Abstract
Background
HIV discordant heterosexual couples are faced with the dual challenge of preventing sexual HIV transmission and unplanned pregnancies with the attendant risk of perinatal HIV transmission. Our aim was to examine uptake of two long-acting reversible contraceptive (LARC) methods – intrauterine devices (IUDs) and hormonal implants – among HIV discordant couples in Rwanda and Zambia.
Study Design
Women were interviewed alone or with their partner during routine cohort study follow-up visits to ascertain fertility goals; those not pregnant, not infertile, not already using LARC, and wishing to limit or delay fertility for ≥3 years were counseled on LARC methods and offered an IUD and implant on-site.
Results
Among 409 fertile Rwandan women interviewed (126 alone, 283 with partners), 365 (89%) were counseled about LARC methods and 130 (36%) adopted a method (100 implant, 30 IUD). Of 787 fertile Zambian women interviewed (457 alone, 330 with partners), 528 (67%) received LARC counseling, of whom 177 (34%) adopted a method (139 implant, 38 IUD). In both countries, a woman’s younger age was predictive of LARC uptake. LARC users reported fewer episodes of unprotected sex than couples using only condoms.
Conclusions
Integrated fertility-goal based family planning counseling and access to LARC methods with reinforcement of dual-method use prompted uptake of IUDs and implants and reduced unprotected sex among HIV-discordant couples in two African capital cities.
Keywords: contraception, family planning, HIV, intrauterine devices, implant
1. Introduction
Sub-Saharan Africa remains the region most heavily affected by HIV, accounting for 68% of the global HIV burden [1]. In 2008, 70% of worldwide new HIV infections occurred in the region, and more than 14 million children had lost one or both parents to AIDS [1]. Of the estimated 430,000 annual global new infections among children under 15 years of age, over 90% occurred in Sub-Saharan Africa [1]. This high HIV prevalence coincides with a high regional total fertility rate (TFR) of 4.8 children per woman [2]. The region also has the lowest contraceptive prevalence in the world due to multiple factors including inadequate and unreliable availability of contraceptives, insufficient health care infrastructure, a large rural population with limited access to health services, poor economic development, misconceptions and knowledge gaps regarding contraceptive methods, and cultural norms which value high fertility [3]. Consequently, three issues relating to fertility and the HIV/AIDS epidemic continue to plague the region: the high proportion of new infections occurring in marriage [4], the risk of HIV-positive births from unintended pregnancies, and the lack of ‘dual method use’ (combining condoms with an effective modern contraceptive method for added protection against unplanned pregnancy) in cohabiting couples. HIV/AIDS prevention programs must enable the prevention of unplanned pregnancies among HIV-affected couples to mutually leverage prevention of perinatal and heterosexual HIV.
While many studies have investigated factors associated with contraceptive uptake in African countries [5], fewer data are available on family size preferences and contraceptive use among HIV-infected women. Contraceptive use by HIV-positive women can prevent unplanned HIV-positive births and reduce unsafe abortions and maternal and child mortality [6]. In addition, the contraceptive needs of discordant couples (in which one partner is HIV-positive and the other is HIV-negative) have gone largely unrecognized. It is essential to focus HIV prevention efforts on these couples as they are faced with the dual burden of high heterosexual HIV infection risk and the subsequent risk of mother-to-child transmission. The challenge is to provide discordant couples with family planning choices that effectively manage both their risk of HIV transmission and their fertility goals.
Long-acting reversible contraceptives (LARCs), which include intrauterine devices (IUDs) and hormonal implants, are more efficacious and cost-effective than short-acting methods such as injectables and oral contraceptives for pregnancy prevention since they are less dependent on user adherence and consistent supply chains and have lower discontinuation rates and longer duration of action [7]. In most African countries, the two most common contraceptive methods are injectables and oral contraceptives. Few health providers have been trained to insert IUDs and implants, and access to surgical sterilization is restricted to facilities with doctors and operating rooms. Previous studies have demonstrated that increasing access to LARC methods improves their uptake among HIV-positive women. In Rwanda, providing information and access to hormonal contraceptives resulted in increased use among both HIV-positive and HIV-negative women [8], and when access to LARC was provided on-site, a substantial portion of HIV-positive women requested hormonal implants postpartum [9]. In Zambia, improving access to non-barrier methods among HIV-concordant-positive and HIV-discordant couples already using condoms increased dual-method use three-fold [10], and a video-based intervention resulted in significantly increased uptake of LARC in discordant and concordant HIV-positive couples [11].
An estimated 70-90% of new adult HIV infections in sub-Saharan Africa are acquired in marriage [4], and numerous studies have demonstrated the positive impact of couples’ voluntary counseling and testing (CVCT) in reducing the incidence of HIV [12-17]. However, to date, no studies have examined the relationship between fertility intentions and LARC use or dual method use among HIV discordant couples. We investigated factors associated with IUD and implant uptake among HIV discordant couples who have undergone CVCT in two African cities using a short questionnaire to ascertain fertility intentions and offering targeted LARC counseling and methods on-site.
1.1 HIV prevalence and contraceptive use in Rwanda and Zambia
In 2007, HIV prevalence in Rwanda for persons aged 15-49 years was estimated at 2.8%, with higher rates in urban (7.3%) versus rural areas (2.2%) [18]. The total number of AIDS orphans was 220,000 [18]. The TFR was 5.5 in 2007-2008 [19]. Contraceptive use among married women was 36.4% for any method and 27.0% for modern methods, with injectables (15.2%) and pills (6.4%) being the most common forms of modern contraceptive [19]. Prevalence of IUD use was 0.2% and of implants was 1.6% [19].
