Abstract
Electrophysiological techniques make use of Ag/AgCl electrodes that are in direct contact with cells or bath. In the bath, electrodes are exposed to numerous experimental conditions and chemical reagents that can modify electrode voltage. We examined voltage offsets created in Ag/AgCl electrodes by exposure to redox reagents used in electrophysiological studies. Voltage offsets were measured in reference to an electrode separated from the solution by an agar bridge. The reducing reagents Tris-2-carboxyethly-phosphine, dithiothreitol (DTT), and glutathione, as well as the oxidizing agent H2O2 used at experimentally relevant concentrations reacted with Ag in the electrodes to produce voltage offsets. Chloride ions and strong acids and bases produced offsets at millimolar concentrations. Electrolytic depletion of the AgCl layer, to replicate voltage clamp and sustained use, resulted in increased sensitivity to flow and DTT. Offsets were sensitive to electrode silver purity and to the amount and method of chloride deposition. For example, exposure to 10 μM DTT produced a voltage offset between 10 and 284 mV depending on the chloride deposition method. Currents generated by these offsets are significant and dependent on membrane conductance and by extension the expression of ion channels and may therefore appear to be biological in origin. These data demonstrate a new source of artifacts in electrophysiological recordings that can affect measurements obtained from a variety of experimental techniques from patch clamp to two-electrode voltage clamp.
Keywords: oxidation, reduction, electrode, Ag/AgCl, silver chloride, electrophysiology, ion channels, motion artifacts
electrophysiological measurements are feasible by the use of electrodes with defined redox (oxidation/reduction) reactions that allow the passage of current in solution. Current is passed in these electrodes by the generation and consumption of electrons following oxidation and reduction of the cathode and anode, respectively. An example of such electrodes is the Ag/AgCl electrode (silver chloride electrode). Ag/AgCl electrodes are universally used in techniques including two-electrode voltage-clamp, chop-stick, or epithelial volt-Ohm measurements and patch-clamp and Ussing chamber measurements (27, 31, 39, 41). Ag/AgCl electrodes are considered “nonpolarizable” and therefore have very stable potentials at a variety of currents (8). They are also reversible, meaning that they can pass current bidirectionally (11, 17). Ag/AgCl electrodes are represented by the following reversible chemical reaction in Eq. 1A and a second half reaction in Eq. 1B, which occurs at the anode when electrolytically chloriding Ag/AgCl electrodes, resulting in the complete reaction in Eq. 1C:
| (1A) |
| (1B) |
| (1C) |
Ag/AgCl electrodes are usually stable and largely insensitive to the amount and duration of current measured in a biological experiment owing to the small magnitudes of the currents and voltages used in these measurements and the larger redox potential of the above reaction (222 mV; Ref. 26). However, given their reversibility, they are susceptible in prolonged experiments to exposure of metallic silver leading to junctional effects, electrode instability and motion artifacts (48). Further, to make electrical contact, Ag/AgCl electrodes are also exposed to the bath solution and to any of the reagents contained in this bath. In this case, these electrodes and the observed voltages are sensitive to reagents that can modify the redox reaction of Ag resulting in large (in terms of biological measurements) voltage offsets. Indeed, galvanic reactions with silver are established components of batteries (18) and are of potential interest in biological applications where Ag/AgCl electrodes are routinely exposed to a variety of redox modifying reagents. Unaccounted, these galvanic reactions and the ensuing offsets that can develop during the course of an experiment can lead to artificial currents, changes of conductance in rectifying channels, and changes of membrane holding potential. As shown below, these artifacts can under some circumstances be erroneously interpreted as biological in origin.
Redox reactions are an important tool to study protein structure and function. When combined with electrophysiology, this can yield real time measurements of the interactions of amino acids within an ion channel or transporter. Redox reactions are important in the study of these integral membrane proteins because oxidation and reduction play a major role in protein folding and hence function (23). Cysteines are particularly important for protein folding because of the sensitivity of thiol groups to redox reactions, as in the formation of disulfide bonds. Ion channels undergo dynamic changes in their conformation that alters the open probability and the single channel conductance. Therefore, redox reactions are an important tool for studying the functional significance of specific regions within ion channels.
A powerful tool to probe protein structure is to modify the interaction and accessibility of amino acids. Most common are those involving cysteines, and numerous reagents exist to oxidize or reduce this amino acid. In this case, bath electrodes are exposed to such reagents and are consequently sensitive to galvanic reactions with Ag/AgCl.
