Abstract
Smoking among people living with HIV, particularly women living with HIV, is associated with higher morbidity and mortality rates when compared to non-smoking individuals with HIV. Despite patients’ higher risk of adverse health outcomes, in particular preventable smoking-related diseases for smokers living with HIV, few smoking cessation interventions have been examined with this population. The aim of the current study was to test the potential efficacy of a brief motivational intervention for smoking cessation with HIV infected women smokers. Participants (N = 30) were randomly assigned to receive a single session of Motivational Interviewing or Prescribed Advice. The primary outcome was 7-day point prevalence abstinence at the one-month follow-up interview. Secondary outcome measures included mean cigarettes smoked per day, desire to quit smoking, perceived difficulty in quitting smoking, and expectation of success. We detected no significant differences between intervention and control groups in self-reported 7-day point prevalence abstinence at the one-month follow-up. However, participants in the MI condition reported a significant decrease in the mean cigarettes smoked per day when compared to the Prescribed Advice condition. There were no significant between-group differences in participants’ desire to quit, perceived difficulty and expectation of success. The results of this pilot study indicate that MI may be an effective smoking cessation intervention for HIV positive women smokers and should be studied further in a larger clinical trial.
INTRODUCTION
Overall rates of smoking have decreased in recent years.1 Smoking rates among the general population are estimated to have dropped to about 20%,2 and are much lower than the estimated 50–70% smoking rates among individuals living with HIV.3–5 Despite the high rates of smoking among people living with HIV/AIDS, there is a paucity of studies examining the efficacy of smoking cessation interventions targeting HIV-infected populations.
The lack of evidence-based smoking cessation interventions among HIV populations is particularly concerning given the higher risk for adverse health consequences. While AIDS-related causes of death have decreased as a result of effective antiretroviral therapy (ART), non-AIDS related causes of death have increased.6 In a study of 867 HIV-infected patients enrolled in Veterans Affairs Medical Centers7 mortality was higher among smokers when compared to nonsmokers, even after controlling for age, CD4 count, and viral load. Moreover, current smokers with HIV/AIDS have an increased risk for bacterial pneumonia,8, 9 oral candidiasis,10 oral lesions,11 non-AIDS related cancers, and cardiovascular disease, when compared to nonsmokers.9
Women smokers living with HIV/AIDS seem to be particularly susceptible to the negative consequences of smoking. Women smokers living with HIV/AIDS have a 36% higher risk for developing AIDS and 53% higher mortality when compared to nonsmokers with HIV/AIDS.12 Women smokers on ART display a poorer viral response, poorer immunologic response, and have a greater risk of viral or immunologic failure when compared to nonsmokers living with HIV/AIDS. These increased health risks may be attributed to the poorer rates of ART adherence among smokers or ART may be less effective in smokers.12 Finally, research studies in non-HIV samples indicate that women have less favorable smoking cessation treatment outcomes when compared to men.13, 14 Smoking cessation interventions are needed among HIV-affected women, however few studies have addressed smoking in HIV+ populations and none were specific to HIV+ women.
Effective and brief interventions exist for smoking cessation in the general population. Motivational interviewing (MI), a directive, client-centered approach, is recommended in smoking cessation clinical practice guidelines15 and has been found to be effective with a variety of health behaviors including alcohol and drug use and diet/exercise adherence.16 MI’s effectiveness with smoking cessation has been documented in three recent meta-analyses.17–19 MI focuses on eliciting a patient’s reasons for and benefits of change, while understanding that patients may be ambivalent about the change process. Key MI skills include asking open questions, affirming the patient’s strengths and attempts at change, emphasizing the patient’s control, asking advice before providing information or advice, and using reflective listening.
Preliminary research indicates that motivational interventions combined with nicotine replacement therapy (NRT) have been effective in reducing cigarette smoking among HIV+ smokers but have not demonstrated a significant advantage over standardized control conditions.20, 21 Prior studies have not focused exclusively on women, although research suggests that brief interventions, including MI, may be more effective in samples of women smokers.22 The aim of this study was to examine the potential efficacy of a brief motivational interview versus prescribed advice among 30 HIV+ female smokers.
