Abstract
While blood vessels have long been implicated in diverse pain syndromes (e.g., migraine headache, angina pectoris, vasculitis, and Raynaud’s syndrome), underlying mechanisms remain to be elucidated. Recent evidence supports a contribution of the vascular endothelium in endothelin-1 induced hyperalgesia, and its enhancement by repeated mechanical stimulation; a phenomenon referred to as stimulus-induced enhancement of (endothelin) hyperalgesia (SIEH). SIEH is thought to be mediated by release of ATP from endothelial cells, to act on P2X3 receptors on nociceptors. In the present study we evaluated the ability of another vasoactive hyperalgesic agent, epinephrine, to induce endothelial cell dependent hyperalgesia and SIEH. We found that epinephrine also produces hyperalgesia and SIEH. Both a P2X3 receptor antagonist, A317491 and octoxynol-9, which attenuate endothelial cell function, eliminated SIEH without affecting epinephrine hyperalgesia. We further evaluated the hypothesis that members of two important classes of drugs used to treat migraine headache, whose receptors are present in endothelial cells - the triptans and beta blockers - have a vascular component to their anti-hyperalgesic action. For this, we tested the effect of ICI-118,551, a β2-adrenergic receptor antagonist and sumatriptan, an agonist at 5-HT1B and 5-HT1D receptors, on nociceptive effects of endothelin and epinephrine. ICI-118,551 inhibited endothelin SIEH, and attenuated epinephrine hyperalgesia and SIEH. Sumatriptan inhibited epinephrine SIEH and inhibited endothelin hyperalgesia and SIEH, while having no effect on epinephrine hyperalgesia or the hyperalgesia induced by a prototypical direct-acting inflammatory mediator, prostaglandin E2. These results support the suggestion that triptans and beta-blockers interact with the endothelial cell component of the blood vessel to produce anti-hyperalgesia.
Keywords: Endothelin, Epinephrine, Hyperalgesia, Triptan, β-blocker, Endothelial cell
INTRODUCTION
Clinical evidence supports a role of endothelial mechanisms in vascular pain. Genetic studies in patients, have described an association between migraine and both ET-AR and ET-BR endothelin receptors (Tzourio et al., 2001, Lemos et al., 2011) and elevated plasma levels of endothelin have been found during acute migraine attacks (Farkkila et al., 1992, Kallela et al., 1998). β -blockers and triptans (Diener et al., 2008, Chiam, 2012), two important and effective classes of drugs used to treat migraine headache have their targets, β-adrenergic and 5HT1B and 5HT1D receptors (Diener and Limmroth, 2005, Evans et al., 2008), on endothelial cells (McLeod and Piper, 1992a) as well as on nociceptors (Potrebic et al., 2003).
We have recently shown that mechanical hyperalgesia induced by the potent vasoconstrictor, endothelin-1, is enhanced by repeated mechanical stimulation (Joseph et al., 2011). This stimulus-induced enhancement of endothelin hyperalgesia is not produced by action of endothelin-1 on the nociceptor, as antisense to mRNA for the ET-AR endothelial receptor, administered intrathecally so that nociceptors are the only cell at the site of nociceptive testing exposed to it, inhibited endothelin-1 hyperalgesia but not its enhancement by mechanical stimuli (Joseph E.K. et al., 2012, in review). Conversely, injury to endothelial cells, which line the blood vessel lumen, eliminated stimulus-induced enhancement of endothelin-1 hyperalgesia, without attenuating its hyperalgesia (Joseph E.K. et al., 2012, in review).
In the present experiments, we have tested the hypothesis that the hyperalgesia induced by β2-adrenergic receptor agonists, which are vasoactive, is also enhanced by mechanical stimulation and that this enhancement is also endothelial cell dependent. We also tested the hypothesis that representatives of two major classes of anti-migraine drugs, triptans and beta-blockers, are capable of inhibiting stimulus-induced enhancement of the hyperalgesia produced by endothelin-1 and the β-adrenergic agonist epinephrine, and that endothelial cells play a role in these actions.
METHODS
Animals
Experiments were performed on adult male Sprague Dawley rats (200–250 g; Charles River, Hollister, CA). Animals were housed three per cage, under a 12-h light/dark cycle, in a temperature and humidity controlled environment. Food and water were available ad libitum. All behavioral nociceptive testing was performed between 10:00 am and 4:00 pm. Rats were acclimatized to the experimental area and behavioral procedures prior to the experiment. To acclimatize rats to the testing environment, they were brought to the experimental area in their home cages and left in the cages for 15–30 min after which they were placed in a restrainer (cylindrical transparent acrylic tubes that have openings on their sides, to allow extension of the hind legs from the restrainer, for nociceptive testing). Rats were left in the restrainer for another 15–30 min before nociceptive testing was started. This acclimatization procedure consistently results in baseline paw withdrawal thresholds of 100 – 120 g for the body weight range for the rats used in this study. All experimental protocols were approved by the UCSF Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Nociceptive testing
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter® (Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat's hind paw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its hind paw. Baseline nociceptive thresholds, was defined as the mean of three readings taken at 5-min intervals, determined prior to all experiments. The mean of these three readings was considered to be the baseline paw-withdrawal threshold before drug administration. Mechanical threshold was re-determined at four time points (15, 20, 25 and 30 min) after treatments and this value was used to calculate the percentage change from the baseline threshold for each paw. Each paw was treated as an independent measure; both paws of the same rat received the same treatment (Aley and Levine, 1997, Khasar et al., 1998, Aley and Levine, 1999, Joseph and Levine, 2006). Each experiment was performed on separate groups of rats. Each animal acted as its own control, with inhibitor injected intradermally into both hind paws 15 min prior to the administration of endothelin-1 or epinephrine and paw withdrawal thresholds compared, pre- and post-drug treatment. Hyperalgesia was defined as a decrease in mechanical nociceptive threshold, here presented as percent reduction from baseline [% reduction in threshold = (pretreatment threshold − post-treatment threshold)/ (pretreatment threshold)] × 100.
