Abstract
Oxidative stress and endoplasmic reticulum (ER) stress are emerging as crucial events in the etiopathology of many neurodegenerative diseases. While the neuroprotective contributions of the dietary compound curcumin has been recognized, the molecular mechanisms underlying curcumin's neuroprotection under oxidative and ER stresses remains elusive. Herein, we show that curcumin protects HT22 from oxidative and ER stresses evoked by the hypoxia (1% O2 or CoCl2 treatment) by enhancing peroxiredoxin 6 (Prdx6) expression. Cells exposed to CoCl2 displayed reduced expression of Prdx6 with higher reactive oxygen species (ROS) expression and activation of NF-κB with IκB phosphorylation. When NF-κB activity was blocked by using SN50, an inhibitor of NF-κB, or cells treated with curcumin, the repression of Prdx6 expression was restored, suggesting the involvement of NF-κB in modulating Prdx6 expression. These cells were enriched with an accumulation of ER stress proteins, C/EBP homologous protein (CHOP), GRP/78, and calreticulin, and had activated states of caspases 12, 9, and 3. Reinforced expression of Prdx6 in HT22 cells by curcumin reestablished survival signaling by reducing propagation of ROS and blunting ER stress signaling. Intriguingly, knockdown of Prdx6 by antisense revealed that loss of Prdx6 contributed to cell death by sustaining enhanced levels of ER stress-responsive proapoptotic proteins, which was due to elevated ROS production, suggesting that Prdx6 deficiency is a cause of initiation of ROS-mediated ER stress-induced apoptosis. We propose that using curcumin to reinforce the naturally occurring Prdx6 expression and attenuate ROS-based ER stress and NF-κB-mediated aberrant signaling improves cell survival and may provide an avenue to treat and/or postpone diseases associated with ROS or ER stress.
Keywords: apoptosis, ER stress, peroxiredoxin 6, oxidative stress, antioxidant, curcumin
insults to neuronal cells induced by hypoxia-mediated oxidative stress lead to initiation and progression of many neurodegenerative disorders, including Alzheimer's and Parkinson's diseases (43, 67, 77). Recently, an inverse correlation between antioxidant levels and neuronal cell injury and malfunction has been reported (7, 24, 43, 82). However, the physiological role of antioxidants depends on their levels of expression and activity in cells. Overproduction of reactive oxygen species (ROS) has been shown to be associated with a decline in the antioxidant defense system during aging and/or in cell facing environmental or cellular stress that leads to failure of cellular homeostasis (15, 25, 28, 91). Furthermore, several studies have demonstrated that hypoxia or its mimic cobalt chloride (CoCl2) can induce ROS expression-dependent apoptosis in many cells such as PC12 (19), rat C6 glioma cells (99), and retinal ganglion cells (24, 93). This injurious process is accelerated during aging due to reduced expression of antioxidants (21, 25, 55, 79, 80, 92, 94, 100). We have shown that reduced expression or deficiency of antioxidants such as peroxiredoxin 6 (Prdx6) can lead to uncontrolled overproduction of ROS and lipid peroxidation (LPO), resulting in spontaneous apoptosis and dysregulation of survival signaling (11, 15). Similarly, hypoxia-evoked, ROS-induced oxidative stress is a known cause of LPO, protein, and DNA oxidation, which contribute to neurodegeneration (60, 76, 93).
Moreover, physiological levels of ROS are maintained by cellular levels of antioxidant expression and activities (24, 25, 28). We previously reported that Prdx6 is essential for cellular protection and cell survival (15, 28). Prdx6, a 1-Cys Prdx, is a bifunctional protein that acts both as glutathione peroxidase and calcium-independent phospholipase A2 (aiPLA2; Refs. 25, 29, 62, 63, 95). The mammalian Prdxs family is composed of six members, Prdx1–6. Prdxs 1–5 have two catalytically active cysteines, while Prdx6 is the sole 1-Cys member (15, 25, 27, 48, 63, 95, 97). Prdxs function together to detoxify ROS and thus provide cytoprotection from internal and external environmental stress (28, 29, 62). The protein Prdx6 is different from other members of the Prdx family bearing aiPLA2 activity (14). Recently, the role of Prdx6 in various forms of cancer has been documented (13, 38, 40, 53, 72, 74). In addition, Prdx6 is known to protect both mouse and human lens epithelial cells against various oxidative stresses, such as H2O2, Paraquat, UVB, heat stress, and endoplasmic reticulum (ER) stress (15, 24, 28). Furthermore, Prdx6 is a potent cytoprotective enzyme in the epidermis (51) and is required for blood vessel integrity in wounded skin (52). Prdxs are distributed in the normal mammalian brain (33). Aon-Bertokino et al. (3) reported a relatively intense Prdx6 staining in the hippocampus of the rat brain (42). The defensive role of Prdx6 has been shown in neurodegenerative disorders (33, 75, 79). The physiological expression of Prdx6 appears to be critical for maintaining cellular integrity and cellular protection within the cellular microenvironment, and these qualities make it an ideal molecule for therapeutic uses. However, despite its recognized functions, little is known about how Prdx6 performs its protective role in neuronal cells. Based on our previous work (15, 28), we believe that Prdx6 may inhibit ROS-based aberrant ER stress signaling in such cells.
In HT22 neuronal cell line, hypoxia has been reported to induce apoptosis as evidenced by DNA fragmentation and up- and downregulation of Bax, a proapoptotic molecule (36). It is not fully investigated whether ER stress-induced signaling was overstimulated and turned to deleterious signaling in cells having reduced level of Prdx6. Recently, considerable evidence indicates that increased ROS production induced by hypoxia or the hypoxia mimic CoCl2-mediated oxidative stress causes ER stress in various cell lines (19, 28, 93, 99). ROS-based oxidative stress-induced apoptotic cell death has been implicated in neuronal cell death (16, 53). Oxidative stress is an inducer of ER stress that activates specific signaling, wherein C/EBP homologous protein (CHOP) participates in ER stress-mediated apoptosis by suppressing Bcl-2 activation (35). We have shown that Prdx6 deficiency initiates ROS-induced ER stress that leads to cell death. We also found repression of Prdx6 in lens cells, one cause of ER stress because of accumulation of ROS (28). However, alterations in cellular environment have been shown to be associated with the modulation in the activity of several transcription factors including NF-κB (78, 83, 88). On the basis of our previous work as well as other published reports (26, 31) showing that NF-κB is regulator of Prdx6 transcription, it seems reasonable that ROS induced by hypoxic stress at certain levels could work as a biosignal and thereby alter the activity of NF-κB and influence Prdx6 expression in cell. Furthermore, in normal physiological condition, NF-κB is inactive (85, 86). Moreover, upon cell stimulation, IκB become phosphorylated and release NF-κB and translocate into nucleus and modulate its target genes. In present study we have found that NF-κB is activated in cells facing hypoxia. However, the proapoptotic and antiapoptotic roles of NF-κB depend on cell types and cellular microenvironment as well as levels of intracellular ROS (69). Moreover, curcumin, a biologically active component of turmeric (Curcuma longa), has a variety of anticarcinogenic, antibacterial, antifungal, antiviral, anti-inflammatory, antiproliferative, and antiapoptotic effects (56, 65, 71, 73). Curcumin is associated with regulation of several antioxidant genes by restoring their activity and expression levels (1, 12, 15, 61). Curcumin is known to enhance the cellular antioxidant defense system using different pathways including NF-κB and thereby protect cells against oxidative stress (71). We think that curcumin may enhance the Prdx6 expression in neuronal cells by optimizing activity of NF-кB, a regulator of Prdx6 gene during stress.
The study presented here was designed to characterize and document cell damage induced by CoCl2- and ROS-based ER stress and to test the ability of curcumin in enhancing Prdx6 expression and reducing death signaling produced by hypoxic stress. We discovered a previously unrecognized regulation and function for Prdx6, as an essential molecule protecting mouse hippocampal neuronal cells, HT22, by using curcumin to combat ROS-mediated ER stress-based cell death during hypoxia. We showed that ROS-evoked aberrant signaling of ER stress was initiated due to repression of Prdx6 expression, which was associated with NF-κB's repressive activity during hypoxia. Prdx6 knockdown experiments in HT22 cells confirmed that Prdx6 deficiency was a cause for initiation of ROS-evoked aberrant ER signaling, and the process was accelerated during hypoxia. Interestingly, HT22 cells pretreated with curcumin, when faced with hypoxia, showed elevated expression of Prdx6 with beneficial activity of NF-κB. That was correlated with reduced expression of ROS and attenuation or deceleration of ER stress-specific apoptotic signaling. Collectively, these findings should contribute to a basis for rational use of dietary supplements to induce expression of natural antioxidant defense molecules such as Prdx6. Such “inductive therapy” might be used to treat or prevent/delay neurodegenerative diseases, while avoiding difficulties related to DNA/protein delivery to cells and tissues.
MATERIALS AND METHODS
Cell culture.
Hippocampal cells (HT22) were purchased from ATCC maintained in DMEM with 10% FBS, 100 μg/ml streptomycin, and 100 μg/ml penicillin in 5% CO2 environment at 37°C as described previously (25). Cells were harvested and cultured in 96-, 24-, 48-, or 6-well plates and 60- or 100-mm petri dishes according to the requirement of the experiment. To examine the effect of curcumin, cells were treated with different concentrations (1, 2, or 5 μM in complete medium) of curcumin for variable time intervals. A stock solution of curcumin (10 mM) was prepared in DMSO and diluted in culture medium keeping the final DMSO concentration at <0.05%, and the same concentration of DMSO was used as vehicle. Curcumin (catalog no. C7727) was purchased from Sigma-Aldrich.
Generation of hypoxic stress (1% O2 or CoCl2).
Cells were cultured in 96-well plates or 60- or 100-mm petri dishes according to the requirements of experiment. For each assay, HT22 cells were cultured in DMEM containing 10% FBS for 24 h. For hypoxic treatment, cells were either exposed to 1% O2 using hypoxic chamber or treated with the hypoxia mimicking agent cobalt chloride (CoCl2) for different concentration and time interval as described previously (28). Tunicamycin, a known ER stress inducer, was used as positive control.
Cell survival assay (MTS assay).
A colorimetric MTS assay (Promega, Madison, MI) was performed as described earlier (15). Briefly, 1 × 104 cells were cultured in 48-well plate and pretreated with curcumin (2 μM), and after 12 h, cells were exposed to O2 (1% ) or CoCl2 (100 and 200 μM) for 24, 48, or 72 h. This assay of cellular proliferation uses 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2 to 4-sulfophenyl-2H-tetrazolium salt (Promega). Upon being added to medium containing viable cells, MTS is reduced to a water-soluble formazan salt. The A490 nm value was measured after 4 h with an ELISA reader.
Assay for intracellular redox state.
Intracellular redox state levels were measured using the fluorescent dye H2-DCF-DA as described earlier (15). Cells (5 × 103) were cultured in 96-well plates and pretreated with curcumin, and after 12 h cells were subjected to O2 (1%) or CoCl2 (100 and 200 μM) for 24, 48, and 72 h. Cells were washed once with HBSS and incubated in the same buffer containing 10 μM of H2-DCF-DA. It is a nonpolar compound that is converted into a polar derivative (dichlorofluorescein) by cellular esterase following incorporation into cells. After 30 min of incubation at room temperature, intracellular fluorescence was detected with excitation at 485 nm and emission at 530 nm (ex 485/em 530) using Spectra Max Gemini EM (Molecular Devices, CA).
LPO assay.
LPO assay was carried out according to the manufacture's protocol (Lipid Peroxidation Microplate Assay Kit; Oxford Biomedical Research, MI) and our published study (15). The assay is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole (R1), with malondialdehyde (MDA) and 4-hydroxyalkenals at 45°C. One molecule of either MDA or 4-hydroxyalkenal reacts with two molecules of reagent R1 to yield a stable chromophore with maximal absorbance at 586 nm. Briefly, HT22 (7 × 105) cells were seeded in 100-mm plates and treated with curcumin, and after 12 h of treatment cells were exposed with O2 (1%) or CoCl2 (200 μM) for 48 h. Cells washed twice with ice-cold PBS, and the total cell lysates were prepared as described previously (15). Equal amounts of protein were used for the assay. Optical density (OD) was measured at 586 nm.
