Abstract
Background
Alterations in inflammatory mediators are an important finding in neonates who develop bronchopulmonary dysplasia (BPD); however, there is a lack of research examining the relationship between multiple inflammatory mediators in premature neonates and the development of BPD.
Objective
This study investigated whether the distribution of 12 inflammatory mediators detected in the tracheal aspirate (TA) of neonates within 24 hours of birth could differentiate between neonates who did and did not develop BPD.
Study Design
TA samples were collected from 27 very low birth weight neonates (BPD+ =11) and the concentrations of 12 biomarkers associated with BPD were determined. Linear discriminate analysis (LDA) was used to classify neonates into two outcome groups.
Result
LDA based on the 12 measured biomarkers displayed a significant level of discriminant function (p=0.007).
Conclusion
Using linear discriminant analysis, predictive models of BPD can be generated. Our results suggest that multiple inflammatory mediators collected within 24 hours of birth may be used to classify neonates into who will and will not develop BPD.
Keywords: cytokines, chronic lung disease, premature neonate
Introduction
The use of antenatal steroids, surfactant therapy, and a more conservative approach to respiratory support have allowed an increasing number of smaller, extremely premature neonates to survive. Additionally, it has led to a change in the pathophysiology of neonates who develop bronchopulmonary dysplasia (BPD) since severe lung injury in larger and more mature infants has been minimized. 1 Significant morbidity in very low birth weight neonates remains attributed to the development of BPD as up to 42% of extremely premature neonates who develop BPD, making it is the most common illness associated with premature birth.2
BPD is characterized by an alteration or arrest of lung development, and is defined as supplemental oxygen requirement at 36 weeks postmenstrual age. 1 Pulmonary inflammation plays an important role in the pathogenesis of BPD. Current research suggests that an imbalance between pro-inflammatory and anti-inflammatory mediators in the developing neonatal lung contributes to the development of BPD. 3 The diagnosis of this condition in very low birth weight neonates has been linked to the alteration of various interrelated biomarkers in the neonatal lung. Biomarker alterations indicated in the development of BPD include markers of poor endothelial integrity, increased fibrinolytic activity, increased oxidative stress, and abnormal lung repair. 4,5
A more in-depth understanding of the mechanisms controlling the inflammatory environment in the developing lung remains a priority for advances in the prevention, diagnosis, and treatment of BPD. 1 Furthermore, early identification of the at-risk premature newborn would enable advances in patient-specific interventions to minimize the development of BPD.
While previous studies have examined the role of specific biomarkers in the lungs of neonates who develop BPD, little current research has studied the relationship between the levels of multiple inflammatory mediators in the tracheal aspirate (TA) of very low birth weight infants prior to their development of BPD. 6 The purpose of this study was to investigate whether the concentrations of inflammatory mediators detected in the TA of neonates within 24 hours of birth could discriminate between very low birth weight neonates who did and who did not develop BPD. We hypothesized that there would be a different relationship among inflammatory mediators in the TA collected 24 hours postnatally in very low birth weight neonates who developed BPD as compared to the inflammatory mediators in the TA of very low birth weight neonates who did not develop BPD.
Patients and methods
Study Design
This was a translational study. TA samples collected and banked as part of a study examining neonatal alveolar macrophage maturation were utilized in the current study. A literature search was used to determine which biomarkers have been demonstrated to be significantly linked to the development of BPD in previous studies and these were included in this analysis. Literature searches were made using combinations of the following text words: [BPD, bronchopulmonary dysplasia] AND [cytokine(s), inflammatory mediator(s)]. Search was limited to studies published in English and after 1990 with human subjects. Manuscripts identified with these search criteria were reviewed. Inflammatory mediators associated with the development of BPD in multiple studies were identified.
Human participants
After approval from the Emory IRB, subjects were enrolled from Emory University Hospital Midtown and Grady Memorial Hospital in Atlanta, GA from November 2006–November 2008. All neonates weighing less than 1,500 grams admitted to the NICU between the gestational ages of 23–31 weeks requiring intubation and mechanical ventilation were eligible for enrollment into the study. Premature newborns with multiple congenital anomalies on physical exam were excluded due to the possible syndromic associations with macrophage function. Patients with clinically suspected or confirmed chromosomal abnormality were excluded for similar reasons. Neonates deemed non-viable by the attending neonatologist were not approached for enrollment. Patients with maternal HIV history were excluded because of the potential risk to laboratory personnel in the sample handling and fluid analysis.
The outcome of the study was the diagnosis of BPD as documented in the medical record by the attending neonatologist. The diagnosis of BPD was defined as supplemental oxygen requirement at 36 weeks postmenstrual age.
