jun also in part prevented cryptotanshinone-induced cell death. iii) Expression of constitutively active mitogen-activated protein kinase kinase 1 (MKK1) conferred resistance to cryptotanshinone inhibition of Erk1/2 phosphorylation and induction of cell death [30]. Furthermore, we observed that cryptotanshinone-induced cell death was attributed to induction of reactive oxygen species (ROS), as N-acetyl-L-cysteine (NAC), a ROS scavenger, attenuated cryptotanshinone activation of p38/JNK, inhibition of Erk1/2, and induction of cell death [30]. Similarly, cryptotanshinone induction of ROS triggered endoplasmic reticulum (ER) stress and activated mitogen-activated protein kinase (MAPK) cascade, leading to apoptosis in hepatoma (HepG2) and breast carcinoma (MCF7) cells [35]. Through inducing ER stress, cryptotanshinone also synergized the anticancer activity of TNF-α, Fas, cisplatin, etoposide or 5-fluorouracil [35], suggesting a therapeutic potential for human hepatoma and breast cancer.
4.3. Inhibition of cell motility and invasion
Cancer metastasis is a process by which cancer cells spread to other parts in the body, which is a leading cause of death in cancer patients [36]. Tumor cell migration and invasion are prerequisite for metastasis [36]. It has been described that cryptotanshinone inhibited complement 5a (C5a) or macrophage inflammatory protein-1α (MIP-1α)-induced motility in RAW264.7 and primary human macrophages [37, 38]. The anti-migratory effect of cryptotanshinone was attributed to its inhibition of C5a or MIP-1α-induced F-actin polymerization and filopodia formation [37]. Furthermore, cryptotanshinone inhibition of C5a or MIP-1α-induced PI3K-p110γ membrane translocation as well as phosphorylation of Akt and Erk1/2 also contributed to the anti-migratory activity [38]. Moreover, in our studies we have observed that cryptotanshinone inhibited cell motility in rhabdomyosarcoma (Rh30) cells as well, although the underlying mechanism is unknown.
In addition, cryptotanshinone was found to inhibit basic fibroblast growth factor (bFGF)-stimulated invasion in bovine aortic endothelial cells (BAECs) in culture [39]. Interestingly, under the same condition, tanshinone IIA did not show such activity [39]. Analysis of the structure-activity relationship revealed that the only structural difference between the two tanshinones was double bond at C-15 position of the dihydrofuran ring [39]. Therefore, it has been proposed that the absence of double bond at C-15 position is the key for the inhibitory effect on cell invasion [39]. In addition, cryptotanshinone was found to inhibit tumor necrosis factor-α (TNF-α) induced invasion in human aortic smooth muscle cells by inhibition of expression of matrix metallopeptidase 9 (MMP-9) at transcriptional level [40]. This was possibly related to cryptotanshinone inhibition of TNF-induced activation of Erk1/2, p38 and JNK signaling pathways, which led to downregulation of the transcriptional activities of activator protein 1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [40]. Apparently, the effect of cryptotanshinone on MAPK cascade in human aortic smooth muscle cells [40] is in sharp contrast to that in cancer cells (Rh30 and DU145), as described above [30]. Further studies are required to determine whether this is due to different cell lines or stimuli used.
4.4. Anti-angiogenesis
Angiogenesis is a process that new capillaries forms with endothelial cells and sprouts from preexisting vessels [41]. It is regulated by a number of factors involving endothelial cells and other cell types (e.g. monocytes/macrophages, fibroblasts, smooth muscle cells, pericytes), cytokines, molecules of the extracellular matrix and adhesion molecules [42]. More importantly, due to playing a crucial role in tumor growth and metastasis, angiogenesis has been regarded as a promising target for cancer therapy [41, 42]. Bevacizumab (Avastin, Genentech/Roche), a humanized monoclonal antibody that inhibits vascular endothelial growth factor (VEGF) and slows angiogenesis, has been used to treat various types of cancer, including colorectal, lung, breast, kidney and ovarian cancer, as well as glioblastoma [43]. Increasing evidence suggests that cryptotanshinone possesses anti-angiogenic activity as well. For instance, cryptotanshinone was found to inhibit proliferation in BAECs in culture, with an IC50 of 10 μM [39]. Also, cryptotanshinone at 10 μM inhibited bFGF-stimulated invasion and tube formation in BAECs [39]. Furthermore, cryptotanshinone inhibited formation of microvessels in chick embryo chorioallantoic membrane [44]. Obviously, it is necessary to perform more in vivo studies in animals to validate the anti-angiogenic activity of cryptotanshinone. Also, further investigations are required to understand how cryptotanshinone functions as an anti-angiogenic agent.
