Abstract
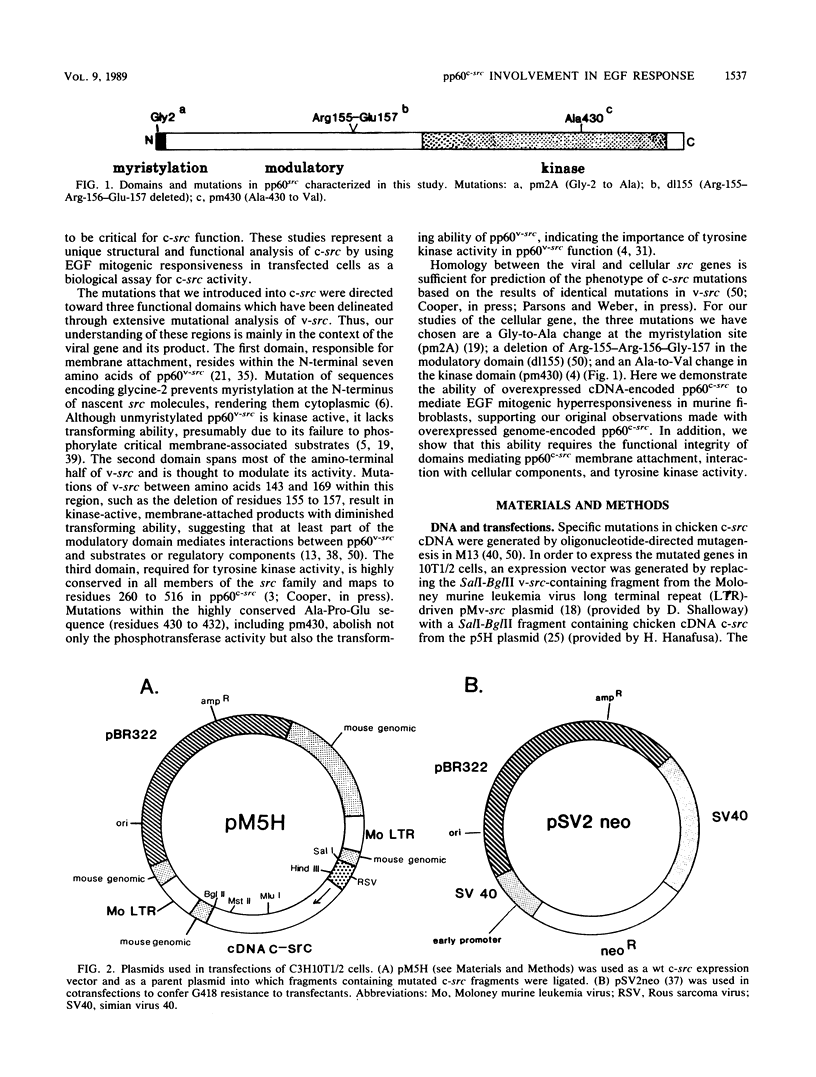
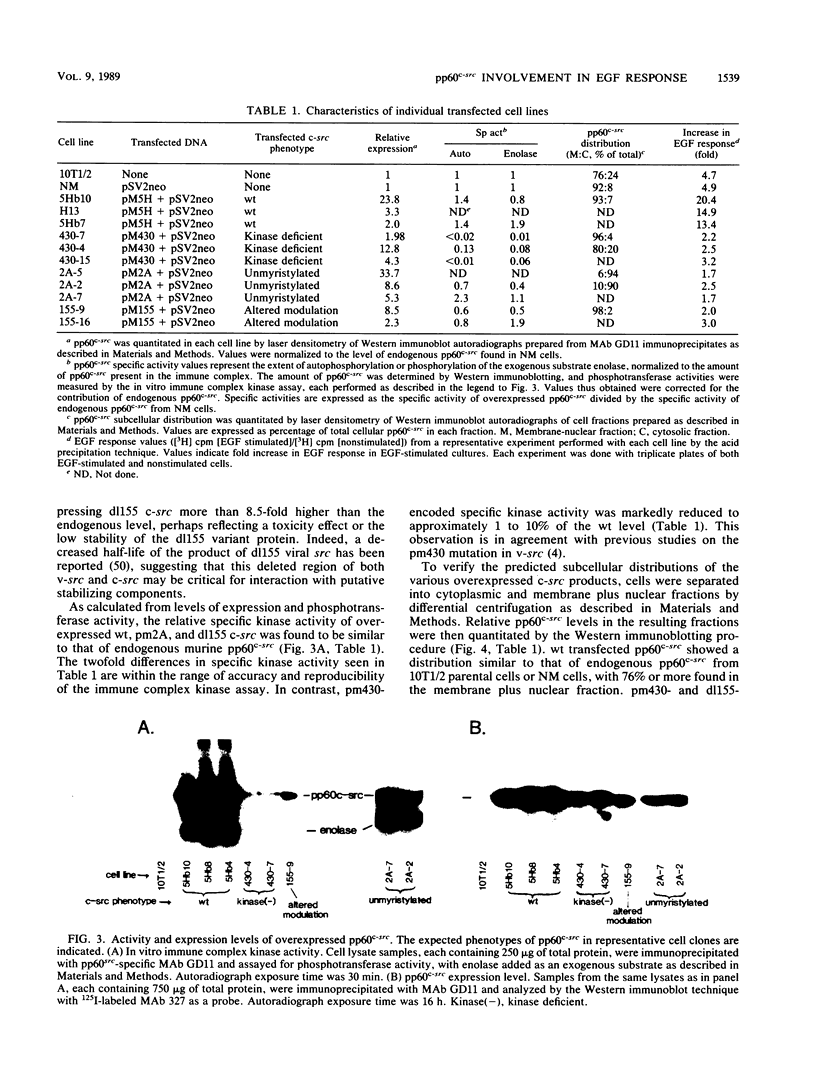
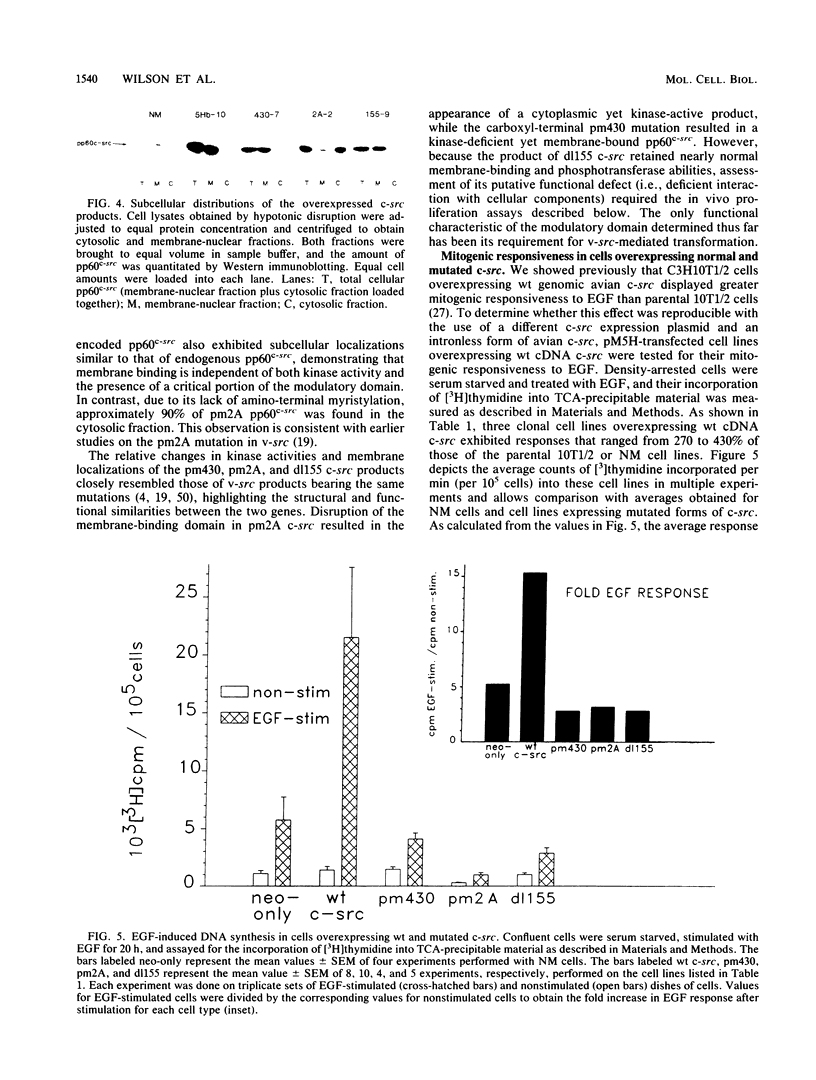
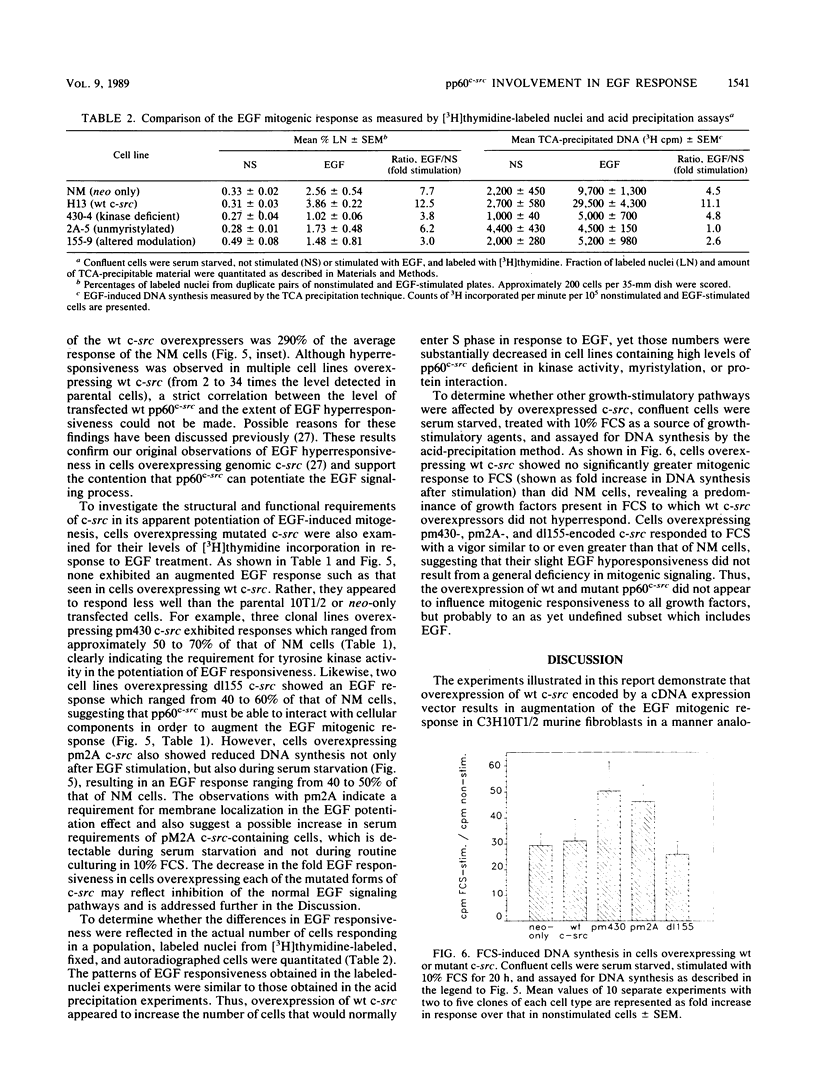
In previous studies examining the potential role of pp60c-src in cellular proliferation, we demonstrated that C3H10T1/2 murine embryo fibroblasts overexpressing transfected chicken genomic c-src displayed an epidermal growth factor (EGF)-induced mitogenic response which was 200 to 500% of the response exhibited by parental control cells (Luttrell et al., Mol. Cell. Biol. 8:497-501, 1988). In order to examine specific structural and functional requirements for pp60c-src in this event, 10T1/2 cells were transfected with chicken c-src genes encoding pp60c-src deficient in tyrosine kinase activity (pm430), myristylation, (pm2A), or a domain hypothesized to modulate the interaction with substrates or regulatory components (dl155). Neomycin-resistant clonal cell lines overexpressing each of the mutated c-src genes were assayed for EGF mitogenic responsiveness by measuring [3H]thymidine incorporation into acid-precipitable material or into labeled nuclei. The results were compared with those obtained with lines overexpressing the cDNA form of wild-type (wt) c-src or control cells transfected with the neomycin resistance gene only. As previously described for cells overexpressing wt genomic c-src (Luttrell et al., 1988), clones overexpressing wt cDNA c-src also exhibited enhanced EGF mitogenic responses ranging from approximately 300 to 400% of the control cell response. In contrast, clones overexpressing unmyristylated, modulation-defective, or kinase-deficient c-src not only failed to support an augmented response to EGF but also exhibited EGF responses lower than that of the control cells. Furthermore, there were no significant differences in the mitogenic responses to 10% fetal calf serum among any of the cells tested. These results indicate