Abstract
Middle-aged Americans have higher obesity rates than any other age group, yet little is known about age-related changes in central taste function during this critical time. Research on taste and aging has primarily focused on psychophysical responses, and on older adults. Central taste processing in middle-age has not been investigated. In the current study, we compared fMRI activation of young and middle-aged adults during hedonic evaluation of a sweet and a bitter taste. A 2 (age group) by 2 (tastant) analysis of variance (ANOVA) on fMRI activation revealed: (1) a main effect of age (young adults > middle-aged adults) in the bilateral anterior cingulate, lentiform nucleus, putamen, caudate, and right precentral gyrus; (2) a main effect of taste (sweet > bitter) in the bilateral pre- and postcentral gyri, anterior cingulate and right middle frontal gyrus; qualified by (3) an age by taste interaction. Further inspection of the age by taste interaction revealed that there was a significant effect of age (greater activation in young adults) in sensory (insula) and reward (lentiform nucleus) regions during hedonic evaluation of the sweet taste; however, there was no age effect in the bitter taste condition. Further, young adults had greater responses during hedonic evaluation of sucrose than of caffeine in several sensory and motor processing regions (pre- and postcentral gyri, insula), but there were no taste-related differences in activation in the middle-aged adults. We speculate that these results might reflect early age-related differences in central taste processing that occur prior to deficits in gustatory function observed in old age, and this might have important implications for weight changes that occur during middle-age.
Keywords: aging, fMRI, gustatory, reward value, taste
Introduction
Aging is often associated with weight changes and nutritional problems. Increased adiposity and decreased energy expenditure, metabolism, and muscle mass frequently contribute to health problems that accompany the aging process (Muller et al., 1996; Jensen and Rogers, 1998; Baumgarter et al., 2005). Unfortunately, unhealthy adiposity during middle age is not only linked to deleterious comorbidities, but also to poor health outcomes including coronary heart disease-related mortality, incidence of breast, colon, and uterine cancer (Folsom et al., 2000), and increased risk of dementia in later life (Whitmer et al., 2005). Caloric intake plays a key role in weight gain at any age (Sherwood et al., 2000), and the gustatory system is critical to flavor perception. However, little is currently known about possible changes in the taste system during middle age.
Chemosensory function declines with age (Murphy, 1979; Gilmore and Murphy, 1989; Schiffman and Gatlin, 1993; Murphy et al., 2002; Nordin et al., 2003; Schiffman, 2009). The literature suggests that older adults have reduced taste sensitivity, with some qualities being affected more than others (for reviews see Schiffman and Gatlin, 1993; Murphy, 1993). Although age-related declines in taste sensitivity vary according to quality, it has been estimated that on average, older adults require 4.7 times more molecules or ions to detect and recognize a tastant compared to their younger counterparts (Schiffman, 2009). Considerable data suggests the independence of threshold and suprathreshold intensity perception (Pepino, Finkbeiner, Beauchamp and Mennella, 2010). Declines in intensity discrimination have also been reported in older adults (Schiffman and Graham, 2000), and there is evidence to suggest that these declines vary with the stimulus (Gilmore and Murphy, 1989; Nordin et al., 2003). Specifically, Weber Ratios (WRs), or the ratio of the just noticeable difference to the stimulus intensity, are higher for older than young adults for caffeine (bitter), NaCl (salty), and citric acid (sour), but no different for sucrose (sweet) taste stimuli. Taste identification also appears to decline in old age (Ship and Weiffenbach, 1993; Boesveldt et al., 2011), to a greater degree for citric acid and quinine-hydrochloride (bitter) than for sucrose and NaCl (Nordin et al., 2007). One might speculate that age-related changes observable in late life are likely to begin during middle age. As such, there is a gap in the literature on taste function across the lifespan that warrants investigation.
Research in the area of age-related gustatory changes has primarily focused on peripheral structures of the taste system. The early hypothesis that aging involves a significant loss of taste buds (Arey et al., 1935), has not received unequivocal support (Arvidson, 1979; Bradley et al., 1985; Miller, 1988). However, aging has been linked to decreases in taste bud size, taste bud density, and taste cell density (Shimizu, 1997), suggesting that some peripheral physiological changes may be associated with observed taste declines. Importantly, little is currently known about the effects of aging on central mechanisms of taste processing.
Our laboratory has used functional magnetic resonance imaging (fMRI) to investigate age-related functional changes in central gustatory processing by comparing fMRI responses of young and healthy older adults (aged 65+) during taste stimulation and hedonic evaluation (Jacobson et al., 2010). Activation in both age groups was demonstrated in prototypical taste regions including the insula. However, more robust activation of regions involved in emotion, motivation, and the rewarding aspects of taste stimuli including the orbitofrontal cortex, amygdala, and caudate nucleus was demonstrated in older relative to young adults during the hunger condition. We suggested that these findings may reflect a process of functional reorganization as a compensatory strategy in older adults who may have some peripheral sensory loss.
The impact of aging on neural pathways important for evaluating the affective properties of taste is also unknown. Importantly, food reward plays an integral role in dietary selection. Foods with a high energy density [i.e., amount of energy (e.g., kilocalories) in a unit weight (e.g., gram) of food] are sought out and consumed, often to excess, because they are highly rewarding (Drewnowski, 1998; Ledikwe et al., 2005). Taste can provide important information about the nutritive properties of food (Scott & Verhagen, 2000). Sweet taste is generally an indicator of energy dense foods, while bitter taste can warn against harmful or poisonous foods that should be avoided. Additionally, food reward is modulated by physiological state; food “tastes” better when an individual is hungry relative to when he or she is satiated (Cabanac, 1971; Rolls et al., 1989; Small et al., 2001; Kringelbach et al., 2003; Haase et al., 2009). This decrease in the experience of food reward from hunger to satiety is a physiological signal to terminate energy consumption.