Apoptosis assay.
Apoptosis and cell cycle assays were carried out using flow cytometry to quantify the levels of oxidative stress-induced apoptotic cells. The annexin V assay was performed using the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences). Briefly, HT22 (7 × 105) were seeded in 100-mm plates and treated with curcumin (2 μM) or 4-PBA, and after 12 h cells were exposed to O2 (1%) or cobalt chloride (100 or 200 μM) for 48 h. Cells were washed twice with ice-cold PBS solution and resuspended in 1 × 105/100 μl of binding buffer and incubated with 5 μl of Annexin V-FITC and 5 μl of propidium iodide (PI) for 15 min at room temperature in the dark. After 15 min of incubation, 400 μl of binding buffer were added to make 500-μl final volumes and flow cytometry was performed within 1 h.
Flow cytometry for cell cycle analysis.
HT22 cells were harvested in 100-mm plates and pretreated with curcumin for 12 h followed by CoCl2 treatment. After 48 h of CoCl2 treatment, cells were fixed with 5 ml of ice-cold 70% ethanol (4°C). Cell pellets were collected by centrifugation and resuspended in 400 μl of PBS, 50 μl of PI solution (0.6 mM), and 50 μl of RNaseA (1 mg/ml). After 30 min, cells were analyzed for DNA content using FACS flow cytometer. Fluorescence from the PI-DNA complex was estimated on a minimum of 20,000 cells per samples and analyzed with Cell Quest Pro Software.
Construction of Prdx6 antisense.
A human lens epithelial cell cDNA library was used to isolate Prdx6 cDNA having a full-length open reading frame. A full-length Prdx6 antisense (Prdx6-As) construct was made by subcloning Prdx6 cDNA into a pcDNA3.1/NT-GFP-TOPO vector in reverse orientation. Plasmid was amplified following TOP 10 bacterial cells transformation as described earlier (25, 28).
Protein expression assay.
Cytoplasmic and nuclear extracts were prepared in ice-cold RIPA lysis buffer as described previously (15, 28). Equal amounts of protein samples were loaded into a 7.5 or 10 or 4–20% SDS-gel, blotted onto polyvinylidene fluoride membrane (PerkinElmer Life Sciences), and immunostained with primary antibodies following suitable dilution: Prdx6 monoclonal antibody (Lab Frontier, Seoul, Korea), Bip (cat. no. 3183; Cell Signaling Technology), calreticulin polyclonal (cat. no. 2891; Cell Signaling Technology), CHOP (sc-7351; Santa Cruz Biotechnology), caspase 3 (cat. no. 9665; Cell Signaling Technology), caspase 9 (sc-7885; Santa Cruz Biotechnology), caspase 12 (cat. no. 2202, Cell Signaling Technology), Bax (sc-7480, Santa Cruz Biotechnology), Bcl2 (cat. no. 2876; Cell Signaling Tech), NF-кB/p65 (sc-7151, Santa Cruz Biotechnology), IкB rabbit polyclonal (sc-371, Santa Cruz Biotechnology), pIкB monoclonal (sc-8404; Santa Cruz; Biotechnology), tubulin monoclonal antibody (ab44928; Abcam), and β-actin rabbit polyclonal (Abcam). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,500 dilutions). Specific protein bands were visualized by incubating the membrane with luminol reagent (Santa Cruz Biotechnology) and exposing to film (X-Omat; Eastman Kodak) or recorded with a FUJIFILM-LAS-4000 luminescent image analyzer (FUJIFILM Medical Systems). To ascertain comparative expression and equal loading of the protein samples, the membrane stained earlier was stripped and reprobed with tubulin or β-actin antibody or above mentioned antibodies.
mRNA expression analysis (real-time PCR).
Total RNA was isolated using the single-step guanidine thiocyanate/phenol/chloroform extraction method (TRIzol reagent; Invitrogen) and converted to cDNA using Superscript II RNAase H-Reverse Transcriptase. Quantitative real-time PCR (qPCR) was performed with SYBR Green Master Mix (Roche Diagnostic, Indianapolis, IN) in a Roche LC480 Sequence detector system (Roche Diagnostic). PCR conditions consisted of 10-min hot start at 95°C, followed by 45 cycles of 10 s at 95°C, 30 s at 60°C, and 10 s at 72°C. Primer sequence was as follows: Prdx1 forward: 5′-GTGAGACCTGTGGCTCGAC-3′ and reverse: 5′-TGTCCATCTGGCATAACAGC-3′; Prdx2 Forward: 5′-GACGAGCATGGGGAAGTCT-3′ and reverse: 5′-TCCTTGCTGTCATCCACATT-3′; Prdx3 forward: 5′-GTGCCTCTTGCGTGCTCT-3′ and reverse: 5′-ACTTGCATGACGAGCAACC-3′; Prdx4 forward: 5′-TGACAAGCATGGAGAAGTCTG-3′ and reverse: 5′-CAGCTGGATCTGGGATTATTG-3′; Prdx5 forward: 5′-GATTGAAGAGTGGGGTCGAG-3′ and reverse: 5′-TCTGTCGCCTTCCCAAAG-3′; Prdx6 forward: 5′-TTTCAATAGACAGTGTTGAGGATCA-3′ and reverse: 5′-CGTGGGTGTTTCACCATTG-3′; NF-кB forward: 5′-TCCGGTTACGTAATGAGTGGT-3′ and reverse: 5′-GATCTGGTTCTCTTTCCGAAGTC-3′; and β-actin forward: 5′-CTAAGGCCAACCGTGAAAAG-3′ and reverse: 5′-ACCAGAGGCATACAGGGACA-3′. Expression levels of target genes were normalized to the levels of β-actin as an endogenous control in each group. The comparative Cp method was used to calculate relative fold expression levels using the Light Cycler 480 software release 1.5.0SP3. The Cps of target genes was normalized to the levels of β-actin as an endogenous control in each group.
Determination of NF-кB activation using HIV-1LTR-CAT.
HIV-1LTR-CAT constructs (a kind gift from Dr. Carole Kretz-Remy) were used to transfect cells. The HIV-1 promoter contains binding sites for many transcriptional factor NF-кB, and can be upregulated 12- to 150-fold following various stresses, including oxidative stress (26). We used pLTR-CAT WT and pLTR-CAT PstI (where NF-кB sites are disrupted) to monitor the activation of NF-кB. We transfected these constructs to the cells and treated with curcumin followed by CoCl2 exposure. Forty-eight hours later, CAT activity was monitored using CAT-ELISA as described earlier (26).
Transfection and CAT assay.
The CAT assay was performed using a CAT-ELISA kit (Roche Applied Science). HT22 were transfected/cotransfected using Superfectamine with Prdx6-CAT reporter constructs (4 μg), treated with different concentrations of curcumin or SN50, a cell-permeable inhibitory peptide of NF-κB translocation (cat. no. 481480; Calbiochem), and then exposed to CoCl2. After 48 h of incubation, cells were harvested, extracts were prepared, and protein was normalized. CAT-ELISA was performed to monitor CAT activity following the manufacturer's protocol. Absorbance was measured at 405 nm using a microtiter plate ELISA reader. Transactivation activities were adjusted for transfection efficiencies using GFP/SEAP values (25).
Statistical method.
For all quantitative data collected, statistical analysis was conducted by Student's t-test and is presented as means ± SD of the indicated number of experiments. A significant difference between control and treatment group was defined as *P < 0.05 and **P < 0.001 for three or more independent experiments.
RESULTS
Curcumin rescued HT22 cells by elevating Prdx6 expression and blunting ROS levels, apoptosis, and cell growth arrest affected by hypoxic stress, 1% O2, or cobalt chloride, a hypoxia-mimicking agent.
Based on our recent work indicating that pretreatment with curcumin activates Prdx6-dependent survival pathways (15) and protects lens epithelial cells, we undertook further examination of the role of curcumin/Prdx6 survival signaling in the murine hippocampal cell line HT22 in response to hypoxia-induced ROS signaling. We first determined effective noncytotoxic concentrations (0–5 μM) of curcumin and then assessed cell growth at different time points (24, 48, and 72 h). A concentration of 2 μM of curcumin appeared ideal, as this concentration produced no inhibition of cell growth; instead, growth was normal or mildly increased (Fig. 1, A and C) along with increased expression of Prdx6 protein (Fig. 1B). In contrast, cells treated with a 5-μM concentration showed growth inhibition (Fig. 1, A and C). Hence, the nontoxic 2-μM concentration of curcumin was used in all experiments unless otherwise indicated.
Fig. 1.
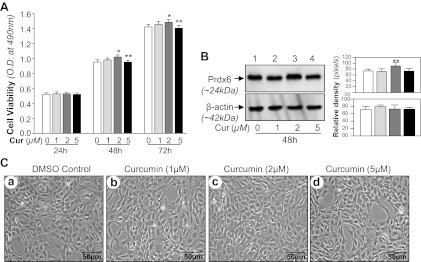
Effects of curcumin (Cur) on HT22 cells. A: MTS assay showing the effects of curcumin on viability of HT22 cells. HT22 cells were cultured in 48-well plate in DMEM containing 10% FBS. After 12 h, cells were washed and treated with different concentration of curcumin for different time periods to determine the nontoxic effect of curcumin on cells. B: HT22 cells were treated with DMSO vehicle or curcumin. After 48 h, total cell lysate was isolated and peroxiredoxin 6 (Prdx6) protein was measured through Western analysis. C: HT22 cells were treated with DMSO (a) or different concentrations of curcumin (b, c, and d) for 48 h. OD, optical density. Photomicrographs are representative of 3 independent experiments. Results are means ± SD of 3 individual experiments. *P < 0.05, **P < 0.001.
Next, to examine curcumin-induced Prdx6-dependent protection against hypoxic stress, we used hypoxic chamber for O2 (1%) or utilized cobalt chloride (CoCl2), a hypoxia-mimicking agent, to induce ROS-driven oxidative stress. Based on our earlier report (28, 93), we selected 1% O2 and optimal concentrations of CoCl2 in HT22 cells by using different concentrations of CoCl2 for different time intervals, assessing cell viability (1% O2 and CoCl2), Prdx6 expression, and ROS expression (1% O2 and CoCl2; data not shown). Data revealed that maximum 200 μM concentrations of CoCl2 can be used for the study. Moreover, we observed that cells exposed to lower concentrations of CoCl2 did not alter expression of protective protein Prdx6 and instead mildly increased Prdx6 expression, a finding consistent with previous reports showing that mild hypoxia is protective (34, 93). Furthermore, higher concentrations led to cell death in time-dependent fashion by increasing ROS production and reducing levels of antioxidant Prdx6 protein (data not shown).
We next examined whether curcumin treatment was able to rescue the HT22 cells from 1% O2- or CoCl2-induced cytotoxicity. Indeed, HT22 cells pretreated with curcumin showed resistance to hypoxia (1% O2 or CoCl2) -induced cell death. Figure 2, A and C, illustrates the time-dependent enhanced viability of curcumin-treated HT22 cells (Fig. 2, A and C, dark gray vs. black bars); these cells also displayed reduced expression of ROS (Fig. 2, B and D, dark gray vs. black bars) when exposed to O2 (1%) or CoCl2 (200 μM), respectively. Data were normalized with absorbance of untreated control(s).
Fig. 2.