In this study we systematically test the hypothesis that galvanic reactions can occur in electrodes used in electrophysiology resulting in large offsets created by experimentally relevant concentrations of redox reagents commonly used to study ion channels. We further test factors such as Ag purity and chloriding methods to determine their contribution to these galvanic offsets and demonstrate that electrical depletion of AgCl or poor chloriding can lead to larger offsets and the appearance of flow-induced voltage changes. We further demonstrate that under voltage-clamp conditions, these voltage artifacts can lead to currents that are dependent on membrane conductance, leading to the appearance of a specific current of apparent biological origin. The effects we describe have the potential to impact and contaminate many electrophysiological recordings and provide a guideline for avoiding such artifacts.
MATERIALS AND METHODS
Chloriding.
All electrodes were freshly chlorided immediately before use, unless otherwise indicated. Electrically chlorided electrodes were prepared by sanding 99.9 or 99.99% pure silver wire (Pierce, Rockford IL, and World Precision Instruments, Sarasota FL) depending on experimental conditions with fine crocus paper until lustrous. Unless otherwise noted, electrical deposition of chloride was performed by a 20-s immersion in 0.1 M HCl and application of a 9-V potential between the wire and a silver cathode. Electrodeposition occurs at voltages above the redox potential due to the two half-reactions described in Eq. 1.
With an HCl concentration of 0.1 M, these half-reactions have redox potentials E0 of 0.222 V (Eq. 1A) and 0 V (Eq. 1B). To calculate the cell potential we use the Nernst equation, which results in a final cell potential of 0.222 V.
| (2A) |
| (2B) |
| (2C) |
where 1 is the activity of the solid Ag and R, T, z, and F have their usual meaning and values. When driven above 222 mV, this reaction produces hydrogen gas at one electrode and a white AgCl coating at the other.
Chemically chlorided wire was produced by sanding as above followed by a 30-min immersion (unless otherwise noted) in 6% sodium hypochlorite, NaOCl (Clorox, Oakland CA). Chloriding through this method occurs through a redox reaction whereby the silver is oxidized in the wire and the hypochlorite ion is reduced in the bulk solution. This reaction occurs spontaneously and is shown below.
| (3A) |
| (3B) |
| (3C) |
Chloriding through this method produces a dark brown/grey color on the surface of the wire. A likely explanation for this difference from the electrolytic method of chloriding is a reaction producing AgOCl and other complexes between Ag and the chlorate (ClO3−) and perchlorate (ClO4−) ions that contaminate NaOCl (4). The first possibility, AgOCl, is likely shared with electrically chlorided silver as these wires tend to turn grey with time after the AgCl is oxidized to AgOCl. Sintered Ag/AgCl pellets of shape E206 were purchased from Warner Instruments (Hamden, CT). By definition, and unless otherwise not applicable, anode refers to the half reaction where electrons are generated, while cathode refers to the half reaction where electrons are consumed.
Chamber and recordings, electrode offsets.
In experiments that did not utilize live cells, solutions were washed through a chamber with a capacity of 0.5 ml at a rate of 3.3 ml/min, with ∼0.5 cm of a 0.5-mm diameter electrode exposed to solution. Except for experiments examining Cl− and H+ concentration ([Cl−] and [H+]), solutions were based on ND-94 solution (94 mM NaCl, 2 mM KCl, 1.8 mM CaCl, 1 mM MgCl2, and 5 mM HEPES, pH 7.4) to replicate experimentally relevant conditions and to obtain electrical continuity. In the remaining experiments, NaCl, HCl, and NaOH were dissolved in distilled water.
Voltage offsets were measured with an A/D converter (Dataq D-158u and D-149, Akron, OH). Measurements were made between the silver-based electrode and a reference electrode consisting of a Ag/AgCl electrode separated from the experimental solution by an agar bridge made with 3 M NaCl and 3% agar. No voltage offsets were observed if both the measurement and reference electrodes were Ag/AgCl agar bridges (not shown). Electrode and reference resistances were <1 kΩ and did not affect the measured voltage offsets, as expected from measurements of voltage under open circuit conditions. Simulated data and calculations relied on data obtained from expression of the epithelial Na+ channel in Xenopus oocytes; see below and Awayda (5).
Chamber and recordings, Xenopus oocytes.
