Abstract
Lysyl-ubiquitination has long been known to target cytoplasmic proteins for proteasomal degradation, and there is now extensive evidence that ubiquitination functions in vacuolar/lysosomal targeting of membrane proteins from both the biosynthetic and endocytic pathways. G protein-coupled receptors (GPCRs) represent the largest and most diverse family of membrane proteins, whose function is of fundamental importance both physiologically and therapeutically. In this review we discuss the role of ubiquitination in the vacuolar/lysosomal downregulation of GPCRs through the endocytic pathway, with a primary focus on lysosomal trafficking in mammalian cells. We will summarize evidence indicating that mammalian GPCRs are regulated by ubiquitin-dependent mechanisms conserved in budding yeast, and then consider evidence for additional ubiquitin -dependent and -independent regulation that may be specific to animal cells.
Keywords: Seven-transmembrane receptor, G-protein, signaling, endocytosis, ubiquitin, ESCRT, multivesicular body, downregulation
Introduction
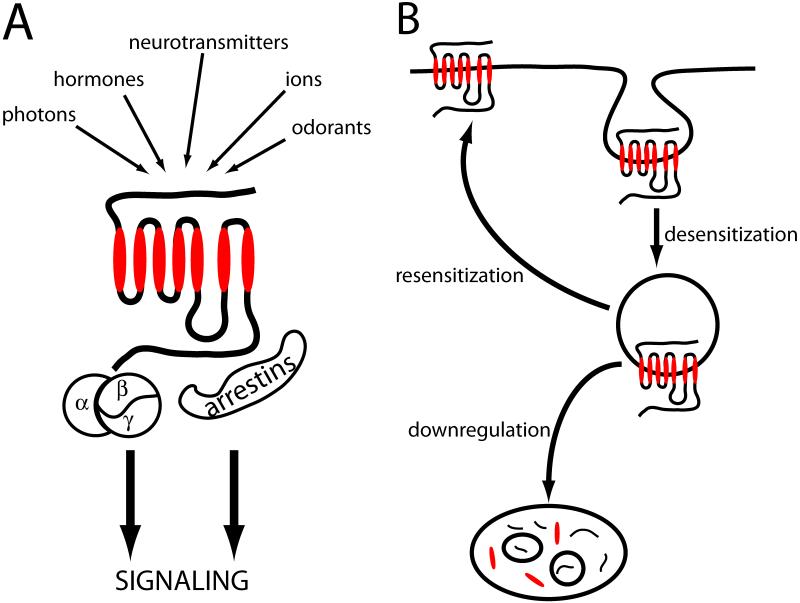
G protein-coupled receptors (GPCRs, also known as 7-transmembrane receptors or 7TMRs) represent the largest family of signaling receptors and integral membrane proteins expressed in animals. There are estimated to be ~700 functional GPCRs encoded by the human genome, approximately half of which are odorant receptors. GPCRs represent the largest class of therapeutic drug targets, with ~30% of drugs presently used in clinical medicine binding directly to particular members of this receptor family (1, 2). GPCRs respond to a diverse array of stimuli, ranging from photons, to small molecules and large glycoproteins, and a considerable number of these GPCRs have unknown endogenous activators (‘orphan’ GPCRs). GPCRs mediate many of their physiological effects by functioning as guanine nucleotide exchange factors for heterotrimeric G proteins, thereby producing diverse effects on downstream signaling networks. In addition, there is evidence that some GPCRs can signal by G protein-independent mechanisms (3-6) (Fig.1A).
Figure 1. Major GPCR signaling responses and regulation by endocytosis.
A. Simplified schematic depicting the range of signaling responses mediated by GPCRs in mammals. B. Major trafficking itineraries of GPCRs that have been linked to functional regulation of cellular signaling. Endocytosis of receptors can produce functional desensitization of cellular responsiveness by physically removing receptors from access to extracellular ligands, and preventing access to plasma membrane-delimited signaling mediators. Recycling of internalized receptors can function, conversely, to resensitize cellular responsiveness. Trafficking of internalized receptors via the MVB/lysosome pathway causes a prolonged attenuation of cellular responsiveness by promoting proteolytic downregulation of receptors.
A general feature of GPCR-linked signaling networks is that they are extensively regulated, particularly at the level of the receptor itself. Perhaps the most highly conserved mechanism of GPCR regulation is by endocytosis. Traditionally, endocytosis of GPCRs is thought to contribute to signal attenuation (or ‘desensitization’), by physically removing receptors from access to extracellular ligands and signaling mediators. After endocytosis, molecular sorting of GPCRs between divergent membrane pathways can confer very different functional consequences, thus contributing to receptor-selective regulation. Recycling of GPCRs back to the plasma membrane, for example, is typically thought to restore (or ‘resensitize’) cellular signaling responsiveness. Trafficking of internalized GPCRs to lysosomes promotes their proteolytic destruction (or ‘downregulation’), thus producing an essentially opposite effect on cellular responsiveness (Fig.1B). Closely related GPCRs can differ greatly in rate and ligand-dependence of endocytosis, and subsequently in their trafficking itinerary after endocytosis. Elucidating mechanisms that determine, and regulate, the specificity of GPCR membrane traffic in the endocytic pathway thus represents an important goal of both basic and pharmaceutically-oriented research.
Ubiquitin
Reversible post-translational modification represents a key principle by which GPCR trafficking itineraries are specified and regulated. Phosphorylation of GPCRs has long been known to influence receptor function and trafficking, and has been extensively reviewed elsewhere (7-9). The evolutionarily conserved process of ubiquitination is another class of post-translational modification that is now well established to specify or regulate membrane trafficking in the vacuolar/lysosomal pathway. Ubiquitin forms stable adducts through isopeptide bond formation with the ε-amino group of lysine residues, or less commonly to the N-terminal amine. There is also evidence for ubiquitin forming thiolester linkages with cysteine residues, and ester linkages with serine or threonine residues (10). Based on the data available to date, however, the primary (or possibly only) linkage relevant to endocytic trafficking of GPCRs is lysyl ubiquitination. The process by which ubiquitin is attached to lysine residues has been well established and is discussed in a number of excellent reviews (11-13). Briefly, a cascade of three ligases is required with the E1 and E2 ligases activating the ubiquitin molecule, via a thiolester bond, before the E3 ligases then control the transfer of the ubiquitin molecule from the E2 to the substrate, thereby controlling specificity. Ubiquitin chains can be removed by various ubiquitin-specific proteases or deubiquitinating enzymes (USPs or DUBs), which themselves have profound effects on cellular function (14). The ubiquitin molecule itself contains 7 lysine residues, a number of which can have further ubiquitin molecules conjugated to form polyubiquitin chains. Lys48 linked polyubiquitin chains are generally thought to target proteins for proteosomal processing, whereas Lys63 linked chains are involved in endocytic processes, although other polyubiquitin chains have also been demonstrated and remain the focus of ongoing research (15).
The last 15 years have seen an explosion of information regarding the role of ubiquitin in the endocytic pathway. Here we outline current views regarding the function of ubiquitin in specifying and regulating the endocytic membrane trafficking of GPCRs. We will start by discussing core features of ubiquitin-dependent trafficking that are conserved in budding yeast, and then focus on GPCR trafficking in mammalian cells, where there is evidence both for the operation of core mechanisms and for the existence of additional specificity and regulation.
Roles of GPCR ubiquitination in Endocytosis
The first reported role for ubiquitination in endosomal trafficking of GPCRs was in facilitating the efficient endocytosis of the Ste2p in the budding yeast Saccharomyces cerevisiae (16). Hicke and Riezman demonstrated that Ste2p undergoes significant ubiquitination following addition of its physiological agonist, α-mating factor, and went on to demonstrate that ubiquitination of a single lysine residue within the C-terminal internalization signal was required for rapid endocytosis of Ste2p and this bound ligand (16). It was later demonstrated that the fusion of a single ubiquitin moiety was sufficient to drive the endocytosis of both Ste2p (17), and the related a-factor GPCR Ste3p (18). Ste3p exhibits a relatively high rate of constitutive (ligand-independent) endocytosis, as well as a ligand-induced component. Ubiquitination of Ste3p is required for constitutive endocytosis of receptors but, remarkably, not for ligand-induced endocytosis (19).