METHODS
Participants
Participants were 30 HIV+ female smokers recruited from an urban public hospital-based HIV primary care clinic serving patients living with HIV/AIDS in San Francisco, CA. Inclusion criteria were: 1) 18 years of age or older; 2) biologically female; 3) self-report of daily smoking at least 5 out of 7 days in the previous week and interest in quitting smoking; 4) English speaking; and 5) HIV+. Exclusion criteria included: 1) being pregnant; 2) not able to give informed consent; 3) cognitive impairment as assessed by the investigator. The Institutional Review Board at the University of California, San Francisco, approved this study.
Procedure
Participants were recruited via flyers and by referrals from clinic staff. Individuals were assessed for study eligibility either in-person at the clinic or over the phone. Eligible participants were invited to attend a baseline interview where they provided written informed consent. Individuals who were not eligible were referred to other local smoking cessation programs.
Assessment
Participants attended a baseline interview during which they were asked to complete measures related to their smoking behaviors. The Center for Epidemiological Studies Depression Scale (CESD)23 was used to detect major clinical depression. The Timeline Follow-back Interview for alcohol and drug use24 was administered to assess the quantity and frequency of alcohol and illicit drug use in the previous month. The Smoking History Questionnaire25 queried the age participants smoked their first cigarette, years of smoking, number of quit attempts, and number of cigarettes smoked in the previous day. The Fagerström Test of Nicotine Dependence26 (FTND) was used to measure nicotine dependence. Finally, the Smoking Stage of Change measure27 was used to categorize participants into one of the Stages of Change (Precontemplation, Contemplation, Preparation, or Action) and the Thoughts about Abstinence questionnaire28 was used to assesses the participants’ desire to quit smoking, abstinence self-efficacy, and perceived difficulty of quitting smoking. Participants were paid $25 for their participation in the baseline interview.
Randomization
Allocation assignment was determined prior to the start of the study using permuted block randomization.29 After the participant completed the baseline interview, the interviewer opened a sealed envelope indicating which condition the participant had been randomized to receive. The intervention took place immediately after the baseline interview.
Interventions
Participants in both the MI and PA conditions met with a therapist (JKM) for a single session. Both conditions were designed to reduce smoking and encourage use of nicotine replacement therapy and other tobacco cessation treatment options. At the end of the session, participants in both conditions were referred to nicotine replacement therapy programs within the hospital and other community resources if they indicated that they were willing to receive these resources and/or referrals.
Brief Motivational Interview (MI)
The MI session was patient-centered, directive and intended to evoke the participants’ potential reasons for and benefits of change.30
Prescribed Advice (PA)
The PA sessions were based on the pamphlet, “You Can Quit Smoking” developed by the National Cancer Institute.31 Together, the therapist and participant reviewed the smoking cessation pamphlet and discussed the recommended smoking cessation strategies. This intervention focused on giving the participants advice and advocating for them to change, rather than eliciting as in the MI condition.
Follow-up
Participants were asked to complete a one-month follow-up interview. A trained research assistant (RA) conducted follow-up assessments. The RA was blind to the participants’ treatment condition. Patients who indicated that they were abstinent from tobacco products at the follow-up interview were asked to provide a urine sample for confirmation of abstinence. Testing for nicotine and cotinine, biomarkers of nicotine exposure, was performed in the medical toxicology laboratory at San Francisco General Hospital using a qualitative liquid chromatography-tandem mass spectrometry method. Participants were paid $25 for the follow-up interview.
Treatment Fidelity
All sessions were recorded and coded by a research assistant, trained to use the Motivational Interviewing Treatment Integrity (MITI)32 coding system, a widely used measure of MI treatment fidelity (see the MITI coding manual for details on MITI training procedures). To our knowledge, there is not a standardized way of evaluating Prescribed Advice sessions; thus both the MI and PA sessions were coded using the MITI to evaluate if they significantly differed on key measures of MI treatment fidelity.
The MITI consists of five global ratings and seven behavior counts. Global ratings are measured on a 1–5 Likert scale and capture the general overview of the session. Behavior counts are a measure of the frequency of specific therapist behaviors. The MITI global behaviors include: Evocation, Collaboration, Autonomy/Support, Direction, and Empathy. Behavior counts include: 1) Giving information, 2) MI-Adherent statements (affirmations, statements that emphasize the patient’s control, supportive statements, or asking permission before giving advice or information to the patient), 3) MI-Nonadherent statements (confrontational statements, directing the patient or advising the patient without his/her prior permission), 4) simple reflections, 5) complex reflections, 6) closed questions, and 7) open questions.