Stimulus-induced enhancement of hyperalgesia protocol
In general, the mechanical thresholds determined at four time points (15, 20, 25 and 30 min) after treatments are similar. However, in endothelin and epinephrine treated rats, there appeared further enhancement of hyperalgesia with repeated testing of mechanical nociceptive threshold (i.e., further decrease in paw withdrawal threshold with each repeated application of the stimulus), which we refer to as stimulus-induced enhancement of hyperalgesia. To differentiate the stimulus-induced enhancement of hyperalgesia from the mechanical hyperalgesia (1st, 2nd, 3rd and 4th reading, taken at 5 min intervals (15–30 min)) induced by both endothelin-1 and epinephrine, the paw withdrawal thresholds were measured (single reading) at an early (5 min) and late (30 min) time point, in different groups of rats, and these results are also shown in the relevant figure.
Drugs
The drugs employed in this study were: endothelin-1, epinephrine, ICI-118,551 (β2-adrenergic receptor antagonist), sumatriptan (5HT1B and 5HT1D agonist), A-317491, octoxynol-9, and prostaglandin E2 (all from Sigma-Aldrich, St. Louis, MO). Drug doses employed in this study were based on dose response curves generated in our previous studies (Aley and Levine, 1999, Khasar et al., 1999, Joseph et al., 2011) or the dose response curves performed as part of the present study.
All drugs except octoxynol-9 were administered intradermally (i.d.) in a volume of 5 µl using a 30-gauge hypodermic needle attached to a micro-syringe (Hamilton, Reno, NV) by PE-10 polyethylene tubing. Octoxynol-9 (0.5% V/V, 1 ml/kg weight) was administered intravenously through a tail vein. All inhibitors were administered 15 min prior to endothelin-1, epinephrine or prostaglandin E2 (PGE2) and nociceptive thresholds measured (four times), at 15, 20, 25 and 30 min post endothelin-1, epinephrine or prostaglandin E2 administration. Also, paw withdrawal thresholds were determined by a single reading at 5’ and 30’ post endothelin and epinephrine administration, to differentiate the time course of mechanical hyperalgesia from the enhancement of hyperalgesia induced by repeated stimulation. The per se effect of all the inhibitors including octoxynol-9 were separately evaluated and none had significant effect on the basal paw-withdrawal threshold of the naïve rats (data not shown). All drugs were dissolved in saline.
Endothelial cell injury
In the cardiovascular and renal literature, a role of endothelial cells in vascular function has been evaluated, in vivo and in situ, using brief exposure to octoxynol-9. As shown by light and electron microscopy, the intravenous or intra-arterial administration of octoxynol-9 selectively injures the blood vessel endothelial cell (McLeod and Piper, 1992b, Bourreau et al., 1993). Octoxynol-9 is used both in vivo (Pridgen et al., 2011, Enanche and Volanschi, 2012) and in vitro (Eddy et al., 2012, Murphy et al., 2012, Soni et al., 2012). While new to the field of pain research, octoxynol-9 has been used for experimental intervention in cardiovascular studies for many years (Connor et al., 1989, Sarvazyan, 1998, Mink et al., 2007).
To evaluate the role of the endothelial cell in stimulus-induced enhancement of hyperalgesia, rats received an intravenous injection, through a tail vein, of a 0.5% solution of octoxynol-9, at a volume of 1 ml/kg body weight (Joseph EK et al., 2012, in review). ET-1 was injected 15 minutes later, and the animals evaluated for hyperalgesia and stimulus-induced enhancement of this hyperalgesia. Injection of saline (diluent for octoxynol-9) served as the control. Rats showed no indication of distress throughout the period of the experiment following administration of octoxynol-9.
Statistical analysis
In all experiments, the dependent variable was change in paw withdrawal threshold, represented as percentage change from the pre-treatment baseline threshold or from the corresponding controls. Group data are represented as mean ± SEM. Statistical significance was determined by two-way repeated measures ANOVA followed by Bonferroni post hoc test comparing results within and between groups at different time points. P values <0.05 were considered statistically significant.
RESULTS
Beta2-adrenergic agonist
We have previously demonstrated that while endothelin-1 induces mechanical hyperalgesia that is enhanced by the mechanical stimulus used to test nociceptive threshold (Joseph et al., 2011), multiple other hyperalgesia-inducing mediators (i.e., prostaglandin E2 (PGE2), nerve growth factor (NGF), glia-derived neurotrophic factor (GDNF), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα)) are not associated with stimulus-induced enhancement of their hyperalgesia (Joseph et al., 2011). β-adrenergic agonists produce mechanical hyperalgesia (Khasar et al., 1999), and since β-adrenergic receptors are present on endothelial cells (Queen et al., 2006, Grueb et al., 2008), we tested the hypothesis that mechanical stimulation also enhances hyperalgesia induced by epinephrine, an endogenous ligand for β-adrenergic receptors.