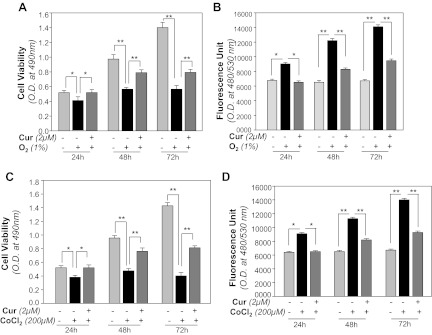
Curcumin protects HT22 cells against hypoxic stress, by optimizing reactive oxygen species (ROS) levels. A and C: curcumin enhanced HT22 cell survival against 1% O2- or CoCl2-induced cytotoxicity. Cells were treated with 2 μM of curcumin or DMSO (a control vehicle). After 12 h cells were submitted to O2 (1%) or CoCl2 (200 μM), and the effects on cell growth and viability were determined after 24, 48, and 72 h by MTS assay. Histogram is representative of means ± SD of 3 experiments. *P < 0.05; **P < 0.001. B and D: effect of curcumin on lowering the ROS expression. HT22 cells were treated with curcumin (2 μM) or with DMSO. Twelve hours later, these cells were exposed to O2 (1%) or CoCl2 (200 μM). ROS were quantified with H2-DCF-DA dye at 24, 48, and 72 h as described in materials and methods. Data represent means ± SD of 3 experiments.*P < 0.05; **P < 0.001.
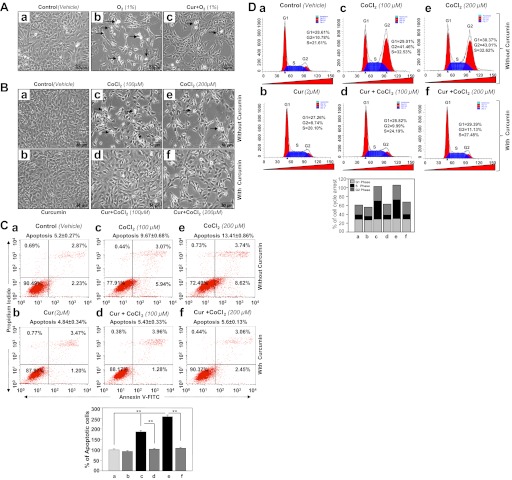
We also determined curcumin's ability to postpone hypoxia-induced apoptotic cell death or growth inhibition and cell cycle arrest. Cells treated with 2 μM of curcumin and untreated cells were submitted to hypoxic stress induced by O2 (1%) or CoCl2 (100 or 200 μM). After 48-h photomicrographs were taken (Fig. 3, A and B). Cells treated with 2 μM of curcumin were resistant to hypoxia-induced cell death as evidenced by lower cell density in untreated cells than in treated cells (Fig. 3, Aa vs. Ab and Ab vs. Ac; Fig. 3B, top, Ba, Bc, and Be vs. bottom, Bb, Bd, and Bf). To determine if these cells underwent apoptosis, CoCl2-exposed cells were processed for annexin V-FITC binding assay followed by FACS analysis (15, 28). As shown in Fig. 3C, the percentage of apoptotic cells was increased with an increase of CoCl2 concentration (Fig. 3, Ca, control; Cc, 100 μM; and Ce, 200 μM) while apoptosis was significantly reduced in cells treated with curcumin (Fig. 3C, Ca vs. Cb; Cc vs. Cd; Ce vs. Cf). These data demonstrate curcumin's ability to postpone CoCl2-induced cell injury as evidenced by histograms (dark gray vs. black bars). To examine whether curcumin affects cell cycle arrest exerted by CoCl2, HT22 cells were pretreated with curcumin followed by different concentrations of CoCl2. After 48 h, cells were stained with Telford reagent followed by FACS analysis (6, 15). We found a significant increase in cell arrest at S-phase following CoCl2 treatment (Fig. 3, Ca, 21.61% vs. Cc, 32.53%, and Ce, 32.63%) and at G2 phase (Fig. 3, Ca, 10.78% vs. Cc, 41.46%, and Ce, 43.01%). Interestingly, we found that curcumin treatment broke the cell arrests at the S phase (Fig. 3, Cc, 32.53% vs. Cd, 24.19% and Ce, 32.62% vs. Cf, 27.48%) as well as at the G2 phase (Fig. 3, Cc, 41.46% vs. Cd, 9.99% and Ce, 43.01% vs. Cf, 11.13%). Figure 3D, bottom, shows the percentage of cell arrest in both the S phase and G2 phase. As a whole, the data demonstrate that curcumin provided HT22 cell protection by attenuating apoptosis and postponing cell arrest, thereby promoting cell growth during hypoxic stress.
Fig. 3.
Curcumin treatment of HT22 cells attenuated apoptosis and released cell cycle arrest during hypoxic stress. A: photomicrograph of HT22 exposed to 1% O2 with or without curcumin treatment. Curcumin (2 μM) or DMSO vehicle-treated cells were placed inside hypoxic chamber to expose to 1% O2. Forty-eight hours later, photomicrographs were taken. Arrows denote rounded white and rounded dead cells (a: control; b: 1% O2; c: Cur + 1% O2). B: photomicrograph of HT22 exposed to CoCl2 with or without curcumin treatment. Curcumin (2 μM) or DMSO vehicle-treated cells were exposed to 100 or 200 μM of CoCl2. Forty-eight hours later, photomicrographs were taken. Arrows denote rounded white dead cells (a: control; b: Cur only; c: 100 μM CoCl2; d: Cur + 100 μM CoCl2; e: 200 μM CoCl2; f: Cur + 200 μM CoCl2). C: curcumin negatively regulated the vulnerability of HT22 cells to CoCl2-evoked apoptotic cells death. Cells untreated (a: vehicle control), treated with different concentration of CoCl2 alone (c: 100 μM; e: 200 μM) or pretreated with curcumin (b: 2 μM curcumin) followed by CoCl2 treatment (d: 100 μM; f: 200 μM). After 48 h of treatment, apoptosis was evaluated using annexin V-FITC/propidium iodide (PI) staining followed by flow cytometry. Top: representative plots showing annexin V-FITC/PI staining of HT22 cells. The proportion of late or apoptotic cells is shown. Bottom: histogram showing a significant increase of apoptotic cells (compare gray vs. black bars). Data represent means ± SD of 3 independent experiments. **P < 0.001. D: curcumin released CoCl2-induced S-phase and G2-M-phase cell cycle arrest in HT22 cells. Cells were cultured in 100-mm plate and treated with curcumin or DMSO (vehicle) for 12 h and then subjected to different concentrations of CoCl2. Cells were harvested and hypotonically lysed in a PI solution to stain the DNA. Nuclei were analyzed for DNA content by flow cytometry. a–f: same as in C. Percentage of cell population at the G1, S, and G2 phases is shown in histogram.
Curcumin protected HT22 cells by reducing hypoxia-induced LPO.
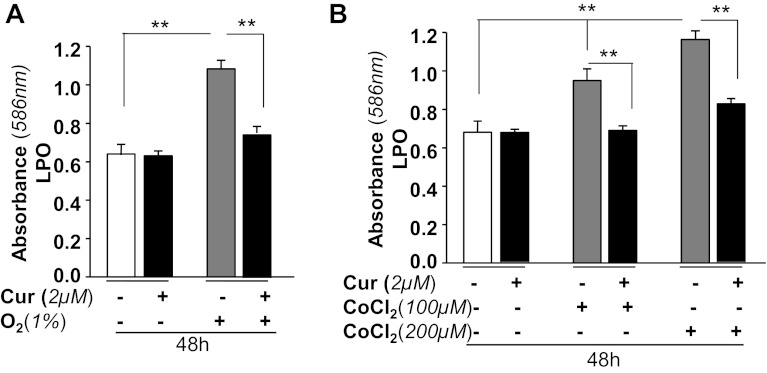
LPO-induced damage has been implicated as a major event in the initiation and progression of neuronal cell injury during hypoxia (81). In previous work, we showed that curcumin attenuates the progression of LPO in lens cells by enhancing expression of ROS quencher Prdx6 (15). Since the activity of curcumin is specific to cell type, we wanted to test the anti-LPO activity of curcumin in HT22 cells. Cells untreated or pretreated with curcumin (2 μM) were exposed to 1% O2 (Fig. 4A) or CoCl2 (Fig. 4B). After 48 h, the cells were processed for LPO assay following the manufacturer's protocol, and the levels of MDA and 4-hydroxyalkenals in unstable lipid peroxide decomposition were monitored. The results indicated a significant increased LPO levels in O2 (1%)-exposed cells or a dose-dependent increase in LPO levels in cells exposed to CoCl2. In contrast, LPO levels were dramatically decreased in cells treated with curcumin (Fig. 4, A and B, black vs. gray bars). Results indicate that curcumin inhibited LPO induced by the hypoxia or hypoxia-mimicking agent CoCl2.
Fig. 4.
Curcumin treatment of HT22 cells attenuated 1% O2- or CoCl2-induced lipid peroxidation process. Cells were either treated with DMSO (a control vehicle) or curcumin (2 μM) and then either exposed to O2 (1%) or CoCl2 (100 or 200 μM) to generate hypoxic stress. After 48 h, cells were assessed for levels of lipid peroxidation (LPO) using LPO assay as described earlier (15). Histogram values are means ± SD of 3 independent experiments. **P < 0.001, statistically significant difference.
HT22 expressed all Prdxs (1–6), while curcumin selectively enhanced expression of Prdx1, Prdx4, and Prdx6 mRNA and protein.
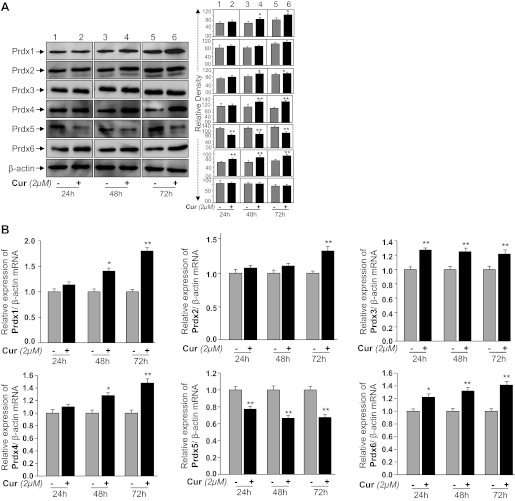
We assessed if curcumin exerts its protective activity by regulating Prdxs expression, and, if so, which of the Prdx(s) is (are) target for curcumin-mediated regulation in HT22 cells. We monitored the expression levels of all six members of the Prdx family in HT22 using Western and quantitative (q) real-time PCR analysis as described earlier (15, 28). In line with the Western and qPCR results, we found that protein (Fig. 5A) and mRNA (Fig. 5B) expression levels of Prdx4 and Prdx6 were significantly increased at all time points (24, 48, or 72 h), while Prdx1 and Prdx2 expression in HT22 cells was increased after 48 and 72 h, respectively, following curcumin treatment. Surprisingly, curcumin adversely affected expression of Prdx5 in HT22 cells. These data demonstrate that curcumin differentially regulated Prdxs, at least in HT22 cells. Because Prdx 1, 2, and 4 were also upregulated in curcumin-treated HT22 cells, we wished to identify the role of Prdx6 in those cells. To this end we conducted Prdx6 overexpression and Prdx6 knockdown experiments by using pGFP-Prdx6 and antisense specific to Prdx6 as shown in Figs. 6 and 7, respectively.
Fig. 5.
A: expression analysis showing curcumin-mediated regulation of Prdxs 1–6 in HT22 cells; 3 × 105 cells were seeded in 60-mm plate and treated with curcumin for 24, 48, and 72 h. At each time interval, total cell lysates were prepared, proteins were resolved on 10% SDS-PAGE, and Western analysis was done. In each experiment, β-actin was used as an internal marker (bottom). B: quantitative real-time PCR (qPCR) showing mRNA levels of Prdxs1–6 in presence of curcumin; 3 × 105 cells were seeded in 60-mm plate and treated with curcumin for 24, 48, and 72 h. Total RNA was isolated using TRIzol method and transcribed into cDNA. qPCR was performed using specific primers as described in materials and methods. mRNA expression of each Prdxs was adjusted to the mRNA copies of β-actin. *P < 0.05, **P < 0.001, statistically significant difference.
Fig. 6.