Sample collection and treatment
After admission to the NICU and verbal consent from the mother, TA samples were collected during routine, clinically indicated endotracheal tube suctioning by respiratory therapists within 24 hours of birth. For the suctioning procedure, bacteriostatic saline (~1 cc) was instilled into the trachea and after several ventilator breaths the sample was retrieved into a closed, sterile Leukins trap. The sample was immediately placed on ice and transported to the Gauthier laboratory for further analysis. The samples were centrifuged at 1200 rpm for 8 minutes at 4°C to separate supernatant and cellular fractions. The supernatant was removed and divided into aliquots. Aliquots were stored at −80°C prior to analysis. Samples were frozen within ~1 hour of collection.
Multiplex analysis
Concentrations of inflammatory mediators in the TA supernatant were determined using a quantitative bead-based Milliplex MAP Human multiplex assay (Millipore Corporation, Billerica, MA). For the controls, each kit includes a low and high quality control. Standard curves were generated and the results extrapolated based on a point-by-point regression in 96-well plates. Plates were incubated overnight as per manufacturer’s protocol. Data were analyzed using a BioRad Bio-Plex System (Bio-Rad Laboratories, Hercules, CA) with gates of 4,335 and 10,000. The concentrations of the samples were determined using a 5-point logistic curve fitting algorithm (Bio-Plex Manager 3.0 Software; Bio-Rad Laboratories).
Data treatment and statistical analysis
Patients were stratified to those who were diagnosed with BPD (designated BPD+) and those who were not diagnosed with BPD (designated BPD−) as determined in the medical record. Raw biomarker concentrations were expressed in pg/mL and were logarithmically transformed prior to statistical analysis given their non-normal distribution. 7 Categorical variables were compared between the two groups using a two-tailed Fisher’s exact test. Continuous variables were compared between the two groups using the Mann-Whitney U Test.
To examine the relationship between the 12 biomarkers measured and the diagnosis of BPD, a logistic regression analysis was performed with BPD (yes/no) being the outcome and the levels of biomarkers as predictors. A stepwise method with an entry and removal probabilities of 0.05 and 0.10 respectively was used. Bivariate Pearson correlations were used to examine associations between the predictors.
Linear discriminant analysis (LDA) using the Fisher method was used to classify patients into two outcome groups based on biomarker levels. While LDA does assume normality of the data, it has been shown to be relatively robust against departures from normality.8 LDA, a high-dimensional data reduction technique, is a supervised learning method of classifying observations based on predictors that has been used in previous studies to classify patients into disease groups based on biomarker concentrations.9 The LDA function aims to minimize within group variation and maximize between group variation. A function was generated from the linear combination of all 12 biomarker levels in order to calculate a discriminate score for each study case. Each subject was placed into a classification group based on which side of the cut point they fell. As LDA is a data classification and dimensionality reduction technique, positive predictive value, specificity, and sensitivity are not able to be determined with this technique.
To measure the strength of the relationship of the predictors (cytokines) to the outcome (BPD), the equation η2 = 1– λ1/3 was used to determine the effect size.10
All data were analyzed with SPSS® for Windows software (Version 18.0, SPSS Inc., Chicago, IL). A significant difference was defined as p ≤ 0.05.
Results
Identification of Biomarkers
There were 122 publications returned by the literature search. Multiple publications returned by the search were not applicable to the study as they did not identify biomarker alterations associated with the development of BPD. Forty publications were returned by the search which was used to identify inflammatory mediators associated with BPD. There were 12 inflammatory mediators identified by the literature search including: IL-1ra, IL-1b, IL-4, IL-6, IL-8, IL-10, GM-CSF, VEGF, MCP-1, MIP-1a, MIP-1b, and TNF-alpha. The primary activity of each biomarker included in this is study is highlighted in Table 1.
Table 1.
Primary activities of biomarkers included in the study.
| Biomarker | Primary activity |
|---|---|
| IL-1ra | Inhibits the activities of IL-1b and IL-1a |
| IL-1b | Induces pro-inflammatory cytokines (IL-6, IL-8, and TNF-alpha) |
| IL-4 | Aids in production, differentiation and proliferation of B cells and macrophages and inhibits pro-inflammatory cytokine production |
| IL-6 | Increases inflammatory cytokine production |
| IL-8 | Chemotactic for neutrophils and aids in neutrophil activation and degranulation |
| IL-10 | Inhibitor of macrophages |
| GM-CSF | Promotes clonal maturation of neutrophil and macrophage progenitors and increases functional activity |
| VEGF | Stimulates angiogenesis |
| MCP-1 | Chemotactic for monocytes and lymphocytes |
| MIP-1a | Activates granulocytes |
| MIP-1b | Activates granulocytes |
| TNF-alpha | Activates macrophages and aids in leucocyte endothelial activation |
Characteristics of Study Population
The study included 11 neonates with BPD (BPD+) and 16 neonates without a diagnosis of BPD (BPD−). The demographic and clinical characteristics of the 27 study neonates are shown in Table 2. No significant differences were detected between the two groups when gestational age, gender, race, and birth weight were compared.