Epithelial Na+ channel (ENaC) expressing oocytes were obtained and used in two-electrode voltage-clamp recordings as previously described by Awayda (5). Oocytes were impaled with glass electrodes with an ∼2-MΩ resistance. Reference electrodes were either electrolytically chlorided Ag/AgCl electrodes or the same electrodes coupled to the chamber by a pair of agar bridges as described above for measurements of voltage offsets. Oocyte experiments utilized the same ND-94 recording solution.
Reagents.
Tris-2-carboxyethly-phosphine (TCEP) and dithiothreitol (DTT) were obtained from Pierce. TCEP and DTT were selected because of their relevance to physiological experiments. Both reagents are used to reduce disulfide bonds in the study of ion channel chemistry and structure-function. TCEP is sometimes preferred over DTT for reducing disulfide bonds because of its greater strength of reduction, hydrophilicity, and irreversibility (19).
Hydrogen peroxide (H2O2) was obtained at 50% purity from Fisher Scientific (Pittsburg, PA). Hydrogen peroxide is used in studies of oxidation of ion channels and especially of cysteine residues. It is also used in studies of oxidative stress and is present in most cells as a metabolic byproduct (20).
Glutathione and zinc acetate were obtained from Sigma-Aldrich (St. Louis, MO). Glutathione can reduce or oxidize based on its own oxidation state and therefore functions as a redox buffer in biological systems. Glutathione was selected to test whether biological amino acid-based solutes are capable of reacting with Ag/AgCl electrodes in the same way as nonbiological reagents. Zinc is also known to modify histidines and is used to study the role of these amino acids in protein structure and function. Zinc provides an oxidizing agent that can modify the redox status of silver.
Statistical significance was determined using Student's t-test. A P < 0.05 was deemed as the point of significance.
RESULTS
Effects of TCEP and glutathione.
Ag/AgCl electrodes are the single most common type of electrode used in electrophysiology. We systematically tested the effects of oxidation and reduction reagents on four types of silver-based recording electrodes. These electrodes include the following: 1) electrically chlorided silver, 2) chemically chlorided silver, 3) sintered silver chloride pellets, and 4) bare silver wires. A fifth method of producing Ag/AgCl electrodes involves dipping Ag into a molten AgCl. However, this method was not tested because of the rarity of its use in electrophysiology.
We tested each of these electrodes with the reducing reagent TCEP at 500 μM (6, 45) to determine if the method of chloriding affected the offset produced by reduction. TCEP is used in electrophysiology experiments to reduce disulfide bonds in situations where a reducing reagent, which is less membrane permeable than DTT, is required. Moreover, TCEP provides a stronger reducing reagent than DTT at pHs <8 (19).
The range of electrode conditions used experimentally also includes poorly chlorided electrodes that may have been left in solution overnight or never chlorided at all. To encompass the maximum range of experimental conditions, we also tested electrically chlorided Ag/AgCl electrodes left in solution for 10–24 h (aged electrodes) in addition to bare Ag wire. Leaving the Ag/AgCl electrodes in solution replicates the multiple use scenario where the Ag/AgCl electrodes are not changed in between individual experiments rendering this electrode susceptible to photo reduction, dissolution of AgCl salt into the solution, and partial depletion of the AgCl layer from electrolytic reversal of the reaction in Eq. 1A during the course of experiments.
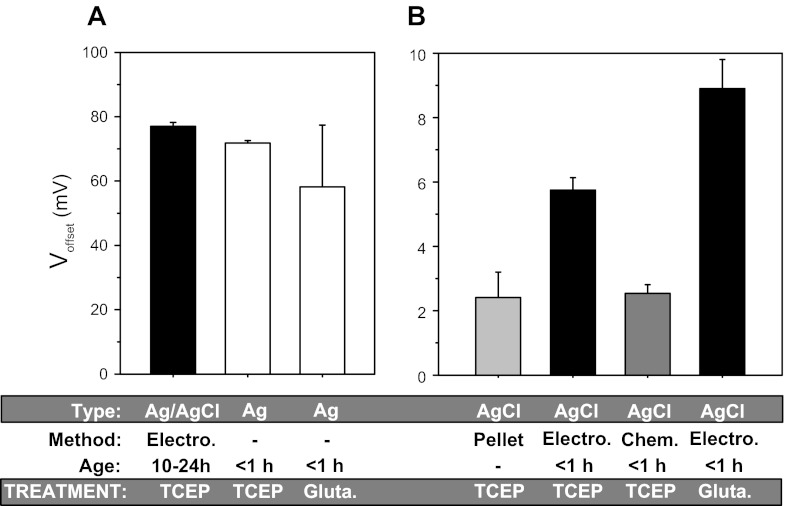
As shown in Fig. 1, TCEP caused a voltage offset of 77 ± 1.2 mV (n = 8) and 71.8 ± 0.75 mV (n = 8) for the aged and bare Ag/AgCl electrodes and Ag wire, respectively. This indicates that poorly chlorided or aged and used electrodes behave very similar to bare silver electrodes. These offsets were much larger than those observed with freshly chlorided Ag/AgCl electrodes and sintered pellets, indicating that TCEP likely reacts with metallic silver or its silver oxide byproduct and that the AgCl coating is likely protective from the effects of TCEP on silver and that this protection degrades with exposure to aqueous solutions.