4.5. Anti-lymphangiogenesis
Lymphangiogenesis refers to the formation of lymphatic vessels from pre-existing lymphatic vessels, which not only plays a critical role in regulating physiological homeostasis, metabolism and immunity, but also promotes tumor metastasis [45, 46]. Anti-lymphangiogenesis has been considered as a potential tactic for cancer treatment and prevention [45, 46]. Recently, we have demonstrated that cryptotanshinone inhibited tube formation in murine lymphatic endothelial cells, an in vitro model of lymphangiogenesis, suggesting that cryptotanshinone has anti-lymphangiogenic activity [47]. The anti-lymphangiogenic effect of cryptotanshinone was partly attributed to its downregulating protein expression of VEGF receptor 3 (VEGFR-3), as overexpression of VEGFR-3 conferred resistance to cryptotanshinone inhibition of the tube formation [47]. Furthermore, cryptotanshinone functioned as an anti-lymphangiogenic agent, in part by suppressing VEGFR-3-mediated Erk1/2 phosphorylation, and in part by inhibiting protein expression and activities of the small GTPases, such as Rac1 and Cdc42 [47]. More in vivo studies are expected to substantiate the in vitro results.
4.6. Anti-oxidant activity
Oxidative stress generates plenty of free radicals, involved in multiple diseases such as aging [48], cancer [49], obesity [50], and Alzheimer’s disease [51]. Studies have shown that cryptotanshinone exhibited inhibitory effects on xanthine oxidase activity [52] and pyrogallol autoxidation [53]. Cryptotanshinone improved the ability of learning and memory in APP/PS1 transgenic mice, protected rat cortical neurons from glutamic acid-induced neurotoxicity [54], and relieved acute liver damage induced by carbon tetrachloride [10], by suppression of lipid peroxidation. Cryptotanshinone was found to enhance the activity of anti-oxidant enzymes, such as superoxide dismutase, glutathione peroxidase and catalase, relieving carbon tetrachloride-induced liver injury in vivo, and reducing production of intracellular ROS in vitro [10]. Moreover, cryptotanshinone was able to directly clear hydroxyl free radicals in vitro [55]. Cryptotanshinone (10 μg/ml, corresponding to 3.37 μM) protected primary rat hepatocytes from bile acid-induced apoptosis by inhibiting JNK phosphorylation [56]. Also, cryptotanshinone (1 μM) directly inhibited hydrogen peroxide-induced NF-κB luciferase reporter gene activity in human umbilical vein endothelial cells [57]. The aforementioned data strongly suggest that cryptotanshinone is a potent anti-oxidant. However, our recent studies have shown that cryptotanshinone is also an oxidant, as treatment with cryptotanshinone was able to induce ROS in a time- and concentration-dependent manner in rhabdomyosarcoma (Rh30) and prostate cancer (DU145) cells [30]. Of note, in our studies, cryptotanshinone did not significantly increase ROS level in Rh30 and DU145 cells until 5 μM. Therefore, CPT acts as an anti-oxidant at low concentrations (<5 μM), whereas likely exerts oxidized activity at high concentrations (>5 μM). From publications, it appears very common that a natural product may act as an anti-oxidant or oxidant, depending on concentrations and environmental conditions [58]. Typical examples include (-)-epigallocatechin-3-gallate (EGCG) [59, 60], curcumin [61-63], and resveratrol [64, 65]. Definitely, the oxidant or anti-oxidant issue of cryptotanshinone warrants further investigation.
4.7. Targeting androgen receptor signaling
Development and progression of prostate cancer are intimately associated with androgen receptor (AR) signaling [66]. Anti-androgen agents that reduce or prevent androgen binding to AR are widely used to suppress AR-mediated prostate cancer growth [66, 67]. Recent studies have shown that cryptotanshinone targets AR signaling as well [68-70]. For instance, on days 16-18 of pregnancy, rats were injected subcutaneously with testosterone propionate continuously for 3 days, and then treated with cryptotanshinone by gavage for 14 days. It was found that cryptotanshinone reduced the serum level of 17α-hydroxy progesterone in male offspring, suggesting inhibition of androgen synthesis [71]. Further studies revealed that cryptotanshinone reversed the reproductive and metabolic disturbances in prenatally androgenized rats via regulating protein expression of insulin receptor substrate-1/2, PI3K p85α, glucose transporter-4, Erk-1, and 17α-hydroxylase in the ovaries [69]. Similarly, cryptotanshinone also reduced the levels of androgens by downregulating expression of the key enzymes for androgen synthesis in Akt-/- mice [72].