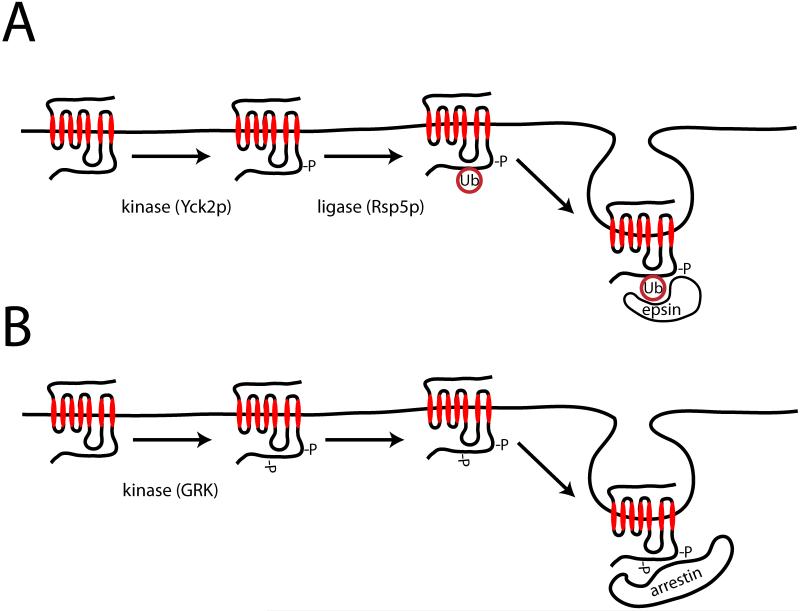
In mammalian cells, it is generally thought that direct ubiquitination is not required for efficient endocytosis of GPCRs via clathrin-coated pits. The details of how GPCR endocytosis is regulated can vary substantially, with respect to yeast GPCRs and relative to other mammalian membrane receptors (reviewed elsewhere, see (7-9, 20)). Superficially, however, the initiating steps in endocytosis of yeast and mammalian GPCRs are similar. In both systems, regulated endocytosis is stimulated by phosphorylation of the receptor following agonist addition; this is mediated by the casein kinase I homologue Yck2p in yeast (21, 22) and by a variety of kinases, most notably a family of kinases called GRKs (for GPCR kinases), in mammalian cells. Phosphorylation of yeast Ste2p promotes ubiquitination of the cytoplasmic tail, which in turn promotes receptor endocytosis. It has been proposed that the ubiquitin tag behaves as an endocytic signal by linking receptors with UIM domain-containing endocytic adaptor proteins such as epsin (Ent1p) and Eps15 (Ede1p), which represent major structural components of clathrin coated pits (22, 23). While there is significant evidence that Ent1p and Ede1p function as classical endocytic adaptors, ubiquitin-linked connectivity with these proteins also appears to function more generally in physical organization or assembly of the endocytic coat ((24), Fig.2A). Ligand-induced phosphorylation of many mammalian GPCRs, by GRKs but also by other kinases (25-27), promotes receptor interaction with cytoplasmic proteins typically called β-arrestins (or ‘non-visual’ arrestins, so-named to distinguish them from the founding arrestin family member that functions in photoreceptors). β-arrestins, which are conserved throughout metazoa but not in yeast, can act as endocytic adaptors by binding directly to the clathrin heavy chain (28) and to the β-subunit of AP-2 (29), as well as by interacting with PtdIns-4,5-P2 (30). β-arrestins mediate additional effects on GPCR signaling including switching off G-protein mediated signaling (desensitization), and switching on non-canonical receptor signaling (5). It appears that mammalian GPCRs do not require ubiquitination for efficient endocytosis and, in fact, prevention of receptor ubiquitination has been shown to have little if any effect on the endocytosis of a number of GPCRs including the β2-adrenergic receptor (β2AR) (31, 32), δ-opioid neuropeptide receptor (DOR) (33), and neurokinin receptor (NK1R) (34).
Figure 2. Comparison of regulated endocytosis of GPCRs in budding yeast and mammalian cells.
A. Ligand-induced endocytosis of Ste2p in budding yeast is initiated by receptor phosphorylation by Yck2p, a casein kinase I -like enzyme, which promotes lysyl-ubiquitination of the cytoplasmic tail by Rsp5p. Ubiquitination is thought to promote endocytosis by linking receptors to the epsins Ent1p and 2p, and interaction with the Eps15 homologue Ede1p, all of which contain ubiquitin-binding domains. As discussed in the text, ubiquitination of Ste3p is required for constitutive receptor endocytosis but not for the ligand-induced component. B. Regulated endocytosis of many mammalian GPCRs is initiated by ligand-induced phosphorylation of receptors by a family of GPCR kinases (GRKs), which promote receptor interaction with the endocytic adaptor proteins β-arrestin-1 and -2 (arrestin 2 and 3). This mechanism does not require ubiquitination of the GPCR although, as discussed in the text, it can be influenced by ubiquitination of arrestin.
The protease activated receptor, PAR1, is unusual among mammalian GPCRs in that neither its constitutive nor ligand induced endocytosis requires β-arrestin. Instead, both processes are wholly (constitutive) or partially (ligand induced) dependent on the endocytic adaptor protein AP-2 (35). Interestingly, PAR1 appears to be basally ubiquitinated in the absence of ligand and, upon agonist activation, undergoes rapid de-ubiquitination. Preventing PAR1 ubiquitination by mutating all cytoplasmic lysine residues had little effect on agonist-induced endocytosis; however, the rate of constitutive endocytosis was significantly increased. This suggests that, contrary to its previously defined role in promoting endocytosis of GPCRs in yeast, ubiquitination of the mammalian PAR1 may have an inhibitory effect.
Although it is generally agreed that direct ubiquitination is not essential for promoting endocytosis of mammalian GPCRs, there is evidence for significant ‘indirect’ regulation via ubiquitination of β-arrestin. Shenoy and coworkers demonstrated that, following agonist-induced activation of the β2AR, β-arrestin-2 undergoes transient ubiquitination. They further demonstrated that β-arrestin-2 interacts with the E3 ligase Mdm2 and that this interaction is required for efficient ubiquitination of β-arrestin-2. Either RNAi-mediated depletion of Mdm2, or over-expression of a catalytically inactive mutant version, inhibited endocytosis of the β2AR (31). Further, lysine mutations preventing β-arrestin-2 ubiquitination inhibited the ability of this protein to promote ligand-induced endocytosis of both the β2AR and V2R (36) (Fig.2B).