HIV prevalence in Zambia in 2007 was 15.2% among persons aged 15-49 years, with urban rates (23.1%) twice the rural rates (10.8%) [20]. There are currently 600,000 AIDS orphans living in the country [20]. Zambia’s TFR was 5.9, and 40.8% of married women reported using a contraceptive method, with 32.7% reporting use of a modern method (including sterilization, pills, IUDs, injectables, implants, and condoms) [21]. Among currently married women, the most commonly used modern method was the pill (11.0%), followed by injectables (8.5%) [21]. Prevalence of IUD and implant use was extremely low at 0.1% and 0.4%, respectively [21].
2. Methods
2.1 Rwanda Zambia HIV Research Group
The Rwanda Zambia HIV Research Group (RZHRG), based at Emory University in Atlanta, Georgia, operates clinical research sites in Africa, including Projet San Francisco (PSF) in Kigali, Rwanda, and the Zambia-Emory HIV Research Project (ZEHRP) in Lusaka, Zambia. Couples receiving joint, same-day HIV counseling and testing at weekend CVCT services in government clinics were invited to participate in ongoing cohort studies based at the two research centers. Participating couples returned every one to three months for follow-up visits during which behavioral and clinical data were collected and couples were provided with condom skills training and condom counseling. At enrollment in the clinical studies and during routine follow-up visits, couples were offered the full range of contraceptive methods (oral contraceptive pills, injectables, IUDs, implants, tubal ligation, vasectomy/hysterectomy, and condoms), sexually transmitted infection (STI) screening and treatment, and referral for antiretroviral (ARV) for the HIV-positive partner. Condoms were advised for both STI/HIV and pregnancy prevention, and an additional modern contraceptive was recommended for added protection against unplanned pregnancy (‘dual method use’). Women’s self-reported data on sexual exposures with their partners, both with and without condoms, were collected at follow-up visits.
Research clinics were staffed by rotating University physicians and senior nurses, and participants were provided with the government health insurance plan to cover off-hours and inpatient care. Further details regarding CVCT recruitment, enrollment, and retention methods and procedures, as well as HIV results and demographic characteristics of RZHRG study participants, have been published previously [12, 22-24].
2.2 Study population
Discordant couples enrolled in a longitudinal cohort study of heterosexual transmission completed a short supplemental questionnaire between August 6, 2009 and December 15, 2010 in Kigali and July 9, 2009 and December 18, 2010 in Lusaka. Women aged 18-45 years and men aged 18-65 years at enrollment were eligible to participate. Additionally, those who were not pregnant, not infertile (due to menopause, vasectomy, hysterectomy, or tubal ligation), not on IUD/implant, and who expressed a desire to have no more children or wait at least 3 years for their next child received information about IUDs and implants and point-of-service insertion. Couples were interviewed together, or the woman was interviewed alone if her partner was not present for that visit.
2.3 Data collection
The questionnaire was administered during routine follow-up visits to ascertain when the woman last gave birth, what family planning method was currently being used, and fertility intentions. The WHO Medical eligibility criteria for contraceptive use [25] were applied to determine the woman’s eligibility for LARC. Eligible couples or individual women desiring to limit further childbearing or birth spacing of at least 3 years were counseled about the Copper-T IUD and Jadelle implant and were reminded that these LARC methods were available on-site. Those expressing interest could elect to have the method inserted immediately or at a later follow-up appointment. Prior to insertion, urine pregnancy tests were performed, and in the case of IUD, a gynecologic exam was performed to rule out STI. Women and couples who wanted a child within the next 3 years were counseled on the benefits of birth spacing and the full range of contraceptive options available at the clinic was reviewed. Though they were not interviewed further for this sub-study, contraceptive use data from the parent cohort studies is presented.
2.4 Data analysis
We defined the outcome of interest as IUD/implant uptake, either on the day of questionnaire administration or at a subsequent visit. Women interviewed at least once with their partners were categorized as ‘interviewed as a couple.’ Data were analyzed using SAS version 9.2 (SAS Institute, Cary, North Carolina, USA). In bivariate analyses, differences in characteristics between those who adopted an IUD, implant, or neither method were calculated using chi-square (or Fisher’s exact) tests for categorical variables and ANOVA for continuous variables. A statistically significant difference was considered to be a p-value <0.05. The multivariate logistic regression analysis modeling predictors of LARC uptake included significant predictors in the bivariate analyses and variables with a scientific rationale for inclusion. Associations are reported as odds ratios (ORs) with 95% confidence intervals (CIs). We limited our sample in the bivariate and multivariate analyses to couples that were currently not pregnant, not infertile, not on IUD or implant, and who wanted to limit or delay fertility for at least 3 years. The final sample size for the bivariate and multivariate analyses is thus 365 for Kigali and 528 for Lusaka. Differences in the mean number of reported unprotected sexual encounters among women on LARC and those using condoms as their only form of contraception were assessed using independent sample t-tests for single comparison and Bonferroni adjustment for multiple comparisons. All available follow-up intervals for LARC users and condom-only users were included.
2.5 Ethics
Participants signed written informed consent in Kinyarwanda (Rwanda) or Nyanja (Zambia) approved by the Office of Human Research Protections-registered Institutional Review Boards at Emory University, the National Ethics Committee of Rwanda, and the University of Zambia Research Ethics Committee.