The objective of the current study was to investigate central taste and reward processing during the middle of the lifespan, in healthy adults with no chemosensory complaints. Specifically, we examined age-related differences in neural correlates of taste stimulation during hedonic evaluation in young and middle-aged adults using fMRI responses to taste stimuli during the physiological state of hunger, when pleasant tastes are the most rewarding (Cabanac, 1971). To increase the salience of the hedonic aspects of the taste stimuli, we used a task involving pleasantness evaluation in the fMRI scanner and we used taste stimuli at the two ends of the pleasantness spectrum (sweet and bitter).
We hypothesized that there would be an age (young vs. middle-aged) by taste (sweet vs. bitter) interaction on fMRI activation such that several areas involved in food reward and hedonics would be more activated in the young relative to the middle-aged adults in response to the pleasant sweet taste. Due to the ecological importance of having an aversive response to a bitter taste, we further hypothesized that there would be no difference between the age groups in response to the bitter stimulus.
Experimental Procedures
A detailed description of the protocol and the system for delivering taste stimuli in the fMRI environment used in the study are outlined in the Journal of Neuroscience Methods (Haase et al., 2007).
Participants
Twelve young adults, (6 males and 6 females), ranging from 19 to 26 years of age (M = 23.08, S.D. = 2.23), and twelve middle-aged adults, (6 males and 6 females), ranging from 45 to 54 years of age (M = 49.5, S.D. = 2.91), were recruited from the San Diego community. Participants gave informed consent and received monetary compensation for their participation. The Institutional Review Boards at both San Diego State University and the University of California, San Diego gave approval for the study. Each subject participated in two separate sessions (1 screening session and 1 fMRI session) detailed below.
Screening Session
During the first session, participants were screened for exclusionary criteria including ageusia, anosmia, and upper respiratory infection or allergies within the prior two weeks (Murphy et al., 2002). Taste thresholds for all participants were assessed using a forced choice procedure with a series of varying concentrations (.0032M to .36M) of sucrose solutions (Murphy et al., 1990). Odor threshold was assessed using a forced-choice procedure with varying concentrations of n-butyl alcohol presented monorhinically (Murphy et al., 1990). Each participant also completed the Three-Factor Eating Questionnaire [TFEQ; Stunkard & Messick, 1985)] to screen for restrained eating.
We have recently reported an association between body mass index (BMI) and cortical activation during hedonic evaluation of a sweet taste in older adults (Green et al., 2011). Therefore, no obese (defined as a BMI of over 30) participants were included in the study. Body mass index (BMI) was calculated by dividing each participant’s measured weight by the square of his or her measured height (Kg/cm2). The young adults had a mean BMI of 23.00 with a standard deviation of 2.66, and the middle-aged adults had a mean BMI of 25.00 with a standard deviation of 2.63. Waist circumference was also measured in centimeters at the midpoint between the highest point of the iliac crest and the lowest point of the rib cage. The young adults had a mean waist circumference of 82.67 with a standard deviation of 9.86, and the middle-aged adults had a mean waist circumference of 90.67 with a standard deviation of 13.87. One-Way Analyses of Variance (ANOVAs) confirmed no significant group differences in BMI, F(1, 22) = 3.43, p =.08, or waist circumference, F(1,22) = 2.70, p = .12 (See Table 1).
Table 1.
Demographics and Psychophysics
| Mean (SD) | ||||
|---|---|---|---|---|
| Demographics | Young Adults | Middle Aged Adults | F | Significance |
| Height (cm) | 174.4(12.68) | 169.77(7.95) | 1.15 | p = .29 |
| Weight (kg) | 71.67(14.29) | 72.79(10.64) | .048 | p = .83 |
| BMI | 23.00 (2.66) | 25.00(2.63) | 3.43 | p = .08 |
| Waist Circumference | 82.67(9.86) | 90.67(13.87) | 2.65 | p = .12 |
| TFEQ - Restraint | 8.92 (4.83) | 9.67(5.55) | .125 | p = .73 |
| Odor threshold L | 7.21(1.60) | 6.17(1.70) | 2.59 | p = .12 |
| Odor threshold R | 6.25(1.71) | 7.08(2.11) | 1.13 | p = .30 |
| Taste threshold | .005 (.008) | .009(.02) | .49 | p = .49 |
| Hunger | 34.08(22.30) | 32.33(26.102) | .031 | p = .83 |
| Fast duration | 14.57(2.48) | 14.64(1.97) | .004 | p = .95 |
Greater activation during hedonic evaluation of sweet than bitter in young Similar activation during hedonic evaluation of sweet and bitter in middle aged Activation in middle-aged less than young during hedonic evaluation of sucrose Activation in middle-aged less in sensory-motor and reward areas Middle-age changes in CNS processing of taste: possible implications for weight gain
Neuroimaging Procedure
The neuroimaging session was conducted at the University of California, San Diego Center for Functional Magnetic Resonance Imaging (fMRI). Participants fasted for a minimum of 12 hours prior to the scan to ensure all participants were scanned during a similar physiological state of hunger. Outside of the scanner, participants reported psychophysical ratings of hunger, pleasantness and intensity of the two taste stimuli (specified below), using modified versions of the General Labeled Magnitude Scale (gLMS; Bartoshuk et al., 2004; Green et al., 1996; Green et al., 1993).