Fig. 1.
The reducing agent Tris-2-carboxyethly-phosphine (TCEP) and glutathione cause large voltage offsets in Ag/AgCl electrodes. Summary of the voltage offsets produced by 500 μM TCEP and 5 mM glutathione with various silver-based electrodes. Electrodes were made from electrically chlorided silver wire (electro.), chemically chlorided silver wire (chem.), and sintered Ag/AgCl pellets (pellet). Electrodes were either freshly made (<1 h) or left overnight in amphibian Ringer's solution (10- to 24-h aged electrodes). A: bare, or aged wire. B: chlorided wire. Note the much larger y-axis scale in A; n = 4–8 in each group as described in the text.
The sintered Ag/AgCl pellet and chemically chlorided wire produced offsets with TCEP of 2.4 ± 0.8 and 2.5 ± 0.5 mV, respectively (n = 8 both groups). The electrically chlorided wire produced an offset of 5.7 ± 0.4 mV (n = 8). No offset was seen if the Ag/AgCl electrode was separated from the solution by an agar bridge (not shown). In general, all of the methods we tested for the production of Ag/AgCl electrodes were subject to significant offsets. Ag/AgCl electrodes are therefore susceptible to voltage offsets produced by reduction by TCEP with the largest offsets produced by electrodes that expose metallic silver or its byproduct silver oxide.
Many experiments also may make use of biological reducing reagents. To examine the effect of such reagents on Ag/AgCl electrodes we tested the effects of glutathione; a tripeptide with a cysteine at its center. In glutathione's oxidized form, this cysteine acts as an electron donor and thus reducing agent. Biologically, glutathione can act as either a reducing or oxidizing agent depending on if it is itself already oxidized by forming a disulfide bond with another glutathione peptide. Glutathione acts as an endogenous biological buffer to modify the redox reactions and disulfide bond formation and protein folding in the endoplasmic reticulum (14, 34). Therefore, glutathione is commonly used to examine the redox state of proteins. Five mM glutathione produced an offset of 8.9 ± 0.9 mV (n = 4) with a chemically chlorided electrode (Fig. 1) and 58.18 ± 19.23 mV for bare wire. This indicates that biological molecules, which may contain suitable reactive amino acids, may also react with Ag/AgCl electrodes yielding appreciable offsets.
Effects of DTT.
Cleland's reagent or DTT is another reducing agent commonly used in electrophysiological experiments. DTT is used in the range of 0.5 to 10 mM to reduce disulfide bonds (12, 24, 36). At 10 mM, the offsets produced were too large to allow accurate recording in our setup and therefore we examined the lower end of commonly used concentrations and chose to test the effects of 1 mM DTT.