3. Results
3.1 IUD/implant uptake in Rwanda
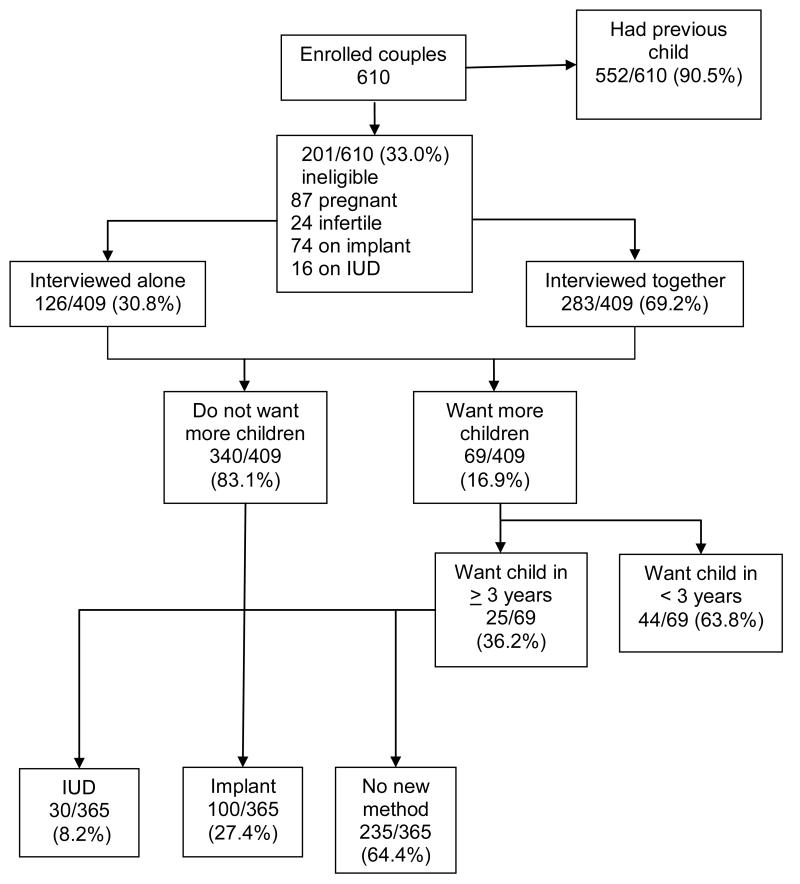
Of 610 discordant couples who completed the LARC study questionnaire, 552 (90.5%) had previously had a child; one hundred eleven women were ineligible due to pregnancy (n=87) or infertility (n=24) (Fig. 1). Of 499 fertile couples, 74 (14.8%) were using a hormonal implant, and 16 (3.2%) were using an IUD that they had previously requested at the research clinic. Of the remaining 409 couples, 126 (30.8%) women were interviewed alone, and 283 (69.2%) were interviewed with their partners. Of these, 340 couples (83.1%) did not want more children, 25 (6.1%) wanted to wait at least 3 years, and 44 (10.8%) wanted a child within the next 3 years. Of the 365 couples wanting to limit or delay childbearing for ≥3 years, 130 requested a LARC method, with 30 (8.2%) choosing IUDs and 100 (27.4%) receiving implants. Of 44 women/couples who wanted a child in the next 3 years, 5 were using injectables, 1 was using oral contraceptive pills (OCP), and 38 were using only condoms. After the sub-study questionnaire, 4 women either switched or started a new method (1 OCP, 2 injectables, and 1 implant), one injectable user stopped all non-barrier methods while one continued with the current method, and 38 remained using only condoms.
Fig. 1.
Enrollment and uptake of LARC among HIV discordant couples in Kigali, Rwanda (August 6, 2009 – December 15, 2010).
3.2. IUD/implant uptake in Zambia
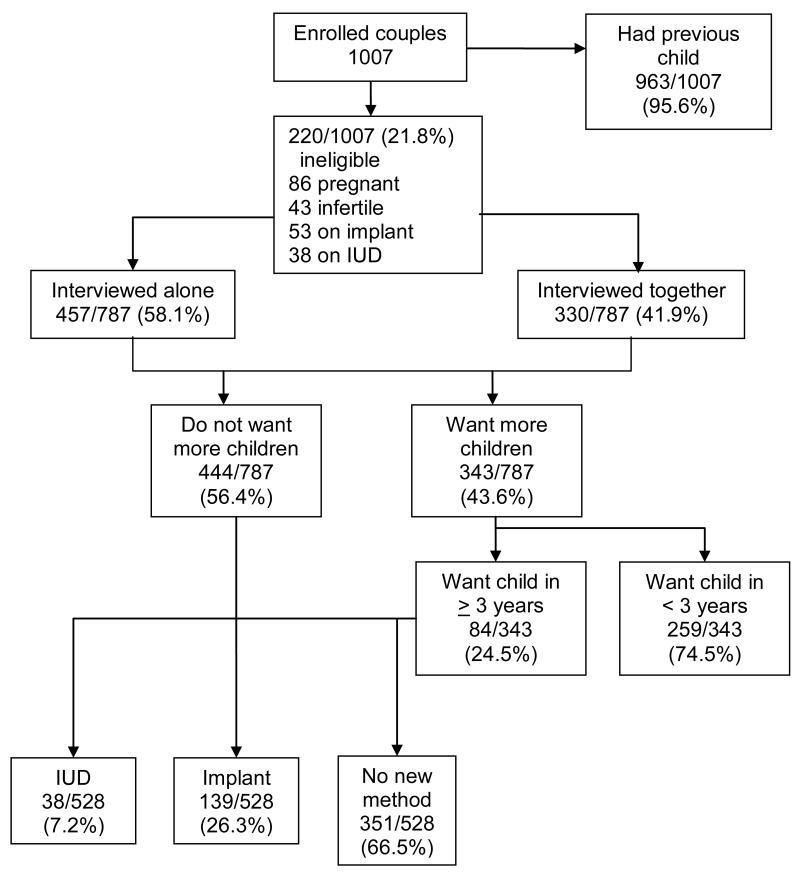
In Zambia, 963/1007 (95.6%) discordant couples completing the LARC study questionnaire had previously had a child. One hundred twenty-nine women were ineligible due to pregnancy (n=86) or infertility (n=43) (Fig. 2). Of 878 fertile couples, 53 (6.7%) had previously requested the hormonal implant, and 38 (4.8%) were using IUDs provided at the research clinic. Of the remaining 787 couples, 457 (58.1%) women were interviewed alone, and 330 (41.9%) were interviewed with their partners. Of these, 444 couples (56.4%) did not want more children, 84 (10.7%) wanted to wait at least 3 years, and 259 couples (32.9%) wanted a child within the next 3 years. A total of 528 eligible couples were counseled on IUDs and implants: 177 requested a LARC method with 38 women (7.2%) receiving IUDs and 139 (26.3%) having implants inserted. Of 259 women/couples wanting a child in the next 3 years, 21 were using injectables, 14 were on OCPs, and 224 were using only condoms. After the sub-study questionnaire, 28 women switched or started a new method (8 OCP, 8 injectables, 10 implants, 1 IUD, and 1 tubal ligation), 2 injectable and 2 OCP users ceased all non-barrier methods, 31 continued with current method, and 196 remained using condoms alone.