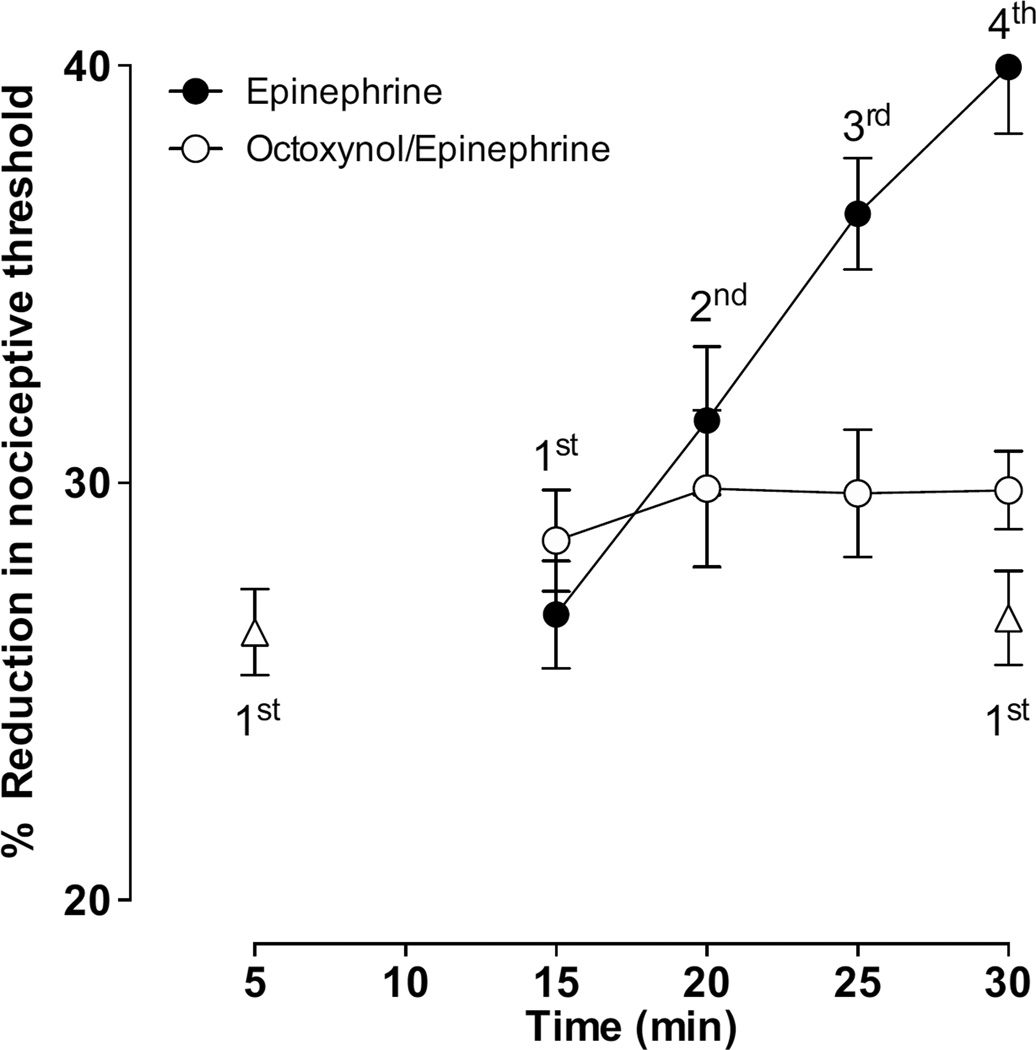
The intradermal injection of epinephrine (100 ng) produced mechanical hyperalgesia and stimulus-induced enhancement of epinephrine hyperalgesia. A single reading of the paw withdrawal threshold at 5 min or 30 min following the epinephrine administration produced a similar degree of hyperalgesia, whereas repeated readings (indicated by 1st, 2nd, 3rd and 4th taken at 5 min intervals, over a similar time period after injection of epinephrine) produce a significant increase from that of a single reading (Fig. 1). To confirm that this stimulus-induced enhancement of epinephrine hyperalgesia was, like that induced by endothelin-1, endothelial cell mediated, a group of rats were pretreated with octoxynol-9, which eliminates the stimulus-induced enhancement of endothelin-1 hyperalgesia (Joseph E.K. et al., 2012, in review). In this group of rats, while epinephrine-induced hyperalgesia was unaffected, epinephrine failed to produce stimulus-induced enhancement of epinephrine hyperalgesia (Fig. 1, n = 8/group).
Figure 1. Effect of octoxynol-9 on epinephrine-induced mechanical hyperalgesia and stimulus-induced enhancement of epinephrine hyperalgesia.
Intradermal administration of epinephrine (100 ng) produced mechanical hyperalgesia (P < 0.001, n = 8). Hyperalgesia reached at 5’after epinephrine administration (single reading) was similar to that observed after a single reading at 30’ measured in a separate group of rats (triangular symbols). Repeated readings (15–30’) of paw withdrawal thresholds at 5 min intervals, caused enhancement of hyperalgesia (further decrease in paw withdrawal threshold on repeated application of the threshold stimulus). Intravenous administration of octoxynol-9 (0.5%, V/V, 1 ml/kg) 15 min prior to epinephrine, abolished stimulus-induced enhancement of epinephrine hyperalgesia (P < 0.001, two way repeated measures ANOVA followed by Bonferroni post test, comparing results within and between groups at different time points, n = 8/group) without affecting the epinephrine hyperalgesia.