Stimulus Delivery
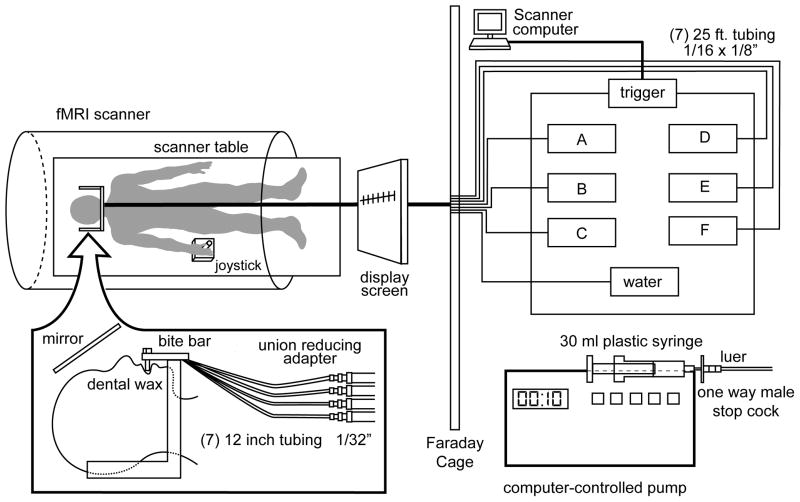
The current study was designed to investigate fMRI activation to taste stimuli that fall at the two ends of the hedonic spectrum: pleasant and aversive. Therefore, we analyzed activation in response to pleasantness evaluation of sucrose (0.64M; sweet) and caffeine (0.02M; bitter) presented as aqueous solutions. Participants lay supine in the scanner and were fitted with a bite bar to minimize head movement, including that associated with swallowing, and to allow the tubing for taste delivery to rest comfortably between the lips (Figure 1). The stimuli were individually filled in syringes and delivered to the tongue of the participant through 25-foot long tubing connected to programmable pumps located in the operator room. The syringe pumps were triggered at the beginning of the scan. The pumps were computer-programmed to push the syringes so that 0.3 ml of solution was presented in 1 sec from each syringe at the appropriate time (Figure 1).
Figure 1.
Illustration of the taste delivery system and setup in the fMRI environment. The participant lies supine in the scanner and tastes are delivered to the mouth via tubing connected to programmable pumps. The participant employs a joystick to rate the pleasantness of the taste by placing a curser on the modified gLMS (pleasantness scale) displayed on the screen in front of them. Participants are able to view the screen via a mirror above the head. Figure reproduced from Haase, Cerf-Ducastel, Buracas, and Murphy (2009), with permission.
Two functional scans were performed on the day of scanning. Each stimulus was delivered 8 separate times for each functional run, presented pseudo-randomly with a 10 second inter-stimulus interval (ISI). Distilled water was presented twice after each stimulus, the first time as a rinse and the second as a baseline for data analysis. A minimum of 30 seconds elapsed before the same stimulus was presented again (except for water delivery, no stimulus was presented twice in a row). This procedure was designed to minimize habituation and adaptation of the gustatory system.
During the scan, taste stimulation was paired with a hedonic evaluation task. Specifically, functional data were collected during the 10-second period coinciding with each taste presentation and allowing time for participants to rate the pleasantness of the taste. The 10-second ISI allowed 1 second for taste delivery, 2 seconds for swallowing (with a cue “please swallow” presented on a screen), and 7 seconds reserved for instructions (with a cue “please rate pleasantness” presented on the screen) and providing a magnitude estimate of pleasantness of the taste. A joystick was employed by participants in order to place a crosshair on a number corresponding to a magnitude estimate of the pleasantness on the gLMS. This whole process was completed with the use of an interactive computer interface displayed on a screen, visible to the participants via a mirror (see Haase et al. 2007 for more detail). The image acquired at the first repetition time (TR) was dropped during processing to control for head movement during swallowing. Thus, functional data were analyzed during the hedonic evaluation task (i.e., determining the number on the labeled scale best describing the pleasantness of the taste and moving the joystick to reach that number). Because we chose to remove the first TR (2 seconds) from data analysis, we are effectively eliminating the “basic perception” component of the task and focusing on the time period during which the participant considers the stimulus and rates its pleasantness.
Image Acquisition
The neuroimaging sessions were performed using a 3T GE Signa EXCITE Short-Bore research scanner. Structural images for anatomical localization of functional images were collected before the functional scans using a high-resolution T1-weighted whole-brain FSPGR sequence (Field of view (FOV) = 25.6cm, slice thickness = 1mm, resolution 1×1×1 mm3, echo time (TE) = 30ms, Locs per slab = 190, flip angle = 15°). A whole brain gradient echo planar pulse sequence was used to acquire T2*-weighted functional images (32 axial slices, FOV = 19.2cm, matrix size = 64X64, spatial resolution = 3X3X3 mm3, flip angle = 90°, echo time (TE) = 30ms, repetition time (TR) = 2000ms).
Image Analysis
Functional data were processed using Analysis of Functional NeuroImage (AFNI) software (Cox, 1996) and FMRIB Software Library (FSL; Smith et al., 2004). The data were first preprocessed through motion correction and alignment of the anatomical image and functional runs. An automated in vivo shimming method using 3-dimensional field maps was employed to correct for heterogeneity of the magnetic field and reduce signal dropout using FSL. Images were spatially smoothed to 4 full width at half maximum, automasked to clip voxels outside of the brain, and normalized to Talaraich space to control for individual structural differences using AFNI. The two functional runs were individually rescaled to a baseline of 100 and concatenated for each participant.
A deconvolution was run on each individual’s concatenated run using 3dDeconvolve within AFNI (Cox, 1996). Deconvolution is a multiple regression analysis used for fMRI data with the purpose of fitting specific time points with distinct coefficients representing an estimate of the impulse response function for each voxel. Deconvolution was used to fit each voxel’s time series to an activation model (based on the specified input contrasts, such as sucrose minus water) and then test these models for significance. This estimate was given as an output statistic (for each voxel) called the fit coefficient, which is synonymous with a beta coefficient. Additionally, activation during the second water presentation after each taste was used as the baseline and this was specified in the deconvolution analysis. Because the hedonic evaluation task was also employed during the water baseline condition, potential age-related differences in joystick proficiency were controlled for.