In addition, cryptotanshinone was also found to inhibit the growth of AR-positive prostate cancer cells [68]. This was attributed to cryptotanshinone inhibition of the transcriptional activity of AR, thereby downregulating expression of several AR-target genes at the mRNA and the protein levels [68]. Mechanistically, cryptotanshinone disrupted the interaction between AR and lysine-specific demethylase 1 (LSD1), and suppressed the AR-LSD1 complex to the promoter of AR target genes without influencing the protein degradation and translocation of AR [68]. Cryptotanshinone enhanced the mono-methyl and di-methylation of histone H3 lysine 9 (H3K9), a repressive histone marker that is demethylated and activated by LSD1 [68].
Furthermore, cryptotanshinone, similar to dihydrotestosterone (DHT) in structure, was also found to inhibit prostate cancer cell growth by suppressing DHT-induced AR transactivation, which resulted in reduced expression of the DHT-mediated AR target genes (PSA, TMPRSS2, and TMEPA1) in both androgen responsive prostate cancer cells, LNCaP cells and castration resistant CWR22rv1 cells [70]. At molecular level, cryptotanshinone blocked DHT-induced AR dimerization and AR-coregulator complex formation [70]. Moreover, cryptotanshinone effectively inhibited CWR22Rv1 cell growth and expression of AR target genes in a xenograft model [70]. Collectively, these findings highlight that cryptotanshinone is a promising therapeutic agent for prostate cancer.
5. Inhibition of mTOR signaling
mTOR, a conserved and ubiquitously expressed serine-threonine kinase, lies downstream of type I insulin-like growth factor (IGF-1) receptor and PI3K, and functions at least as two complexes (mTORC1 and mTORC2) in mammalian cells [73, 74]. In response to growth factors, mTORC1 and mTORC2 phosphorylates p70 ribosomal protein S6 kinase 1 (S6K1)/eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and Akt, respectively [73, 74]. Although the cellular functions of the mTOR complexes remain to be determined, current data indicate that mTOR is a central controller for cell growth, proliferation, survival, motility, invasion, angiogenesis and lymphangiogenesis [73-75]. As discussed above, cryptotanshinone functions as an anticancer agent by inhibiting cell proliferation, inducing cell death, suppressing cell motility/invasion, and inhibiting angiogenesis/lymphangiogenesis. In particular, cryptotanshinone has been found to target certain proteins, such as Stat3 [26], cyclin D1 [11], NF-κB [40], MMP9 [40], Akt [38, 76, 77], VEGF [78], VEGFR-3 [47], and small GTPases [47], which all are directly or indirectly regulated by mTOR [79-88]. This prompted us to hypothesize that the effects of cryptotanshinone on those proteins and the cellular events may be a consequence of inhibition of mTOR. Our recent findings [11] support this hypothesis. In the studies, we found that treatment of serum-starved Rh30 cells with cryptotanshinone for 2 h inhibited IGF-1-stimulated phosphorylation of S6K1 and 4E-BP1, in a dose- and time-dependent manner [11]. After 2 h exposure, cryptotanshinone obviously inhibited IGF-1-stimulated phosphorylation of S6K1 (Thr389) starting at 2.5 μM; and at 10 μM, cryptotanshinone profoundly suppressed this phosphorylation event within 2 h in Rh30 cells [11]. Similarly, cryptotanshinone also inhibited IGF-1-stimulated phosphorylation of 4E-BP1 in the cells [11]. Furthermore, cryptotanshinone did not apparently affect expression of total protein levels of S6K1 and 4E-BP1 [11]. In addition, we found that cryptotanshinone also inhibited IGF-1-stimulated phosphorylation of mTOR at Ser2448, a site phosphorylated by S6K1 [89], in a dose- and time-dependent manner [11]. Similar results were also noticed in DU145 and MCF-7 cells, and Rh30 cells grown in the normal culture medium containing 10% fetal bovine serum [11]. Interestingly, other tanshinones, including tanshinone I, tanshinone IIA and dihydrotanshinone, did not obviously affect phosphorylation of S6K1, 4E-BP1 and mTOR [11], which was in good agreement with the findings that cryptotanshinone, but not tanshinone I, tanshinone IIA and dihydrotanshinone, potently inhibited cancer cell proliferation [11]. Besides, expression of constitutively active mTOR conferred high resistance to cryptotanshinone inhibition of cyclin D1 expression and cell proliferation in Rh30 and DU145 cells [11].