Effects of overexpression of GFP-Prdx6 on cell viability and removing ROS in HT22 cells against the hypoxic stress. HT22 cells were transfected with pEGFP-vector or pGFP-Prdx6 and divided into predefined experimental group as shown, and then an equal number of cells was cultured for assays to avoid transfection effect. Transfection efficiency was equalized with GFP optical density values. After 12 h, cells were exposed with hypoxic stress [1% O2 (A) or CoCl2 (C)] for 48 h and MTS assay was conducted as described in materials and methods. B and D: histogram showing the ROS levels in HT22 cells after exposure of 1% O2 (B) or CoCl2 (D). Data represent means ± SD of 3 independent experiments. *P < 0.05 **P < 0.001. E: Prdx6 overexpression normalized aberrant expression of endoplasmic reticulum (ER) stress-related proteins, Bip, and C/EBP homologous protein (CHOP) during hypoxic stress. Cells were cultured in 60-mm plates and transfected with pEGFP-vector or pGFP-Prdx6. Forty-eight hours later, total cell lysates were prepared and were resolved on 10% SDS-PAGE and immunoblotted as indicated. Membranes were striped/restriped and immunostained with antibodies as shown. Tubulin was used as an internal marker (E). Protein bands were quantified and values are presented as histograms. **P < 0.001, statistically significant difference.
Fig. 7.
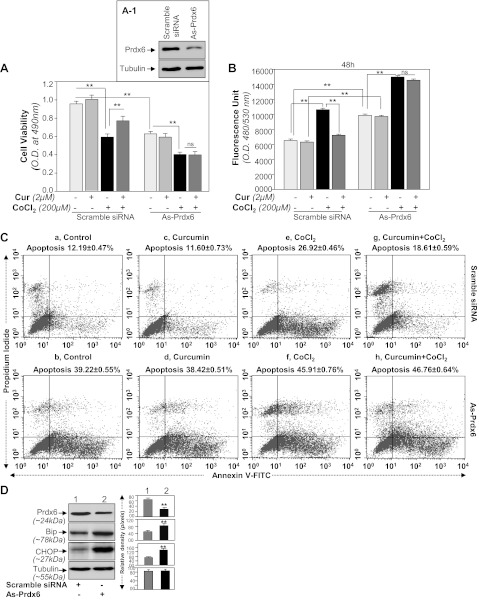
Prdx6 knockdown assay revealed that curcumin exerted its cytoprotective activity through Prdx6. A: MTS assay showing higher susceptibility of As-Prdx6-transfected cells to CoCl2 treatment. Cells were transfected with scramble siRNA or As-Prdx6 and processed for immunoblotting (inset A1). Cells were divided into predefined experimental group as shown, and then equal number of cells was cultured for assays to avoid transfection effect. Curcumin-treated or untreated HT22 cells were exposed to CoCl2. At different time intervals, MTS assay was conducted as described in materials and methods. Figure is representative of 48 h. A significant decrease in survival of As-Prdx6-transfected cells was observed compared with scramble control DNA-transfected cells, suggesting that Prdx6 is essential to protect cells from hypoxia-induced damage. B: H2-DCF-DA assay showing modulation in ROS levels in curcumin-treated or untreated As-Prdx6-transfected cells after CoCl2 treatment. Dye was added to the cells with or without CoCl2 treatment and fluorescent intensity was measured. C: scramble siRNA (a, c, e, and g) or As-Prdx6 (b, d, f, and h)-transfected HT22 cells were treated with curcumin followed by CoCl2 exposure. Forty-eight hours later, cells were analyzed for apoptosis using annexin V-FITC/PI staining following company's protocol (Apoptosis Detection Kit, BD Biosciences). Plots displaying annexin V-FITC/PI staining of control or As-Prdx6-transfected HT22 cells without (e and f) or with (g and h) curcumin treatment exposed to CoCl2. D: Prdx6 knockdown cells displayed prevalence of ER response. Western analysis showing reduced Prdx6 expression and increased expression of Bip and CHOP expression in As-Prdx6-transfected HT22 cells. Cells were cultured in 60-mm plates and transfected with As-Prdx6 for 48 h, cell lysates were prepared, proteins were resolved on 10% SDS-PAGE, and Western analysis was done. Membranes were striped/restriped and immunostained with antibodies as shown. Tubulin was used as an internal marker (D). Protein bands were quantified and presented as histograms. **P < 0.001, statistically significant difference.
HT22 cells overexpressing Prdx6 encounter resistance against hypoxia-driven oxidative stress-induced cytotoxicity.
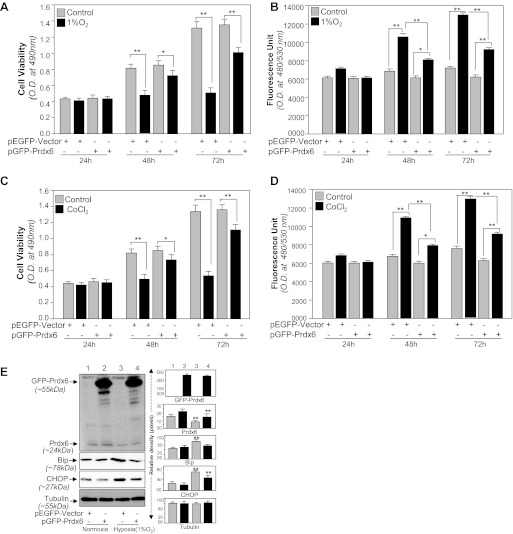
Cellular expression of Prdx6 provides cytoprotection against various stressors (15, 24, 25, 28, 29, 50, 62). In the present study we found that hypoxic stress causes HT22 cell death by upregulating ROS levels. Prdx6 provides cytoprotection by removing ROS. Thus next we performed experiments on HT22 cells overexpressing Prdx6. Cells were transfected with pEGFP-vector or pGFP-Prdx6 and exposed to 1% O2 or CoCl2 (Fig. 6) for different time intervals. MTS assay coupled with ROS assay revealed that Prdx6 overexpression enhanced cell viability (Fig. 6, A and C) and reduced the expression of ROS expression (Fig. 6, B and D). Transfection efficiency was normalized with OD value of GFP (OD: ex 485/em 530). Next, we examined the effect of Prdx6 overexpression on the Bip and CHOP expression levels, markers of ER stress in cells facing hypoxia (1% O2). Figure 6E demonstrated that cells overexpressed with Prdx6 abated hypoxia-induced aberrant ER stress signaling, and these cells displayed reduced levels of ER markers [Fig. 6E, top: GFP-Prdx6 (∼55 kDa); bottom: Bip and CHOP]. Taken together, data argued that Prdx6 attenuated ROS-based ER stress mediated adverse signaling.
Curcumin failed to protect Prdx6-knockdown HT22 cells against CoCl2-induced cytotoxicity.
From our current results as shown in Figs. 1–3 and 5, it was evident that curcumin upregulated Prdx6 and curcumin-treated cells conferred resistance against hypoxia. However, it was not clear if Prdx6 is a requisite for curcumin-mediated cytoprotection. To evaluate if curcumin exerts its protective activity by Prdx6 upregulation, we used antisense of Prdx6 to knock down Prdx6 expression. Expression level of Prdx6 was validated using Western blotting (Fig. 7, A1), and these Prdx6-depleted HT22 cells were divided into four groups as shown in Fig. 7. Cells with scramble siRNA or As-Prdx6 (Prdx6-depleted) were treated or untreated with curcumin and submitted to CoCl2 exposure. Cell viability assay at 24, 48, or 72 h revealed curcumin's ability to protect cells against CoCl2-induced death (Fig. 7A). Quantification of ROS levels in Prdx6 knockdown HT22 cells by the oxidative conversion of DCF-DA to highly fluorescent DCF (25, 28) revealed that curcumin treatment did not lower the expression of ROS (Fig. 7B). Furthermore, the transfection caused significant cell death due to reduce expression of Prdx6 (Fig. 7, Ca vs. Cb), and cell death further increased following CoCl2 exposure (Fig. 7, Ce vs. Cf) and cells could not be protected even in presence of curcumin (Fig. 7, Cg vs. Ch). These cells cell underwent apoptosis when subjected to CoCl2 exposure as shown by annexin V-FITC assay (Fig. 7, Ca vs. Cb, Cc vs. Cd, Ce vs. Cf, and Cg vs. Ch), suggesting that cells lacking or reduced levels of Prdx6 did not respond to curcumin, and it was Prdx6 through which curcumin acted to protect cells.
Prdx6 knockdown HT22 cells displayed ROS-based ER stress signaling.
Recent evidence has shown that ROS is a crucial regulator of ER stress and that ROS-induced oxidative stress and ER stress worked concurrently (57, 58). Prdx6 optimizes intracellular ROS and thereby provides cytoprotection. However, previous work from our laboratory showed a close link between expression levels of Prdx6 and ROS-based ER stress (28). We presume that HT22 cells with reduced expression of Prdx6 may have abnormal ROS-based ER stress signaling due to increased expression of ROS. We observed that indeed Prdx6-knockdown HT22 cells had aberrant ER stress signaling, as Prdx6-depleted cells displayed elevated expression of the ER stress markers Bip and proapototic factor CHOP (Fig. 7D). Thus our data of Western analysis and the relative densitometry of protein bands as shown in Fig. 7D revealed that Prdx6 deficiency in HT22 may have initiated ER stress signaling.
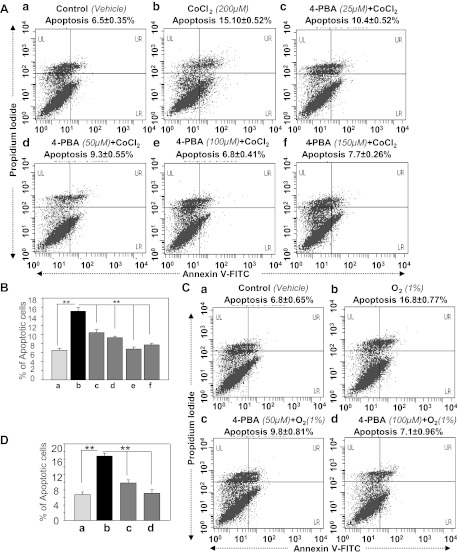
Sodium 4-PBA, a chemical chaperone, inhibited CoCl2-induced apoptosis in HT22 cells.
Sodium 4-PBA is known to reverse mislocalization or aberrant accumulation of misfolded proteins in ER and thus prevent unfolded protein response (UPR)/ER stress-induced cell death (32, 98). To examine if apoptosis in HT22 exposed to 1% O2 or CoCl2 is due to ER stress, cells were treated with increasing concentrations of 4-PBA (25, 50, 100, and 150 μM), and after overnight incubation, cells were exposed to hypoxia (CoCl2, Fig. 8, A and B; 1% O2, Fig. 8, C and D) for 48 h and analyzed by annexin V-FITC/PI binding. We found that 4-PBA treatment blunted apoptosis process in HT22 exposed to hypoxia (CoCl2 or 1% O2; Fig. 8, B and D, black vs. gray bars) in concentration-dependent fashion, demonstrating the presence of ER stress-induced signaling in those cells. Moreover, none of the 4-PBA concentrations tested in the experiments provided absolute protection, suggesting the possible involvement of some other type of ER stress-induced apoptotic signaling that is not attenuated by the supply of 4-PBA. However, the same was the case in cucumin-induced Prdx6-mediated protection of HT22 against CoCl2-induced apoptosis, arguing that Prdx6-mediated protection may belong to ROS-based ER stress-driven apoptotic signaling.
Fig. 8.
A treatment of sodium phenylbutyrate (4-PBA), an ER stress inhibitor, abated apoptotic cell death in HT22 cells induced by CoCl2 (A and B) or 1% O2 (C and D) in vitro. Cells were cultured in DMEM supplemented with increasing concentrations of 4-PBA. Twelve hours later, cells were exposed to CoCl2 or 1% O2. Forty-eight hours later, cells were analyzed for apoptosis using annexin V-FITC/PI staining following the company's protocol (Apoptosis Detection Kit, BD Biosciences). A: representative plots displaying annexin V-FITC/PI staining of HT22 cells treated with CoCl2 alone (b: 200 μM) or pretreated with 4-PBA (c: 25 μM; d: 50 μM; e: 100 μM; f: 150 μM) followed by 200 μM of CoCl2 treatment or untreated control (a). B: results showing inhibition of apoptosis induced by CoCl2 in HT22 cells in the presence of 4-PBA (gray vs. black bars). C: results showing the annexin V-FITC/PI staining of HT22 cells treated with 1% O2 (b) or pretreated with 4-PBA (c: 50 μM; d: 100 μM) followed by 1% O2 treatment or untreated control (a). D: histogram showing inhibition of apoptosis induced by hypoxia in HT22 cells in presence of 4-PBA (gray vs. black bars). Experiments were done 3 times, and values are represented as means ± SD. **P < 0.001, statistically significant difference.