In order to examine the interaction between age and taste (bitter v. sweet) on fMRI activation during a physiological state of hunger, a 2 group × 2 taste ANOVA was run with subjects as a random factor and fMRI activation as the dependent variable. Group statistical maps were thresholded to protect a whole-brain probability of false positives at an alpha of 0.05, corrected for multiple comparisons at the cluster level using the AFNI program AlphaSim (Cox, 1996). AlphaSim uses Monte Carlo simulation to compute the probability of the generation of a random field of noise and determines the cluster size necessary to control for false positives at a specific alpha level. Therefore, significant clusters met an individual voxel of p < 0.01 (F ≥ 7.95, df = 1,22 for main effect of age, main effect of taste, and group x category interaction), and consisting of at least 17 contiguous voxels. Significant clusters surviving the threshold for the interaction effect were explored further using voxelwise t-tests to investigate the 4 possible simple effects: (1) young adults versus middle-aged adults in the sucrose condition; (2) young adults versus middle-aged adults in the caffeine condition; (3) sucrose versus caffeine in young adults; (4) and sucrose versus caffeine in middle-aged adults.
Results
Demographics and Psychophysical Data
One-Way Analyses of Variance (ANOVAs) were run to examine potential demographic differences between the young and middle-aged adults. These data are presented in Table 1. There were no significant group differences in height, (F(1, 22) = 1.15, p =.29) or weight, (F(1, 22) = .05, p =.83), between young and middle-aged adults. There were also no differences in restraint on the Three Factor Eating Questionnaire, (F(1, 22) = .13, p =.73), taste threshold, (F(1, 22) = .49, p =.49), or for the left nostril, (F(1, 22) = 2.59, p =.12), or right nostril’s odor threshold, (F(1,22) = 1.13, p = .30). Importantly, there were also no differences between the age groups on hunger ratings prior to the scan, (F(1,22) = .031, p = .83). All participants fasted for a minimum of 12 hours prior to the scan. Data on the duration of the fast was collected for all but 5 of the participants. There were no differences in the mean duration (hours) of the pre-scan fast between the age groups (F(1,19) = 0.004, p = .95).
Participants rated the pleasantness and intensity of the caffeine and sucrose solutions before and after the scan, using a modified version of the gLMS. A repeated-measures ANOVA was used to compare pleasantness ratings for the two taste stimuli (caffeine and sucrose), between the age groups over time (pre- and post scan). There was a main effect for taste, (F(1, 22) = 35.49, p < .001); sucrose (M = 57.06, SE = 2.51) was considered to be more pleasant than caffeine (M = 36.21, SE = 2.66) by both age groups averaged across time. There was no main effect for age group, (F(1, 22) = 58.59, p = .686); and time point, (F(1, 22) = 3.005, p = .097)) did not reach statistical significance. Additionally, no significant taste by age group, (F(1, 22) = 1.825, p = .190), taste by time point (F(1, 22) = .143, p = .709), or taste by time point by age group (F(1, 22) = 1.401 p = .249), interactions were found.
Similarly, a repeated-measures ANOVA was run on intensity ratings of sucrose and caffeine for the young and middle-aged adults over the two time points. There was no significant main effect of taste, (F(1, 22) =.002, p =.968); and time point, (F(1, 22) = 3.368, p = .08) and age group, (F(1, 22) = 3.251, p = . 085) did not reach statistical significance. Additionally, there was no significant taste by age, (F(1, 22) = .009, p = .925), time point by age, (F(1, 22) = .759, p = .393), or taste by time point by age group interactions, (F(1, 22) = .057, p = .814).
fMRI Analyses: ANOVA
A 2 factorial mixed-effects ANOVA was run on fMRI activation with age group and taste as the two factors in order to investigate: (1) main effects of age, (2) main effect of taste (bitter v. sweet), and (3) the interaction between age and taste on fMRI activation. The results of the ANOVA including F values, cluster sizes, and anatomical locations of significant clusters of activation are reported in Table 3. First, a main effect of age was revealed in the bilateral anterior cingulate, lentiform nucleus, putamen, caudate head and body, cingulate gyrus, left insula, left middle frontal gyrus, and right precentral gyrus. There was also a main effect of taste in the bilateral postcentral gyri, precentral gyri, and anterior cingulate when collapsed over age group. There was also an age by taste interaction in five regions: (1) the left insula; (2) the left precentral and postcentral gyrus; (3) the right precentral and postcentral gyrus; (4) the right lentiform nucleus and putamen; and (5) the left lentiform nucleus and putamen.
To investigate this interaction further, the four possible simple effects [(1) young adults vs. middle-aged adults in the sucrose condition; (2) young aged adults vs. middle-aged adults in the caffeine condition; (3) sucrose vs. caffeine in young adults, and (4) sucrose vs. caffeine in middle-aged adults] were calculated using voxelwise t-tests in AFNI (Cox et al., 1996). Again, the resulting statistical maps were thresholded at p < .05 using the same voxel and cluster thresholds as described above (t ≥ 3.786, df = 1,22 for contrasts 1 and 2 and t ≥ 2. 818, df = 1,22 for contrasts 3 and 4). Subsequently, all voxels that did not meet the threshold for a significant age by taste interaction from the ANOVA (see above) were masked out; that is, excluded from the simple effects statistical map.