However, in the studies, we also observed that cryptotanshinone did not affect expression of total Akt, but increased phosphorylation of Akt (Ser473) in Rh30 and DU145 cells [11]. Our result was in good consistence with the findings in C2C12 myotubes [76] and primary rat cortical neurons [77], but was in great contrast to the observation in macrophages [38]. Treatment with cryptotanshinone resulted in elevated phosphorylation of Akt (Ser473) in C2C12 cells [76]. Similarly, cryptotanshinone (5 μM) rapidly and transiently activated phosphorylation of Akt (Ser473) in primary rat cortical neurons [77]. Time course studies revealed that cryptotanshinone induced obvious activation of Akt as early as at 5 min, and maximal at 30 min before returning to the baseline at 3 h in primary rat cortical neurons [77]. Dose response studies indicated that cryptotanshinone was able to activate Akt at 0.1 μM, and the activation reached to the maximal level at 5 μM in the neurons [77]. Cryptotanshinone did not affect the expression either in C2C12 cells or in primary rat cortical neurons [76, 77]. In addition, it has been described that cryptotanshinone activation of Akt appeared to be mediated by PI3K in primary rat cortical neurons, since this could be blocked by LY294002 and wortmannin, two PI3K inhibitors [77]. In contrast, cryptotanshinone-induced Akt activation in C2C12 cells was independent of PI3K, and instead, this was through activation of AMP-activated protein kinase (AMPK), as Compound C, an AMPK inhibitor, profoundly attenuated cryptotanshinone-induced Akt activation [76]. It remains unclear how cryptotanshinone activates Akt in Rh30 and DU145 cells. However, cryptotanshinone was found to inhibit complement 5a (C5a) or macrophage inflammatory protein-1α (MIP-1α)-induced phosphorylation of Akt in RAW264.7 and primary human macrophages [38]. This was likely associated with inhibition of PI3K, as cryptotanshinone inhibited C5a or MIP-1α-induced PI3K-p110γ membrane translocation [38].
Taken together, current data suggest that cryptotanshinone inhibits mTORC1-mediated phosphorylation of S6K1 and 4E-BP1, but may inhibit or activate mTORC2-mediated Akt, depending on cell lines. It would be important to identify the underlying molecular mechanisms. The new data would help understand how cryptotanshinone acts as a new anticancer agent. The findings may also lead to design of novel tumor-selective treatments.
Conclusions and perspectives
Unlike tanshinone IIA that has been investigated comprehensively for a long time, cryptotanshinone was really concerned only in recent years, albeit they are very similar structurally. Based on what mentioned above, cryptotanshinone exerts diverse activities in vitro and in vivo. Increasing evidence indicates that cryptotanshinone is a potential anticancer agent. However, because of its poor bioavailability, cryptotanshinone has not been in clinical trials for any cancer therapy. Some attempts have been made to improve its bioavailability, but none of them proves effective enough. Clearly, more efforts are needed to solve the problem. Beside modification of its structure to increase the solubility and stability, nano-cryptotanshinone is a promising strategy. In addition, it is essential to further elucidate the molecular mechanisms by which cryptotanshinone functions as an anticancer agent. As mTOR is a master kinase that regulates cell growth/proliferation, survival, and motility, autophagy, as well as angiogenesis/lymphangiogenesis, understanding how cryptotanshinone inhibits mTOR signaling would shed new insights on the design and development of novel therapies.
Acknowledgments
This work was supported in part by the Open Project Program of National First-Class Key Discipline for Traditional Chinese Medicine of Nanjing University of Chinese Medicine (2011ZYX4-002), National Natural Science Foundation of China (30801545 to W. Chen; 81173174 to Y. Lu; 21162009 to G. Chen), Jiangsu Natural Science Foundation (BK2012854 to W. Chen), NIH (CA115414 to S. Huang), and American Cancer Society (RSG-08-135-01-CNE to S. Huang).