Ubiquitination and Sorting after Endocytosis
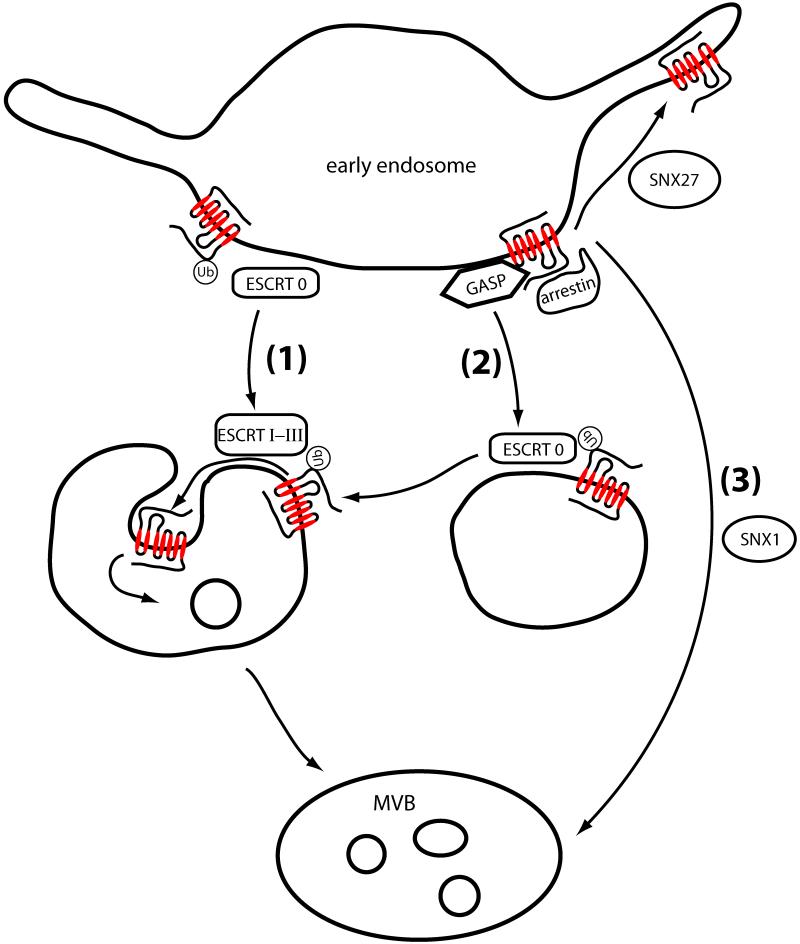
GPCR ubiquitination clearly affects receptor trafficking after endocytosis. This too was originally demonstrated in yeast, where the same lysine mutation that prevented endocytosis of Ste3p reduced vacuolar targeting of internalized receptors and increased receptor recycling to the plasma membrane (19). Ubiquitin is thought to direct membrane cargo, delivered from both the endocytic and biosynthetic pathways, to the intralumenal space within late endosomes by a process of multivesicular body (MVB) formation. Mechanisms of MVB biogenesis and sorting have been extensively studied, in both yeast and mammalian systems, and are reviewed elsewhere (37-40). While other mechanisms may exist, at least in mammalian cells (41, 42), a highly conserved protein machinery functioning in MVB biogenesis has been defined both genetically and biochemically (Fig.3). A large number of genes, identified in a subset (class E) of vacuolar protein sorting (Vps) mutants, encode proteins that can be isolated from yeast extracts as a set of endosome-associating protein complexes; thus, in pioneering studies carried out primarily by Emr and co-workers, these proteins were defined collectively as the ‘endosomal sorting complex required for transport’ (ESCRT) (43). Together with several additional gene products not identified in Vps screens, the ESCRT is presently viewed as a group of four complexes - typically called ESCRT 0, I, II, and III. These proteins are conserved in mammalian cells and are thought to function in a coordinated manner, both in generating intralumenal vesicles (ILVs) within MVBs and in directing ubiquitinated cargo to ILVs. It is generally agreed, as demonstrated in mammalian cells by Stenmark and colleagues (44), that cargo selection begins by ubiquitin-dependent binding to the ESCRT 0 complex containing HRS and STAM (Vps27 and Hse1 in yeast), each of which contains ubiquitin-binding domains. ESCRTs I - III are thought to link to ESCRT 0 and some of their components also contain ubiquitin-binding domains. ESCRTs I - III function in physically generating ILVs, and there are presently various models for how they do so. One model, based on in vitro reconstitution studies carried out in the Hurley lab, proposes that ESCRTs I and II cause membrane deformation leading to inward budding and stabilization of the bud neck. Subsequently, ESCRT III proteins act in concert to cinch the neck closed (45), using mechanical energy supplied by the AAA-ATPase Vps4 that also promotes complex disassembly allowing multiple packaging cycles (46, 47). While the importance of ubiquitin in endocytic sorting was first demonstrated using a GPCR as cargo, and yeast GPCRs require the ESCRT for efficient vacuolar sorting (see below), much of the work on ESCRT-dependent sorting in mammalian cells has focused on the EGF receptor tyrosine kinase; role(s) of ubiquitination and ESCRT in MVB/lysosome sorting of mammalian GPCRs remain incompletely defined. As discussed later in this review, there may also be significant additions or differences in the regulation of mammalian GPCRs, relative to the canonical model.
Figure 3. Mechanisms implicated in sorting mammalian GPCRs between recycling and lysosomal itineraries.
Mechanism (1) depicts the canonical ubiquitin and ESCRT-dependent mechanism, similar to that described in yeast and for the EGF receptor tyrosine kinase in mammalian cells. This mechanism is proposed to mediate ubiquitination-dependent lysosomal sorting of a number of mammalian GPCRs, as discussed in the text. Mechanism (2) depicts proposed roles of additional machinery effectively upstream of the ESCRT, as demonstrated for several mammalian GPCRs including the delta opioid receptor. Receptor interactions with GASP, and possibly also with arrestins, inhibits receptor recycling and promotes traffic to the late endocytic pathway. Efficient recycling of receptors requires an active sorting process mediated by receptor interaction with distinct cellular proteins such as sorting nexin 27 (SNX27). None of these sorting steps requires receptor ubiquitination but, for receptors directed to the late endocytic pathway by GASPs, later steps in the proteolytic pathway are ESCRT-dependent and regulated by receptor ubiquitination. Mechanism (3) illustrates an alternate mechanism of GPCR trafficking to lysosomes, proposed for PAR1, which does not require receptor ubiquitination or the ESCRT and instead requires sorting nexin 1 (SNX1).
Passive recycling by ‘lack’ of sorting
The prevailing view is that ubiquitin-directed sorting of cargo from the limiting membrane to ILVs functions as a ‘geometrical’ sorting operation, which effectively removes membrane cargo from the recycling pathway by sequestering them in the intralumenal space. Accordingly, one might suppose that disrupting cargo ubiquitination would prevent this sorting, resulting in recycling essentially by bulk membrane flux. This appears to be the case for GPCRs in yeast, and some but not all GPCRs in mammalian cells. Mutating all intracellular lysine residues in Ste3p to arginine (a strategy often used to prevent receptor ubiquitination) prevented sorting of this GPCR to the vacuole, and accordingly increased receptor recycling to the plasma membrane (19). Similar experiments and results have been reported for several mammalian GPCRs, including the chemokine receptor CXCR4, the NK1R and a distinct (from PAR1) protease activated receptor called PAR2 (34, 48, 49). Studies of the β2AR provided early clues to the existence of additional complexity in the endocytic sorting of mammalian GPCRs. The β2AR is rapidly ubiquitinated in an agonist-stimulated manner (31, 50); however, the major endocytic trafficking itinerary of this GPCR in many mammalian cell types is rapid and non-destructive recycling to the plasma membrane. Further, the β2AR can recycle efficiently irrespective of the presence or absence of cytoplasmic lysine residues (32). These considerations raise the question of whether there exist additional mechanism(s) contributing to the sorting of mammalian GPCRs in the early endocytic pathway.
The first direct evidence for additional sorting was the discovery that efficient recycling of the β2AR requires a short sequence present in the receptor’s cytoplasmic tail, which does not contain lysine residues and conforms to a consensus PDZ motif (51). Fusion of this motif to the cytoplasmic tail of the DOR, a distinct mammalian GPCR that normally traffics to lysosomes after endocytosis, is sufficient to re-route receptors into the recycling pathway (52). The β2AR-derived PDZ motif can bind to a family of PDZ proteins whose founding member is EBP50 or NHERF1; a primary cellular function of NHERF/EBP50-family proteins is to mediate indirect connectivity of cognate motif-bearing integral membrane proteins to actin filaments (53). Engineered actin connectivity can indeed direct plasma membrane recycling of mutant GPCRs from which the native PDZ motif has been deleted (54), but the major PDZ protein essential for efficient recycling of the wild type β2AR is sorting nexin 27 (SNX27) (55). A number of other mammalian GPCRs also contain distinct cytoplasmic determinants that are essential for efficient recycling and some, but not all, correspond to PDZ motifs. Further, it is evident that not all GPCR ‘recycling sequences’ work by the same mechanism (56-59). Efficient recycling of the μ-opioid neuropeptide receptor (MOR), for example, requires a 12-residue cytoplasmic sequence that does not conform to a PDZ motif (60), and recycling directed by this sequence is insensitive to depletion of SNX27 (55). These data suggest the existence of a potentially diverse array of cis-acting sorting sequences that can specifically promote efficient recycling even of GPCRs that are subject to extensive ubiquitination.