Curcumin delivery abated ROS-mediated overstimulation of ER stress signaling in HT22 cells by upregulating Prdx6 during 1% O2- or CoCl2-induced hypoxia.
We next examined whether curcumin delivery to HT22 cells would prevent ROS-based overstimulation of ER stress signaling evoked by 1% O2 (Fig. 9, A and B) or CoCl2 (Fig. 10, A and B). Hypoxia is known to generate ROS, leading to oxidative stress-induced cell injury (5, 28, 92, 100). We found that HT22 cells exposed to O2 (1%) or CoCl2 (200 μM) showed increased expression of CHOP, Bip, and calreticulin, markers for anaberrant ER-stress pathway, as well as activated caspases 3, 9, and 12 with upregulation of Bax and decrease of Bcl2. These changes were directly correlated with downregulated expression of Prdx6 as shown in Figs. 9A and 10A, top, Prdx6. Densitometric analysis of protein bands showed elevation of Bip and calreticulin, but these chaperon proteins were unable to counteract cell death, suggesting that ER stress occurs downstream of oxidative stress. Furthermore, it was sticking to note that activated ER stress-induced apoptotic signaling in HT22 cells induced by the hypoxia mimic CoCl2 could be subsided by curcumin treatment. We found that the suppression of expression of proapoptotic protein CHOP and Bax and activation of caspases in curcumin-treated HT22 cells were directly related to expression of Prdx6 (Figs. 9A and 10A, top, Prdx6; lanes 1, 2, 3 vs. lanes 1, 2, 3 and B, lanes 1, 2, 3).
Fig. 9.
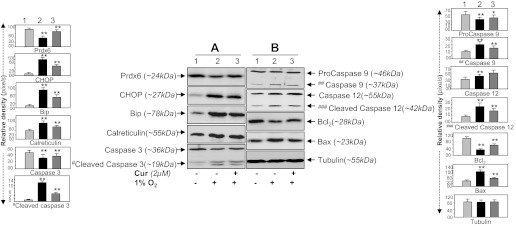
Hypoxia-induced aberrant expression of ER stress-responsive proteins in HT22 could be reversed by curcumin. A and B: Western analysis showing reduction of ER stress-responsive proteins in curcumin-treated HT22 cells expressing elevated Prdx6 expression after CoCl2 treatment. Cells were pretreated with curcumin for 12 h followed by O2 (1%) exposure. After 48 h, cell lysate was prepared and immunoblotted using different antibodies as shown. An increased expression pattern of Bip, CHOP, calreticulin, and activated forms of caspase 3, caspase 9, and caspase 12 was observed in 1% O2 exposed cells while in presence of curcumin expression of ER stress-responsive protein reduced (A, compare lane 1 vs. 3 and B, lane 1 vs. 3). A, top; Prdx6 expression following curcumin treatment (lane 3). Protein bands were quantified; histograms are shown at left and right of the protein blot. *P < 0.05, **P < 0.001, statistically significant difference.
Fig. 10.
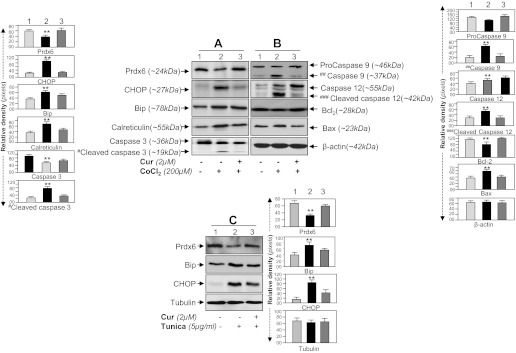
Hypoxia mimic agent, CoCl2, or an ER stressor, tunicamycin, induced aberrant expression of ER stress-responsive proteins in HT22 could be reversed by curcumin. A and B: Western analysis showing reduction of ER stress-responsive proteins in curcumin-treated HT22 cells expressing elevated Prdx6 expression after CoCl2 treatment. Cells were pretreated with curcumin for 12 h followed by CoCl2 (200 μM) exposure. After 48 h, cell lysate was prepared and immunoblotted using different antibodies as shown. An increased expression pattern of Bip, CHOP, calreticulin, and activated forms of caspase 3, caspase 9, and caspase 12 was observed in CoCl2 exposed cells while in presence of curcumin expression of ER stress-responsive protein reduced (A, compare lane 1 vs. 3 and B, lane 1 vs. 3). A, top: Prdx6 expression following curcumin treatment (lane 3). Protein bands were quantified using a densitometer and levels were normalized to corresponding β-actin levels; histograms are shown at left and right of the protein bands. **P < 0.001, statistically significant difference. C: HT22 cells were pretreated with curcumin for 12 h followed by tunicamycin treatment for 48 h. Total cell lysate was prepared and resolved onto 10% SDS gel, and the expression of the ER markers Bip and CHOP was measured. Experiments were done 3 times, and values are represented as means ± SD. **P < 0.001, statistically significant difference.
To test validity of Prdx6 potential in abating aberrant ER stress signaling, we next performed the experiments by exposing HT22 cell to known ER stress inducer tunicamycin. The cell displayed elevated expression of Bip and CHOP (Fig. 10C) and showed reduced expression of Prdx6 (Fig. 10C, top, lanes 1 vs. 2) with increased levels of ROS (data not shown). In contrast, cells treated with curcumin restored the expression of Prdx6 and these cells showed reduced expression of CHOP and Bip (Fig. 10C), suggesting that loss of Prdx6 in cells treated with tunicamycin causes initiation of ROS-mediated ER stress. Collectively, the results revealed that by reinforcing expression of endogenous Prdx6 by curcumin can attenuate ROS-based ER stress-induced cell death.
Curcumin restored Prdx6 mRNA and suppressed NF-κB mRNA expression in HT22 cells facing hypoxic stress.
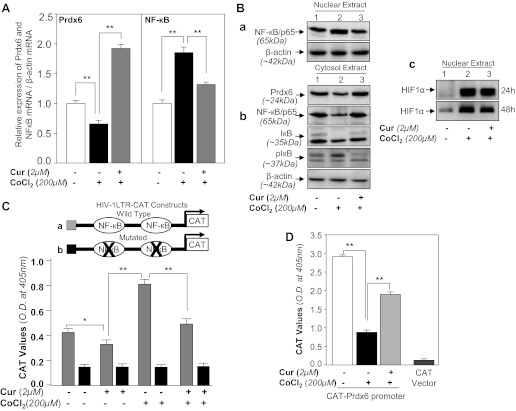
The results shown in Figs. 9 or 10 reveal that expression of Prdx6 protein under the hypoxic condition or tunicamycin treatment was downregulated, while it was restored by curcumin. However, whether the changes in expression were due to modulation in the translation or the transcription level of Prdx6 remained unclear. Thus we wished to know if the curcumin-induced increase in expression of Prdx6 mRNA in HT22 cells during hypoxia was associated with NF-кB mRNA expression. Cells treated with curcumin alone or curcumin followed by CoCl2 treatments for 48 h were processed for real-time PCR as described in materials and methods. The data demonstrated that treatment with CoCl2 reduced the expression of Prdx6 mRNA and activated the NF-кB expression (Fig. 11A, open bar vs. black bar). While curcumin-treated HT22 cells exposed to CoCl2 displayed restored the expression of Prdx6 and decreased expression of NF-кB (Fig. 11A, gray bar vs. black bar), indicating that CoCl2-induced repression of Prdx6 mRNA could be attenuated by curcumin, a known blocker of stimulated activity of NF-кB (85, 96).
Fig. 11.
A: curcumin restored Prdx6 mRNA expression and suppresses NF-кB mRNA in HT22 cells facing hypoxic stress. HT22 cells untreated or treated with curcumin were exposed to CoCl2-induced hypoxic stress. After 48 h of CoCl2, total RNA was isolated and submitted to real-time PCR using specific primers as described in materials and methods. mRNA expression of Prdx6 and NF-кB was normalized with β-actin mRNA. B–D: repression of Prdx6 transcription was associated with NF-κB activation in HT22 cells during hypoxia and was reversed by curcumin treatment. HT22 cells treated with curcumin or DMSO vehicle were exposed to CoCl2 stress. After 48 h of CoCl2 exposure cytosol or nuclear extract was isolated and immunoblotted using anti-NF-κB or anti-IκB or anti-Prdx6 antibody as shown (Ba and Bb). HIF-1α level was measured in nuclear extract showing cells were in hypoxic stress (Bc). β-Actin was used as internal control. C: transactivation of HIV-1LTR showed the presence of active NF-кB signaling in CoCl2 exposed HT22 cells. Relative CAT activity was measured in HT22 cells using HIV-1LTR promoter constructs (C, top). HT22 cells were transiently transfected with either wild type pLTR-CAT (a, where NF-кB sites are open) or pLTR-CAT-Pst1 (b, where NF-кB sites are mutated) and treated with curcumin. After 12 h cells exposed with CoCl2 for 48 h and promoter activity was monitored using CAT-ELISA. D, HT22 cells were transfected with CAT empty Vector or Prdx6 wild-type construct and treated with curcumin for 12 h, and then exposed to the CoCl2. After 48 h, total cell lysate were prepared and CAT activity was measured. Experiments were performed 3 times, and values are represented as means ± SD. *P < 0.05; **P < 0.001.
NF-кB is involved in repression of Prdx6 transcription in HT22 cells during hypoxia and process was attenuated by curcumin treatment.
Several studies indicate that NF-κB plays both proapoptotic and antiapoptotic roles depending on cell types and cell background (41, 84). It has been reported that NF-κB is activated during hypoxia. To examine if NF-кB plays a repressive role in Prdx6 transcription under hypoxia and process can be attenuated by curcumin, HT22 cells were pretreated with curcumin, and 12 h later these cells were exposed to 200 μM of cobalt chloride. Nuclear and cytosolic extracts were immunoblotted using NF-кB (p65; Fig. 11, Ba and Bb) and IкB phosphorylation/degradation (Fig. 11Bb) antibodies. Furthermore, in unstimulated resting cells, NF-кB is inactive through interactions with an inhibitor protein IкB. During cell stimulation, the IкB is phosphorylated and subsequently ubiquitinylated and releases NF-κB, which translocates into the nucleus and modulates target genes. In the present study, cells treated with cobalt chloride showed decreased expression of IкB (Fig. 11Bb, IкB, lane 2), with an increase in phospho-IкB levels (Fig. 11Bb, pIкB, lane 2). We found that nuclear fraction of CoCl2 exposed HT22 was enriched with NF-κB, while curcumin-pretreated HT22 cells followed by CoCl2 contained NF-κB, which was indistinguishable to control vehicle (Fig. 11B, top, lane 1 vs. lane 3). Collectively, the results revealed that kinetics of the increase in p65 in nucleus mirrors the kinetics for phosphorylation and degradation of IкB in cytoplasmic fraction in hypoxia-exposed and/or unexposed HT22 cells. In addition, data demonstrated that curcumin treatment restores the expression of Prdx6 by attenuating NF-кB activation. Further activation of NF-кB was confirmed by transactivation assay; HT22 transfected with pLTR-CAT construct, which consists of two NF-кB sites (26), were subjected to hypoxic stress (200 μM CoCl2) for 48 h. CAT activity assessed with CAT-ELISA as described in materials and methods revealed activation of NF-кB (Fig. 11C, gray bar). Collectively, the findings demonstrate activation of NF-кB by CoCl2, a hypoxia-mimicking agent. Because hypoxic stress is known to activate hypoxia-inducible factor-1α (HIF-1α), next, we examined expression of HIF-1α in cells exposed to hypoxia. We found that this molecule is elevated in HT22 cells facing hypoxia. Results revealed that expression of HIF-1α declined after 48 h (Fig. 11Bc). Upregulation of HIF-1α indicated that indeed these cells were under hypoxic stress. Furthermore, curcumin attenuates the hypoxic expression of HIF-1α-induced genes by blocking HIF-1 activity (17). Our data revealed that expression of HIF-1α was reduced. However, through this result, we could not know the role of HIF-1α in context with Prdx6 expression, and further work is required if HIF-1α is involved during hypoxic signaling in Prdx6 regulation.