Fig. 2.
Enrollment and uptake of LARC among HIV discordant couples in Lusaka, Zambia (July 9, 2009 – December 18, 2010).
3.3. Bivariate analyses
Among couples wishing to limit fertility or delay conception for ≥3 years, LARC uptake was similar among women interviewed alone and those interviewed with their partner present. Couples with HIV-positive men and those with HIV-positive women had similar LARC selection patterns in Zambia, while the distribution of the methods chosen by Rwandan couples differed by the serostatus of the woman partner, with HIV-positive women being more likely to select the implant than couples with HIV-negative women (39% vs 22%, p=0.05: Table 1). ARV use, baseline contraceptive use (condoms only, injectable contraception, or oral contraception) and reproductive goals (limiting vs delaying pregnancy) were not associated with LARC uptake in either city. In Kigali, women who chose implants were significantly younger than IUD or non-LARC users (implant=27.5, IUD=29.6, no LARC=30.0, p=0.003) (Table 1).
Table 1.
Demographics, fertility, health, and contraceptive characteristics of discordant couples eligible for LARC in Kigali, Rwanda (August 6, 2009 to December 15, 2010) and Lusaka, Zambia (July 9, 2009 to December 18, 2010)
| Kigali, Rwanda | Lusaka, Zambia | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N=365) |
IUD (N=30) | Implant (N=100) |
No LARC (N=235) |
p- value |
Total (N=528) |
IUD (N=38) |
Implant (N=139) |
No LARC (N=351) |
p- value |
|||||||||
|
Questionnaire
administered to: |
N | % | N | % | N | % | N | % | 0.63 | N | % | N | % | N | % | N | % | 0.38 |
| Woman alone | 111 | 30.4 | 7 | 6.3 | 30 | 27.0 | 74 | 66.7 | 333 | 63.1 | 21 | 6.3 | 93 | 27.9 | 219 | 65.8 | ||
| Couple | 254 | 69.6 | 23 | 9.1 | 70 | 27.6 | 161 | 63.4 | 195 | 36.9 | 17 | 8.7 | 46 | 23.6 | 132 | 67.7 | ||
|
Couple’s HIV serostatus
at baseline |
0.05* | 0.79 | ||||||||||||||||
| Discordant (man is HIV positive) |
166 | 45.5 | 17 | 10.2 | 36 | 21.7 | 113 | 68.1 | 242 | 45.8 | 18 | 7.4 | 67 | 27.7 | 157 | 64.9 | ||
| Discordant (woman is HIV positive) |
199 | 54.5 | 13 | 6.5 | 64 | 32.2 | 122 | 61.3 | 286 | 54.2 | 20 | 7.0 | 72 | 25.2 | 194 | 67.8 | ||
|
HIV-positive partner on
antiretroviral therapy |
0.25 | 0.77 | ||||||||||||||||
| Yes | 79 | 21.6 | 3 | 3.8 | 21 | 26.6 | 55 | 69.6 | 86 | 16.3 | 6 | 7.0 | 20 | 23.3 | 60 | 69.8 | ||
| No | 286 | 78.4 | 27 | 9.4 | 79 | 27.6 | 180 | 62.9 | 442 | 83.7 | 32 | 7.2 | 119 | 26.9 | 291 | 65.8 | ||
| Reproductive intentions | 0.68 | 0.52 | ||||||||||||||||
| Want a child in ≥3 years | 25 | 6.8 | 2 | 8.0 | 5 | 20.0 | 18 | 72.0 | 83 | 15.7 | 4 | 4.8 | 25 | 30.1 | 54 | 65.1 | ||
| Do not want more children |
340 | 93.2 | 28 | 8.2 | 95 | 27.9 | 217 | 63.8 | 445 | 84.3 | 34 | 7.6 | 114 | 25.6 | 297 | 66.7 | ||
|
Current contraceptive
method |
0.41 | 0.60 | ||||||||||||||||
| Condoms only | 289 | 79.2 | 25 | 8.7 | 72 | 24.9 | 192 | 66.4 | 414 | 78.4 | 33 | 8.0 | 114 | 27.5 | 267 | 64.5 | ||
| Injectables | 65 | 17.8 | 5 | 7.7 | 23 | 35.4 | 37 | 56.9 | 51 | 9.7 | 2 | 3.9 | 10 | 19.6 | 39 | 76.5 | ||
| Oral contraceptives | 11 | 3.0 | 0 | 0.0 | 5 | 45.5 | 6 | 54.5 | 63 | 11.9 | 3 | 4.8 | 15 | 23.8 | 45 | 71.4 | ||
| Women age, mean (SD) | 29.2 | 6.1 | 29.6 | 5.8 | 27.5 | 5.4 | 30.0 | 6.3 | 0.003* | 29.7 | 6.3 | 29.6 | 6.7 | 28.6 | 6.0 | 30.1 | 6.4 | 0.06 |
| Men age, mean (SD) | 34.8 | 7.9 | 34.8 | 8.1 | 34.0 | 8.3 | 35.1 | 7.7 | 0.49 | 36.0 | 7.5 | 35.3 | 8.2 | 35.2 | 7.7 | 36.4 | 7.3 | 0.24 |
|
Number of previous
pregnancies, mean (SD) |
3.0 | 2.0 | 2.8 | 2.0 | 2.7 | 1.8 | 3.2 | 2.1 | 0.10 | 3.7 | 2.1 | 3.8 | 2.2 | 3.5 | 2.1 | 3.8 | 2.1 | 0.48 |
Statistically significant (p<0.05) with “No LARC” as the reference group.