Analysis Approach
Data were analyzed using SPSS software. Means, standard deviations and frequencies were utilized to characterize the demographic and smoking variables for the sample. Inter-rater reliability for the MITI coding was assessed for each global rating and behavior count using intra-class correlation coefficients (ICCs). Wilcoxon signed-rank tests were used to compare the MITI scores for the MI and PA condition. Mann-Whitney U tests were conducted to determine if participants in the MI and PA conditions differed in their report of smoking behavior, desire to quit smoking, perceived difficulty and expectation of success at the one-month follow-up.
RESULTS
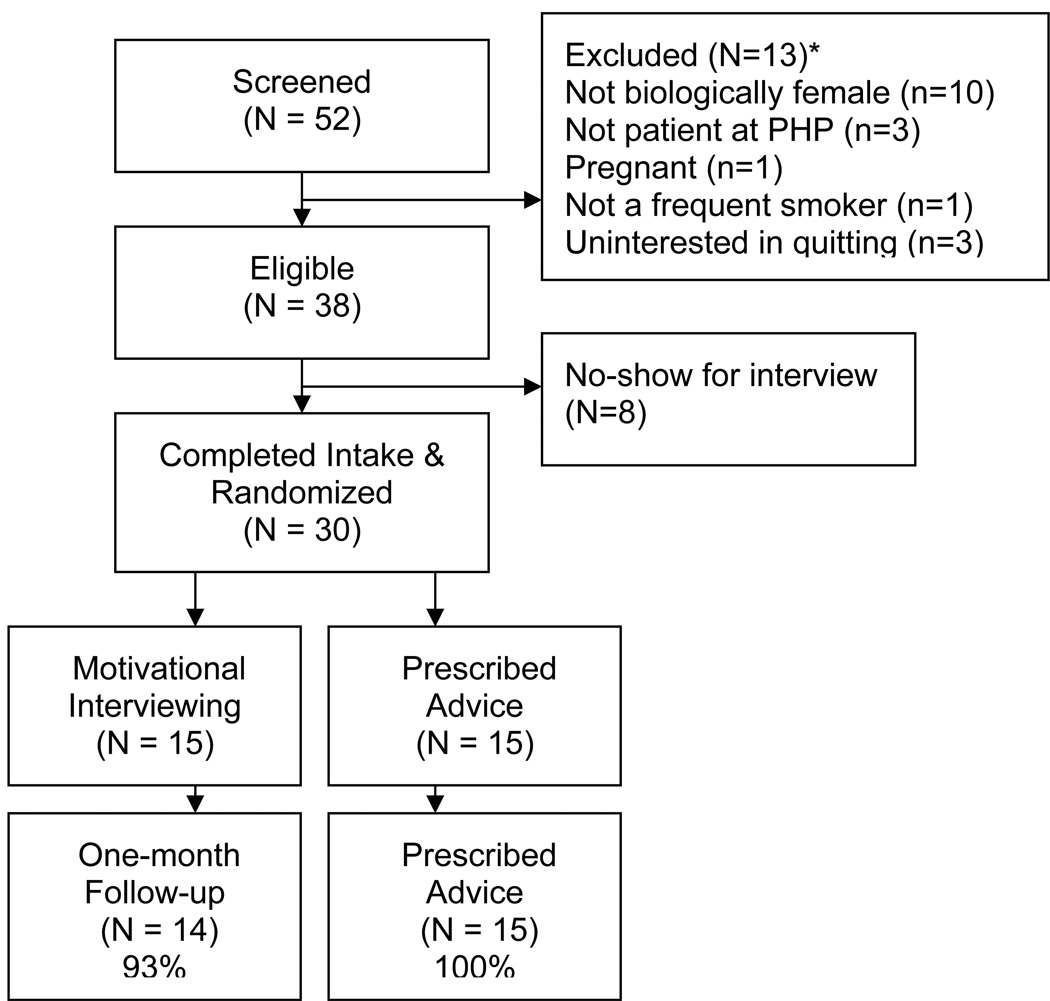
A total of 52 individuals contacted the study requesting to be screened, and 38 were eligible to participate. Of those meeting eligibility, 38 scheduled a baseline interview, and 30 attended the baseline interview. See Figure 1 for details regarding reasons for ineligibility, participant recruitment, and follow-up rates.
Figure 1.