Furthermore, NF-κB is known to exert diverse function ranging from cell survival and cell death depending on cell types and cell background. The antiapoptotic and proapoptic roles of NF-кB depend on its target gene and DNA binding activities. In current study, we think that NF-κB regulation of Prdx6 is associated with its repressive activity, which leads to repression of Prdx6 transcription in HT22 during hypoxic stress. Since curcumin can attenuate NF-кB aberrant function, we posit that curcumin treatment can restore repression of Prdx6 transcription in HT22 cells during hypoxia. To this end, we examined Prdx6 promoter activity. HT22 cells were transfected with pCAT-Prdx6 and treated with 2 μM curcumin for 12 h followed by 200-μM CoCl2 exposure. After 48 h of CoCl2 exposure, total cell lysates were extracted and processed for CAT-ELISA assay. Data revealed that CoCl2 treatment suppressed CAT activity of Prdx6 promoter (Fig. 11D, black bar); in contrast, curcumin restored the activity of Prdx6 promoter activity, suggesting that NF-κB is functionally involved in repression of Prdx6 promoter in HT22 cells facing hypoxic stress. However, in earlier work we found that in lens epithelial cells facing oxidative stress, NF-кB is one regulator of Prdx6 and is involved in fine tuning of Prdx6 expression in favor of cell survival (26). However, our current study demonstrates that during acute stress hypoxia-evoked ROS-induced activation of NF-κB-mediated repressive signaling is involved in suppression of Prdx6 expression that leads to apoptotic signaling in HT22 cells, and this adverse signaling can be blocked by curcumin.
SN50, a NF-кB inhibitor, experiments revealed the involvement of NF-кB in repressing the transcription of Prdx6 in HT22 cells during acute hypoxic stress.
To determine involvement of NF-κB activation in repression of Prdx6 expression in HT22 cells during acute hypoxic stress, we assessed NF-κB's activity by using SN50, a cell permeable inhibitor peptide that inhibits translocation of the NF-кB active complex into the nucleus. HT22 cells were pretreated with SN50 as described in materials and methods. After 12 h cells were exposed to 200 μM of CoCl2 and nuclear and cytosolic extracts were examined by Western blot. Results indicated that NF-кB translocalized into nucleus after CoCl2 exposure (Fig. 12A, lane 2). While cells pretreated with SN50 blocked CoCl2-induced migration of NF-κB to nucleus (Fig. 12A, lane 3). In addition, we found that expression levels of Prdx6 were increased in SN50-pretreated HT22 cells (Fig. 12A, bottom, lanes 1 vs. 2 vs. 3). Consequently, we assessed whether activation of NF-κB induced by hypoxia (CoCl2 treatment) was involved in repression of Prdx6 transcription. We conducted real-time PCR and CAT-promoter assay in HT22 cells treated or untreated with SN50 followed by hypoxia as described in materials and methods. The upregulation of Prdx6 mRNA (Fig. 12B, gray bars) and Prdx6 promoter activity (Fig. 12C, gray bars) in SN50-treated HT22 facing hypoxia was observed. In the absence of SN50, however, Prdx6 transcription was repressed, suggesting that involvement of NF-κB in repression of Prdx6 expression. Thus the hypoxic stress-evoked repression of Prdx6 via NF-κB activation is one cause of HT22 injuries, and this deleterious process can be blocked by supply of curcumin as described in Fig. 13.
Fig. 12.
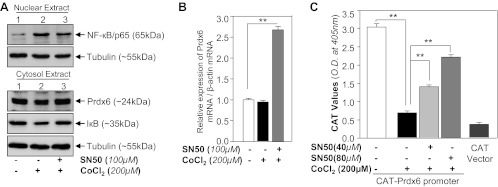
SN50, a blocker for NF-κB, treatment revealed the involvement of NF-кB in repressing the Prdx6 in HT22 cells during hypoxic stress. A and B: HT22 cells were treated with or without SN50 for 2 h followed by 200 μM CoCl2. After 12 h, cytosolic or nuclear extracts and total RNA were isolated and proceed for Western analysis (A) and real-time PCR analysis (B). C: HT22 cells were transfected with pCAT-Prdx6 promoter construct and treated with SN50 for 2h followed by 200 μM CoCl2 exposure. After 48 h, total proteins were isolated and proceed for CAT ELISA. Experiments were done 3 times, and values are represented as means ± SD. **P < 0.001, statistically significant difference.
Fig. 13.
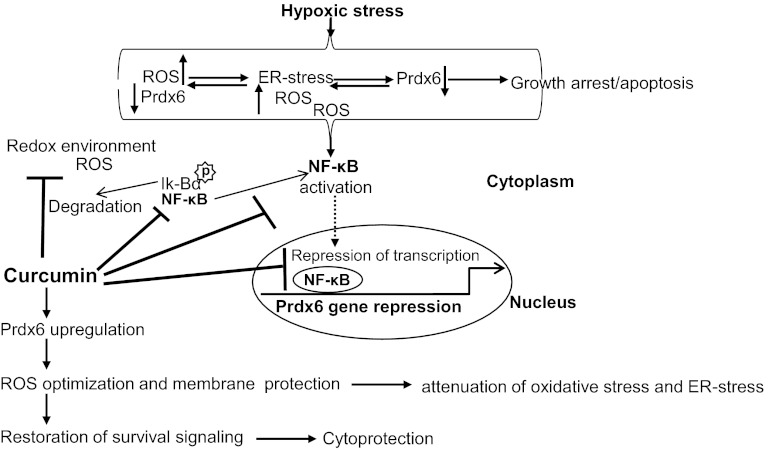
Schematic diagram showing plausible mode of action of curcumin in protecting HT22 cells and cellular response during hypoxic stress in vitro. Hypoxia-driven ROS generation evokes oxidative stress and reduced expression Prdx6 that leads to ER stress. In the cellular redox environment, cytoplasmic IκBα is phosphorylated and degraded. NF-κB translocates to the nucleus and binds to Prdx6 gene promoter, which leads repression of Prdx6 gene transcription. In HT22 cells, curcumin inhibits NF-κB signaling pathway and relieves Prdx6 transcription that in turn blocks feed-forward deleterious signaling due to cellular abundance of Prdx6.
DISCUSSION
Recent evidence reveals that hypoxia-induced excessive generation of ROS and ROS-based ER stress death signaling are major factors in the initiation and progression of neurodegenerative disease and neuronal cell degeneration (9, 28, 44, 45, 47, 57, 58, 70, 93, 104). Using HT22 cells and the hypoxia-mimicking agent CoCl2 as a model system, we found that ROS and ROS-induced ER stress signaling, coupled with reduced expression of antioxidants such as Prdx6, create a major deleterious process during hypoxia. They produce a vicious feed-forward process (hypoxia→ROS↔ER stress) in the hypoxic cellular microenvironment that causes cell degeneration or cell death and, if this process prolongs, results in a disease state. Moreover, the antioxidant protein Prdx6 negatively regulates death signaling by optimizing ROS expression (15, 25, 28, 48) and attenuates ROS-based aberrant ER stress signaling in lens cells (28). However, therapeutic application of Prdx6 has not been widely adopted, due to difficulties in delivering it to target tissues/organs. In an effort to circumvent these difficulties, in the present study, we reenforced the expression of naturally occurring Prdx6 by means of a dietary supplement, curcumin. We found that curcumin did indeed stimulate endogenous Prdx6 in the murine hippocampal neuronal cell line HT22 at a normal physiological condition as well as during hypoxic stress (Figs. 1 and 2), thus abating the ROS- and ER stress-induced apoptosis (Figs. 3, 9, and 10). In the current work we found that curcumin treatment elevated expression of Prdx6 in HT22 cells and thereby stabilized ROS expression and attenuated LPO. However, we repeatedly observed that curcumin protected HT22 cells against CoCl2, a hypoxia mimic (Fig. 1), suggesting that such protection may involve upregulation of antioxidants. Furthermore, we found that a low concentration of curcumin was able to mount protection of HT22 cells against 1% O2- or CoCl2-induced hypoxic stress; the curcumin acted by optimizing ROS level (Fig. 2, B and D) and thereby inhibiting apoptosis (Fig. 3C), restoring cell cycle in the G2 and S phases (Fig. 3D), and attenuating the process of LPO (Fig. 4). Furthermore, ER stress has been shown to cause key pathologic events in neurological disease processes and neurological cell death (4, 8). We have reported that Prdx6 attenuates ER stress-induced apoptosis (28). Inconsistent with our earlier results, we found that curcumin blocks tunicamycin as well as CoCl2-induced apoptotic cell death in HT22 by upregulating Prdx6 thereby removing ROS, suggesting the plausible involvement of ROS-based ER stress death signaling in CoCl2-induced apoptosis in HT22 cells (Figs. 7 and 10).
Hypoxia-induced oxidative stress or ER stress has been found to be a major culprit(s) in the etiopathology of many diseases including neurodegenerative diseases (19, 24, 28, 93). Neuronal cells are particularly susceptible to ROS-induced injury, as they contain more lipids and require more oxygen than other cells. In addition, these cells have only a feeble antioxidant defense, due to their low levels of endogenous antioxidant enzymes (20). Recent reports emphasize that among the major events in neurological diseases are significant biological changes related to oxidative stress caused by deficiency of antioxidants in brain tissues (64, 66, 87). Aging itself is seen as a major event in neuronal cell death, as a decline in antioxidant proteins in cells leaves them susceptible to oxidative stress (23). Our laboratory has found that cells lacking the antioxidant protein Prdx6 show spontaneous apoptosis and LPO and become more susceptible to environmental stresses (15, 28, 48). We also found that the level of Prdx6 declines with aging and the decline is associated with increased ROS expression, LPO, and apoptosis (15, 25, 28, 48, 49). Collectively, the findings provide strong evidence that a neuroprotective antioxidant should be considered a potential approach to postponing or delaying neuronal cell loss or loss of function (9, 28, 93). Curcumin is known to have diverse activities, including activation and expression of antioxidants (10, 15). We previously showed that curcumin protects lens epithelial cells by upregulating Prdx6 (15). In the present work, we studied whether curcumin protects neuronal cells by elevating the expression of Prdx6. Figures 1–5 and 9 illustrate that, indeed, curcumin enhanced expression of Prdx6 and the increased expression was correlated with prevention of hypoxia-induced LPO and apoptosis in the HT22 cells. This was consistent with our previous report that cells overexpressing Prdx6 gained protection against hypoxic stress (28). A similar conclusion regarding the protective role of Prdx6 in various cells and tissues has been documented (29, 51, 62, 74, 79).