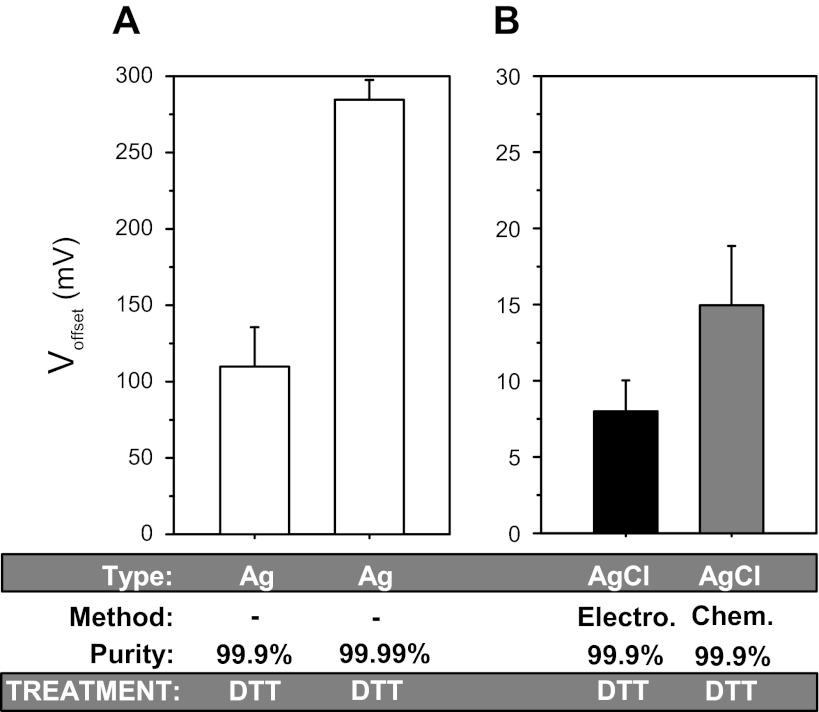
As shown in Fig. 2A, 1 mM DTT caused offsets of 109 ± 25 mV (n = 8) and 284 ± 13 mV (n = 8) in 99.9 and 99.99% pure silver wire. This indicates that besides the reaction of DTT with Ag, impurities in the silver wire (e.g., copper; Ref. 10) may also react with DTT to form offsets of the opposite direction thereby slightly reducing the overall observed voltage. Nonetheless, and irrespective of the reaction with impurities, these results demonstrate a marked effect of this reducing reagent on poorly chlorided or bare silver wires.
Fig. 2.
The reducing agent dithiothreitol (DTT) causes large voltage offsets in Ag/AgCl electrodes. Summary of the voltage offsets produced by 1 mM DTT. Two wire purities of 99.9 and 99.99% silver were examined. As in Fig. 1, bare wire (A), which simulates the use and poor chloriding scenarios, caused much larger offsets than chlorided wire (B). Note the much larger y-axis scale in A. See text and Fig. 1 legend for additional details; n = 4–8 in each group.
Smaller, but significant, offsets were also observed with freshly chlorided silver wires (Fig. 2B). These offsets averaged 7 and 15 mV for electrically and chemically chlorided wires, respectively. These data indicate that like TCEP, DTT is also capable of producing large offsets in even the more carefully produced and freshly chlorided Ag/AgCl electrodes.
Time sensitivity-.
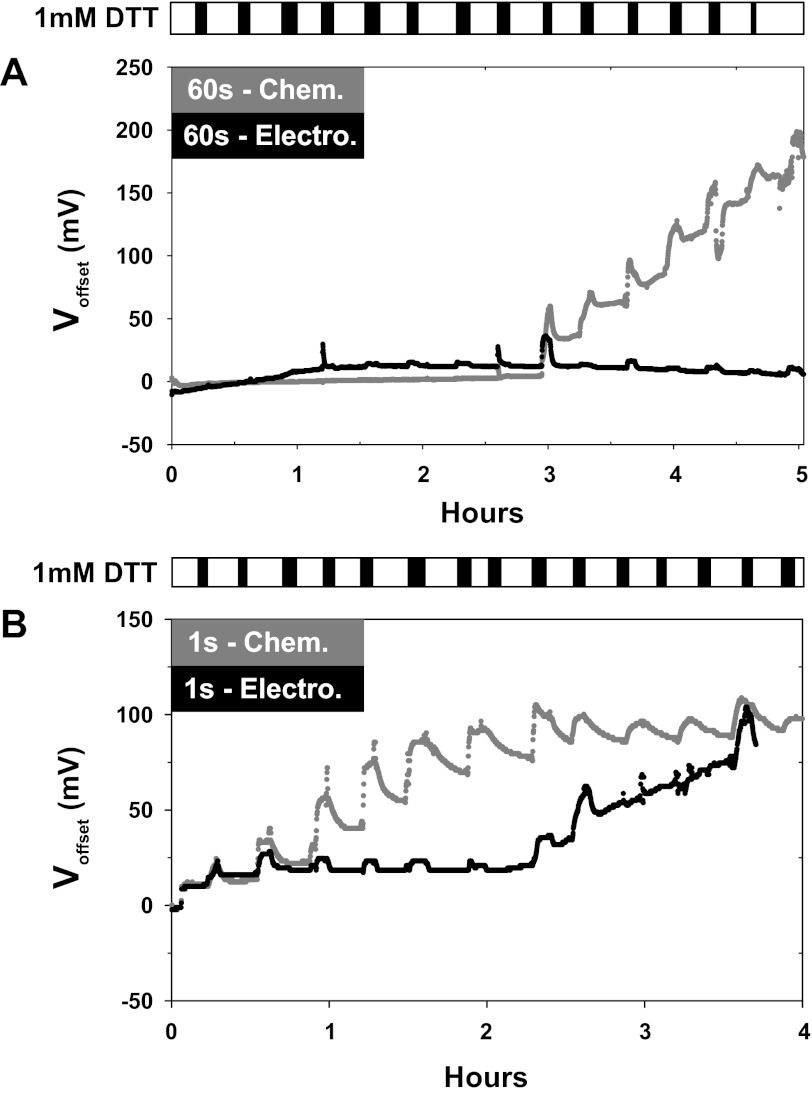
Because the reaction of Ag/AgCl electrodes to DTT showed appreciable time sensitivity, we tested the change in voltage offsets over time. To simulate a voltage clamp and electrical usage of the deposited layer of AgCl, a −80-μA current was applied to electrodes in solution over the course of 5 h as voltage was measured. To determine how the sensitivity to redox reagents changed over time, 1 mM DTT was washed in and out of the chamber at ∼20-min intervals for ∼5 min (Fig. 3). Electrodes were chlorided for 1 or 60 s. In those chlorided for 60 s (Fig. 3A) and after ∼3 h, the chemically chlorided Ag/AgCl electrodes developed a voltage offset. The magnitude of this offset increased at a rate of ∼100 