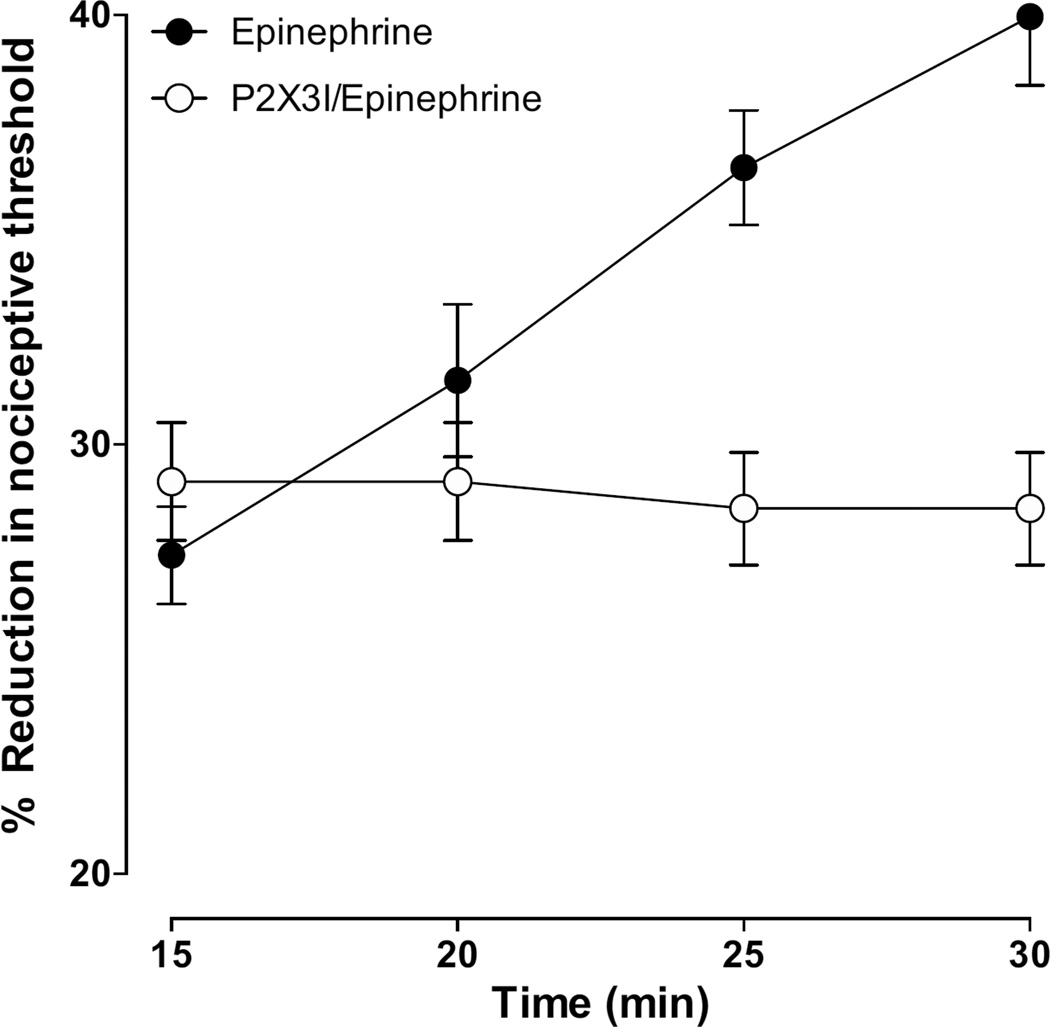
To further confirm that this stimulus-induced enhancement of epinephrine hyperalgesia was, like that induced by endothelin-1, P2X3 or P2X2/3 receptor dependent, we tested the effect of A-317491, a P2X2/3 inhibitor on stimulus-induced enhancement of epinephrine hyperalgesia. A group of rats were treated with A-317491 (1 µg, intradermal), 15 min prior to epinephrine. In this group of rats, stimulus-induced enhancement of epinephrine hyperalgesia was abolished, without affecting epinephrine hyperalgesia (Fig. 2, n = 8/group).
Figure 2. Effect of A-317491 (P2X2/3 inhibitor) on epinephrine-induced mechanical hyperalgesia and stimulus-induced enhancement of epinephrine hyperalgesia.
Intradermal administration of A-317491 (1 µg/paw) 15 min prior to epinephrine, abolished stimulus-induced enhancement of epinephrine hyperalgesia (P < 0.001, two way repeated measures ANOVA followed by Bonferroni post test, comparing results within and between groups at different time points, n = 8/group) without affecting the epinephrine hyperalgesia.
Beta2-adrenergic receptor antagonism
Beta-blockers, such as propranolol, which are antagonists at both β1 and β2-adrenergic receptors, are amongst the most commonly used drugs for the treatment of migraine headache (Firnhaber and Rickett, 2009, Supiot, 2009), suggesting that tonic activity at β-adrenergic receptors contributes to the predisposition to migraine. In support of this hypothesis, stress is generally recognized as the most common antecedent to a migraine attack (Martin et al., 2005, Tuncel et al., 2008).
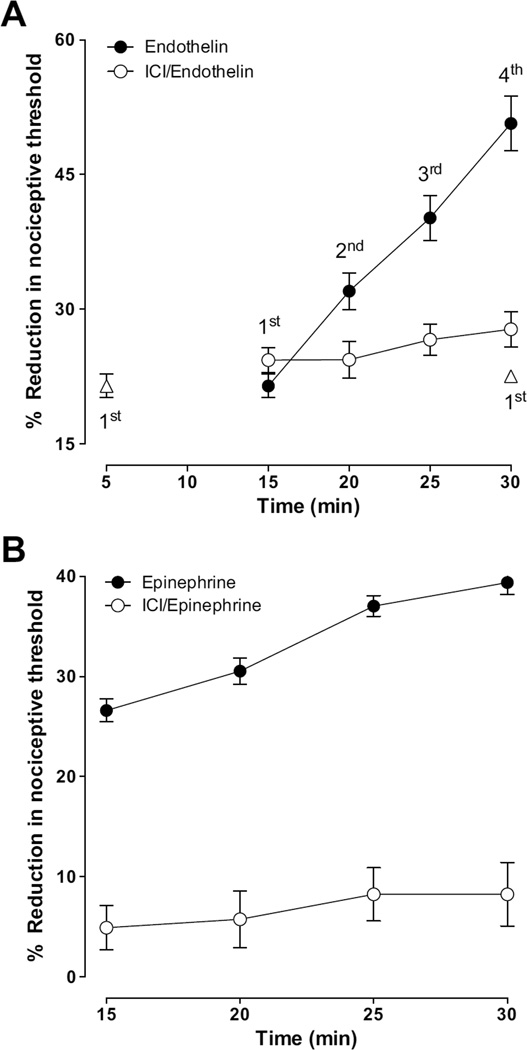
We found that the β2-adrenergic receptor selective antagonist, ICI-118,551, attenuated stimulus-induced enhancement of endothelin-1 hyperalgesia (Fig. 3 A, n = 8/group), which is compatible with the suggestion that tonic β2-adrenergic receptor activity in endothelial cells contributes to peripheral pain mechanisms. While its effect was limited to abolishing the stimulus-induced enhancement of ET-1 hyperalgesia, ICI-118,551 significantly inhibited both epinephrine-induced hyperalgesia and stimulus-induced enhancement of epinephrine hyperalgesia (Fig. 3 B, n = 12/group).