Table 2.
Baseline characteristics of the 11 neonates who did (BPD+) and the 16 neonates who did not (BPD−) develop BPD. Gender and race were compared using Fisher’s exact test. Gestational age and birth weight were compared using the Mann-Whitney U Test. No significant difference was found between BPD+ and BPD− groups. Data represent the mean ± SD or the frequency (%).
| BPD+ | BPD− | P-value | |
|---|---|---|---|
|
| |||
| Gestational age (wks) | |||
| Mean ± SD (range) | 26.95 ± 2.63 (24–31) | 26.18 ± 1.86 (24–29) | 0.512 |
|
| |||
| Gender | |||
| Number (%) | |||
| Male | 4 (36) | 11 (69) | 0.130 |
| Female | 7 (64) | 5 (31) | |
|
| |||
| Race | |||
| Number (%) | |||
| Caucasian | 3 (27) | 2 (13) | 0.573 |
| Black | 7 (64) | 13 (81) | |
| Hispanic | 1 (9) | 1 (6) | |
|
| |||
| Birthweight (gm) | |||
| Mean ± SD (range) | 817 ± 263 (480–1390) | 872 ± 245 (590–1320) | 0.610 |
Inflammatory Mediator Concentrations in TA
Table 3 displays the mean log transformed mediator concentrations obtained from analysis of the TA samples. The histograms for this data (data not shown) suggested that log transformation of the values did not completely normalize the data. Mean concentrations of the two groups were compared using Mann-Whitney U test and were not found to be statistically significant (Table 3).
Table 3.
Median inflammatory mediator values for neonates who did (BPD+) and did not develop (BPD−). Data represent median (25th %, 75th %) and are measured in pg/mL after log transformation. P-values from the comparisons of individual biomarkers between the BPD groups are displayed.
| Inflammatory Mediator | Median (25th – 75th %) | P-value | |
|---|---|---|---|
| BPD− | BPD+ | ||
| IL-1ra | 8.42 (6.14, 9.53) | 8.87 (6.96, 9.68) | 0.730 |
| IL-1b | 6.23 (3.84, 8.25) | 5.92 (4.23, 8.51) | 0.961 |
| IL-4 | 4.11 (3.22, 5.77) | 3.71 (3.47, 4.44) | 0.324 |
| IL-6 | 8.62 (8.13, 10.09) | 8.95 (8.40, 9.84) | 0.921 |
| IL-8 | 10.16 (10.04, 10.24) | 10.18 (10.06, 10.21) | 0.921 |
| IL-10 | 5.20 (3.37, 6.96) | 4.38 (3.91, 6.15) | 0.961 |
| GMCSF | 5.96 (4.76, 6.96) | 5.56 (4.45, 6.15) | 0.199 |
| MCP-1b | 10.18 (10.07, 10.22) | 10.17 (10.12, 10.22) | 0.844 |
| MIP-1a | 4.63 (4.96, 10.17) | 8.62 (5.14, 9.86) | 0.521 |
| MIP-1b | 7.83 (4.57, 9.55) | 7.77 (5.30, 9.02) | 0.969 |
| TNF-alpha | 6.28 (4.04, 8.48) | 5.83 (4.35, 9.04) | 0.961 |
| VEGF | 6.43 (5.02, 7.95) | 6.59 (4.72, 6.84) | 0.675 |
Linear Association of Biomarkers with Disease Outcome
No predictors were included in the model as none were found to be individually significant to remain in the model.
Correlations between Biomarkers
A Pearson correlation matrix demonstrated significant positive correlations between most of the 12 biomarkers of pulmonary inflammation (p < 0.05). The only biomarker pairs not significantly correlated were IL-4 and IL-8, IL-10 and IL-8, IL-10 and MCP-1, and MCP-1 and IL-4 (p > 0.05 for all comparisons).