mV/h. Concurrently, sensitivity to reduction by DTT also increased and approached that observed with the bare silver wire (P < 0.005). This effect was not observed with the electrodeposition method of forming AgCl. We hypothesized that these electrodes remained stable until enough AgCl was depleted to expose bare silver to DTT. In this case, the chemically chlorided wire had a much lower capacity for AgCl and earlier depletion time than that electrically chlorided for 60 s.
Fig. 3.
Time and use dependent increase in the offsets caused by DTT. Both electrically and chemically chlorided Ag/AgCl electrodes were examined. To simulate use and deplete the layer of deposited AgCl, a −80-μA current was applied to each electrode for the 4–5 h experimental duration. At ∼15-min intervals, DTT was applied for 3–5 min as shown by the scale bar at the bottom. A: electrodes chlorided for 60 s showed no initial effects of DTT for up to 3 h. After this time period, the chemically chlorided Ag/AgCl electrodes developed a >200-mV offset with no effects on the electrically chlorided electrode. B: electrodes chlorided for 1 s showed large offsets in response to DTT with the chemically chlorided electrode developing an offset first. In both cases the electrodes developed offsets were similar in magnitude to those observed with bare silver wires; n = 4 in each group.
This hypothesis was tested by examining the time-dependent offset in electrodes chlorided for 1s (Fig. 3B). Under these conditions, the chemically chlorided wire began to show an offset first followed by the electrically chlorided wire, and both eventually developed a 100-mV offset. These data indicate that the sensitivity of Ag/AgCl electrodes to reduction by DTT is dependent on the amount of exposed silver, which is in turn dependent on the time that the electrodes have been in use and the duration and method of chloriding. Therefore, it is expected that based on the degree of exposed silver, electrodes that may appear to be visually chlorided may develop very large offsets capable of producing appreciable currents under voltage-clamp conditions as outlined in the discussion.
Effects of chloride.