Furthermore, there is accumulating evidence that the initiation and progression of neurodegenerative diseases are associated with oxidative stress and protein misfolding (30). We believe that neuronal cell death or apoptosis may be mediated by ROS-induced oxidative and ER stresses. In earlier studies, we found that cells with a deficiency of Prdx6 or cells facing oxidative stress display upregulation of ER stress markers and these abnormal processes are blocked by the delivery of Prdx6 (28). In the current work we found that HT22 cells facing CoCl2 stress displayed higher levels of ROS and aberrant expression of ER stress proteins such as Bip, CHOP, and calreticulin (Figs. 9 and 10). These adversely regulated processes were blunted by curcumin-evoked expression of Prdx6 (Figs. 9 and 10). Recent literature indicates that oxidative and ER stresses are interrelated, but how they are linked requires further investigation. The present work found that HT22 cells showed that higher expression of ROS with reduced expression of Prdx6 as one cause of ER stress. The process was accelerated during hypoxia, which further increased ROS production, leading to acceleration of ER stress, further increasing ROS, and so on, forming a vicious feed-forward process. We believe that this abnormal process can be blocked by stimulating expression of the endogenous antioxidant protein Prdx6. In fact, ROS-based activation of aberrant ER-stress signaling in HT22 cells was postponed by increased expression of Prdx6 resulting from curcumin delivery to cells (Figs. 9 and 10). It would be worthwhile to mention that recent studies have now demonstrated that antioxidant deficiency as well as hypoxia-evoked oxidative stress is among the causes of ER stress-mediated death signaling. Earlier we showed that a supply of the antioxidant protein Prdx6 prevents ER stress signaling by optimizing cellular ROS level in lens cells (28). Also, we found that the function of curcumin was dependent on upregulation of Prdx6 expression, as evidenced by Prdx6 overexpression as well as Prdx6 knockdown experiments showing that Prdx6 ectopic expression gained resistance, while Prdx6-depleted HT22 cells did not gain resistance against hypoxic stress. As shown in Fig. 7, knockdown of Prdx6 enhanced ROS-based ER stress signaling, and the abnormal apoptotic signaling could not be blocked by curcumin. In contrast, cells overexpressing Prdx6 nullified hypoxic express (Fig. 6). This suggested that other antioxidants may not be as essential as Prdx6 for curcumin-mediated protection of HT22 cells. Further, we were also interested in learning whether curcumin alters the expression of other Prdxs in HT22 cells. Expression analysis revealed that curcumin altered the expression levels of Prdx1 and Prdx4, which were upregulated. However, the upregulation of these two did not save the HT22 from 1% O2- or CoCl2-induced hypoxic stress, suggesting curcumin's mode of protective action is through Prdx6. This was supported by As-Prdx6 knockdown experiment showing that curcumin failed to prevent cell death induced by the hypoxic stress (Fig. 7A) and was not able to normalize the increased ROS level (Fig. 7B) as shown in FACS analysis assay (Fig. 7C). Fatma et al. (28) reported that the loss of Prdx6 causes initiation of UPR, and this process becomes overstimulated in response to stressors. We found increased expression of the ER markers Bip and CHOP in As-Prdx6-transfected HT22 cells (Fig. 7D) (16, 18, 102), suggesting that reduced expression of Prdx6 initiated ER stress in HT22 cells. Furthermore, our experiments revealed that cells treated with hypoxia displayed higher expression of ER stress and/or apoptosis markers (Figs. 9 and 10). We observed a time-dependent increase in Bax protein levels and activation of caspases during hypoxic stress with reduced expression of Prdx6. Hypoxia stress is associated with increased production of ROS. Accumulation of unfolded proteins triggers ER stress and is considered a part of cellular response to hypoxia. Expression analysis revealed that in stressed cells, the level of the ER stress-related genes Bip, CHOP, caspase 3, caspase 9, and caspase 12 were overstimulated significantly compared with cells pretreated with curcumin (Figs. 9A and 10A). We also found increased Bax and decreased Bcl2 expression in HT22 cells exposed to O2 or CoCl2 but found decreased Bax and increased Bcl2 expression in the presence of curcumin. Moreover, mitochondria have been proposed as a primary source of ROS production during hypoxic stress. Prdx6 has been found to be translocated into mitochondria during ischemia, suggesting that Prdx6 may eliminate hypoxia-induced ROS-mediated cell injury (25, 28, 48, 97). This was further supported by the finding that hypoxia-induced abnormal ER signaling was blocked in cells treated with 4-PBA (Fig. 8), a result in agreement with previously published studies (28, 98). As a whole, our data demonstrate that curcumin inhibits ROS-based ER stress signaling via upregulation of Prdx6. Furthermore, in cells, Prdx6 participates in oxidative defense by removing excess of ROS and thereby optimizing them at cellular physiological level to maintain cellular homeostasis. ROS is diffusible and can be present in cellular components, including ER or Golgi-body, and therefore will hinder normal functioning of these organelles due to a lack of Prdx6-reduced expression. Emerging literature provides evidence that oxidative stress is integrated with ER stress (39, 54, 59, 101, 103) and ROS function in the upstream of UPR. Prdx6 play a pivotal in maintaining the cytosolic environment at physiological level. Recently, Prdx6 expression and localization have been reported in mitochondria (22), plasma membrane (2), as well as in the endoplasmic reticulum or lysosomal organelles (89, 90). However, the molecular mechanism involved has not established how Prdx6 stabilize ER stress homeostasis. It has been very well established by our group as well as other laboratories that Prdx6 localizes to cytoplasm and mitochondria and exerts its function via controlling ROS expression. Furthermore, this is a smaller molecule and may diffuse into other organelles and provide cytoprotection by removing ROS. As whole, the role of Prdx6 in maintaining survival signaling pathways and its recent identification of expression in different organelles including ER (90) argue that the expression level may influence both the UPR-mediated survival and apoptotic death responses to ER stress. We believe that reduced expression or loss of Prdx6 may initiate ER stress due to cellular redox-environment and ER stress can be overstimulated during hypoxia or oxidative stress, leading to apoptotic signaling. Prdx6 antisense and/or Prdx6 overexpression (Figs. 6 and 7) supports the notion that loss of Prdx6 expression initiates ER-stress signaling. It is also known that oxidative stress causes ER dysfunction (37). We further posit that lack of cytoplasmic Prdx6 will cause higher levels of ROS, and diffusible ROS will disrupt ER quality control functionality. Therefore, it is logical to surmise that ectopic expression of Prdx6 or induction of Prdx6 by a means dietary supplement, curcumin, can attenuate ROS-based ER stress apoptotic signaling (Fig. 13).
Finally, our present study demonstrates that curcumin plays its protective role in HT22 cells by inducing the expression of the antioxidant enzyme Prdx6. There is evidence that one of curcumin's protective modes of action is upregulation of antioxidant proteins in various cell types against stressors (1, 10, 15, 46, 61, 68). In the hypoxic condition, ROS generation and LPO significantly increased with decreased antioxidant enzymes and activation of NF-кB. Curcumin has been reported to attenuate over activated deleterious activity and thereby restore normal signaling (46, 85, 86). Our results also vividly demonstrate that addition of curcumin in HT22 cells enhances Prdx6 mRNA expression (Fig. 11A). We think that curcumin-mediated increased expression of Prdx6 leads to removal of ROS that in turn stabilizes NF-кB activity (feed-back process), since SN50, an inhibitor of NF-кB treatment of HT22 cells, increased expression of Prdx6 mRNA (Fig. 12B). Interestingly, similar results has been reported by Gallagher and Phelan (31) where they have shown repressive role of NF-кB in Prdx6 regulation. However, previously we have also shown that regulatory role of NF-кB in Prdx6 transcription wherein NF-кB controls Prdx6 expression depending on cellular need (26). In this scenario, we think that NF-кB inactivation in curcumin-treated HT22 under hypoxic conditions involves the increased expression of Prdx6 and reduced expression of ROS and ER stress (Fig. 13). Furthermore, cells exposed to hypoxia showed increased expression of HIF-1α validating that HT22 cells faced hypoxic stress, and during hypoxia the level of Prdx6 is reduced after 24 h. In this study, however, we could not unveil the role of HIF-1α in regulation of Prdx6. HIF-1α can be inactivated by repressing its transcriptional activity or by reducing its abundance. We found that curcumin-treated cells showed reduced abundance of HIF-1α. However, more work is required to unveil role of HIF-1α for Prdx6 regulation during hypoxia.
In summary, we found that curcumin delivery protects cells from both oxidative stress and ER stress induced by hypoxia. This function is mediated through Prdx6 expression in HT22 cells. However, when HT22 cells were subjected to hypoxic stress, Prdx6 downregulation was restored in curcumin-treated cells, which in turn normalized the cellular signaling. In addition, our study has disclosed the presence of all six known Prdxs in HT22 cells, but only Prdx6 is a prerequisite for curcumin-mediated protection against hypoxia, as evidenced by Prdx6 knockdown experiments. Downregulation of Prdx6 during hypoxic stress leads to subsequent overproduction of ROS that in turn induces ROS-based ER-stress death signaling, finally resulting in a vicious feed-forward cyclical process within the cellular microenvironment (Fig. 13). If not blocked, the process results in cell degeneration and disease. We have taken initial steps toward the ultimate goal of developing antioxidant-based “inductive therapy” to reinforce the naturally occurring protective Prdx6 molecule by 1) identifying curcumin-mediated survival signaling involved in upregulating Prdx6, and 2) determining the protective efficacy of curcumin in relation to Prdx6 regulation and expression in HT22 cells facing hypoxia.
GRANTS
Grants were provided by the National Eye Institute (EY-013394 and EY-017613 to D. P. Singh) and Research for Preventing Blindness are gratefully acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.C., N.F., E.K., and D.P.S. conception and design of research; B.C. and P.R. performed experiments; B.C., N.F., E.K., and D.P.S. analyzed data; B.C., N.F., E.K., and D.P.S. interpreted results of experiments; B.C. prepared figures; B.C. and D.P.S. drafted manuscript; B.C., N.F., E.K., and D.P.S. edited and revised manuscript; B.C., N.F., E.K., S.P.S., and D.P.S. approved final version of manuscript.