Participant Enrollment and Retention
Participants were all female, with a mean age of 49 years (SD=5.78). Thirteen percent (n=4) reported being of Hispanic ethnicity. Nearly half (n=14) were African-American, 30% (n=9) were Caucasian, and 23% (n=7) were of other or more than one or other ethnicity. Of the sample, 40% (n=12) were single, 30% (n=9) divorced, 20% (n=6) married and 10% (n=3) widowed. Most of the sample (67%, n=20) reported their sexual orientation as heterosexual, 30% (n=9) bisexual, and 3% (n=1) homosexual. Almost half (47%; n=14) of the sample lived in their own house or apartment while the remaining participants reported living with friends, in a therapeutic community, a single room occupancy hotel, or that they were homeless. Many (67%; n=20) of the participants reported an annual income of less than $10,000, while the remaining participants reported receiving $11,000-$20,000 annually. Most (90%, n=27) of the sample was unemployed with the remaining 10% (n=3) reporting student status. Participants in the PA condition reported higher scores of depression at the baseline interview (mean=24.41; SD=11.52) than participants in the MI condition (16.09, SD=11.12), a difference that approached significance F(1, 27) = 3.91, p=.058.
Baseline Tobacco Use
At the baseline interview, participants reported smoking a mean of 16.13 (SD=9.82, range 3–40) cigarettes per day. Of the sample, 43% (n=13) were light smokers (smoking 10 or less cigarettes per day or CPD), 3% (n=1) were moderate smokers (11–19 CPD), and 53% (n=16) were heavy smokers (20 or more CPD). Participants reportedly first tried smoking at 12.50 (SD=5.37) years of age and began smoking regularly at 16.17 (SD=6.50) years of age. Participants indicated they had smoked for a mean of 31.87 (SD=10.22) years. Applying the Prochaska and Diclemente’s (1983) stage of change model to baseline tobacco use, 3.3% (n=1) reported that they were in the Precontemplation phase, 60% (n=18) in the Contemplation phase, while and 36.7% (n=11) were in the Preparation phase.
A third (33% of the sample; n=10) indicated that a mental health professional had previously advised them to quit smoking and most (87%; n=26) said that a health professional had advised them to quit smoking. Fifty-seven percent (n=17) of the sample had previously tried to quit smoking. Of those who had attempted to quit, 33% (n=10) reported that they had tried to quit cold turkey, 20% (n=6) had used a nicotine patch, 7% (n=2) bupropion, 10% (n=3) nicotine gum, 13% (n=4) gradually cut down, 10% (n=3) took a free class, and 3% (n=1) took a fee-based class.
Treatment conditions did not differ on FTND scores of tobacco dependence, the mean number of cigarettes smoked per day, desire to quit smoking, expectation of success, or perceived difficulty of quitting smoking at the baseline interview. Descriptive statistics for these variables are shown in Table 1.
Table 1.
Baseline and Follow-up Measures of Smoking
| Variable | Motivational Interviewing Mean(SD) |
Prescribed Advice Mean(SD) |
|---|---|---|
| Fagerström Test of Nicotine Dependence | ||
| Baseline | 4.07(2.52) | 5.00(2.07) |
| Follow-up | 3.43(2.85) | 4.14(1.75) |
| Cigarettes per Day | ||
| Baseline | 15.53(11.10) | 16.73(8.71) |
| Follow-up | 7.00 (8.62)* | 15.79(14.02)* |
| Desire to Quit Smoking (1–10 Range) | ||
| Baseline | 7.50(1.70) | 8.20(2.15) |
| Follow-up | 6.79(3.62) | 7.73(3.01) |
| Expectation of Success (1–10 Range) | ||
| Baseline | 6.93(2.17) | 5.60(2.38) |
| Follow-up | 7.43(3.50) | 6.67(2.61) |
| Perceived Difficulty (1–10 Range) | ||
| Baseline | 7.93(2.09) | 7.33(2.55) |
| Follow-up | 6.50(2.82) | 7.73(1.91) |
p < .05.