Discrimination of Study Cases into BPD+ and BPD− Groups using LDA
Given the high correlation of many of the biomarkers in the analysis, a Linear Discriminant Analysis (LDA) was performed to contrast BPD− versus BPD+ patients. The model displayed a significant level of discriminant function with a Wilk’s Lambda = 0.24 and p = 0.007. Figure 1 displays the discriminant scores of all cases. The average discriminant score for patients without BPD was 1.42, while the average score for patients with BPD was −2.06. Cases with a positive value for discriminant score were classified into the BPD− group while cases with negative discriminant scores were classified into the BPD+ group. The effect size was determined to be large, 0.379, suggesting a strong relationship between the predictors and the outcome.
Figure 1.
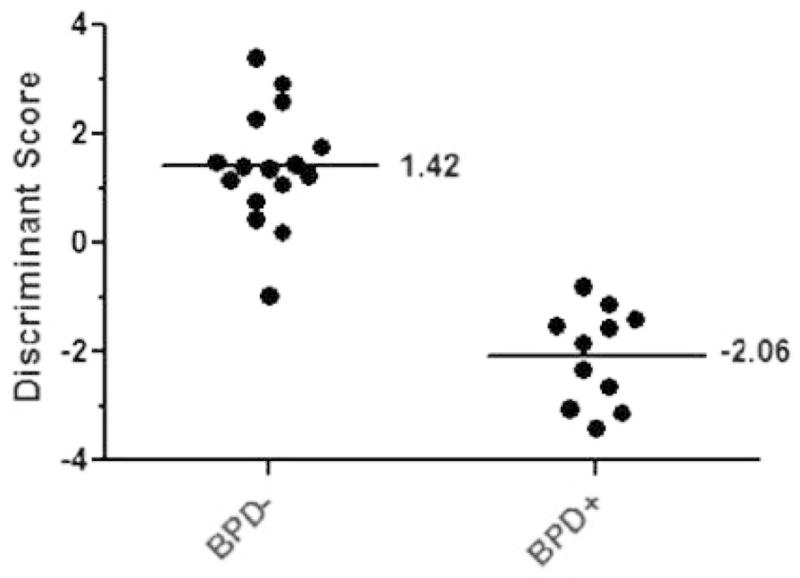
Discriminant score scatter plot displaying the discriminant score for each patient in the study. Each dot corresponds to a patient’s discriminate score determined by the 12-predictor LDA function. On the X-axis the patients are grouped by clinical BPD diagnosis, which is plotted against their discriminative score on the Y-axis. The average discriminant score for patients without BPD (BPD−) was 1.42 and −2.06 for patients diagnosed with (BPD+).
Classification of Study Cases Using the LDA Function
Using the 12-predictor model determined by LDA, the cases were classified based on the predictors (Table 4). 96% of all of the cases were correctly classified. All of the patients diagnosed with BPD were classified into the BPD+ group, meaning all BPD+ cases were correctly classified by the function. One of the BPD− cases was incorrectly classified as BPD+ and 15 of these cases were correctly classified as non-BPD cases. The incorrectly classified case was a 25 week neonate whose birthweight was 670 grams.
Table 4.
Classification of study patients using the original 12-predictor model determined by LDA with BPD status as the outcome. Actual group membership reflects the patient’s BPD diagnosis represented as number of cases (% of study patients in corresponding BPD group). The shaded cell represents misclassified cases.
| Actual Group Membership | Group Membership Predicted by Function | ||
|---|---|---|---|
| BPD− | BPD+ | ||
| Original | BPD− | 15(93.8%) | 1 (6.3%) |
| BPD+ | 0(0%) | 11 (100%) | |
Cross-validation classification was performed by classifying each case by a function, using 12 mediators as predictors, derived from all cases other than that case (Table 5). Analysis demonstrated 81% of the cross-validated grouped cases were correctly classified. Of the patients who were diagnosed with BPD, 18.2% of the cases were misclassified. Of the patients who were not diagnosed with BPD, 18.8% of the cases were incorrectly classified as BPD+.
Table 5.
Cross-validation classification of study patients using 12-predictor models determined by LDA with BPD status as the outcome. Cross-validation was performed by classifying each case by a function, using all 12 mediators as predictors, derived from all cases other than that case. Actual group membership reflects the patient’s BPD diagnosis represented as number of cases (% of study patients in corresponding BPD group). Shaded cells represent misclassified cases.
| Actual Group Membership | Group Membership Predicted by Function | ||
|---|---|---|---|
| BPD− | BPD+ | ||
| Cross-validated | BPD− | 13(81.3%) | 3 (18.8%) |
| BPD+ | 2(18.2%) | 9(81.8%) | |
Discussion
This is the only study which has utilized high-dimensional data reduction methods on inflammatory biomarkers in TA to examine the development of BPD in very low birth neonates. By using linear discriminant analysis, we were able to suggest that there is a different inflammatory cytokine milieu at 24 hours of life in the lungs of neonates who develop BPD. While the comparison of individual inflammatory biomarkers did not differ, when combined these biomarkers provided a definite separation of BPD+ and BPD− neonates. These results suggest the development of BPD in the premature lung is associated with distinctive alterations in these inflammatory biomarkers leading to pulmonary injury.