Simple effect of age for sucrose and caffeine
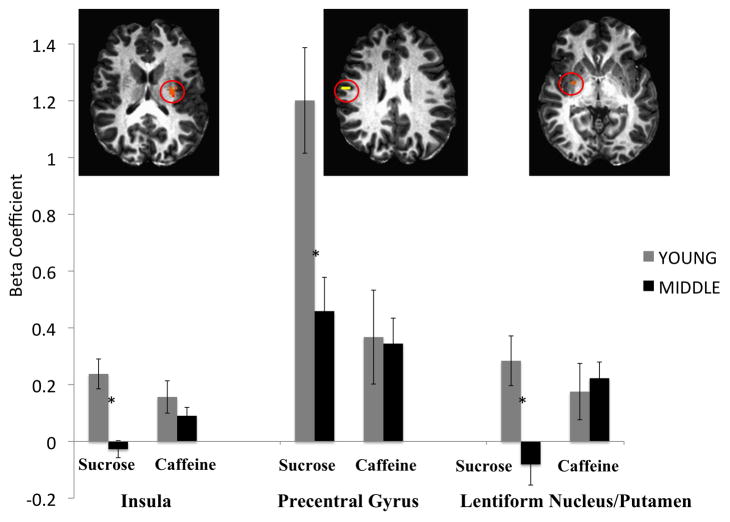
Further investigation of the simple effects of age in response to sucrose and caffeine separately revealed no differences between the age groups in response to caffeine, but significantly greater activation of the left insula, right precentral gyrus, and right lentiform nucleus and putamen in young relative to middle aged adults when evaluating the pleasantness of sucrose. The three regions reaching significance in this condition are displayed in Figure 2. It has been suggested that using region of interest (ROI) analyses as follow up tests to ANOVA in fMRI are non-independent analyses (Vul et al., 2009). Note, however, in this study, there were no follow-up statistical tests conducted on the ROIs displayed in Figure 2; they are only displayed in order to provide a graphical illustration of brain activation in the sucrose condition.
Figure 2.
Age by taste interaction: Simple effects of age. Activation during pleasantness evaluation of sucrose was greater in young adults relative to middle-aged adults in the left insula, right precentral gyrus, and right lentiform nucleus/putamen. There were no simple effects of age on fMRI activation during hedonic evaluation of caffeine.
Simple effect of taste for young and middle-aged adults
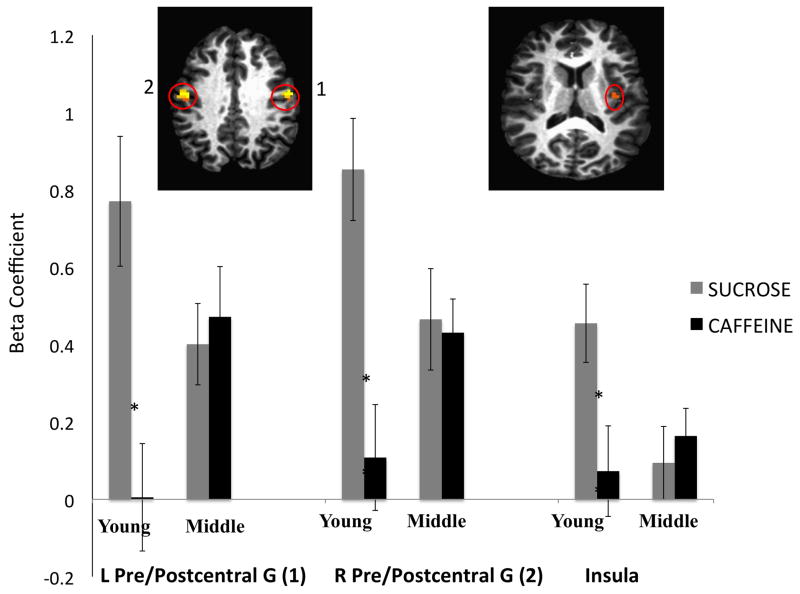
Further investigation of the simple effects of taste for young and middle-aged adults revealed greater activation in young adults in response to hedonic evaluation of sucrose relative to caffeine in three regions: the bilateral pre- and postcentral gyri, and the left insula. These regions are displayed in Figure 3.
Figure 3.
Age by taste interaction: simple effects of taste for young adults. Pleasantness evaluation of sucrose activated the bilateral pre- and postcentral gyri, and left insula to a greater degree than pleasantness evaluation of caffeine in young adults. There were no significant differences between the pleasantness evaluation of sucrose or caffeine in the middle-aged adults. L = Left; R = Right; G = Gyrus.
There was no difference between fMRI activation during hedonic evaluation of sucrose and caffeine in the middle-aged adults.
fMRI Analyses: Correlation between brain activation and BMI
In the present study, we restricted the range of BMI in our sample by using obesity (BMI ≥ 30) in our exclusionary criteria because we have recently reported strong associations between BMI and fMRI activation to sucrose in the caudate nucleus, nucleus accumbens, and amygdala during hedonic evaluation (Green et al., 2011). However, in order to determine if BMI should be included as a covariate in further statistical analyses, we ran correlations between brain activation in anatomically defined regions of interest (ROIs; amygdala, caudate head, body, and tail, nucleus accumbens) and BMI in our sample. Our rationale was that if BMI was influencing brain activation significantly in this sample, the regions mentioned above would be the most likely to show significant associations with BMI because we have demonstrated this previously using a very similar paradigm. We found no significant correlations between BMI and brain activation in the aforementioned regions in this restricted sample (r ranged from .03 to −.35, all p > .05). Therefore, we conducted the voxelwise ANOVA and follow up t-tests without including BMI as a covariate.
Discussion
Middle-aged Americans have the highest obesity rates of any age group in the United States (Ogden et al., 2006), yet little is known about changes in central gustatory pathways during this critical period of life. Consuming more energy than is expended leads to weight gain, so understanding mechanisms relating to dietary selection and caloric consumption is crucial to understanding the growing obesity rates in this age group. To date, most of what is known about taste processing in the aging brain has been focused on potential changes in the peripheral taste system in older adults. Thus, the aim of the present study was to examine central taste processing in middle-aged adults.