Further evidence for additional sorting specificity comes from studies identifying a discrete role of ESCRT 0 in the recycling of mammalian GPCRs. Hanyaloglu and co-workers demonstrated that depletion of cellular HRS, or disruption of ESCRT 0 by HRS overexpression, inhibits recycling of both the β2AR and the MOR (32). Similar results have been shown for two other mammalian GPCRs, the PAR2 and calcitonin receptor-like receptor (CLR) (61). One interesting aspect of this mechanism is that neither the ubiquitination status of the receptor, nor the UIM domain of HRS, is important for HRS-dependent recycling. Another interesting aspect is that Tsg101 (an essential component of ESCRTI) is not required for efficient recycling of these GPCRs, suggesting that the recycling function of HRS is independent of its canonical role in the ubiquitin-directed sorting by the ESCRT. These observations provide additional evidence for distinct features in the endocytic sorting of mammalian GPCRs and, in particular, suggest an elaboration of sorting machinery that operates at an early stage in the pathway and likely interfaces with ESCRT 0.
Sorting of Mammalian GPCRs by the ubiquitin-ESCRT pathway
The current model of ubiquitin-directed vacuolar/lysosomal downregulation of GPCRs via the ESCRT/MVB pathway is predicated on three principle experimental criteria: 1) that lysosomal/vacuolar proteolysis requires direct receptor ubiquitination; 2) that this dowregulation requires the ESCRT; and 3) that both ubiquitination and the ESCRT are required for localization of internalized receptors to ILVs. As discussed briefly herein, and reviewed extensively elsewhere (62-64), all three criteria have been convincingly established for GPCRs in yeast. For mammalian GPCRs, in contrast, most of the available evidence is limited to the first criterion. Ubiquitination of mammalian GPCRs was initially established for rhodopsin and opioid receptors, where it was implicated in degradation of misfolded receptors from the biosynthetic pathway and in agonist-induced downregulation by proteasomes (65-67). The Lefkowitz laboratory used lysyl mutation to demonstrate ubiquitination-dependence in agonist-induced downregulation of the β2AR by an undefined pathway (31). Marchese and Benovic, in studies of the CXCR4 chemokine receptor, established that ubiquitination of a mammalian GPCR promotes its agonist-induced down-regulation specifically by endocytic trafficking to lysosomes (48). This was established both by lysyl mutation of the receptor’s cytoplasmic tail and the demonstration that AIP4, a HECT-domain ubiquitin ligase related to yeast Rsp5, promotes lysosomal downregulation of the wild type CXCR4 (68). Similar effects of lysyl mutations have since been found for the V2R (69), PAR2 (49), NK1R (34) and κ-opioid receptor (KOR) (70). Conversely, the β1-adrenergic receptor has been shown to elude downregulation by not being ubiquitinated (50). Specific ubiquitin ligase requirements were identified for some but not all of these examples (Table). To what degree the conserved ESCRT machinery, or a subset thereof, is required for downregulation has been investigated only for a few mammalian GPCRs. Downregulation of the CXCR4 requires the ESCRT components HRS and Vps4, and internalized CXCR4s localize to HRS-associated endosome membrane microdomains (68). Lysosomal downregulation of the DOR was shown to require HRS and Vps4 (71), and downregulation of the PAR2 and CLR was shown to require HRS (61). With regard to the third criterion, several mammalian GPCRs have been shown to localize to ILVs (including the M4 acetylcholine receptor (72), CXCR4 (73) amongst others), and elegant studies from the Marsh laboratory have established ILV localization and trafficking of virally-encoded chemokine receptors (74). We are not aware, however, of any evidence directly addressing the ubiquitination-dependence for ILV localization of GPCRs in mammalian cells.
Table.
Role of Ubiquitin in GPCR Downregulation
| Receptor | Abbr. | Ubiquitinated? | Ligase | DUB | ESCRT | Other Proteins Implicated in Regulating Lysosomal Trafficking |
Comments | Refs. |
|---|---|---|---|---|---|---|---|---|
| β1-adrenergic Receptor |
β1AR | NO | N/A | N/A | N/D | Resistant to downregulation |
50 | |
| β2-adrenergic Receptor |
β2AR | YES | Nedd4-1 | USP33 USP20 |
N/D | SNX27 Arrestins ARRDC3 |
Downregulates very slowly |
31, 55, 75, 84, 119 |
| Calcitonin Receptor-like Receptor |
CLR | NO | N/A | N/A | YES | RAMP1 ECE |
Still undergoes lysosomal downregulation |
61, 93 ,97 |
| Cannabinoid Receptor 1 |
CB1 | N/D | N/D | N/D | N/D | GASP1 | 105 | |
| Chemokine Receptor 4 |
CXCR4 | YES | AIP4 | N/D | YES | Arrestins |
48, 68, 78, 79, 80 |
|
| δ-opioid Receptor | DOR | YES | AIP4 | AMSH UBPY |
YES | GASP Dysbindin |
Ubiquitination not required for downregulation |
33, 71, 98, 99, 111 |
| Dopamine Receptor 2 |
D2R | N/D | N/D | N/D | N/D | GASP1 Dysbindin |
103, 111 | |
| κ-opioid Receptor | KOR | YES | N/D | CYLD | N/D | Lys63 specific polyubiquitin |
70 | |
| Neurokinin Receptor 1 |
NK1 | YES | N/D | N/D | N/D | ECE | 34, 92 | |
| Platelet Activating Factor |
PAF | N/D | c-Cbl | N/D | N/D | 77 | ||
| Proteolytically Activated Receptor 1 |
PAR1 | De-ubiquitinated | N/A | N/D | NO | SNX1 | 94, 95 | |
| Proteolytically Activated Receptor 2 |
PAR2 | YES | c-Cbl | AMSH UBPY |
YES | 49, 61 | ||
| Vasopressin Receptor 2 |
V2R | YES | N/D | N/D | N/D | Arrestins | Ubiquitinated at single residue |
69 |
N/A – Not Applicable, N/D – Not Determined
Specificity and regulation
A very important issue in mammalian cells, particularly considering the diversity of GPCR family members that are often co-expressed, is how specificity is conferred on the endocytic trafficking of particular GPCRs. A related and equally important issue is how precise regulation is achieved because, for proper physiological homeostasis, downregulation of signaling receptors is often controlled by the activation state of that particular receptor or by a specifically linked signaling pathway. In budding yeast, essentially all ubiquitin-dependent functions in endocytic trafficking depend on Rsp5. In mammalian cells there are clearly multiple ubiquitin ligases involved, and individual GPCRs exhibit ligase-specificity in their endocytic trafficking. The HECT domain ligase AIP4 was shown to promote downregulation of the CXCR4, and this was specific because the related HECT ligase Nedd4 did not affect this process (68). Nedd4, on the other hand, was reported in another study to promote downregulation of the β2AR (75). Downregulation of the PAR2 is dependent on c-Cbl (49), a RING domain ligase shown previously to function in downregulation of the EGF receptor tyrosine kinase (76). c-Cbl also functions in downregulation of the platelet-activating factor GPCR (77), however. Thus, while there is evidence for considerable specificity in the ligase ‘code’ determining downregulation of particular mammalian GPCRs, this specificity is not absolute.