References
- 1.Scalbert A, Andres-Lacueva C, Arita M, Kroon P, Manach C, Urpi-Sarda M, Wishart D. Databases on food phytochemicals and their health-promoting effects. J. Agric. Food Chem. 2011;59(9):4331–48. doi: 10.1021/jf200591d. [DOI] [PubMed] [Google Scholar]
- 2.Tan BKH, Bay B, Zhu Y. Novel compounds from natural products in the new millennium: potential and challenges. World Scientific; Singapore: 2004. p. 183. [Google Scholar]
- 3.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005;45(12):1345–59. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 4.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol. Sin. 2000;21(12):1089–94. [PubMed] [Google Scholar]
- 5.Clebsch B. The New Book of Salvias: sages for every garden. Timber Press; Portland: 2003. p. 196. [Google Scholar]
- 6.Hu P, Luo GA, Zhao Z, Jiang ZH. Quality assessment of radix salviae miltiorrhizae. Chem. Pharm. Bull (Tokyo) 2005;53(5):481–6. doi: 10.1248/cpb.53.481. [DOI] [PubMed] [Google Scholar]
- 7.Zhao K, Guo XH, Song J, Wang RT, Yang XJ, Lin GB, Yan ZY. Nationwide survey and analysis of the Salvia production. Lishizhen Med. Materia Medica Res. 2010;21(9):2307–10. [Google Scholar]
- 8.Cheng TO. Cardiovascular effects of Danshen. Int. J. Cardiol. 2007;121(1):9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen LN, Zhu XX. Advances in study of the pharmacological effects of danshen on hemorheology. Zhongguo Zhong Yao Za Zhi. 2005;30(8):630–3. [PubMed] [Google Scholar]
- 10.Park EJ, Zhao YZ, Kim YC, Sohn DH. Preventive effects of a purified extract isolated from Salvia miltiorrhiza enriched with tanshinone I, tanshinone IIA and cryptotanshinone on hepatocyte injury in vitro and in vivo. Food Chem. Toxicol. 2009;47(11):2742–8. doi: 10.1016/j.fct.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Luo Y, Liu L, Zhou H, Xu B, Han X, Shen T, Liu Z, Lu Y, Huang S. Cryptotanshinone inhibits cancer cell proliferation by suppressing Mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev. Res. (Phila) 2010;3(8):1015–25. doi: 10.1158/1940-6207.CAPR-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takiura K. Study on components of Danshen: the structure of cryptotanshinone. J. Pharm. Soc. Japan. 1941;61(12):482–90. [Google Scholar]
- 13.Takiura K. Study on components of Danshen: isolation of pigments. J. Pharm. Soc. Japan. 1941;61(12):475–82. [Google Scholar]
- 14.Yang YB. The determination of stability of cryptotanshinone. Yunnan J. Tradit. Chin. Med. Materia Medica. 1990;11(3):24–5. [Google Scholar]
- 15.Dong C, Qiao JL, Yang P. Study on the fluorescence properties of cryptotanshinone and tanshinone IIA and their molecular structures in different acidic media. Chin. J. Spectrosc. Lab. 2000;17(4):369–72. [Google Scholar]
- 16.Inouye Y, Kakisawa H. Total Syntheses of Tanshinone-I, Tanshinone-II and Crytotanshinone. Bull Chem. Soc. 1969;42(11):3318–23. [Google Scholar]
- 17.Xie MZ, Shen ZF. Absorption, distribution, excretion and metabolism of cryptotanshinone. Yao Xue Xue Bao. 1983;18(2):90–6. [PubMed] [Google Scholar]
- 18.Xue M, Cui Y, Wang HQ, Luo YJ, Zhang B, Zhou ZT. Pharmacokinetics of cryptotanshinone and its metabolite in pigs. Yao Xue Xue Bao. 1999;34(2):81–4. [Google Scholar]
- 19.Xue M, Cui Y, Wang HQ, Hu ZH, Zhang B. Reversed-phase liquid chromatographic determination of cryptotanshinone and its active metabolite in pig plasma and urine. J. Pharm. Biomed. Anal. 1999;21(1):207–13. doi: 10.1016/s0731-7085(99)00098-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Huang M, Guan S, Bi HC, Pan Y, Duan W, Chan SY, Chen X, Hong YH, Bian JS, Yang HY, Zhou S. A mechanistic study of the intestinal absorption of cryptotanshinone, the major active constituent of Salvia miltiorrhiza. J. Pharmacol. Exp. Ther. 2006;317(3):1285–94. doi: 10.1124/jpet.105.100701. [DOI] [PubMed] [Google Scholar]
- 21.Pan Y, Bi HC, Zhong GP, Chen X, Zuo Z, Zhao LZ, Gu LQ, Liu PQ, Huang ZY, Zhou SF, Huang M. Pharmacokinetic characterization of hydroxylpropyl-beta-cyclodextrin-included complex of cryptotanshinone, an investigational cardiovascular drug purified from Danshen (Salvia miltiorrhiza) Xenobiotica. 2008;38(4):382–98. doi: 10.1080/00498250701827685. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y, Xue M, Luo YJ, Hu ZY, Zhang B, Zhou ZT, Shi YB, Xia WJ. Pig oral cryptotanshinone excretion data analysis. Chin. J. Vet. Sci. Tech. 1999;29(12):30–2. [Google Scholar]