We used functional magnetic resonance imaging during hedonic evaluation of a pleasant, (sweet) and unpleasant, (bitter) taste to examine potential differences in central processing of gustatory information during a motivational state (hunger) in young and middle-aged adults. In order to increase the salience of the hedonic aspects of each taste, participants were asked to actively evaluate each taste (including the water baseline), by providing on-line magnitude estimation of pleasantness during the functional scans (Haase et al., 2007; Cerf-Ducastel et al., 2012), and data were analyzed during the hedonic evaluation component of the task.
Psychophysical ratings of intensity and pleasantness were collected prior to and following the fMRI scan. The purpose of collecting these data was to investigate possible differences in the subjective pleasantness or intensity of the tastes between the age groups or over time. There were no differences in psychophysical ratings of intensity and pleasantness of the taste stimuli between the age groups or over time that reached statistical significance.
A 2(age) × 2(taste) ANOVA revealed a main effect of age (greater activation in young relative to middle-aged adults) in the bilateral anterior cingulate, caudate head and body, lentiform nucleus, putamen, left middle frontal gyrus, and right precentral gyrus. Additionally, there was a main effect of taste (greater activation in response to sucrose than caffeine) in the bilateral pre- and postcentral gyrus, anterior cingulate, and right middle frontal gyrus.
As expected, there was a significant age by taste interaction. Simple effects analyses revealed that, although there was a significant effect of age (young > middle-aged) on activation of the left insula, right precentral gyrus, and right lentiform nucleus during pleasantness evaluation of sucrose, the age groups did not differ in brain response during evaluation of caffeine in these regions (See Figure 2). Investigation into the simple effects of taste revealed that in young adults, fMRI activation during pleasantness evaluation of sucrose was greater than during pleasantness evaluation of caffeine in the bilateral pre- and postcentral gyri and left insula. However, there were no differences between brain activation during evaluation of sucrose or caffeine in these regions for the middle-aged group (See Figure 3). Thus, the main effects were qualified by the significant interaction.
The lentiform nucleus is a region of the basal ganglia complex that is made up of the globus pallidus and putamen. It receives dopaminergic projections from midbrain nuclei and efferent projections from the ventral striatum (Haber and Knutson, 2010). The putamen especially, is considered to be important for food reward signaling (Berthoud & Morrison, 2008; Volkow et al., 2008). Further, age-related declines in dopamine concentration and receptor density have been documented in the putamen (Antonini et al., 1993; Bäckman and Farde, 2005). Certainly, a larger dopamine reward response would be expected in response to a sweet, rewarding taste relative to a bitter, aversive taste, which could partially explain why there were no age differences in response to caffeine.
Dopamine levels play a role in modulating emotional processes, including positive affect and the experience of reward. Striatal activation in humans has been linked with both primary (food-related stimuli, drug administration) and secondary (monetary) rewards (for reviews see Delgato, 2007 and Balleine et al., 2007). Differential activation of reward circuitry has been linked to both changes in motivational state (Haase et al., 2009; Small et al., 2001) and levels of body fat (Stice et al., 2009). In fact, there is recent evidence to suggest that there is a stronger association between adiposity and decreased activation of dopaminergic brain circuitry in older relative to young adults (Green et al., 2011).
Notably, there were no significant differences in pleasantness ratings of sucrose or caffeine between the age groups. Several studies have noted differential activation of reward networks according to physiological state (Green et al., 2011; Haase et al., 2009) or group membership (Cowdrey et al., 2011; Felsted et al., 2010; Jacobson et al., 2010; McCabe et al., 2009) that does not correspond to differences in subjective hedonic ratings. Thus, a lack of significant age effect on pleasantness ratings of the taste stimuli (specifically sucrose) suggests that potential differences between the age groups in the subjective experience of reward may be very subtle. Additionally, if we conceptualize differences in activation of reward networks as resulting from a culmination of small changes that occur across the lifespan, then we might speculate that middle-aged adults may have already begun the process of habituating to a world where sensory stimuli are all less rewarding.
We conducted an exploratory analysis investigating potential links between brain activation during pleasantness evaluation of sucrose and the recorded pleasantness ratings of sucrose. Specifically, we examined associations between fMRI activation in anatomically-defined regions of interest (ROI) that are involved in taste hedonics and reward (i.e., orbitofrontal cortex/Brodmann Area 47, amygdala, and caudate nucleus) and the difference between pleasantness ratings of sucrose from time point 1 (prior to scanning) to time point 2. We found a positive correlation approaching significance in the left and right orbitofrontal cortex (r(22) = .36, ps = .08) suggesting that a positive association between fMRI activation of the orbitofrontal cortex during pleasantness evaluation of sucrose and pleasantness change scores.
A region of the mid-dorsal insula/rolandic operculum was activated to a greater degree during pleasantness evaluation of sucrose than during pleasantness evaluation of caffeine in young, but not middle-aged adults. The anterior insula/frontal operculum receives projections from the ventroposteromedial (VPMpc) nucleus of the thalamus, is considered to be the first cortical site to process taste information and has been termed the primary gustatory cortex (Yaxley et al., 1988; Yaxley et al., 1990; Scott & Plata-Salaman, 1999). Notably, the insular cortex is large in volume, relative to other regions of the brain. Recent evidence also suggests a role for specific regions of the insula in processing the affective properties of taste and sensory information (Haase et al., 2009; Small et al., 2001). The notion that young adults may have different responses in this region in response to pleasant versus unpleasant stimuli also supports this hypothesis.
In young adults, evaluation of sucrose also elicited a greater response than evaluation of caffeine in the bilateral pre- and postcentral gyri. The precentral and postcentral gyri are generally considered to be involved in the early stages of sensory and motor processing and discussion of the function of these regions in the central taste literature is limited. However, the postcentral region may be involved in processing oral somatosensory information (Boling et al., 2002). It has been suggested that the postcentral gyri receive afferent projections from the VPMpc (Faurion et al., 2005; Kobayakawa et al., 1996; Ogawa et al., 2005) and taste-related activation of the postcentral gyri has been consistently reported in the taste neuroimaging literature (for a meta-analysis, see Veldhuizen et al., 2011). Using the same task, we have previously reported consistent activation in both the precentral and postcentral gyri during pleasantness evaluation of sweet taste in young adults (Green & Murphy, 2012), which, combined with the current findings, suggests that these regions may respond preferentially to sweet or pleasant taste stimuli in young adults.