Treatment Fidelity
A total of 28 sessions were examined for treatment fidelity by a trained coder who was blinded to the study hypotheses. One session was not recorded due to error with the digital recorder, and one session was deemed inaudible. Six sessions were randomly selected for double-coding, conducted by a trained coder at the University of New Mexico. Inter-reliability between the two coders was calculated using intra-class correlation (ICCs) for each global score and behavior count. Reliability estimates ranged from poor to excellent.33 Simple reflections were removed from further analyses due to poor reliability estimates (ICC = .−006). The reliability estimates for global scores fell in the excellent range with estimates from .76 – 1.00. An ICC could not be calculated for the Direction global due to restricted range and the small sample of double-coded sessions. As an alternative, we calculated a difference score to examine coder reliability on the Direction variable as reported in a previous behavioral coding study with restricted range among variables34. For the Direction variable, there was exact agreement on 83% (n = 5) of the double-coded sessions. The remaining session had a one-point discrepancy (on a 1–5 scale). See Table 2 for MITI means, standard deviations, and ICC calculations.
Table 2.
Motivational Interviewing Treatment Fidelity Ratings
| MITI Code | Motivational Interviewing Mean(SD) |
Prescribed Advice Mean(SD) |
ICC1 | Wilcoxon Signed Rank Test |
|---|---|---|---|---|
| Global Rating (1–5 Range) | ||||
| Evocation | 4.79(.43)* | 2.07(.48)* | 1.00 | Z = −4.805, p = .000 |
| Collaboration | 5.00(.00)* | 2.00(.00)* | .878 | Z = −5.196, p = .000 |
| Autonomy/Support | 4.86(.36)* | 2.79(.58)* | .762 | Z = −4.904, p = .000 |
| Direction | 4.93(.27) | 5.00(.00) | ** | Z=−1.00, p=.317 |
| Empathy | 4.93(.27)* | 1.43(.65)* | .906 | Z=−4.842, p=.000 |
| Behavior Counts (Average Frequency) | ||||
| Giving Information | 8.36(5.84) | 9.36(2.74) | .234 | Z=−1.572, p=.116 |
| MI Adherent | 9.43(3.20)* | 2.23(1.69)* | .645 | Z=−3.140, p=.002 |
| MI Nonadherent | .86(.69)* | 6.93(3.45)* | .740 | Z=−3.199, p=.001 |
| Closed Questions | 5.93(2.73)* | 8.29(3.41)* | .320 | Z=−2.120, p=.034 |
| Open Questions | 10.86(4.07)* | 6.00(3.16)* | .909 | Z=−3.102, p=.002 |
| Complex Reflections | 23.64(10.50)* | 3.07(2.65)* | .910 | Z=−4.513, p=.000 |
| MITI Summary Scores | ||||
| Reflections/Questions | 1.72 (.66)* | .42(.32)* | .672 | Z=−4.32, p=.000 |
| Percent MI Adherent | .90 (.08)* | .28 (.25)* | .560 | Z = −3.14, p = .002 |
= ICC stands for Intraclass Correlation;
Indicates a significant between-group difference;
Due to the restricted range for this variable, an ICC could not be calculated.
As expected, MI and PA sessions significantly differed from each other on four of five global measures, including therapist Evocation, Collaboration, Autonomy/Support, and Empathy. The sessions did not differ significantly on the global measure of Direction, indicating that both the MI and PA sessions were focused on smoking cessation with minimal deviation to other unrelated topics. MI and PA sessions also significantly differed on the MITI behavior count codes. MI sessions had significantly more MI-Adherent statements, open questions, and complex reflections whereas the PA sessions had significantly more MI Non-adherent statements and closed questions. There was a significant difference in session length in the two conditions (F(1, 26) = 17.78, p < .001), with MI sessions averaging of 26.67 (SD=8.43) minutes and the PA sessions averaging 15.09 (SD=5.88) minutes. Due to the significant difference in session length, we calculated MI summary scores: the ratio of reflections to questions (total number of simple and complex reflections/total number of open and closed questions) and the Percent MI-Adherent statements (number of MI-Adherent statements/MI Adherent + MI Nonadherent statements), as detailed in the MITI coding manual.32 Again, MI sessions contained a significantly higher ratio of reflections to questions and percent MI Adherent statements.
Outcome Analyses
Three participants in the MI condition reported seven-day point prevalence abstinence at the one-month follow-up; no participants in the PA condition reported abstinence. This difference was not significantly different (χ2(1, N = 28) = 3.36, p = .067). Urinalysis testing for nicotine and cotinine was performed on two urine screens. Of the two urine samples analyzed, only one screen confirmed nicotine and tobacco abstinence.