- 23.Song M, Hang TJ, Zhang Z, Chen HY. Effects of the coexisting diterpenoid tanshinones on the pharmacokinetics of cryptotanshinone and tanshinone IIA in rat. Eur. J. Pharm. Sci. 2007;32(4-5):247–53. doi: 10.1016/j.ejps.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Sun JH, Yang M, Ma XC, Kang J, Han J, Guo DA. Microbial biotransformation of cryptotanshinone by Cunninghamella elegans and its application for metabolite identification in rat bile. J. Asian Nat. Prod. Res. 2009;11(6):482–9. doi: 10.1080/10286020902877754. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Lu Y, Zheng SZ, Wang AY. Research progress on pharmacological activities of cryptotanshinone. China J. Tradit. Chin. Med. Pharmacy. 2010;25(11):1839–41. [Google Scholar]
- 26.Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ, Han DC, Kwon BM. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69(1):193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Zheng SZ, Sun ZG, Wang AY, Huang CH, Punchard NA, Huang SL, Gao X, Lu Y. Cryptotanshinone has diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. Cancer Chemother. Pharmacol. 2011;68(1):17–27. doi: 10.1007/s00280-010-1440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H, Blackburn GL, Zhou JR. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer. 2011;129(5):1042–52. doi: 10.1002/ijc.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama H, Sen S. Aurora kinase inhibitors as anticancer molecules. Biochim. Biophys. Acta. 2010;1799(10-12):829–39. doi: 10.1016/j.bbagrm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Liu L, Luo Y, Odaka Y, Awate S, Zhou H, Shen T, Zheng S, Lu Y, Huang S. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev. Res. (Phila) 2012;5(5):778–87. doi: 10.1158/1940-6207.CAPR-11-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TJ. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest. 2001;107(3):351–62. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101(4):1535–42. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuda M, Wakasaki T, Suzui M, Toh S, Joe AK, Weinstein IB. Stat3 orchestrates tumor development and progression: the Achilles’ heel of head and neck cancers? Curr. Cancer Drug Targets. 2010;10(1):117–26. doi: 10.2174/156800910790980197. [DOI] [PubMed] [Google Scholar]
- 35.Park IJ, Kim MJ, Park OJ, Choe W, Kang I, Kim SS, Ha J. Cryptotanshinone induces ER stress-mediated apoptosis in HepG2 and MCF7 cells. Apoptosis. 2012;17(3):248–57. doi: 10.1007/s10495-011-0680-3. [DOI] [PubMed] [Google Scholar]
- 36.Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat. Rev. Cancer. 2011;11(3):177–87. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- 37.Chiou WF, Don MJ. Cryptotanshinone inhibits macrophage migration by impeding F-actin polymerization and filopodia extension. Life Sci. 2007;81(2):109–14. doi: 10.1016/j.lfs.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Don MJ, Liao JF, Lin LY, Chiou WF. Cryptotanshinone inhibits chemotactic migration in macrophages through negative regulation of the PI3K signaling pathway. Br. J. Pharmacol. 2007;151(5):638–46. doi: 10.1038/sj.bjp.0707271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIA inhibits angiogenesisin in vitro. Exp. Mol. Med. 2005;37(2):133–7. doi: 10.1038/emm.2005.18. [DOI] [PubMed] [Google Scholar]
- 40.Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, Jeon SJ, Son KH, Chang YC, Lee YC, Kim CH. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem. Pharmacol. 2006;72(12):1680–9. doi: 10.1016/j.bcp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 42.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 43.Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: overview of the literature. Expert Rev. Anticancer Ther. 2012;12(5):567–80. doi: 10.1586/era.12.13. [DOI] [PubMed] [Google Scholar]
- 44.Bian WP, Xu Y, Wang J, Chen F. The antiangiogenesis effect of cryptotanshinone on chick embryo chorioallantoic membrane. J. Chin. Microcirculation. 2007;11(1):23–6. [Google Scholar]