3.4. Multivariate analysis
The multivariate analysis modeling predictors of LARC uptake included interviewing alone or with the partner, ARV use in the positive partner, couples’ HIV serostatus, the ages of the man and woman, reproductive intentions (desire no more children vs. delay for ≥3 years), current contraceptive method, and number of previous pregnancies. In both cities, a woman’s younger age was a significant predictor of LARC uptake when adjusted for other factors (Kigali: p=0.05, Lusaka: p=0.02, Table 2). With each one year increase in the woman’s age, the odds of the woman adopting LARC decreased by 5% in Kigali (OR=0.95, 95%CI: 0.91-1.00, Table 2) and Lusaka (OR=0.95, 95% CI: 0.92-0.99, Table 2). Current method of contraception was also found to be a significant predictor of LARC uptake in the multivariate; women currently using injectables in Lusaka were half as likely to adopt LARC compared to condom-only users (OR=0.48, 95%CI: 0.24-0.98, Table 2).
Table 2.
Logistic regression model for factors associated with LARC uptake among discordant couples in Kigali, Rwanda (N=365) and Lusaka, Zambia (N=528)
| Kigali, Rwanda | Lusaka, Zambia | |||
|---|---|---|---|---|
| OR (95% CI)≠ | p-value | OR (95% CI)≠ | p-value | |
| Questionnaire administered to: | ||||
| Woman alone | 1.0 (Ref) | 1.0 (Ref) | ||
| Couple | 1.18 (0.72, 1.94) | 0.52 | 0.89 (0.60, 1.33) | 0.58 |
| Couple’s HIV serostatus at baseline | ||||
| Discordant (man is positive) | 1.0 (Ref) | 1.0 (Ref) | ||
| Discordant (woman is positive) | 1.25 (0.79, 1.99) | 0.34 | 0.88 (0.61, 1.29) | 0.58 |
|
HIV-positive partner on antiretroviral
therapy |
||||
| No | 1.0 (Ref) | 1.0 (Ref) | ||
| Yes | 0.84 (0.48, 1.48) | 0.54 | 0.84 (0.50, 1.42) | 0.51 |
| Reproductive intentions | ||||
| Want a child in ≥3 years | 1.0 (Ref) | 1.0 (Ref) | ||
| Do not want more children | 1.84 (0.72, 4.69) | 0.20 | 1.04 (0.62, 1.74) | 0.88 |
| Current contraceptive method | ||||
| None/condoms only | 1.0 (Ref) | 1.0 (Ref) | ||
| Injectables | 1.34 (0.74, 2.40) | 0.33 | 0.48 (0.24, 0.98) | 0.04* |
| Oral contraceptives | 1.51 (0.41, 5.52) | 0.53 | 0.76 (0.42, 1.38) | 0.37 |
| Women age | 0.95 (0.91, 1.00) | 0.05* | 0.95 (0.92, 0.99) | 0.02* |
| Number of previous pregnancies | 0.96 (0.83, 1.11) | 0.56 | 1.04 (0.94, 1.17) | 0.42 |
Adjusted for all other covariates in the table.
Statistically significant (p<0.05).
3.5 Condom use among LARC users
A separate analysis examining LARC use and self-reported incidence of unprotected sex with respect to pregnancy prevention during follow-up revealed that LARC users reported less unprotected sex than couples using only condoms. In Lusaka, women using condoms as their only method of contraception reported an average of 1.44 unprotected sexual encounters per 3-month interval while LARC users reported 1.03 (p=0.02). The average number of unprotected sexual encounters among condom-only users in Kigali was 0.99, while it was 0.65 (p=0.02) among LARC users per 3-month interval. When the two LARC methods were compared separately with condom-only use, both IUD and implant users reported fewer unprotected sexual encounters with respect to pregnancy prevention than condom-only users. However, when the Bonferroni adjustment for multiple comparisons was applied these differences were not significant due to small sample size (results not shown).
4. Discussion
Antiretrovirals are highly effective in preventing perinatal HIV transmission and have offered hope to HIV-positive women who desire children [26, 27]. While a great deal of attention and funding has been focused on this third prong of prevention of mother-to-child transmission (PMTCT), less effort has been devoted to the first two prongs: prevention of HIV in women of reproductive age, and prevention of unintended pregnancies among HIV-infected women. This study examined the impact of a combined intervention including CVCT to prevent heterosexually transmitted HIV infection, followed by family planning counseling and provision of LARC services for HIV discordant couples wishing to limit or delay fertility. The majority of fertile participants – 83% in Rwanda and 56% in Zambia – did not want more children, and another 6-11% wished to delay their next pregnancy for at least 3 years. Among these discordant couples counseled on LARC, 36% of Rwandans and 34% of Zambians requested an implant or an IUD. Condom use remained high, with fewer unprotected sexual exposures reported by LARC users compared with condom-only users. Integration of couples’ HIV testing services, family planning counseling, and provision of LARC services resulted in effective dual method use and mutual leveraging of heterosexual and perinatal HIV prevention.
IUDs and implants had already been offered as part of a comprehensive outpatient care and family planning services provided in the parent study, and a substantial proportion of couples – 15% in Rwanda and 8% in Zambia – had already requested LARC in that context. In the present study, in order to best match family planning goals with the most suitable contraceptive method, additional LARC counseling was provided to fertile couples who wanted to limit or delay fertility for at least 3 years. Providing IUD and implant counseling and services in the context of stated fertility intentions further increased LARC use by 140% in Rwanda and by 200% in Zambia.