At the one-month follow-up, participants in the MI condition reported smoking significantly fewer cigarettes per day than participants in the PA condition z = −2.49, p < .05. Participants in the MI and PA conditions did not significantly differ in their total FTND score, t desire to quit smoking, expectation of success or perceived difficulty to quit and remain abstinent. Therefore, we collapsed across treatment conditions and used Wilcoxon Signed rank tests to determine if participants differed from baseline to the one-month follow-up. Participants’ desire to quit smoking and perception of smoking cessation difficulty did not significantly differ from the baseline to the follow-up interview. Participants did report an increase in their expectation of success from the baseline to the follow-up interview z = −2.04, p < .05.
DISCUSSION
This study compared two smoking cessation interventions for urban, low-income HIV+ women smokers, an underserved and under-studied population. Participants in the MI condition demonstrated a greater reduction in the mean cigarettes smoked per day, however abstinence rates did not vary at the one-month follow-up. Participants in both conditions reported a significant increase in their expected success with quitting smoking. Unexpectedly, participants in both conditions reported a decrease in the desire to quit smoking from the baseline to the follow-up interview.
MI has been found to be an effective smoking cessation intervention.17–19 While this is the first study, to our knowledge, to examine the efficacy of MI with women smokers living with HIV/AIDS, other research studies have investigated the efficacy of MI with samples of both men and women living with HIV.20,21 MI studies have demonstrated that MI was equally effectives as a standardized control condition although neither of these studies reported on standardized measures of MI treatment adherence.20,21 In the current study, MI demonstrated an advantage over Prescribed Advice in reducing the mean number of cigarettes smoked per day.
This study included an objective measure of MI treatment fidelity, the Motivational Interviewing Treatment Integrity coding system.32 Coding data indicate that the MI sessions strongly adhered to the key MI principles and skills. Moreover, the content of the MI sessions was significantly different from the content of the PA sessions.
Limitations
The current study has several limitations, which deserve consideration. First, this was a small sample (n=30), which may have been too small to detect significant differences between the two conditions. As a pilot study, the goal of this work was to examine the potential efficacy of MI when compared to a standardized control condition. Second, this study utilized a single therapist, who is a highly experienced MI clinician, in both treatment conditions. Thus, it is possible that the therapist inadvertently biased the treatment interventions. Third, the discrepancy between participants’ self-report of abstinence and biochemical verification prevents a clear interpretation of the study results. While three participants (20%) in the MI condition reported seven-day point prevalence abstinence, only one participant’s self-reported abstinence was verified by the absence of urine nicotine and cotinine. Taking a conservative approach and utilizing only abstinent rates that have been biochemically verified our abstinent rates decrease to 7% at the one-month follow-up. One potential explanation may be social desirability bias on the part of participants in the MI intervention, even though the interventionist did not conduct the follow-up interviews. Finally, the rates of inter-rater reliability were low for some measures of MITI behavior counts, possibly due to the small number of sessions (n = 6) coded for inter-rater reliability.
Future Directions
This study examined the potential efficacy of MI with HIV+ female smokers. Future studies should examine smoking cessation interventions among a larger sample of HIV-infected individuals, include carbon monoxide monitors for more accurate biological confirmation of abstinence, and follow participants over a longer time period.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (T32 DA007250), the NIDA San Francisco Treatment Research Center (P50 DA009253), and by University of California’s Center for AIDS Prevention (A105651). The authors would like to thank the patients and staff of the UCSF Positive Health Program at San Francisco General Hospital for their assistance with this project. Portions of this manuscript were presented at the 2012 annual meeting of the College on Problems of Drug Dependence.