- 45.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140(4):460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25(4):677–94. doi: 10.1007/s10555-006-9026-y. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y, Chen W, Zhou H, Liu L, Shen T, Alexander JS, Zheng S, Lu Y, Huang S. Cryptotanshinone inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3/ERK and small GTPase pathways. Cancer Prev. Res. (Phila) 2011;4(12):2083–91. doi: 10.1158/1940-6207.CAPR-11-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malinin NL, West XZ, Byzova TV. Oxidation as “the stress of life”. Aging (Albany NY) 2011;3(9):906–10. doi: 10.18632/aging.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawless MW, O’Byrne KJ, Gray SG. Targeting oxidative stress in cancer. Expert Opin. Ther. Targets. 2010;14(11):1225–45. doi: 10.1517/14728222.2010.526933. [DOI] [PubMed] [Google Scholar]
- 50.Van Gaal LF, Zhang A, Steijaert MM, De Leeuw IH. Human obesity: from lipid abnormalities to lipid oxidation. Int. J. Obes. Relat. Metab. Disord. 1995;19(Suppl 3):S21–S26. [PubMed] [Google Scholar]
- 51.Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59(4-5):290–4. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Zhang HD, Huang YL, He JQ. Inhibition of diterpenoid tanshinones from salvia miltiorrhiza bge. on xanthine oxidase activity. Chin. J. Pharmacol. Toxicol. 2007;21(3):174–8. [Google Scholar]
- 53.Zhang HD, Chen M, Ma L, Gu LQ. The inhibition on pyrogallol autoxidation of diterpenoid tanshinones from salvia miltiorrhiza bge. Pharmacol. Clin. Chin. Materia Medica. 2004;20(4):16–9. [Google Scholar]
- 54.Zhang FY, Chen SR, Mei ZR, Liu PQ. Protective effect of cryptotanshinone on glutamaic acid induced injury in cortical neurons. Chin. Pharmaceut. J. 2011;46(16):1245–8. [Google Scholar]
- 55.Xue M, Cui Y, Wang HQ, Shi YB, Zhang B, Luo YJ, Zhou ZT, Xia WJ, Zhao RC. Scavenging effect of diterpenoid tanshinones and some natural products on hydroxyl free radicals. Chin. J. Vet. Drug. 1999;33(2):15–8. [Google Scholar]
- 56.Park EJ, Zhao YZ, Kim YC, Sohn DH. PF2401-SF, standardized fraction of Salvia miltiorrhiza and its constituents, tanshinone I, tanshinone IIA, and cryptotanshinone, protect primary cultured rat hepatocytes from bile acid-induced apoptosis by inhibiting JNK phosphorylation. Food Chem. Toxicol. 2007;45(10):1891–8. doi: 10.1016/j.fct.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Jin YC, Kim CW, Kim YM, Nizamutdinova IT, Ha YM, Kim HJ, Seo HG, Son KH, Jeon SJ, Kang SS, Kim YS, Kam SC, Lee JH, Chang KC. Cryptotanshinone, a lipophilic compound of Salvia miltiorrhiza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur. J. Pharmacol. 2009;614(1-3):91–7. doi: 10.1016/j.ejphar.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 58.Korkina LG, De Luca C, Kostyuk VA, Pastore S. Plant polyphenols and tumors: from mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009;16(30):3943–65. doi: 10.2174/092986709789352312. [DOI] [PubMed] [Google Scholar]
- 59.Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (-) Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J. Nutr. 2005;135(4):707–13. doi: 10.1093/jn/135.4.707. [DOI] [PubMed] [Google Scholar]
- 60.Coyle CH, Philips BJ, Morrisroe SN, Chancellor MB, Yoshimura N. Antioxidant effects of green tea and its polyphenols on bladder cells. Life Sci. 2008;83(1-2):12–8. doi: 10.1016/j.lfs.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan WH, Wu HJ, Hsuuw YD. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann. N Y Acad. Sci. 2005;1042:372–8. doi: 10.1196/annals.1338.057. [DOI] [PubMed] [Google Scholar]
- 62.Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol. Med. 2008;45(10):1403–12. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 63.Chen Q, Wang Y, Xu K, Lu G, Ying Z, Wu L, Zhan J, Fang R, Wu Y, Zhou J. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol. Rep. 2010;23(2):397–403. [PubMed] [Google Scholar]
- 64.Filomeni G, Graziani I, Rotilio G, Ciriolo MR. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2(3):295–305. doi: 10.1007/s12263-007-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose) polymerase-LKB1 pathway. Mol. Pharmacol. 2009;76(4):884–95. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 66.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin. Cancer Res. 2011;17(12):3876–83. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 67.Johnson JJ, Syed DN, Suh Y, Heren CR, Saleem M, Siddiqui IA, Mukhtar H. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev. Res. (Phila) 2010;3(9):1112–23. doi: 10.1158/1940-6207.CAPR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu CY, Hsieh CY, Huang KE, Chang C, Kang HY. Cryptotanshinone down-regulates androgen receptor signaling by modulating lysine-specific demethylase 1 function. Int. J. Cancer. 2011 Nov 2; doi: 10.1002/ijc.27343. doi: 10.1002/ijc.27343. [DOI] [PubMed] [Google Scholar]