REFERENCES
- 1.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 30: 85–94, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ambruso DR, Ellison MA, Thurman GW, Leto TL. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim Biophys Acta 1823: 306–315, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Aon-Bertolino ML, Romero JI, Galeano P, Holubiec M, Badorrey MS, Saraceno GE, Hanschmann EM, Lillig CH, Capani F. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim Biophys Acta 1810: 93–110, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Arduino DM, Esteves AR, Cardoso SM, Oliveira CR. Endoplasmic reticulum and mitochondria interplay mediates apoptotic cell death: relevance to Parkinson's disease. Neurochem Int 55: 341–348, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Asimiadou S, Bittigau P, Felderhoff-Mueser U, Manthey D, Sifringer M, Pesditschek S, Dzietko M, Kaindl AM, Pytel M, Studniarczyk D, Mozrzymas JW, Ikonomidou C. Protection with estradiol in developmental models of apoptotic neurodegeneration. Ann Neurol 58: 266–276, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bhargavan B, Fatma N, Chhunchha B, Singh V, Kubo E, Singh DP. LEDGF gene silencing impairs the tumorigenicity of prostate cancer DU145 cells by abating the expression of Hsp27 and activation of the Akt/ERK signaling pathway. Cell Death Dis 3: e316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourassa MW, Miller LM. Metal imaging in neurodegenerative diseases. Metallomics 4: 721–738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 13: 363–373, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cacciatore I, Baldassarre L, Fornasari E, Mollica A, Pinnen F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid Med Cell Longev 2012: 240146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese V, Bates TE, Mancuso C, Cornelius C, Ventimiglia B, Cambria MT, Di Renzo L, De Lorenzo A, Dinkova-Kostova AT. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res 52: 1062–1073, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Candan N, Tuzmen N. Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. Neurotoxicology 29: 708–713, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Carmona-Ramirez I, Santamaria A, Tobon-Velasco JC, Orozco-Ibarra M, Gonzalez-Herrera IG, Pedraza-Chaverri J, Maldonado PD. Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J Nutr Biochem 24: 14–24, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di GH, Jin W, Ou ZL, Shen ZZ, Shao ZM. Identification of the functional role of AF1Q in the progression of breast cancer. Breast Cancer Res Treat 111: 65–78, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 275: 28421–28427, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Chhunchha B, Fatma N, Bhargavan B, Kubo E, Kumar A, Singh DP. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis 2: e234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi AY, Choi JH, Lee JY, Yoon KS, Choe W, Ha J, Yeo EJ, Kang I. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem Int 57: 143–152, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol 70: 1664–1671, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Choi HK, Choi KC, Yoo JY, Song M, Ko SJ, Kim CH, Ahn JH, Chun KH, Yook JI, Yoon HG. Reversible SUMOylation of TBL1-TBLR1 regulates beta-catenin-mediated Wnt signaling. Mol Cell 43: 203–216, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Ciafre SA, Niola F, Giorda E, Farace MG, Caporossi D. CoCl(2)-simulated hypoxia in skeletal muscle cell lines: role of free radicals in gene upregulation and induction of apoptosis. Free Radic Res 41: 391–401, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689–695, 1993 [DOI] [PubMed] [Google Scholar]
- 21.D'Arceuil H, Rhine W, de Crespigny A, Yenari M, Tait JF, Strauss WH, Engelhorn T, Kastrup A, Moseley M, Blankenberg FG. 99mTc annexin V imaging of neonatal hypoxic brain injury. Stroke 31: 2692–2700, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Eismann T, Huber N, Shin T, Kuboki S, Galloway E, Wyder M, Edwards MJ, Greis KD, Shertzer HG, Fisher AB, Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol 296: G266–G274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev 130: 203–215, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Fatma N, Kubo E, Sen M, Agarwal N, Thoreson WB, Camras CB, Singh DP. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res 1233: 63–78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6-/- mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ 12: 734–750, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Fatma N, Kubo E, Takamura Y, Ishihara K, Garcia C, Beebe DC, Singh DP. Loss of NF-kappaB control and repression of Prdx6 gene transcription by reactive oxygen species-driven SMAD3-mediated transforming growth factor beta signaling. J Biol Chem 284: 22758–22772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatma N, Kubo E, Toris CB, Stamer WD, Camras CB, Singh DP. PRDX6 attenuates oxidative stress- and TGFbeta-induced abnormalities of human trabecular meshwork cells. Free Radic Res 43: 783–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B, Singh DP. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol 301: C954–C967, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A activities. Antioxid Redox Signal 15: 831–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forman MS, Lee VM, Trojanowski JQ. “Unfolding” pathways in neurodegenerative disease. Trends Neurosci 26: 407–410, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Gallagher BM, Phelan SA. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic Biol Med 42: 1270–1277, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, Ryu H, Langley B, Ratan RR, Ferrante RJ, Beal MF. Neuroprotective effects of phenylbutyrate in the N171–82Q transgenic mouse model of Huntington's disease. J Biol Chem 280: 556–563, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Goemaere J, Knoops B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J Comp Neurol 520: 258–280, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Gorgias N, Maidatsi P, Tsolaki M, Alvanou A, Kiriazis G, Kaidoglou K, Giala M. Hypoxic pretreatment protects against neuronal damage of the rat hippocampus induced by severe hypoxia. Brain Res 714: 215–225, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Ha JS, Park SS. Glutamate-induced oxidative stress, but not cell death, is largely dependent upon extracellular calcium in mouse neuronal HT22 cells. Neurosci Lett 393: 165–169, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Helenius A, Marquardt T, Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol 2: 227–231, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Ho JN, Lee SB, Lee SS, Yoon SH, Kang GY, Hwang SG, Um HD. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol Cancer Ther 9: 825–832, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Hotokezaka Y, van Leyen K, Lo EH, Beatrix B, Katayama I, Jin G, Nakamura T. alphaNAC depletion as an initiator of ER stress-induced apoptosis in hypoxia. Cell Death Differ 16: 1505–1514, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Huang CF, Sun ZJ, Zhao YF, Chen XM, Jia J, Zhang WF. Increased expression of peroxiredoxin 6 and cyclophilin A in squamous cell carcinoma of the tongue. Oral Dis 17: 328–334, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Jeyasuria P, Subedi K, Suresh A, Condon JC. Elevated levels of uterine antiapoptotic signaling may activate NFкB and potentially confer resistance to caspase 3-mediated apoptotic cell death during pregnancy in mice. Biol Reprod 85: 417–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin DQ, Lim CS, Hwang JK, Ha I, Han JS. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem Biophys Res Commun 331: 1264–1269, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345: 91–104, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Jung JY, Kim WJ. Involvement of mitochondrial- and Fas-mediated dual mechanism in CoCl2-induced apoptosis of rat PC12 cells. Neurosci Lett 371: 85–90, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Jung SY, Jeon HK, Choi JS, Kim YJ. Reduced expression of FASN through SREBP-1 downregulation is responsible for hypoxic cell death in HepG2 cells. J Cell Biochem 113: 3730–3739, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein downregulation. J Biol Chem 285: 25332–25344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotake-Nara E, Saida K. Characterization of CoCl2-induced reactive oxygen species (ROS): inductions of neurite outgrowth and endothelin-2/vasoactive intestinal contractor in PC12 cells by CoCl2 are ROS dependent, but those by MnCl2 are not. Neurosci Lett 422: 223–227, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol 294: C842–C855, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev 127: 249–256, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Kubo E, Singh DP, Fatma N, Akagi Y. TAT-mediated peroxiredoxin 5 and 6 protein transduction protects against high-glucose-induced cytotoxicity in retinal pericytes. Life Sci 84: 857–864, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Kumin A, Huber C, Rulicke T, Wolf E, Werner S. Peroxiredoxin 6 is a potent cytoprotective enzyme in the epidermis. Am J Pathol 169: 1194–1205, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumin A, Schafer M, Epp N, Bugnon P, Born-Berclaz C, Oxenius A, Klippel A, Bloch W, Werner S. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J Cell Biol 179: 747–760, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SB, Ho JN, Yoon SH, Kang GY, Hwang SG, Um HD. Peroxiredoxin 6 promotes lung cancer cell invasion by inducing urokinase-type plasminogen activator via p38 kinase, phosphoinositide 3-kinase, and Akt. Mol Cells 28: 583–588, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 99: 121–128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lubbad AS, Oriowo MA, Khan I. Curcumin reverses attenuated carbachol-induced contraction of the colon in a rat model of colitis. Scand J Gastroenterol 44: 187–194, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mancuso C, Barone E, Guido P, Miceli F, Di Domenico F, Perluigi M, Santangelo R, Preziosi P. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro. Neurosci Lett 518: 101–105, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med 46: 672–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 38: 1422–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 99: 11599–11604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markesbery WR. The role of oxidative stress in Alzheimer disease. Arch Neurol 56: 1449–1452, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci 29: 632–639, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Moreira PI, Honda K, Liu Q, Santos MS, Oliveira CR, Aliev G, Nunomura A, Zhu X, Smith MA, Perry G. Oxidative stress: the old enemy in Alzheimer's disease pathophysiology. Curr Alzheimer Res 2: 403–408, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Moreira PI, Smith MA, Zhu X, Nunomura A, Castellani RJ, Perry G. Oxidative stress and neurodegeneration. Ann NY Acad Sci 1043: 545–552, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 28: 1303–1312, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Munz B, Frank S, Hubner G, Olsen E, Werner S. A novel type of glutathione peroxidase: expression and regulation during wound repair. Biochem J 326: 579–585, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muratsu-Ikeda S, Nangaku M, Ikeda Y, Tanaka T, Wada T, Inagi R. Downregulation of miR-205 modulates cell susceptibility to oxidative and endoplasmic reticulum stresses in renal tubular cells. PLos One 7: e41462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onder A, Kapan M, Gumus M, Yuksel H, Boyuk A, Alp H, Basarili MK, Firat U. The protective effects of curcumin on intestine and remote organs against mesenteric ischemia/reperfusion injury. Turk J Gastroenterol 23: 141–147, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Ouyang X, DeWeese TL, Nelson WG, Abate-Shen C. Loss-of-function of Nkx31 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res 65: 6773–6779, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Pae HO, Jeong SO, Zheng M, Ha HY, Lee KM, Kim EC, Kim DH, Hwang SY, Chung HT. Curcumin attenuates ethanol-induced toxicity in HT22 hippocampal cells by activating mitogen-activated protein kinase phosphatase-1. Neurosci Lett 453: 186–189, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Pak JH, Choi WH, Lee HM, Joo WD, Kim JH, Kim YT, Kim YM, Nam JH. Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Invest 29: 21–28, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol 682: 12–20, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24: 66–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pereira C, Agostinho P, Moreira PI, Cardoso SM, Oliveira CR. Alzheimer's disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr Drug Targets CNS Neurol Disord 4: 383–403, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, Bours V. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem 378: 1237–1245, 1997 [PubMed] [Google Scholar]
- 79.Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC, Gai WP. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol 115: 611–622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Power JH, Blumbergs PC. Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol 117: 63–73, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Rauchova H, Vokurkova M, Koudelova J. Hypoxia-induced lipid peroxidation in the brain during postnatal ontogenesis. Physiol Res 61, Suppl 1: S89–S101, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Roy A, Ghosh A, Jana A, Liu X, Brahmachari S, Gendelman HE, Pahan K. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson's disease. PLos One 7: e38113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rupec RA, Baeuerle PA. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-kappa B. Eur J Biochem 234: 632–640, 1995 [DOI] [PubMed] [Google Scholar]
- 84.Saile B, Matthes N, El Armouche H, Neubauer K, Ramadori G. The bcl, NFkappaB and p53/p21WAF1 systems are involved in spontaneous apoptosis and in the antiapoptotic effect of TGF-beta or TNF-alpha on activated hepatic stellate cells. Eur J Cell Biol 80: 554–561, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, Ilavazhagan G. Role of oxidative stress and NFкB in hypoxia-induced pulmonary edema. Exp Biol Med (Maywood) 233: 1088–1098, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Sarada SK, Himadri P, Ruma D, Sharma SK, Pauline T, Mrinalini Selenium protects the hypoxia induced apoptosis in neuroblastoma cells through upregulation of Bcl-2. Brain Res 1209: 29–39, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem 8: 721–738, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10: 2247–2258, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sorokina EM, Feinstein SI, Milovanova TN, Fisher AB. Identification of the amino acid sequence that targets peroxiredoxin 6 to lysosome-like structures of lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L871–L880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorokina EM, Feinstein SI, Zhou S, Fisher AB. Intracellular targeting of peroxiredoxin 6 to lysosomal organelles requires MAPK activity and binding to 14–3-3epsilon. Am J Physiol Cell Physiol 300: C1430–C1441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stenger C, Naves T, Verdier M, Ratinaud MH. The cell death response to the ROS inducer, cobalt chloride, in neuroblastoma cell lines according to p53 status. Int J Oncol 39: 601–609, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Tu S, Wang X, Yang F, Chen B, Wu S, He W, Yuan X, Zhang H, Chen P, Wei G. Propofol induces neuronal apoptosis in infant rat brain under hypoxic conditions. Brain Res Bull 86: 29–35, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Tulsawani R, Kelly LS, Fatma N, Chhunchha B, Kubo E, Kumar A, Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci 11: 125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vert P, Daval JL. [Cell death and neurogenesis after hypoxia: a brain repair mechanism in the developing rat?]. Bull Acad Natl Med 190: 469–481; discussion 481–464, 2006 [PubMed] [Google Scholar]
- 95.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 278: 25179–25190, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 10: 12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Ophthalmol Vis Sci 48: 1683–1690, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Yang SJ, Pyen J, Lee I, Lee H, Kim Y, Kim T. Cobalt chloride-induced apoptosis and extracellular signal-regulated protein kinase 1/2 activation in rat C6 glioma cells. J Biochem Mol Biol 37: 480–486, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Yang Y, Li L, Wang YG, Fei Z, Zhong J, Wei LZ, Long QF, Liu WP. Acute neuroprotective effects of extremely low-frequency electromagnetic fields after traumatic brain injury in rats. Neurosci Lett 516: 15–20, 2012 [DOI] [PubMed] [Google Scholar]
- 101.Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J, Kitamura M. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem 283: 4252–4260, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Yoon SW, Kang S, Ryu SE, Poo H. Identification of tyrosine-nitrated proteins in HT22 hippocampal cells during glutamate-induced oxidative stress. Cell Prolif 43: 584–593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med 3: 33–40, 2010 [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng KW, Chen Z, Hao YH, Tan Z. Molecular crowding creates an essential environment for the formation of stable G-quadruplexes in long double-stranded DNA. Nucleic Acids Res 38: 327–338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]