REFERENCES
- 1.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. National Center for Health Statistics. Vital Health Statistics. 2012;10(252) [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Vital signs: Current cigarette smoking among adults aged ≥ 18 years ---United States: 2005–2010. Morbidity and Mortality Weekly Report. 2011;60(35):1207–1212. [PubMed] [Google Scholar]
- 3.Gritz ER, Vidrine DJ, Lazev AB, Amick BC, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine & Tobacco Research. 2004;6(1):71–77. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- 4.Collins RL, Kanouse DE, Gifford AL, Senterfitt JW, Schuster MA, McCaffrey DF, Shapiro MF, Wenger NS. Changes in health-promoting behavior following diagnosis with HIV: Prevalence and correlates in a national probability sample. Health Psychology. 2001;20(5):351–360. [PubMed] [Google Scholar]
- 5.Neumann T, Reinsch N, Esser S, Krings P, Konorza T, Woiwoid T, Miller M, Brockmeyer N, Erbel R. Smoking behavior of HIV-infected patients. Health. 2010;2(8):913–918. [Google Scholar]
- 6.Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD the HIV Outpatient Study Investigators. Mortality in the highly antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. Journal of Acquired Immune Deficiency Syndromes. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 7.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, Gibert CL, Justice AC. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. Journal of General Internal Medicine. 2005;20(12):1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordin FM, Roediger MP, Girard PM, Lundgren JD, Miro JM, Palfreeman A, Slater LN. Pneumonia in HIV-infected Persons: Increased Risk with Cigarette Smoking and Treatment Interruption. American Journal of Respiratory and Critical Care Medicine. 2008;178(6):630–636. doi: 10.1164/rccm.200804-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TRH. Smoking-Related Health Risks Among Persons With HIV in the Strategies for Management of Antiretroviral Therapy Clinical Trial. American Journal of Public Health. 2010;100(10):1896–1903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay A, Caplan DJ, Slade GD, Shugars DC, Tien HC, Patton LL. Risk indicators for oral candidiasis and oral hairy leukoplakia in HIV-infected adults. Community Dentistry and Oral Epidemiology. 2007;33(1):35–44. doi: 10.1111/j.1600-0528.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Sroussi HY, Villines D, Epstein J, Alves MC, Alves ME. Oral lesions in HIV-positive dental patients – one more argument for tobacco smoking cessation. Oral Diseases. 2007;13:324–328. doi: 10.1111/j.1601-0825.2006.01289.x. [DOI] [PubMed] [Google Scholar]
- 12.Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, Anastos K. Association of cigarette smoking with HIV prognosis among women in the HAART era: A report from the women's intergeneracy HIV study. American Journal of Public Health. 2006;96(6):1060–1065. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine & Tobacco Research. 2003;5(1):111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- 14.Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67(4):555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 15.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Wewers ME. Treating Tobacco Use and Dependence: 2008 Update. In: Services USDoHaH, editor. Clinical Practice Guideline. Rockville, MD: 2008. [Google Scholar]
- 16.Hettema JE, Steele J, Miller WR. Motivational Interviewing. Annual Review of Clinical Psychology. 2005;(1):91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 17.Heckman CJ, Egleston B, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tobacco Control. 2010 doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hettema JE, Hendricks PS. Motivation interview for smoking cessation: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(6):868–884. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- 19.Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews. 2010;1 doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine relacement interventions for HIV positive smokers. AIDS Behavior. 2009;13:545–554. doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, Niaura R. Motivational and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction. 2009;104(11):1891–1900. doi: 10.1111/j.1360-0443.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MF, Shapiro D, Windsor R, Whalen P, Rhode R, Miller HS, Sechrest L. Motivational interviewing versus prescriptive advice for smokers who are not ready to quit. Patient Education and Counseling. 2011 doi: 10.1016/j.pec.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale: A self report depressoin scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 24.Sobell LC, Sobell MB. A technique for assessing self-reported alcohol consumption. In: Allen RZLJ, editor. Measuring alcohol consumption: Psychosocial and biological methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 25.Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, Gorecki JA. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. American Journal of Public Health. 2006;96:1808–1814. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 27.Prochaska JO, DiClemente C. Stages and processes of self-change for smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 28.Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress relapse to alcohol, opiates, and nicotine. Journal of Consulting and Clinical Psychology. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- 29.Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Controlled Clinical Trials. 1988;9(4):327–344. doi: 10.1016/0197-2456(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 30.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Vol 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- 31.U.S. Department of Health and Human Services. You can quit smoking: Consumer guide. 2000
- 32.Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in motivational interviewing. Journal of Substance Abuse Treatment. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items. Applications to assessment of adaptive behavior. American Journal of Mental Deficiency. 1981;86:127–137. [PubMed] [Google Scholar]
- 34.Miller WR, Moyers TB, Arcinega L, Ernst D, Forcehimes A. Training, supervision and quality monitoring of the COMBINE Study Behavioral Interventions. Journal of Studies on Alcohol. 2005;S15:188–195. doi: 10.15288/jsas.2005.s15.188. [DOI] [PubMed] [Google Scholar]