While the molecular basis of ligase recognition of mammalian GPCRs remains poorly understood, the data presently available suggest at least two levels of control. The primary level is, of course, that of direct interaction with the GPCR substrate. Some ligases that ubiquitinate mammalian GPCRs bind specifically and directly to the cytoplasmic tail of the receptor, which is one of the most highly divergent domains among GPCRs. HECT domain ligases related to Nedd4, like Rsp5 in yeast, possess multiple WW domains that typically interact with PPXY motifs (and, to a lesser extent, PY motifs) in target proteins (12). No such motifs exist in the C-terminal tail of the CXCR4. Instead it is thought that the WW domains in AIP4 bind to a serine doublet in the receptor’s C-terminal tail, which is phosphorylated upon agonist activation; this strategy confers both specificity and agonist-dependent regulation on CXCR4 ubiquitination (78).
A second level of specificity is by recruitment of ubiquitin ligases to receptors via intermediate adaptor proteins. β-arrestins, which play a central role in desensitization and endocytosis of mammalian GPCRs, can also bind various E3 ubiquitin ligases. Shenoy and coworkers reported that downregulation and lysosomal targeting of β2AR is regulated by agonist-stimulated ubiquitination of receptors by Nedd4, and this function is dependent on a direct interaction between Nedd4 and β-arrestin-2 (75). Agonist activation increases the interaction of both β-arrestin-2 with Nedd4 and also with β2AR, illustrating how this adaptor strategy can also confer regulation of GPCR ubiquitination. Somewhat surprisingly, a similar adaptor strategy has been reported to recruit AIP4 to the CXCR4, in this case via β-arrestin-1 (79). Interestingly, however, the ubiquitination status of the receptor was unaffected by depleting β-arrestin-1. In fact, it has recently been shown that β-arrestin-1 also interacts with the ESCRT 0 component STAM1, and this interaction plays an important role in regulating CXCR4 trafficking, potentially by controlling the amount of AIP4-mediated ubiquitination of HRS (68, 80). The degree to which the trafficking of mammalian GPCRs depends on ubiquitination of other trafficking proteins remains to be determined, but this may represent another level of specificity and control.
Yeast can deploy a similar strategy of adaptor-mediated recruitment through a group of proteins (called arrestin-like proteins or ARTs) that are distantly related to mammalian β-arrestins, and share similar ‘arrestin domains’ that mediate interaction with other proteins. Arrestin-related proteins in yeast have been implicated in promoting downregulation of a distinct group of polytopic membrane proteins (plasma membrane nutrient transporters) but not, so far, GPCRs. These arrestin-related proteins, unlike mammalian β-arrestins, contain a canonical PPXY motif that interacts with WW domains in Rsp5, explaining how these proteins function as adaptors for Rsp5-mediated ubiquitination (81-83). Arrestin-related proteins are also found in mammals (where they are typically called arrestin domain-containing proteins or ARRDCs). It was reported recently that downregulation of the β2AR by Nedd4 is facilitated by ARRDC3, and that this occurs via interaction of ARRDC3 with both the ligase and GPCR (84). Moreover, arrestin-like proteins have been shown to control a seven-transmembrane protein (Rim21) implicated in pH signaling in the distinct fungal species Aspergillus nidulans, by a mechanism involving direct interaction with ESCRT I (85). Thus it appears that discrete arrestin-related proteins contribute additional specificity and regulation on the ubiquitin-directed trafficking and function of GPCRs, and other membrane proteins, in diverse organisms and possibly by multiple mechanisms.
Additional mechanisms controlling lysosomal trafficking of GPCRs in mammalian cells
While ubiquitin plays a central role in promoting downregulation of many mammalian GPCRs, there is also evidence for the existence of distinct or additional regulation. Here we will first discuss evidence that ubiquitin affects GPCR sorting and function indirectly, via ubiquitination of arrestins. Secondly, we will discuss evidence for lysosomal downregulation of mammalian GPCRs by a mechanism independent of both ubiquitination and the ESCRT. Thirdly, we will discuss evidence for the existence of additional control in ESCRT-mediated downregulation of mammalian GPCRs.
1. Ubiquitin dependent regulation of β-arrestins
There is accumulating evidence that the ubiquitination state of β-arrestin, in addition to influencing the ability of this protein to promote GPCR endocytosis, can affect the trafficking of mammalian GPCRs after endocytosis. In many cases β-arrestins dissociate from GPCRs during or immediately after endocytosis but, in other cases, β-arrestins appear to remain associated (86). This difference in β-arrestin behavior can be determined by the phosphorylation state of the GPCR cytoplasmic tail (87), but also by ubiquitination of the β-arrestin. β-arrestin-2 (31) can be ubiquitinated by Mdm2 (see above) and deubiquitinated by the DUB enzyme USP33 (88). Interestingly, the time course of ubiquitination and deubiquitination of β-arrestin-2 correlates with whether or not β-arrestins remain associated with GPCR-containing endosomes (89). Additionally, β-arrestin-2 is persistently ubiquitinated following activation of the angiotensin-activated GPCR AT1AR, which remains in endosomes for a prolonged period after endocytosis (90). Further, a β-arrestin-2-ubiquitin fusion has been shown to stably interact with the β2AR and increase downregulation of this GPCR (89), and also the M1 and M2 acetylcholine GPCRs (91). It is also increasingly evident that some mammalian GPCRs can signal through distinct G protein-linked and arrestin-linked pathways. One such non-canonical pathway involves β-arrestin-mediated activation of the MAP kinase ERK1/2. Mdm2-mediated ubiquitination of β-arrestin enhances ERK signaling of the β2AR via this pathway, and USP33 mediated deubiquitination decreases ERK signaling (88). An additional role for arrestin-dependent control of trafficking has been demonstrated for the NK1R and CLR. Here, arrestin-receptor stability appears to be regulated by the action of an endosomal enzyme, endothelin converting enzyme (ECE) (92, 93). Bunnett and coworkers demonstrated that active ECE degrades the receptor agonist; this promotes dissociation of arrestin from the receptor, thus terminating ERK signaling. Depletion of ECE prevents this dissociation, enhances ERK signaling, and appears to effectively trap these GPCRs in the early endosome membrane. Together these results suggest that the association/dissociation kinetics of arrestins with mammalian GPCRs are regulated by multiple mechanisms, including ubiquitination of arrestins, with potentially myriad trafficking and signaling consequences.
2. Ubiquitination and ESCRT-independent trafficking of GPCRs to lysosomes
Unlike the PAR2 that exhibits ubiquitination-dependent downregulation, the closely related protease activated GPCR, PAR1, is rapidly deubiquitinated following agonist activation (as discussed above). Further, mutation of all cytoplasmic lysine residues in this mammalian GPCR does not inhibit downregulation of PAR1 (35). Nevertheless, PAR1 is efficiently downregulated via lysosomal proteolysis, but this GPCR appears to utilize a completely different mechanism to do so. Trejo and coworkers showed that downregulation of PAR1 is unaffected by depletion of the ESCRT components HRS or Tsg101 (94) and instead, requires sorting nexin 1 (SNX1). SNX1 localizes to endosomes via PX domain-dependent binding to PtdIns3P, and concentrates via its BAR domain to highly curved or tubular regions. The PAR1 co-immunoprecipitates with SNX1 following receptor activation, and depletion of cellular SNX1 inhibits lysosomal downregulation of this GPCR. The mechanism by which SNX1 promotes PAR1 down-regulation remains unclear but appears to be independent of the ESCRT, and also does not require the ‘retromer’ endosome-to-trans Golgi recycling complex in which SNX1 was shown previously to function (95).