The proportion of women wanting to limit fertility is comparable to previously reported figures of 50-90% among women living with HIV or with HIV-positive partners [9, 10]. The very low proportion of women using LARC nationwide, <2% in Rwanda and <0.5% in Zambia [19, 21], confirms a substantial unmet need for long-acting methods. Among couples who did want more children, one third in Rwanda and one quarter in Zambia wanted to wait at least 3 years, indicating that long-acting methods are also in demand for birth spacing.
Family planning programs in Africa currently rely heavily on oral and injectable hormonal contraception. While these methods are very useful for shorter-term birth spacing, they are not the optimal choices for couples wishing to limit fertility as they require user adherence and consistent access over a period of many years. The high proportion of women initiating LARC confirms that use of these methods is acceptable and feasible if couples are provided with information and services. Although insertion is initially more time consuming than distribution of OCPs or injections, LARC methods require fewer visits over the long term, thus increasing cost-effectiveness and decreasing the burden on clients and providers [28]. IUDs and implants have also been shown to have higher satisfaction and continuation rates than OCPs [29], and IUDs are not associated with HIV seroconversion or increased viral shedding in cervicovaginal fluids [30, 31].
Women who initiated LARC were significantly younger than those who did not. This finding supports an earlier study in Rwanda showing that older age was associated with lack of contraceptive usage [32] and indicates that younger women are more open and willing to initiate LARC while older women may have more reservations about unfamiliar modern methods. Further education is needed for both providers and clients in operational settings.
Discordant couples in Kigali where the woman was HIV-positive were more likely to initiate LARC and favored implants over IUDs compared with their HIV-negative counterparts. Similar results were found in a South African study with HIV-positive women less likely to report childbearing intentions compared with HIV-negative women [33]. This emphasizes the need to provide LARC to support the rights of all women to safely achieve their fertility goals and prevent unintended pregnancies and subsequent perinatal HIV transmission. Injectable users in Zambia were less likely to initiate LARC compared to those solely using condoms, perhaps because they were satisfied with their current method.
In both cities, IUDs were chosen less commonly than implants. A study in South Africa found that providers lack adequate skills and training needed to correctly counsel about and offer the IUD [34]. Insertion of implants is technically simpler than IUD insertion, and discomfort with IUDs may lead to provider bias in favor of implants. Studies in Ghana, Iraq, El Salvador, and the United States revealed that women often hold negative perceptions and fears about the IUD [35-38]. Given the large cost differential ($23/implant vs $0.62/CopperIUD) [39], and the erroneous concerns about the possible effects of hormonal contraception on HIV transmission [40], education and training for providers as well as clients is needed to overcome these obstacles.
We did not observe any difference in LARC uptake between couples interviewed together and women interviewed alone. The parent study had provided joint HIV testing and counseling, and both partners received family planning counseling and access to the full range of contraceptive methods at enrollment. These prior activities may have attenuated the added effect of couple participation in this follow-up questionnaire about fertility goals on method uptake. Many women interviewed alone did state a desire to discuss with their partners before making a decision, reinforcing the importance of involving men in decisions about contraception [41].
We found that self-reported incidence of unprotected sex was lower among discordant couples on LARC compared to those using only condoms. Previous studies have found reduced condom use among LARC users in US women who did not know their own or their partners’ HIV status [42, 43]. However, it is difficult to compare these results with those in our study of jointly counseled discordant couples. Previous work in Zambia confirmed that unprotected sex was under-reported among discordant couples [12-17]. Additionally, based on our results that LARC users appeared to be more compliant with dual method use than couples using only condoms, advocating LARC use for women/couples with a shorter fertility horizon (i.e., those who want a child in the next 3 years) would reduce the risk of unplanned pregnancy and subsequent mother-to-child transmission of HIV. Further research is needed with LARC counseling for couples desiring effective contraception for shorter time frames.
Our results also support integration of family planning and HIV services. Although referral to existing contraceptive services is helpful, integrated services allow counseling based on both HIV test results and fertility goals, and link family planning counseling and services closely in space and time to HIV prevention programs. Previous studies in Haiti, Rwanda, Uganda, and Cambodia have shown that integrating family planning with other HIV services increases contraceptive use and reduces unwanted pregnancies for both HIV-positive and HIV-negative couples [8, 44, 45].
The convenience sample, inclusion criteria, and urban study population limit the generalizability of our study. The couples included in the analysis are a subset of urban couples that volunteered to participate in CVCT, had discordant HIV test results, and enrolled in ongoing research studies. A further limitation of these findings is that only women expressing a ≥3 year fertility window were given focused counseling on LARC. It is possible that if all women (including those with self-professed fertility window of <3 years) had been given similar counseling, LARC uptake might have been higher in that group than was observed. More research is needed to assess the effect of LARC counseling and services in concordant HIV-positive and concordant HIV-negative couples, in couples from rural areas, and in couples who wish to conceive within the next three years.
5. Conclusion
The majority of fertile HIV-discordant couples in this study wished to limit fertility, and among these couples already using condoms to prevent heterosexual HIV transmission, the demand for long-acting, user-independent contraception for added protection against unintended pregnancy was high. After standard family planning counseling and access to the full range of contraceptives had been provided in the parent study, additional counseling based on stated fertility intentions resulted in increased LARC uptake. Involving men in family planning programs, promoting dual method use to ensure both HIV and pregnancy prevention, and integrating LARC into existing HIV services are essential steps in preventing transmission among couples at high risk of HIV infection, unplanned pregnancy, and vertical HIV transmission.
Acknowledgments
Financial Support:
This work was supported by ongoing funding from the US National Institutes of Health (RO1: MH066767, AI040951, HD040125), the AIDS International Training and Research Program (AITRP: FIC D43 TW001042), the Center for AIDS Research at Emory (P30 AI050409), and the International AIDS Vaccine Initiative. Funding was used for employment, travel, and provision of writing assistance, surgical instruments, equipment, and/or administrative support related to the study.