Although the young adults responded differentially to sweet and bitter tastes, the middle aged adults did not demonstrate significant differences in fMRI activation to sucrose and caffeine. Pleasant, sweet tastes are predictive of elevated energy content, while bitter, aversive tastes can serve to signal a warning of food that is dangerous to consume. Age-related declines in detection thresholds have been reported for both sweet and bitter taste (Murphy, 1979; Stevens et al., 1995; Schiffman and Gatlin, 1993). However, Weber Ratios for bitter taste are more affected by age than Weber ratios for sweet taste (Gilmore and Murphy, 1989) and the relative ecological importance of bitter taste may be more salient (warning of poisonous or rotting food). Therefore, we speculate that preserved central activation to bitter taste may serve as compensation for subtle changes in bitter taste processing that may begin to occur during middle age, prior to the onset of observable deficits.
There are limitations to the study. We discuss the results as corresponding to the “pleasantness evaluation task”. This task consisted of both considering the pleasantness of each taste or water stimulus, and also the motor component of manipulating a joystick to place a cursor on the appropriate number on a gLMS. However, we suggest that using water as a baseline and subtracting this condition out allowed us to control for possible individual differences in joystick proficiency. Additionally, acute administration of caffeine can affect the BOLD response (Mulderink et al., 2002). Our participants received only 1.55mg of caffeine 8 times intermittently over a 12-minute period. In comparison, Mulderink et al. administered 200mg of caffeine and waited up to 40 minutes to observe the delayed effects.
In summary, we used fMRI to examine brain activation in young and middle-aged adults during hedonic evaluation of a sweet taste stimulus and a bitter taste stimulus. While young adults had greater activation during hedonic evaluation of sucrose in brain regions involved in sensory-motor and reward processing than the middle-aged group, there were no differences between the age groups in the caffeine evaluation condition. Additionally, although young adults had greater activation of several primary sensory processing regions in the sucrose relative to the caffeine condition, there were no significant differences in activation between evaluation of the two tastes in the middle-aged group. We speculate that differential brain responses during hedonic evaluation of a rewarding taste according to age group might reflect the early age-related declines in central processing of pleasant tastes that may occur prior to observable deficits in gustatory function in old age, and this might have important implications for weight changes that occur during middle age. Importantly, longitudinal studies are warranted to better characterize age-related changes in central taste processing.
Greater activation during hedonic evaluation of sweet than bitter in young
Similar activation during hedonic evaluation of sweet and bitter in middle aged
Activation in middle-aged less than young during hedonic evaluation of sucrose
Activation in middle-aged less in sensory-motor and reward areas
Middle-age changes in CNS processing of taste: possible implications for weight gain
Acknowledgments
This research was supported by NIH grant No. AG004085-25 from the National Institute on Aging to Claire Murphy. Erin Green has been supported by both AG004085-25 and the Rose Marie Pangborn Sensory Science Scholarship. We thank Nobuko Kemmotsu for fMRI expertise and Delaney Downer, Ariana Stickel, Kelli Hayashi, and Sandra Daoud for data acquisition and research assistance.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonini A, Leenders K, Reist H, Thomann R, Beer H, Locher J. Effect of age on D2 dopamine receptors in normal human brain measured by positron emission tomography and 11C-raclopride. Archives of Neurology. 1993;50(5):474–480. doi: 10.1001/archneur.1993.00540050026010. [DOI] [PubMed] [Google Scholar]
- Arey LB, Tremaine MJ, Monzingo FL. The numerical and topographical relations of taste buds to human circumvallate papillae throughout the life span. Anatomical Record. 1935;64:9–25. [Google Scholar]
- Arvidson K. Location and variation in number of taste buds in human fungiform papillae. Scandinavian Journal of Dental Research. 1979;87:435–442. doi: 10.1111/j.1600-0722.1979.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Farde L. The role of dopamine systems in cognitive aging. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2005. pp. 58–84. [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiology & Behavior. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Baumgarter RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obesity Research. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundstrom JN. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 2011;49:324–30. doi: 10.4193/rhino10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling W, Reutens DC, Olivier A. Functional topography of the low postcentral area. Journal of Neurosurgery. 2002;97(2):388–95. doi: 10.3171/jns.2002.97.2.0388. [DOI] [PubMed] [Google Scholar]
- Bradley RM, Stedman HM, Mistretta M. Age does not affect numbers of taste buds and papillae in adult rhesus monkeys. Anatomical Record. 1985;212:246–249. doi: 10.1002/ar.1092120305. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Haase L, Murphy C. Effects of magnitude estimation of pleasantness and intensity on fMRI activation to taste. Chemosensory Perception. 2012;5:100–109. doi: 10.1007/s12078-011-9109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdrey FA, Park RJ, Harmer CJ, McCabe C. Increased neural processing of rewarding and aversive stimuli in recovered anorexia nervosa. Biological Psychiatry. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software analysis and visualization of functional magnetic resonance neuroimages. Computer Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Energy density, palatability, and satiety: Implications for weight control. Nutrition Reviews. 1998;56:347–353. doi: 10.1111/j.1753-4887.1998.tb01677.x. [DOI] [PubMed] [Google Scholar]
- Faurion A, Kobayakawa T, Cerf-Ducastel B. Cerebral imaging in taste. Chemical Senses. 2005;30(Suppl 1):i230–i231. doi: 10.1093/chemse/bjh198. [DOI] [PubMed] [Google Scholar]