3. Additional role(s) of ubiquitination on controlling ESCRT -dependent downregulation
Downregulation of the DOR is important for physiological regulation of opioid responsiveness (96) and, as discussed above, this process is mediated primarily by endocytic trafficking of receptors to lysosomes. Remarkably the DOR, unlike a number of other mammalian GPCRs already discussed, was found to downregulate with nearly wild type kinetics when its ubiquitination was prevented by lysyl mutation (33). Nevertheless, downregulation of both wild-type and ubiquitination-defective (lysyl mutant) DORs is ESCRT-dependent (71). Another mammalian GPCR, the CLR complexed with its accessory protein RAMP1, is not detectably ubiquitinated in its wild type form yet also traffics to the lysosome after endocytosis, and downregulation of both proteins requires functional ESCRT machinery (61, 97). Interestingly, even though ubiquitin-defective DORs can traffic efficiently to lysosomes after regulated endocytosis, the wild type DOR is extensively ubiquitinated in intact cells and its ubiquitination is increased following agonist activation. AIP4, the same ubiquitin ligase promoting downregulation of CXCR4, was identified as a major E3 ligase contributing to ubiquitination of the DOR in intact cells. In contrast to the canonical sorting model, however, AIP4-mediated ubiquitination of the DOR was found to specifically promote later proteolytic events. This function of AIP4-dependent ubiquitination occurs effectively downstream of GPCR delivery to lysosomes, and the occurrence of extensive proteolytic fragmentation that likely renders the receptor nonfunctional (98). This suggests that ubiquitination of some mammalian GPCRs is not necessary to sort receptors into the ESCRT-dependent downregulation pathway, or to effectively destroy them, but can mediate a discrete regulatory function at a later stage of proteolytic processing.
Besides revealing a specific, later consequence of GPCR ubiquitination in the down-regulation pathway, studies of DOR (and CLR) beg the question of how any GPCR can engage the ESCRT pathway absent ubiquitination. This has motivated the search for additional proteins that interact with mammalian GPCRs and influence their endocytic sorting. The first evidence for the existence of such proteins, collectively named GPCR-associating sorting proteins (GASPs or GPRASPs), came from studies of the DOR. GASP1 was identified in a yeast 2-hybrid screen searching for interacting partners with the C-terminal tail of the DOR. GASP1 is a cytoplasmic protein that is broadly expressed in mammalian tissues, and highly in brain, but it is not conserved in yeast. Studies in cultured cells linked GASP1 specifically to downregulation of the DOR, and found no effect of this protein on ubiquitin-directed downregulation of the EGFR (99). GASP1 represents a family of broadly expressed cytoplasmic proteins (100) that share extensive homology in regions mediating binding to the C-terminal tails of a number of GPCRs (101). Further, to a first approximation, GASPs bind preferentially to GPCRs that have a higher propensity to downregulate rather than recycle (102). Besides the DOR, GASP1 has now been implicated in downregulation of the D2R, CB1R, B1R and the US28 viral chemokine receptor (103-106). GASP1 knockout mice have been generated, and these animals have been shown to have reduced tolerance to cannabinoid agonists and also reduced sensitization to cocaine; these are behavioral phenotypes consistent with defective downregulation of the CB1 and D2 GPCRs, respectively (107-109). How GASPs influence receptor trafficking to the lysosome is currently unknown. One possibility is via binding to dysbindin, which is an essential component of the BLOC-1 complex functioning in the biogenesis of specialized lysosome-related organelles (LROs) such as melanosomes (110). Dysbindin is not conserved in yeast but is expressed ubiquitously in mammalian tissues, including in cell types not known to produce LROs. Depletion of cellular dysbindin was found to inhibit downregulation of both the DOR and dopamine D2R (111), and neurons cultured from homozygous dysbindin mutant (Sandy) mice exhibit increased surface levels of D2Rs attributed to enhanced recycling (112). Interestingly, dysbindin was found to co-immunoprecipitate from cell extracts with both HRS and GASP (111). This has motivated a current hypothesis that GASPs function as part of an alternate connectivity network linking particular GPCRs to the ESCRT, thereby providing an additional means for conferring diversity and specificity on the endocytic regulation of GPCRs in mammalian (and likely other metazoan) cells.
Closing Thoughts and Future Directions
It is clear that the ubiquitination of GPCRs, and subsequent interaction with the ESCRT, plays a fundamental role in the endocytic trafficking in both yeast and mammalian cells. Mammalian cells express a larger diversity of GPCRs, for which selective regulation is essential to normal development and tissue physiology. It is therefore important to define precisely how specificity is encoded by GPCR ubiquitination. While a single ubiquitin fusion is sufficient to drive endocytosis and vacuolar targeting of yeast GPCRs, there appears to be considerably more specificity encoded by ubiquitination of GPCRs in mammalian cells. Such complexity is not restricted to GPCRs, as illustrated in elegant recent studies showing distinct effects of ubiquitin branching patterns on trafficking of the MHC class 1 receptor complex (15). The large number of ligases and DUBs expressed in mammalian cells points to considerable potential for specificity and regulation at multiple stages of the endocytic pathway. Accordingly, one important future direction is to better define the various trafficking functions of GPCR ubiquitination in mammalian cells and, related to this, to determine how discrete functions are differentiated biochemically or spatially.
Another important future direction is to determine how DUBs control GPCR endocytic trafficking. Mutation of the yeast DUB, Doa4, inhibits receptor downregulation of Ste2p but this phenotype can be rescued by over-expression of ubiquitin, suggesting that the main function of this DUB is to prevent cellular depletion of free ubiquitin (113, 114). DUBs clearly have additional functions in the regulated endocytic membrane trafficking of signaling receptors in mammalian cells. This was first demonstrated for the EGF receptor, where two endosome associated DUBs have been implicated. The use of RNAi to deplete levels of one of these enzymes, UBPY, inhibited EGFR downregulation, reminiscent of the Doa4 phenotype in yeast. Depletion of another endosome-associated DUB, AMSH, in contrast, increased EGFR downregulation (115-117). Both UBPY and AMSH are required for efficient downregulation of the DOR and PAR2 mammalian GPCRs but these functions are not redundant, as might be expected if their roles were simply to prevent depletion of free ubiquitin (98, 118). Conversely, depletion of both USP33 and USP20 was shown to increase downregulation of the β2AR, suggesting a specific role of these DUBs in regulating recycling of this GPCR, in a manner similar to that described for AMSH on the EGFR (119).
A third direction, which is presently almost completely unexplored, is determining the significance of specific GPCR ubiquitination reactions to integrative mammalian physiology. The functional importance of both GPCR ubiquitination and endocytic trafficking is well established in cell culture models, but little is known about physiological consequences at the whole-animal level. There is already compelling evidence that GPCR endocytosis has significant in vivo effects, based on study of a few mouse models (107, 109, 120-122). However, to our knowledge, the physiological consequences of particular GPCR ubiquitination / de-ubiquitination reactions influencing receptor traffic remain essentially undefined in intact animals. This is clearly an important avenue for future study, and represents an exciting frontier for both physiological and membrane trafficking research. Further, considering the high level of diversity and specificity that is already evident from cell-based studies of ubiquitin-dependent regulation of mammalian GPCRs, we speculate that particular GPCR ubiquitination / de-ubiquitination reactions could represent promising new targets for therapeutic drug development.
Acknowledgments
We thank present and former members of the von Zastrow laboratory, and many colleagues in other laboratories for valuable contributions of published data as discussed in the present review, and for generously sharing unpublished results, ideas and criticism that have guided our thinking. We particularly thank Ms. Anastasia Henry for helpful advice and critical comments on the manuscript. Work in the von Zastrow laboratory is supported by the U.S. National Institutes of Health. JNH received support from the American Heart Association.