Footnotes
Author contributions:
Naw H. Khu, MPH: data management, analyzed data, drafted manuscript
Bellington Vwalika, MD, MSc: oversaw data collection, reviewed manuscript, and gave critical input
Etienne Karita, MD, MSc, MSPH: oversaw data collection, reviewed manuscript, and gave critical input
William Kilembe, MD, MSc: oversaw data collection, reviewed manuscript, and gave critical input
Roger A. Bayingana, MD: oversaw data collection, reviewed manuscript, and gave critical input
Deborah Sitrin, MPH: oversaw data collection, reviewed manuscript, and gave critical input
Heidi Roeber-Rice, MD, MPH: data management, reviewed manuscript and gave critical input
Emily Learner, MPH: oversaw data collection, reviewed manuscript, and gave critical input
Amanda C. Tichacek, MPH: data management, reviewed manuscript and gave critical input
Lisa B. Haddad, MD, MS: reviewed manuscript and gave critical input
Kristin M. Wall, MS: analyzed data, reviewed manuscript, and gave critical input
Elwyn C. Chomba, MD: conceived study, oversaw data collection, reviewed manuscript and gave critical input
Susan A. Allen, MD, MPH: conceived study, oversaw data collection, conceived analysis, reviewed manuscript and gave critical input
References
- [1].Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS Epidemic Update. Geneva, Switzerland: 2009. [Google Scholar]
- [2].UNFPA . State of the World Population 2011. UNFPA; New York, New York: [Google Scholar]
- [3].Centers for Disease Control and Prevention . Family Planning Methods and Practice: Africa. Atlanta, Georgia: 2000. [Google Scholar]
- [4].Iliyasu Z, Abubakar IS, Kabir M, et al. Correlates of fertility intentions among HIV/AIDS patients in northern Nigeria. Afr J Reprod Health. 2009;13:71–83. Epub 2010/08/10. [PubMed] [Google Scholar]
- [5].Stephenson R, Baschieri A, Clements S, et al. Contextual influences on modern contraceptive use in sub-Saharan Africa. Am J Public Health. 2007;97:1233–40. doi: 10.2105/AJPH.2005.071522. Epub 2007/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reynolds HW, Janowitz B, Homan R, et al. The value of contraception to prevent perinatal HIV transmission. Sex Transm Dis. 2006;33(6):350–6. doi: 10.1097/01.olq.0000194602.01058.e1. PubMed PMID: 16505747. Epub 2006/03/01. [DOI] [PubMed] [Google Scholar]
- [7].Mavranezouli I. The cost-effectiveness of long-acting reversible contraceptive methods in the UK: analysis based on a decision-analytic model developed for a National Institute for Health and Clinical Excellence (NICE) clinical practice guideline. Hum Reprod. 2008;23:1338–45. doi: 10.1093/humrep/den091. Epub 2008/03/29. [DOI] [PubMed] [Google Scholar]
- [8].King R, Estey J, Allen S, et al. A family planning intervention to reduce vertical transmission of HIV in Rwanda. AIDS. 1995;9(Suppl 1):S45–51. Epub 1995/07/01. [PubMed] [Google Scholar]
- [9].Dhont N, Ndayisaba GF, Peltier CA, et al. Improved access increases postpartum uptake of contraceptive implants among HIV-positive women in Rwanda. Eur J Contracept Reprod Health Care. 2009;14:420–5. doi: 10.3109/13625180903340584. Epub 2009/11/26. [DOI] [PubMed] [Google Scholar]
- [10].Mark KE, Meinzen-Derr J, Stephenson R, et al. Contraception among HIV concordant and discordant couples in Zambia: a randomized controlled trial. J Womens Health (Larchmt) 2007;16:1200–10. doi: 10.1089/jwh.2006.0238. Epub 2007/10/17. [DOI] [PubMed] [Google Scholar]
- [11].Cooper D, Moodley J, Zweigenthal V, et al. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009;13(Suppl 1):38–46. doi: 10.1007/s10461-009-9550-1. Epub 2009/04/04. [DOI] [PubMed] [Google Scholar]
- [12].Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. Epub 2003/03/21. [DOI] [PubMed] [Google Scholar]
- [13].Antelman G, Smith Fawzi MC, Kaaya S, et al. Predictors of HIV-1 serostatus disclosure: a prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS. 2001;15:1865–74. doi: 10.1097/00002030-200109280-00017. Epub 2001/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Becker S. Couples and reproductive health: a review of couple studies. Stud Fam Plann. 1996;27:291–306. Epub 1996/11/01. [PubMed] [Google Scholar]
- [15].Guthrie BL, de Bruyn G, Farquhar C. HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res. 2007;5:416–29. doi: 10.2174/157016207781023992. Epub 2007/07/14. [DOI] [PubMed] [Google Scholar]
- [16].Padian NS, O’Brien TR, Chang Y, et al. Prevention of heterosexual transmission of human immunodeficiency virus through couple counseling. J Acquir Immune Defic Syndr. 1993;6:1043–8. Epub 1993/09/01. [PubMed] [Google Scholar]
- [17].Painter TM. Voluntary counseling and testing for couples: a high-leverage intervention for HIV/AIDS prevention in sub-Saharan Africa. Soc Sci Med. 2001;53:1397–411. doi: 10.1016/s0277-9536(00)00427-5. Epub 2001/11/17. [DOI] [PubMed] [Google Scholar]
- [18].Epidemiology Fact Sheet on HIV and AIDS: Rwanda - 2008 Update. UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance; Geneva: 2009. [Google Scholar]
- [19].Ministry of Health (MOH) [Rwanda] National Institute of Statistics of Rwanda (NISR) ICF Macro . Rwanda Interim Demographic and Health Survey 2007-08. MOH, NISR, and ICF Macro; Calverton, Maryland, USA: 2009. [Google Scholar]
- [20].Epidemiological Fact Sheet on HIV and AIDS: Zambia - 2008 Update. UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance; Geneva: 2009. [Google Scholar]