that pp60(c-scr) can potentiate mitogenic signaling generated by EGF but not all growth factors. This potentiation requires the utilization of pp60(c-scr) myristylation, and modulatory and tyrosine kinase domains and can me mediated by cDNA-encoded as well as by genome-encoded wt pp60(c-scr).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Barnekow A., Gessler M. Activation of the pp60c-src kinase during differentiation of monomyelocytic cells in vitro. EMBO J. 1986 Apr;5(4):701–705. doi: 10.1002/j.1460-2075.1986.tb04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Darrow D. Analysis of the catalytic domain of phosphotransferase activity of two avian sarcoma virus-transforming proteins. J Biol Chem. 1984 Apr 10;259(7):4550–4557. [PubMed] [Google Scholar]
- Bryant D. L., Parsons J. T. Amino acid alterations within a highly conserved region of the Rous sarcoma virus src gene product pp60src inactivate tyrosine protein kinase activity. Mol Cell Biol. 1984 May;4(5):862–866. doi: 10.1128/mcb.4.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackalaparampil I., Shalloway D. Altered phosphorylation and activation of pp60c-src during fibroblast mitosis. Cell. 1988 Mar 25;52(6):801–810. doi: 10.1016/0092-8674(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Markland W., Markham A. F., Smith A. E. Mutations around the NG59 lesion indicate an active association of polyoma virus middle-T antigen with pp60c-src is required for cell transformation. EMBO J. 1986 Feb;5(2):325–334. doi: 10.1002/j.1460-2075.1986.tb04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Hunter T. Platelet-derived growth factor induces multisite phosphorylation of pp60c-src and increases its protein-tyrosine kinase activity. Mol Cell Biol. 1988 Aug;8(8):3345–3356. doi: 10.1128/mcb.8.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn K. V., Singley J. A., Chavin W. Fetal bovine serum: a multivariate standard. Proc Soc Exp Biol Med. 1975 Jun;149(2):344–347. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Coussens P. M., Danko A. V., Shalloway D. Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol. 1985 May;5(5):1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Parsons S. J., Parsons J. T., Gilmer T. M. Activation of pp60c-src tyrosine kinase specific activity in tumor-derived Syrian hamster embryo cells. Oncogene. 1988 Apr;2(4):327–335. [PubMed] [Google Scholar]
- Kaplan J. M., Mardon G., Bishop J. M., Varmus H. E. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988 Jun;8(6):2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Takeya T., Grandori C., Iba H., Levy J. B., Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986 Dec;6(12):4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell D. K., Luttrell L. M., Parsons S. J. Augmented mitogenic responsiveness to epidermal growth factor in murine fibroblasts that overexpress pp60c-src. Mol Cell Biol. 1988 Jan;8(1):497–501. doi: 10.1128/mcb.8.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- McCarley D. J., Parsons S. J. Reduced tyrosine kinase specific activity is associated with hypophosphorylation of pp60c-src in cells infected with avian erythroblastosis virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5793–5797. doi: 10.1073/pnas.84.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Martinez R., Weber M. J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983 Mar;3(3):380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. J., Creutz C. E. p60c-src activity detected in the chromaffin granule membrane. Biochem Biophys Res Commun. 1986 Jan 29;134(2):736–742. doi: 10.1016/s0006-291x(86)80482-x. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Raymond V. W., Parsons J. T. Localization of conserved and nonconserved epitopes within the Rous sarcoma virus-encoded src protein. J Virol. 1986 Sep;59(3):755–758. doi: 10.1128/jvi.59.3.755-758.