It is well known that pulmonary inflammation is associated with the development of BPD in premature infants.11 The association of BPD with pulmonary inflammation was first suggested with the observation of elevated neutrophil concentrations in the TA of neonates diagnosed with BPD.12 Research continues to identify pulmonary cytokine and growth factor alterations associated with the development of BPD. However, there remains a need for the early identification of such patients to advance the possibility for early identification and the development of successful therapeutic interventions.
As predicted, the results of this study suggested that inflammatory mediators associated with BPD were highly related to one another since most of the mediators statistically correlated with each another. These results further suggested that the development of BPD was linked to an alteration in the total cytokine and growth factor milieu in the neonatal lung as opposed to isolated alterations of select individual mediators. While other studies have found significant differences between the individual biomarkers in patients that did or did not develop BPD, our analysis did not find such significant differences in this study. This is likely due to a small sample size not powered to detect such differences between individual biomarkers.
This study examined the development of BPD without potential postnatal exposures that frequently occur after 24 hours of birth (ventilator use, supplemental oxygen use, infections, and nutritional deficits). The current data demonstrated the ability to discriminate those who develop BPD from those who do not develop BPD at this early time point, suggesting that antenatal exposures (steroids, chorioamnionitis, intrauterine growth restriction, and perinatal inflammatory status) significantly impact the cytokine milieu in the premature lung. Furthermore, these results suggested that early alterations in multiple mediators in the lung may be important in the pathogenesis of BPD. This is in agreement with a prior study that found multiple growth factors were altered within 24 hours of birth in the lungs of neonates developing BPD. 6 While previous studies have identified potential biomarkers the first 24 hours of life, this study suggests that the collective analysis of multiple biomarkers can be potentially used to discriminate neonates into those who do and do not develop BPD early in their NICU course.
The major limitation to this study was the small sampled size, which increased the probability of type II error. Additionally, we were not able to control for potential confounders such as chorioamnionitis or steroid use, given the small sample size. The number of research participants was too small to create a validation data set in order to assess the generalizability of the results to independent data sets. TA samples were collected at one point in time; thus, we were not able to determine a pattern over time. Although levels were examined at one early time point, the study demonstrated that the examination of a collective set of cytokines significantly discriminated between those infants who did and did not develop BPD.
Further study needs to be completed with a larger sample size in order to account for potential confounders. A subset of biomarkers that accurately classifies neonates who develop BPD and those who do not needs to be identified. Additionally, a subset of biomarkers used to classify patients at multiple time points after birth requires examination. Such results might identify a time point where the association of biomarker levels with BPD is the greatest and could potentially serve as a tool to better predict the development of BPD early in the clinical course of the neonates stay in the NICU.
In summary, multiple inflammatory mediators in low birth weight neonates obtained within 24 hours of birth can be used to discriminate between premature newborns that will and will not develop BPD. This approach to interpreting the inflammatory milieu of the developing lung may aid in early identification and potential targeted intervention for those at a higher risk of developing BPD.
Acknowledgments
Statement of financial support. Supported in part by PHS Grant (TL1 RR025010) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (KRS) and the Childrens Healthcare of Atlanta Center for Developmental Lung Biology (TWG and LAB).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001;13(2):124–129. doi: 10.1097/00008480-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11(5):354–362. doi: 10.1016/j.siny.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Thompson A, Bhandari V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark Insights. 2008;3:361–373. doi: 10.4137/bmi.s834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F455–461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 6.Been JV, Debeer A, van Iwaarden JF, Kloosterboer N, Passos VL, Naulaers G, et al. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr Res. 2010;67(1):83–89. doi: 10.1203/PDR.0b013e3181c13276. [DOI] [PubMed] [Google Scholar]
- 7.de Blic J, Midulla F, Barbato A, Clement A, Dab I, Eber E, et al. Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Eur Respir J. 2000;15(1):217–231. doi: 10.1183/09031936.00.15121700. [DOI] [PubMed] [Google Scholar]
- 8.Hand DJ. Discrimination and classification. Wiley; New York: 1981. [Google Scholar]
- 9.Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125(4):851–857. e818. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson B, Trapp RG. Basic & Clinical Biostatistics. 4. McGraw-Hill; New York: 2004. [Google Scholar]
- 11.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008;34(2):174–190. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 12.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, et al. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983;72(2):656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]