Figure 3. Effect of ICI 118,551 (β2 - selective adrenergic antagonist) on endothelin-1 (A) and epinephrine (B) induced mechanical hyperalgesia and stimulus-induced enhancement of their hyperalgesia.
Intradermal administration of ICI 118,551 (1 µg/paw) 15 min prior to endothelin, abolished stimulus-induced enhancement of endothelin hyperalgesia (A, P < 0.001, two way repeated measures ANOVA followed by Bonferroni post test, n = 8/group) without affecting the endothelin hyperalgesia. Intradermal administration of ICI 118,551 (1 µg/paw) 15 min prior to epinephrine abolished both epinephrine hyperalgesia and stimulus-induced enhancement of hyperalgesia (B, for both P < 0.001, two way repeated measures ANOVA followed by Bonferroni post test, comparing results within and between groups at different time points, n = 12/group).
5HT1B and 5HT1D receptor agonism
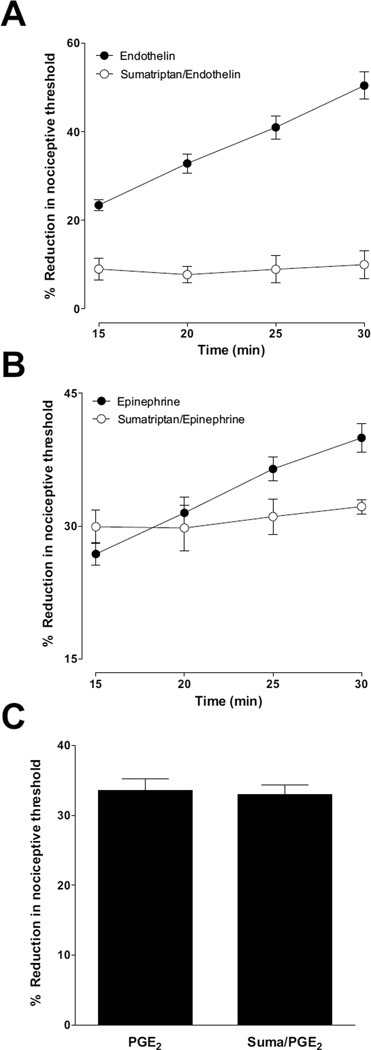
While the effect of triptans on migraine headache is thought to be due to their action on the central and peripheral terminals of the primary afferent nociceptor, which contain 5HT1B and 5HT1D receptors (Hargreaves and Shepheard, 1999, Classey et al., 2010), and/or at sites in the central nervous system (Goadsby, 1998), these receptors are also present on endothelial cells (Seager et al., 1992, Kilbourne and Winneker, 2001, van den Broek et al., 2002). Therefore, we tested the hypothesis that triptans inhibit stimulus-induced enhancement of hyperalgesia produced by the potent vasoconstrictor, endothelin-1 and the β-adrenergic receptor agonist epinephrine. Intradermal injection of sumatriptan (5 µg), 15 min before endothelin-1 (100 ng), not only inhibited stimulus-induced enhancement of endothelin-hyperalgesia, but also endothelin-hyperalgesia (Fig. 4 A). And while sumatriptan also inhibited stimulus-induced enhancement of epinephrine-induced hyperalgesia, it had no effect on epinephrine-induced mechanical hyperalgesia (Fig. 4 B). Triptans also failed to inhibit the mechanical hyperalgesia induced by the prototypical direct-acting pronociceptive inflammatory mediator, prostaglandin E2 (100 ng) (Fig. 4 C). Thus, triptans may produce antinociceptive effects at least in part, by acting at their cognate receptors located on endothelial cells, as well as on the primary afferent nociceptor.
Figure 4. Effect of sumatriptan (5-HT1B/D agonist) on endothelin-1 (A), epinephrine (B) induced mechanical hyperalgesia and stimulus-induced hyperalgesia and on PGE2 (C) induced mechanical hyperalgesia.
Intradermal administration of sumatriptan (5 µg/paw) 15 min prior to endothelin, attenuated endothelin hyperalgesia and stimulus-induced enhancement of endothelin hyperalgesia (A, for both P < 0.001, two way repeated measures ANOVA followed by Bonferroni post test, n = 8/group), whereas sumatriptan treatment 15 min prior to epinephrine, only attenuated stimulus-induced enhancement of epinephrine hyperalgesia (B, P < 0.001, two way repeated measures ANOVA followed by Bonferroni post test, comparing results within and between groups at different time points, n = 8/group). Intradermal administration sumatriptan 15 min prior to PGE2 (100 ng) had no effect on PGE2 hyperalgesia (C, n = 6).
DISCUSSION
Migraine headache is a complex disorder that likely involves central nervous system and peripheral nervous system, as well as vascular mechanisms (Kurth, 2007, Elliott, 2008, Levy, 2012, McCrory and Gray, 2012). A role of neurovascular mechanisms in migraine headache has been suggested based on a number of clinical observations, including the occurrence of vasoconstriction followed by vasodilatation at onset of migraine headaches, decrease in migraine pain with compression of the temporal artery, and precipitation of migraine headache with vasodilators. Furthermore, treatment of migraine with vasoconstrictors and beta-blockers, association of migraine with vascular diseases (e.g., systemic lupus erythematous, patent foramen ovale, angina, Raynaud’s and A–V malformation), and increase in plasma endothelin level at onset of migraine (Watts et al., 1995, Edvinsson et al., 2005), are all compatible with neurovascular involvement in migraine. While more recent studies provide evidence for an important role of the central and peripheral nervous system in migraine headache (Dodick and Silberstein, 2006, Goadsby, 2012, Samsam, 2012), many of these studies also support the suggestion that there is an interaction between the peripheral nervous system and blood vessels (May, 2003, Olesen et al., 2009). While the peripheral contribution has, in general, been related to primary afferent nociceptor function (Shepard et al., 1991), abnormalities in vascular function have also been described (May, 2003). However, how altered vascular function relates to the primary manifestations of migraine headache has not been established. In the present experiments we tested the hypothesis that the representatives of two major classes of drugs used in the treatment of migraine headache inhibit endothelin or epinephrine hyperalgesia, at least in part, by action on endothelial cells.