Abbreviations
- AchR
Acetylcholine Receptor
- AIP4
Atrophin Interacting Protein 4
- ARRDC
Arrestin Domain Containing Protein
- β1AR
beta 1 adrenergic receptor
- β2AR
beta 2 adrenergic receptor
- B1R
Bradykinin 1 receptor
- CB1
Cannabinoid Receptor 1
- CLR
Calcitonin receptor-like Receptor
- CXCR4
CXC chemokine receptor 4
- D2R
dopamine 2 Receptor
- DOR
δ-opioid receptor
- Dub
Deubiquitinating Enzyme
- ECE
Endothelin converting Enzyme
- ESCRT
Endosomal Sorting Complex Required for Transport
- EGFR
Epidermal Growth Factor Receptor
- ERK
Extracellular Regulated-Signal Kinase
- GASP
GPCR Associated Sorting Protein
- GPCR
G-protein coupled receptor
- GRK
G-protein receptor kinase
- ILV
Intralumenal Vesicle
- KOR
κ-opioid receptor
- MAPK
Mitogen Activated Protein Kinase
- MOR
μ-opioid receptor
- MVB
Multivesicular Body
- NK1R
neurokinin 1 receptor
- PAR
proteolytically activated receptor
- Snx27
Sorting Nexin 27
- USP
Ubiquitin Specific Protease
- V2R
vasopressin 2 receptor
References
- 1.Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1(8):761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB, Hokfelt T, Milligan G. G-protein-coupled receptors: an update. Acta Physiol (Oxf) 2007;190(1):3–7. doi: 10.1111/j.1365-201X.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- 3.Brzostowski JA, Kimmel AR. Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem Sci. 2001;26(5):291–297. doi: 10.1016/s0968-0004(01)01804-7. [DOI] [PubMed] [Google Scholar]
- 4.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 5.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62(2):305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106(42):17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53(1):1–24. [PubMed] [Google Scholar]
- 8.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 9.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 10.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309(5731):127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 11.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2(3):195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 12.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10(6):398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 13.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86(2):669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 14.Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16(11):551–559. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Boname JM, Thomas M, Stagg HR, Xu P, Peng J, Lehner PJ. Efficient internalization of MHC I requires lysine-11 and lysine-63 mixed linkage polyubiquitin chains. Traffic. 2010;11(2):210–220. doi: 10.1111/j.1600-0854.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84(2):277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 17.Shih SC, Sloper-Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. Embo J. 2000;19(2):187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth AF, Davis NG. Ubiquitination of the PEST-like endocytosis signal of the yeast a-factor receptor. J Biol Chem. 2000;275(11):8143–8153. doi: 10.1074/jbc.275.11.8143. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Davis NG. Ubiquitin-independent entry into the yeast recycling pathway. Traffic. 2002;3(2):110–123. doi: 10.1034/j.1600-0854.2002.030204.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsao PI, von Zastrow M. Diversity and specificity in the regulated endocytic membrane trafficking of G-protein-coupled receptors. Pharmacol Ther. 2001;89(2):139–147. doi: 10.1016/s0163-7258(00)00107-8. [DOI] [PubMed] [Google Scholar]
- 21.Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. Journal of Cell Biology. 1998;141(2):349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toshima JY, Nakanishi J, Mizuno K, Toshima J, Drubin DG. Requirements for recruitment of a G protein-coupled receptor to clathrin-coated pits in budding yeast. Mol Biol Cell. 2009;20(24):5039–5050. doi: 10.1091/mbc.E09-07-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2002;4(5):389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 24.Dores MR, Schnell JD, Maldonado-Baez L, Wendland B, Hicke L. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic. 2010;11(1):151–160. doi: 10.1111/j.1600-0854.2009.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobin AB, Totty NF, Sterlin AE, Nahorski SR. Stimulus-dependent phosphorylation of G-protein-coupled receptors by casein kinase 1alpha. J Biol Chem. 1997;272(33):20844–20849. doi: 10.1074/jbc.272.33.20844. [DOI] [PubMed] [Google Scholar]
- 26.Torrecilla I, Spragg EJ, Poulin B, McWilliams PJ, Mistry SC, Blaukat A, Tobin AB. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J Cell Biol. 2007;177(1):127–137. doi: 10.1083/jcb.200610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanyaloglu AC, Vrecl M, Kroeger KM, Miles LE, Qian H, Thomas WG, Eidne KA. Casein kinase II sites in the intracellular C-terminal domain of the thyrotropin-releasing hormone receptor and chimeric gonadotropin-releasing hormone receptors contribute to beta-arrestin-dependent internalization. J Biol Chem. 2001;276(21):18066–18074. doi: 10.1074/jbc.M009275200. [DOI] [PubMed] [Google Scholar]
- 28.Goodman OJ, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 29.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A. 1999;96(7):3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaidarov I, Keen JH. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146(4):755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294(5545):1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 32.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. Embo J. 2005;24(13):2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanowitz M, von Zastrow M. Ubiquitination-independent Trafficking of G Protein-coupled Receptors to Lysosomes. J Biol Chem. 2002;277(52):50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- 34.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, Grady EF, Bunnett NW. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem. 2006;281(38):27773–27783. doi: 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe BL, Marchese A, Trejo J. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J Cell Biol. 2007;177(5):905–916. doi: 10.1083/jcb.200610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282(40):29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3(12):893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 38.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 39.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8(5):355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 40.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol. 2010 doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 42.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 43.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106(2):145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 44.Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4(5):394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 45.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136(1):97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458(7235):172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276(49):45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 49.Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280(16):16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- 50.Liang W, Fishman PH. Resistance of the human beta1-adrenergic receptor to agonist-induced ubiquitination: a mechanism for impaired receptor degradation. J Biol Chem. 2004;279(45):46882–46889. doi: 10.1074/jbc.M406501200. [DOI] [PubMed] [Google Scholar]
- 51.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401(6750):286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 52.Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276(48):44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- 53.Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Current Opinion in Cell Biology. 1999;11(1):109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- 54.Lauffer BE, Chen S, Melero C, Kortemme T, von Zastrow M, Vargas GA. Engineered Protein Connectivity to Actin Mimics PDZ-dependent Recycling of G Protein-coupled Receptors but Not Its Regulation by Hrs. J Biol Chem. 2009;284(4):2448–2458. doi: 10.1074/jbc.M806370200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190(4):565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner LA, Naren AP, Bahouth SW. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2007;282(7):5085–5099. doi: 10.1074/jbc.M608871200. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto Y, Morisawa K, Saito H, Jojima E, Yoshida N, Haga T. Muscarinic M4 receptor recycling requires a motif in the third intracellular loop. J Pharmacol Exp Ther. 2008;325(3):947–953. doi: 10.1124/jpet.107.135095. [DOI] [PubMed] [Google Scholar]
- 58.Lahuna O, Quellari M, Achard C, Nola S, Meduri G, Navarro C, Vitale N, Borg JP, Misrahi M. Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. Embo J. 2005;24(7):1364–1374. doi: 10.1038/sj.emboj.7600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li JG, Chen C, Liu-Chen LY. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J Biol Chem. 2002;277(30):27545–27552. doi: 10.1074/jbc.M200058200. [DOI] [PubMed] [Google Scholar]
- 60.Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278(46):45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- 61.Hasdemir B, Bunnett NW, Cottrell GS. Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) mediates post-endocytic trafficking of protease-activated receptor 2 and calcitonin receptor-like receptor. J Biol Chem. 2007;282(40):29646–29657. doi: 10.1074/jbc.M702974200. [DOI] [PubMed] [Google Scholar]
- 62.Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131(3):603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95(6):847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- 64.Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1(2):193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 65.Obin MS, Jahngen-Hodge J, Nowell T, Taylor A. Ubiquitinylation and ubiquitin-dependent proteolysis in vertebrate photoreceptors (rod outer segments). Evidence for ubiquitinylation of Gt and rhodopsin. J Biol Chem. 1996;271(24):14473–14484. doi: 10.1074/jbc.271.24.14473. [DOI] [PubMed] [Google Scholar]