- 69.Yang X, Zhang Y, Wu X, Bae CS, Hou L, Kuang H, Wang Y, Stener-Victorin E. Cryptotanshinone reverses reproductive and metabolic disturbances in prenatally androgenized rats via regulation of ovarian signaling mechanisms and androgen synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300(4):R869–75. doi: 10.1152/ajpregu.00334.2010. [DOI] [PubMed] [Google Scholar]
- 70.Xu D, Lin TH, Li S, Da J, Wen XQ, Ding J, Chang C, Yeh S. Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 2012;316(1):11–22. doi: 10.1016/j.canlet.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li XH, Yang XM, Wu XK. Effects of cryptotanshinone in lowering androgens synthesis for the prenatally androgenized male rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(11):1001–4. [PubMed] [Google Scholar]
- 72.Zhao LL, Zhang YH, Wang NM, Wu XK, Hou LH. Impact of Cryptotanshinone on the reproductivity and metabolism of male mice with Akt2 deletion. Zhonghua Nan Ke Xue. 2011;17(7):662–8. [PubMed] [Google Scholar]
- 73.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009;21(2):209–18. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 74.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou H, Huang S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr. Protein Pept. Sci. 2011;12(1):30–42. doi: 10.2174/138920311795659407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim EJ, Jung SN, Son KH, Kim SR, Ha TY, Park MG, Jo IG, Park JG, Choe W, Kim SS, Ha J. Antidiabetes and antiobesity effect of cryptotanshinone via activation of AMP-activated protein kinase. Mol. Pharmacol. 2007;72(1):62–72. doi: 10.1124/mol.107.034447. [DOI] [PubMed] [Google Scholar]
- 77.Zhang F, Zheng W, Pi R, Mei Z, Bao Y, Gao J, Tang W, Chen S, Liu P. Cryptotanshinone protects primary rat cortical neurons from glutamate-induced neurotoxicity via the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. Exp. Brain Res. 2009;193(1):109–18. doi: 10.1007/s00221-008-1600-9. [DOI] [PubMed] [Google Scholar]
- 78.Dat NT, Jin X, Lee JH, Lee D, Hong YS, Lee K, Kim YH, Lee JJ. Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor 1. J. Nat. Prod. 2007;70(7):1093–7. doi: 10.1021/np060482d. [DOI] [PubMed] [Google Scholar]
- 79.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 2000;10(1):47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 80.Fang P, Hwa V, Rosenfeld RG. Interferon-gamma-induced dephosphorylation of STAT3 and apoptosis are dependent on the mTOR pathway. Exp. Cell Res. 2006;312(8):1229–39. doi: 10.1016/j.yexcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin ER, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. U S A. 2007;104(41):16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J. Biol. Chem. 1998;273(23):14424–9. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- 83.Murooka TT, Rahbar R, Platanias LC, Fish EN. CCL5-mediated T-cell chemotaxis involves the initiation of mRNA translation through mTOR/4E-BP1. Blood. 2008;111(10):4892–901. doi: 10.1182/blood-2007-11-125039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lawrence DM, Singh RS, Franklin DP, Carey DJ, Elmore JR. Rapamycin suppresses experimental aortic aneurysm growth. J. Vasc. Surg. 2004;40(2):334–8. doi: 10.1016/j.jvs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 85.Jundt F, Raetzel N, Muller C, Calkhoven CF, Kley K, Mathas S, Lietz A, Leutz A, Dorken B. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein {beta} and NF-{kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood. 2005;106(5):1801–7. doi: 10.1182/blood-2004-11-4513. [DOI] [PubMed] [Google Scholar]
- 86.Luo Y, Liu L, Rogers D, Su W, Odaka Y, Zhou H, Chen W, Shen T, Alexander JS, Huang S. Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression. Neoplasia. 2012;14(3):228–37. doi: 10.1593/neo.111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X, Huang S. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J. Biol. Chem. 2010;285(49):38362–73. doi: 10.1074/jbc.M110.141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71(9):3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123(4):569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]