In a previous study we established that vascular endothelial cells play a role in pain induced by the potent vasoconstrictor peptide, endothelin-1 (Joseph E.K. et al., 2012 in review). We hypothesized that drugs used in the treatment of migraine headache would inhibit the endothelial cell contribution to endothelin-hyperalgesia. Therefore, we evaluated the effect of two major classes of compounds, a β-adrenergic receptor antagonist, ICI-118551 and a 5HT1B and 5HT1D agonist, sumatriptan, both of which have receptors in endothelial cells, on endothelin hyperalgesia. Both compounds blocked stimulus – induced enhancement of endothelin hyperalgesia, while alone having no effect on nociceptive threshold. Since ICI-118,551 has inverse agonist properties, it could operate to suppress signaling, in part, absent an endogenous ligand, but still presumably by action on the endothelial cell. Furthermore, sumatriptan failed to attenuate the hyperalgesia induced by the direct-acting pronociceptive inflammatory mediator, prostaglandin E2, compatible with a role of endothelial cells in vascular pain.
That endothelial cells may play a role in migraine headache raises some interesting questions. For example, one of the diagnostic tests for migraine headache has been the precipitation of a headache by the administration of nitroglycerin, a potent vasodilator, which increases nitric oxide (NO) in the endothelial cell (Greco et al., 2011). One of the unusual characteristic features of the nitroglycerin provocation test is that in patients with migraine headache, there is a distinct delay from the administration of nitroglycerin and the onset of the migraine headache (Schoonman et al., 2006). We suggest that such a delay might be explained by an indirect mechanism of action, such as by its action to increase NO in endothelial cells, leading to the release of mediators that act on nociceptors to produce headache pain. A second interesting clinical correlation relates to the suggestion that triptans are considered specific for migraine headache (Pym et al., 1991, Classey et al., 2010). In support of this, we observed that sumatriptan did not treat prostaglandin-induced mechanical hyperalgesia. Since the receptors on which the triptans are thought to act, 5HT1B and 5HT1D are present on dorsal root ganglion as well as trigeminal ganglion neurons (Pierce et al., 1996, Classey et al., 2010), triptans may be effective against non-trigeminal vascular pain, by action at both the nociceptors and endothelial cells, and potentially at other levels of the neuraxis.
In a previous study (Joseph EK et al, 2012, in review), we tested the hypothesis that ATP functions as a mediator of stimulus-induced enhancement of hyperalgesia using A-317491, a P2X3 antagonist. We found that A-317491 significantly inhibit stimulus-induced enhancement of hyperalgesia for ET-1. Therefore, a similar hypothesis was tested in this study for stimulus-induced enhancement of hyperalgesia for epinephrine. We found that A-317491 markedly inhibited epinephrine induced SIEH. Endothelial cells lining the vessel lumen are constantly subjected to conditions of varying blood flow and shear stress. The release of vasoactive substances including ATP during conditions of increased shear stress has been extensively documented (Bodin and Burnstock, 1995). A mechanical stimulus (stress) may cause the vesicular release of ATP, which may be responsible for the enhancement of hyperalgesia. However, further research is required to determine the exact mechanisms responsible for this event.
Of note, we found that triptans not only prevented stimulus-induced enhancement of endothelin-1 hyperalgesia but also endothelin-1 hyperalgesia, while the beta-blockers only inhibited stimulus-induced enhancement of endothelin-1 hyperalgesia. This additional effect of the triptans may help to explain the notable efficiency of the triptans for the treatment of migraine headache.
In conclusion, we provide evidence that anti-migraine compounds can produce anti-hyperalgesia by action on endothelial cells. And, while the role of this particular mechanism in the treatment of migraine headache remains to be established, a better understanding of the role of endothelial cells in vascular pain may help identify novel targets for the treatment of migraine headache as well as other vascular related pain syndromes.
Highlights.
Endothelin-1 and epinephrine induce mechanical hyperalgesia.
Mechanical stimulation enhances endothelin-1 and epinephrine-induced hyperalgesia.
Such enhanced hyperalgesia is dependent on P2X3 receptor activation.
Triptan and beta-blocker attenuate stimulus-induced enhancement of hyperalgesia.