- 66.Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome involvement in agonist-induced down-regulation of mu and delta opioid receptors. J Biol Chem. 2001;276(15):12345–12355. doi: 10.1074/jbc.M008054200. [DOI] [PubMed] [Google Scholar]
- 67.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276(6):4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 68.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5(5):709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 69.Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J Biol Chem. 2003;278(46):45954–45959. doi: 10.1074/jbc.M308285200. [DOI] [PubMed] [Google Scholar]
- 70.Li JG, Haines DS, Liu-Chen LY. Agonist-promoted Lys63-linked polyubiquitination of the human kappa-opioid receptor is involved in receptor down-regulation. Mol Pharmacol. 2008;73(4):1319–1330. doi: 10.1124/mol.107.042846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hislop JN, Marley A, Von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J Biol Chem. 2004;279(21):22522–22531. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- 72.Volpicelli LA, Lah JJ, Levey AI. Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies. J Biol Chem. 2001;276(50):47590–47598. doi: 10.1074/jbc.M106535200. [DOI] [PubMed] [Google Scholar]
- 73.Slagsvold T, Marchese A, Brech A, Stenmark H. CISK attenuates degradation of the chemokine receptor CXCR4 via the ubiquitin ligase AIP4. Embo J. 2006;25(16):3738–3749. doi: 10.1038/sj.emboj.7601267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3(3):218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- 75.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem. 2008;283(32):22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12(23):3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupre DJ, Chen Z, Le Gouill C, Theriault C, Parent JL, Rola-Pleszczynski M, Stankova J. Trafficking, ubiquitination, and down-regulation of the human platelet-activating factor receptor. J Biol Chem. 2003;278(48):48228–48235. doi: 10.1074/jbc.M304082200. [DOI] [PubMed] [Google Scholar]
- 78.Bhandari D, Robia SL, Marchese A. The E3 ubiquitin ligase atrophin interacting protein 4 binds directly to the chemokine receptor CXCR4 via a novel WW domain-mediated interaction. Mol Biol Cell. 2009;20(5):1324–1339. doi: 10.1091/mbc.E08-03-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282(51):36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- 80.Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21(14):2529–2541. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10(12):1856–1867. doi: 10.1111/j.1600-0854.2009.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9(12):1216–1221. doi: 10.1038/embor.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135(4):714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 84.Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. s. 2010 doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrador A, Herranz S, Lara D, Vincent O. Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol. 30(4):897–907. doi: 10.1128/MCB.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275(22):17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 87.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276(22):19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 88.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci U S A. 2009;106(16):6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278(16):14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 90.Shenoy SK, Lefkowitz RJ. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem. 2005;280(15):15315–15324. doi: 10.1074/jbc.M412418200. [DOI] [PubMed] [Google Scholar]
- 91.Mosser VA, Jones KT, Hoffman KM, McCarty NA, Jackson DA. Differential role of beta-arrestin ubiquitination in agonist-promoted down-regulation of M1 vs M2 muscarinic acetylcholine receptors. J Mol Signal. 2008;3:20. doi: 10.1186/1750-2187-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roosterman D, Cottrell GS, Padilla BE, Muller L, Eckman CB, Bunnett NW, Steinhoff M. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc Natl Acad Sci U S A. 2007;104(28):11838–11843. doi: 10.1073/pnas.0701910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Padilla BE, Cottrell GS, Roosterman D, Pikios S, Muller L, Steinhoff M, Bunnett NW. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J Cell Biol. 2007;179(5):981–997. doi: 10.1083/jcb.200704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell. 2006;17(3):1228–1238. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Zhou Y, Szabo K, Haft CR, Trejo J. Down-Regulation of Protease-activated Receptor-1 Is Regulated by Sorting Nexin 1. Mol Biol Cell. 2002;13(6):1965–1976. doi: 10.1091/mbc.E01-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103(25):9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem. 2007;282(16):12260–12271. doi: 10.1074/jbc.M606338200. [DOI] [PubMed] [Google Scholar]
- 98.Hislop JN, Henry AG, Marchese A, von Zastrow M. Ubiquitination regulates proteolytic processing of G protein-coupled receptors after their sorting to lysosomes. J Biol Chem. 2009;284(29):19361–19370. doi: 10.1074/jbc.M109.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297(5581):615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 100.Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor associated sorting proteins. J Neurochem. 2004;89(3):766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 101.Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279(52):54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 102.Thompson D, Pusch M, Whistler JL. Changes in G protein-coupled receptor sorting protein affinity regulate postendocytic targeting of G protein-coupled receptors. J Biol Chem. 2007;282(40):29178–29185. doi: 10.1074/jbc.M704014200. [DOI] [PubMed] [Google Scholar]
- 103.Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, Whistler JL. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A. 2005;102(32):11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Enquist J, Skroder C, Whistler JL, Leeb-Lundberg LM. Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol Pharmacol. 2007;71(2):494–507. doi: 10.1124/mol.106.030858. [DOI] [PubMed] [Google Scholar]
- 105.Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. Faseb J. 2007;21(3):802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 106.Tschische P, Moser E, Thompson D, Vischer HF, Parzmair GP, Pommer V, Platzer W, Schwarzbraun T, Schaider H, Smit MJ, Martini L, Whistler JL, Waldhoer M. The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28. Traffic. 2010;11(5):660–674. doi: 10.1111/j.1600-0854.2010.1045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martini L, Thompson D, Kharazia V, Whistler JL. Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology. 2010;35(6):1363–1373. doi: 10.1038/npp.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boeuf J, Trigo JM, Moreau PH, Lecourtier L, Vogel E, Cassel JC, Mathis C, Klosen P, Maldonado R, Simonin F. Attenuated behavioural responses to acute and chronic cocaine in GASP-1-deficient mice. Eur J Neurosci. 2009;30(5):860–868. doi: 10.1111/j.1460-9568.2009.06865.x. [DOI] [PubMed] [Google Scholar]
- 109.Thompson D, Martini L, Whistler JL. Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PLoS One. 2010;5(6):e11038. doi: 10.1371/journal.pone.0011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dell’Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol. 2004;16(4):458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PLoS One. 2010;5(2):e9325. doi: 10.1371/journal.pone.0009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, Lu B. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A. 2009;106(46):19593–19598. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dupre S, Haguenauer-Tsapis R. Deubiquitination step in the endocytic pathway of yeast plasma membrane proteins: crucial role of Doa4p ubiquitin isopeptidase. Mol Cell Biol. 2001;21(14):4482–4494. doi: 10.1128/MCB.21.14.4482-4494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swaminathan S, Amerik AY, Hochstrasser M. The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol Biol Cell. 1999;10(8):2583–2594. doi: 10.1091/mbc.10.8.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCullough J, Row PE, Lorenzo O, Doherty M, Beynon R, Clague MJ, Urbe S. Activation of the endosome-associated ubiquitin isopeptidase AMSH by STAM, a component of the multivesicular body-sorting machinery. Curr Biol. 2006;16(2):160–165. 116. doi: 10.1016/j.cub.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 116.McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166(4):487–492. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Row PE, Clague MJ, Urbe S. Growth factors induce differential phosphorylation profiles of the Hrs-STAM complex: a common node in signalling networks with signal-specific properties. Biochem J. 2005;389(Pt 3):629–636. doi: 10.1042/BJ20050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hasdemir B, Murphy JE, Cottrell GS, Bunnett NW. Endosomal deubiquitinating enzymes control ubiquitination and down-regulation of protease-activated receptor 2. J Biol Chem. 2009;284(41):28453–28466. doi: 10.1074/jbc.M109.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. Embo J. 2009;28(12):1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Filipeanu CM, Zhou F, Lam ML, Kerut KE, Claycomb WC, Wu G. Enhancement of the recycling and activation of beta-adrenergic receptor by Rab4 GTPase in cardiac myocytes. J Biol Chem. 2006;281(16):11097–11103. doi: 10.1074/jbc.M511460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW., 2nd Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci U S A. 2004;101(18):7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-Induced Receptor Endocytosis in a Novel Knockin Mouse Reduces Tolerance and Dependence. Curr Biol. 2008;18(2):129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]