- Felsted JA, Xueying R, Chouinard-Decorte F, Small D. Genetically determined differences in brain response to a primary food reward. The Journal of Neuroscience. 2010;30:2429–2432. doi: 10.1523/JNEUROSCI.5483-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olsen JE, Hong CP, Sellers TA, Lazovich D, Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women. Archives of Internal Medicine. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- Gilmore MM, Murphy C. Aging is associated with increased Weber ratios for caffeine, but not for sucrose. Perception and Psychophysics. 1989;46:555–559. doi: 10.3758/bf03208152. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “Labeled Magnitude Scale” for Measuring Sensations of Taste and Smell. Chemical Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses. 1993;18:683–702. [Google Scholar]
- Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Research. 2011;1386:109–117. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E, Murphy C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiology and Behavior. 2012;107(4):560–567. doi: 10.1016/j.physbeh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. Journal of Neuroscience Methods. 2007;159:98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44:1008–21. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Green E, Murphy C. Age-related functional changes in gustatory and reward processing regions: An fMRI study. Neuroimage. 2010;53:602–610. doi: 10.1016/j.neuroimage.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen GL, Rogers J. Obesity in older persons. Journal of the American Dietetic Association. 1998;98:1301–1311. doi: 10.1016/S0002-8223(98)00293-4. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, et al. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neuroscience Letters. 1996;212:155–158. doi: 10.1016/0304-3940(96)12798-1. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Ledikwe JH, Ello-Martin JA, Rolls BJ. Portion sizes and the obesity epidemic. The Journal of Nutrition. 2005;135(4):905–909. doi: 10.1093/jn/135.4.905. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I. Human taste bud density across adult age groups. Journal of Gerontology. 1988;43:B26–30. doi: 10.1093/geronj/43.1.m26. [DOI] [PubMed] [Google Scholar]
- Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. NeuroImage. 2002;15:37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- Muller DC, Elahi D, Tobin JD, Andres R. The effect of age on insulin resistance and secretion: A review. Seminars in Nephrology. 1996;16:289–298. [PubMed] [Google Scholar]
- Murphy C. The effects of age on taste sensitivity. In: Han DS, Coon DH, editors. Special Senses in Aging. Ann Arbor (MI): University of Ann Arbor, Institute of Gerontology; 1979. pp. 21–33. [Google Scholar]
- Murphy C. Nutrition and chemosensory perception in the elderly. Critical Reviews in Food Science and Nutrition. 1993;33(1):3–15. doi: 10.1080/10408399309527607. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiology of Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, et al. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Nordin S, Bramerson A, Bringlov E, Kobal G, Hummel T, Bende M. Substance and tongue-region specific loss in basic taste-quality identification in elderly adults. European Archives of Oto-Rhino-Laryngology. 2007;264:285–289. doi: 10.1007/s00405-006-0169-9. [DOI] [PubMed] [Google Scholar]
- Nordin S, Razani J, Markison S, Murphy C. Age-associated increases in intensity discrimination for taste. Experimental Aging Research. 2003;29:371–381. doi: 10.1080/03610730303719. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Wakita M, Hasegawa K, Kobayakawa T, Sakai N, Hirai T, et al. Functional MRI detection of activation in the primary gustatory cortices in humans. Chemical Senses. 2005;30:583–592. doi: 10.1093/chemse/bji052. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell M, Tabak C, et al. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal- weight women. Obesity. 2010;18:959–965. doi: 10.1038/oby.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Gatlin C. Clinical physiology of taste and smell. Annual Review of Nutrition. 1993;13:435–436. doi: 10.1146/annurev.nu.13.070193.002201. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. European Journal of Clinical Nutrition. 2000;54(Suppl 3):54–63. doi: 10.1038/sj.ejcn.1601026. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Effects of aging on the human taste system. Annals of the New York Academy of Sciences. 2009;1170:725–729. doi: 10.1111/j.1749-6632.2009.03924.x. [DOI] [PubMed] [Google Scholar]
- Schimizu Y. A histomorphometric study of the age-related changes of the human taste buds in circumvallate papillae. Oral medicine & Pathology. 1997;2:17–24. [Google Scholar]
- Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiology & Behavior. 1999;67(4):489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Scott TR, Verhagen JV. Taste as a factor in the management of nutrition. Nutrition. 2000;16(10):874–885. doi: 10.1016/s0899-9007(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Sherwood NE, Jeffery RW, French SA, Hannan PJ, Murray DM. Predictors of weight gain in the Pound of Prevention study. Int J Obes Relat Metab Disord. 2000;24:395–403. doi: 10.1038/sj.ijo.0801169. [DOI] [PubMed] [Google Scholar]
- Ship JA, Weiffenbach JM. Age, gender, medical treatment, and medication effects on smell identification. Journal of Gerontology. 1993;48:26–32. doi: 10.1093/geronj/48.1.m26. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens T, et al. Advances in functional and structural MR image analysis and implementation in FSL. NeuroImage. 2004;23:8208–8219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Cruz LA, Hoffman JM, Patterson MQ. Taste sensitivity and aging: High incidence of decline revealed by repeated threshold measures. Chemical Senses. 1995;20:451–459. doi: 10.1093/chemse/20.4.451. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald D. Relation of obesity to consummatory and anticipatory food reward. Physiology & Behavior. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research 1985. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundstr JN. Identification of human gustatory cortex by activation likelihood estimation. Human Brain Mapping. 2011;32:2256–2266. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008a;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal based study. British Medical Journal. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. The Journal of Neurophysiology. 1990;63(4):689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ, Scott TR. Satiety does not affect gustatory activity in the nucleus of the solitary tract of the alert monkey. Brain Research. 1985;347(1):85–93. doi: 10.1016/0006-8993(85)90891-1. [DOI] [PubMed] [Google Scholar]