Acknowledgements
the NIH supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aley KO, Levine JD. Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in rat. J Neurosci. 1997;17:8018–8023. doi: 10.1523/JNEUROSCI.17-20-08018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- Bourreau JP, Banijamali HS, Challice CE. Modification of excitation-contraction coupling in cat ventricular myocardium following endocardial damage. Can J Physiol Pharmacol. 1993;71:254–262. doi: 10.1139/y93-040. [DOI] [PubMed] [Google Scholar]
- Chiam PJ. Topical beta-blocker treatment for migraine. Int Ophthalmol. 2012;32:85–88. doi: 10.1007/s10792-012-9516-6. [DOI] [PubMed] [Google Scholar]
- Classey JD, Bartsch T, Goadsby PJ. Distribution of 5-HT(1B), 5-HT(1D) and 5-HT(1F) receptor expression in rat trigeminal and dorsal root ganglia neurons: relevance to the selective anti-migraine effect of triptans. Brain Res. 2010;1361:76–85. doi: 10.1016/j.brainres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Connor HE, Edwards LA, Feniuk W. Neurogenically mediated contractions of dog basilar artery involve the release of a thromboxane-like substance. European journal of pharmacology. 1989;174:205–213. doi: 10.1016/0014-2999(89)90313-0. [DOI] [PubMed] [Google Scholar]
- Diener HC, Katsarava Z, Limmroth V. Current diagnosis and treatment of migraine. Ophthalmologe. 2008;105:501–508. doi: 10.1007/s00347-008-1747-6. quiz 509–510. [DOI] [PubMed] [Google Scholar]
- Diener HC, Limmroth V. Migraine therapy. Internist (Berl) 2005;46:1087–1095. doi: 10.1007/s00108-005-1459-9. [DOI] [PubMed] [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(Suppl 4):S182–S191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Eddy MT, Ong TC, Clark L, Teijido O, van der Wel PC, Garces R, Wagner G, Rostovtseva TK, Griffin RG. Lipid dynamics and protein-lipid interactions in 2D crystals formed with the beta-barrel integral membrane protein VDAC1. Journal of the American Chemical Society. 2012;134:6375–6387. doi: 10.1021/ja300347v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Uddman E, Wackenfors A, Davenport A, Longmore J, Malmsjo M. Triptan-induced contractile (5-HT1B receptor) responses in human cerebral and coronary arteries: relationship to clinical effect. Clin Sci (Lond) 2005;109:335–342. doi: 10.1042/CS20050016. [DOI] [PubMed] [Google Scholar]
- Elliott D. Migraine and stroke: current perspectives. Neurol Res. 2008;30:801–812. doi: 10.1179/174313208X341049. [DOI] [PubMed] [Google Scholar]
- Enanche M, Volanschi E. Spectroscopic investigations of the molecular interaction of anticancer drug mitoxantrone with non-ionic surfactant micelles. J Phar Pharacol. 2012;64:688–696. doi: 10.1111/j.2042-7158.2012.01445.x. [DOI] [PubMed] [Google Scholar]
- Evans RW, Rizzoli P, Loder E, Bana D. Beta-blockers for migraine. Headache. 2008;48:455–460. doi: 10.1111/j.1526-4610.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Farkkila M, Palo J, Saijonmaa O, Fyhrquist F. Raised plasma endothelin during acute migraine attack. Cephalalgia. 1992;12:383–384. doi: 10.1111/j.1468-2982.1992.00383.x. discussion 340. [DOI] [PubMed] [Google Scholar]
- Firnhaber JM, Rickett K. Clinical inquiries. What are the best prophylactic drugs for migraine? J Fam Pract. 2009;58:608–610. [PubMed] [Google Scholar]
- Goadsby PJ. Serotonin receptors and the acute attack of migraine. Clin Neurosci. 1998;5:18–23. [PubMed] [Google Scholar]
- Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol. 2012;15:S15–S22. doi: 10.4103/0972-2327.99993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco R, Meazza C, Mangione AS, Allena M, Bolla M, Amantea D, Mizoguchi H, Sandrini G, Nappi G, Tassorelli C. Temporal profile of vascular changes induced by systemic nitroglycerin in the meningeal and cortical districts. Cephalalgia. 2011;31:190–198. doi: 10.1177/0333102410379887. [DOI] [PubMed] [Google Scholar]
- Grueb M, Bartz-Schmidt KU, Rohrbach JM. Adrenergic regulation of cAMP/protein kinase A pathway in corneal epithelium and endothelium. Ophthalmic Res. 2008;40:322–328. doi: 10.1159/000150446. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26(Suppl 3):S12–S19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Gear RW, Levine JD. Mechanical stimulation enhances endothelin-1 hyperalgesia. Neuroscience. 2011;178:189–195. doi: 10.1016/j.neuroscience.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121:105–114. doi: 10.1016/j.pain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kallela M, Farkkila M, Saijonmaa O, Fyhrquist F. Endothelin in migraine patients. Cephalalgia. 1998;18:329–332. doi: 10.1046/j.1468-2982.1998.1806329.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Miao FJ, Janig W, Levine JD. Vagotomy-induced enhancement of mechanical hyperalgesia in the rat is sympathoadrenal-mediated. J Neurosci. 1998;18:3043–3049. doi: 10.1523/JNEUROSCI.18-08-03043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne EJ, Winneker RC. Serotonin 5-HT1 receptors potentiate histamine and thrombin stimulated prostaglandin synthesis in endothelial cells. Thromb Haemost. 2001;85:924–928. [PubMed] [Google Scholar]
- Kurth T. Migraine and ischaemic vascular events. Cephalalgia. 2007;27:965–975. doi: 10.1111/j.1468-2982.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- Lemos C, Neto JL, Pereira-Monteiro J, Mendonca D, Barros J, Sequeiros J, Alonso I, Sousa A. A role for endothelin receptor type A in migraine without aura susceptibility? A study in Portuguese patients. Eur J Neurol. 2011;18:649–655. doi: 10.1111/j.1468-1331.2010.03239.x. [DOI] [PubMed] [Google Scholar]
- Levy D. Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading epression. Curr Pain Headache Rep. 2012;16:270–277. doi: 10.1007/s11916-012-0255-1. [DOI] [PubMed] [Google Scholar]