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. 1985 Mar 28-Apr 3Nature. 314(6009):374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The product of the protooncogene c-src is modified during the cellular response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7845–7849. doi: 10.1073/pnas.82.23.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond V. W., Parsons J. T. Identification of an amino terminal domain required for the transforming activity of the Rous sarcoma virus src protein. Virology. 1987 Oct;160(2):400–410. doi: 10.1016/0042-6822(87)90011-0. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Reconstitution of the Rous sarcoma virus transforming protein pp60v-src into phospholipid vesicles. Mol Cell Biol. 1988 May;8(5):1896–1905. doi: 10.1128/mcb.8.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Enhancement of polyoma virus middle T antigen tyrosine phosphorylation by epidermal growth factor. Nature. 1983 Aug 25;304(5928):742–744. doi: 10.1038/304742a0. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Drees B., Kornberg T., Bishop J. M. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell. 1985 Oct;42(3):831–840. doi: 10.1016/0092-8674(85)90279-x. [DOI] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Tamura T., Friis R. R., Bauer H. pp60c-src is a substrate for phosphorylation when cells are stimulated to enter cycle. FEBS Lett. 1984 Nov 5;177(1):151–156. doi: 10.1016/0014-5793(84)81001-7. [DOI] [PubMed] [Google Scholar]
- Taparowsky E. J., Heaney M. L., Parsons J. T. Oncogene-mediated multistep transformation of C3H10T1/2 cells. Cancer Res. 1987 Aug 1;47(15):4125–4129. [PubMed] [Google Scholar]
- Wang H. C., Parsons J. T. Deletions and insertions within an amino-terminal domain of pp60v-src inactivate transformation and modulate membrane stability. J Virol. 1989 Jan;63(1):291–302. doi: 10.1128/jvi.63.1.291-302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]