- [21].Central Statistical Office (CSO) Ministry of Health (MOH) Tropical Diseases Research Centre (TDRC) et al. Zambia Demographic and Health Survey 2007. CSO and Macro International Inc.; Calverton, Maryland, USA: 2009. [Google Scholar]
- [22].Chomba E, Allen S, Kanweka W, et al. Evolution of couples’ voluntary counseling and testing for HIV in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:108–15. doi: 10.1097/QAI.0b013e31815b2d67. Epub 2007/11/07. [DOI] [PubMed] [Google Scholar]
- [23].Kempf MC, Allen S, Zulu I, et al. Enrollment and retention of HIV discordant couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:116–25. doi: 10.1097/QAI.0b013e31815d2f3f. Epub 2007/11/22. [DOI] [PubMed] [Google Scholar]
- [24].Allen S, Karita E, Chomba E, et al. Promotion of couples’ voluntary counselling and testing for HIV through influential networks in two African capital cities. BMC Public Health. 2007;7:349. doi: 10.1186/1471-2458-7-349. Epub 2007/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].World Health Organization Medical eligibility criteria for contraceptive use: WHO. 2009 cited 2012 July 13, 2012. Available from: http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf. [PubMed]
- [26].De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. Epub 2000/03/07. [DOI] [PubMed] [Google Scholar]
- [27].Nolan ML, Greenberg AE, Fowler MG. A review of clinical trials to prevent mother-to-child HIV-1 transmission in Africa and inform rational intervention strategies. AIDS. 2002;16:1991–9. doi: 10.1097/00002030-200210180-00003. Epub 2002/10/09. [DOI] [PubMed] [Google Scholar]
- [28].Chiou CF, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception. 2003;68:3–10. doi: 10.1016/s0010-7824(03)00078-7. [DOI] [PubMed] [Google Scholar]
- [29].Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstetrics and Gynecology. 2011;117:1105–13. doi: 10.1097/AOG.0b013e31821188ad. Epub 2011/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morrison CS, Sekadde-Kigondu C, Sinei SK, et al. Is the intrauterine device appropriate contraception for HIV-1-infected women? BJOG. 2001;108:784–90. doi: 10.1111/j.1471-0528.2001.00204.x. Epub 2001/08/21. [DOI] [PubMed] [Google Scholar]
- [31].Richardson BA, Morrison CS, Sekadde-Kigondu C, et al. Effect of intrauterine device use on cervical shedding of HIV-1 DNA. AIDS. 1999 Oct 22;13:2091–7. doi: 10.1097/00002030-199910220-00012. [DOI] [PubMed] [Google Scholar]
- [32].Family Health International . Expanding Contraceptive Use in Rwanda. Family Health International; Research Triangle Park: Dec, 2010. 2010. Report No. [Google Scholar]
- [33].Kaida A, Laher F, Strathdee SA, et al. Childbearing Intentions of HIV-Positive Women of Reproductive Age in Soweto, South Africa: The Influence of Expanding Access to HAART in an HIV Hyperendemic Setting. American Journal of Public Health. 2010 May 13; doi: 10.2105/AJPH.2009.177469. Epub 2010/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gutin SA, Mlobeli R, Moss M, et al. Survey of knowledge, attitudes and practices surrounding the intrauterine device in South Africa. Contraception. 2011;83:145–50. doi: 10.1016/j.contraception.2010.07.009. [DOI] [PubMed] [Google Scholar]
- [35].Osei I, Birungi H, Addico G, et al. What happened to the IUD in Ghana? Afr J Reprod Health. 2005;9:76–91. Epub 2006/02/21. [PubMed] [Google Scholar]
- [36].Alnakash AH. Influence of IUD perceptions on method discontinuation. Contraception. 2008;78:290–3. doi: 10.1016/j.contraception.2008.05.009. Epub 2008/10/14. [DOI] [PubMed] [Google Scholar]
- [37].Katz KR, Johnson LM, Janowitz B, et al. Reasons for the low level of IUD use in El Salvador. International Family Planning Perspectives. 2002;28:26–31. [Google Scholar]
- [38].Gilliam ML, Warden M, Goldstein C, et al. Concerns about contraceptive side effects among young Latinas: a focus-group approach. Contraception. 2004;70:299–305. doi: 10.1016/j.contraception.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [39].Neukom J, Chilambwe J, Mkandawire J, et al. Dedicated providers of long-acting reversible contraception: new approach in Zambia. Contraception. 2011;83:447–52. doi: 10.1016/j.contraception.2010.08.021. [DOI] [PubMed] [Google Scholar]
- [40].Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. Epub 2011/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bii SC, Otieno-Nyunya B, Siika A, et al. Family planning and safer sex practices among HIV infected women receiving prevention of mother-to-child transmission services at Kitale District Hospital. East Afr Med J. 2008;85:46–50. doi: 10.4314/eamj.v85i1.9606. Epub 2008/06/12. [DOI] [PubMed] [Google Scholar]
- [42].Cushman LF, Romero D, Kalmuss D, et al. Condom use among women choosing long-term hormonal contraception. Family Planning Perspectives. 1998;30:240–3. Epub 1998/10/22. [PubMed] [Google Scholar]
- [43].Pazol K, Kramer MR, Hogue CJ. Condoms for dual protection: patterns of use with highly effective contraceptive methods. Public Health Reports. 2010;125:208–17. doi: 10.1177/003335491012500209. Epub 2010/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peck R, Fitzgerald DW, Liautaud B, et al. The feasibility, demand, and effect of integrating primary care services with HIV voluntary counseling and testing: evaluation of a 15-year experience in Haiti, 1985-2000. J Acquir Immune Defic Syndr. 2003;33:470–5. doi: 10.1097/00126334-200308010-00007. Epub 2003/07/19. [DOI] [PubMed] [Google Scholar]
- [45].Family Health International . Case studies: Uganda, Cambodia. 3. Vol. 23. Research Triangle Park; FHI, Network: 2004. [Google Scholar]