- Martin PR, Todd J, Reece J. Effects of noise and a stressor on head pain. Headache. 2005;45:1353–1364. doi: 10.1111/j.1526-4610.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- May A. The trigeminovascular system in the human. Cerebral blood flow, functional imaging and primary headache. Nervenarzt. 2003;74:1067–1077. doi: 10.1007/s00115-003-1578-2. [DOI] [PubMed] [Google Scholar]
- McCrory DC, Gray RN. Oral sumatriptan for acute migraine. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD002915.pub2. CD002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, Piper PJ. Effect of K+ channel-modulating drugs on the vasoconstrictor responses of leukotrienes C4, D4 and angiotensin II in the guinea-pig isolated perfused heart. British journal of pharmacology. 1992a;105:739–743. doi: 10.1111/j.1476-5381.1992.tb09048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, Piper PJ. Effect of removing the endothelium on the vascular responses induced by leukotrienes C4 and D4 in guinea-pig isolated heart. European journal of pharmacology. 1992b;212:67–72. doi: 10.1016/0014-2999(92)90073-d. [DOI] [PubMed] [Google Scholar]
- Mink SN, Cheng ZQ, Bose R, Jacobs H, Kasian K, Roberts DE, Santos-Martinez LE, Light RB. Lysozyme, a mediator of sepsis, impairs the cardiac neural adrenergic response by nonendothelial release of NO and inhibitory G protein signaling. American journal of physiology Heart and circulatory physiology. 2007;293:H3140–H3149. doi: 10.1152/ajpheart.00502.2007. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Xu H, Latchman H, Larkins NT, Gooley PR, Stapleton DI. Single fiber analyses of glycogen related proteins reveal their differential association with glycogen in rat skeletal muscle. American journal of physiology Cell physiology. 2012 doi: 10.1152/ajpcell.00252.2012. [DOI] [PubMed] [Google Scholar]
- Nagaraja S, Iyer S, Liu X, Eichberg J, Bond RA. Treatment with inverse agonists enhances baseline atrial contractility in transgenic mice with chronic beta2-adrenoceptor activation. British journal of pharmacology. 1999;127:1099–1104. doi: 10.1038/sj.bjp.0702645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Xie GX, Peroutka SJ, Levine JD. Dual effect of the serotonin agonist, sumatriptan, on peripheral neurogenic inflammation. Reg Anesth. 1996;21:219–225. [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgen BC, Woon CY, Kim M, Thorfinn J, Lindsey D, Pham H, Chang J. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C methods. 2011;17:818–828. doi: 10.1089/ten.tec.2010.0457. [DOI] [PubMed] [Google Scholar]
- Pym RA, Johnson RJ, Etse DB, Eason P. Inheritance of plasma insulin-like growth factor-I and growth rate, food intake, food efficiency and abdominal fatness in chickens. Br Poult Sci. 1991;32:285–293. doi: 10.1080/00071669108417352. [DOI] [PubMed] [Google Scholar]
- Queen LR, Ji Y, Xu B, Young L, Yao K, Wyatt AW, Rowlands DJ, Siow RC, Mann GE, Ferro A. Mechanisms underlying beta2-adrenoceptor-mediated nitric oxide generation by human umbilical vein endothelial cells. J Physiol. 2006;576:585–594. doi: 10.1113/jphysiol.2006.115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsam M. Central nervous system acting drugs in treatment of migraine headache. Cent Nerv Syst Agents Med Chem. 2012;12:158–172. doi: 10.2174/187152412802430147. [DOI] [PubMed] [Google Scholar]
- Sarvazyan N. A new approach to assess viability of adult cardiomyocytes: computer-assisted image analysis. Journal of molecular and cellular cardiology. 1998;30:297–301. doi: 10.1006/jmcc.1997.0624. [DOI] [PubMed] [Google Scholar]
- Schoonman GG, Sandor PS, Agosti RM, Siccoli M, Bartsch P, Ferrari MD, Baumgartner RW. Normobaric hypoxia and nitroglycerin as trigger factors for migraine. Cephalalgia. 2006;26:816–819. doi: 10.1111/j.1468-2982.2006.01112.x. [DOI] [PubMed] [Google Scholar]
- Seager JM, Clark AH, Garland CJ. Endothelium-dependent contractile responses to 5-hydroxytryptamine in the rabbit basilar artery. British journal of pharmacology. 1992;105:424–428. doi: 10.1111/j.1476-5381.1992.tb14269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard SR, Ghajar JB, Giannuzzi R, Kupferman S, Hariri RJ. Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J Surg Res. 1991;51:417–424. doi: 10.1016/0022-4804(91)90144-b. [DOI] [PubMed] [Google Scholar]
- Soni SK, Singh R, Awasthi A, Singh M, Kalra A. In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environmental science and pollution research international. 2012 doi: 10.1007/s11356-012-1178-4. [DOI] [PubMed] [Google Scholar]
- Supiot F. Migraine in 2009: from attack to treatment. Rev Med Brux. 2009;30:399–403. [PubMed] [Google Scholar]
- Tuncel D, Tolun FI, Gokce M, Imrek S, Ekerbicer H. Oxidative stress in migraine with and without aura. Biol Trace Elem Res. 2008;126:92–97. doi: 10.1007/s12011-008-8193-9. [DOI] [PubMed] [Google Scholar]
- Tzourio C, El Amrani M, Poirier O, Nicaud V, Bousser MG, Alperovitch A. Association between migraine and endothelin type A receptor (ETA −231 A/G) gene polymorphism. Neurology. 2001;56:1273–1277. doi: 10.1212/wnl.56.10.1273. [DOI] [PubMed] [Google Scholar]
- van den Broek RW, Bhalla P, VanDenBrink AM, de Vries R, Sharma HS, Saxena PR. Characterization of sumatriptan-induced contractions in human isolated blood vessels using selective 5-HT(1B) and 5-HT(1D) receptor antagonists and in situ hybridization. Cephalalgia. 2002;22:83–93. doi: 10.1046/j.1468-2982.2002.00295.x. [DOI] [PubMed] [Google Scholar]
- Watts SW, Gilbert L, Webb RC. 5-Hydroxytryptamine2B receptor mediates contraction in the mesenteric artery of mineralocorticoid hypertensive rats. Hypertension. 1995;26:1056–1059. doi: 10.1161/01.hyp.26.6.1056. [DOI] [PubMed] [Google Scholar]