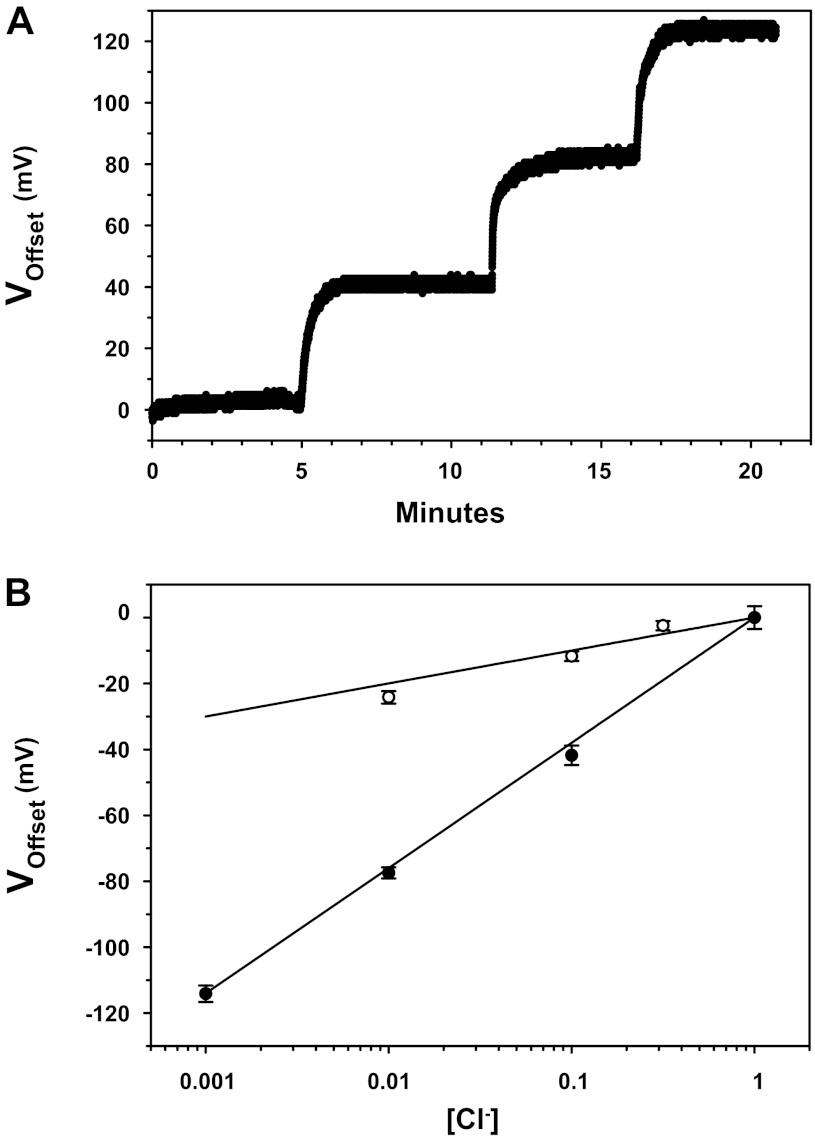
To compensate for offsets generated by changes in chloride concentration in experiments using chloride salts we tested the response of electrically chlorided and bare silver wire to varying concentrations of NaCl. Figure 4A shows the response of an electrically chlorided Ag/AgCl electrode to logarithmic changes in NaCl concentrations. Our measured results for electrically chlorided and bare wire indicate an electrode response of 38 and 10 mV/decade, respectively (Fig. 4B).
Fig. 4.
Offsets produced by chloride on Ag/AgCl electrodes. A: representative voltage trace of the response of an electrically chlorided wire as experimental solution increases in chloride concentration (1 mM, 10 mM, 100 mM, and 1 M). B: semilogarithmic plot of electrode voltage and chloride concentration. Best fit shown represents a line with a slope of 38 mV/decade concentration change for electrically chlorided wire (●), and 10 mV/decade for bare wire (○). See text for more details; n = 4 in each group.
Ag/AgCl electrodes are known to respond to chloride in a manner than can deviate from a pure Nernstian potential based on the binding of Cl− in the aqueous phase to the Ag in solid phase in the electrode (35). This effect holds true for metal-oxide electrodes as well. Therefore, experiments using Cl− compensated for its offset by subtracting the offset due to Cl− as predicted by Eqs. 4A and 4B obtained from the best fit to the experimental data.
| (4A) |
| (4B) |
Effects of H+.
The above data indicate a role of three different reducing agents in creating large offsets in Ag/AgCl electrodes. As evident from the half-cell reactions in Eqs. 1 and 2, many of the intermediates, e.g., Cl− and OH−, can form complexes with H+ and this may cause the reaction to shift further from equilibrium. To test this we examined the effect of acidification.
Shown in Fig. 5 are the effects of [H+] on Ag/AgCl electrodes offset. Acidic pHs used HCl in unbuffered solutions to prevent the formation of secondary Ag salts (silver sulfate or silver nitrate), and therefore, we adjusted our physiological solutions with HCl. Given the known effect of [Cl−] on Ag/AgCl electrodes (see Fig. 4), the effects of acidic solutions were adjusted to account for the offsets due to the chloride ions.
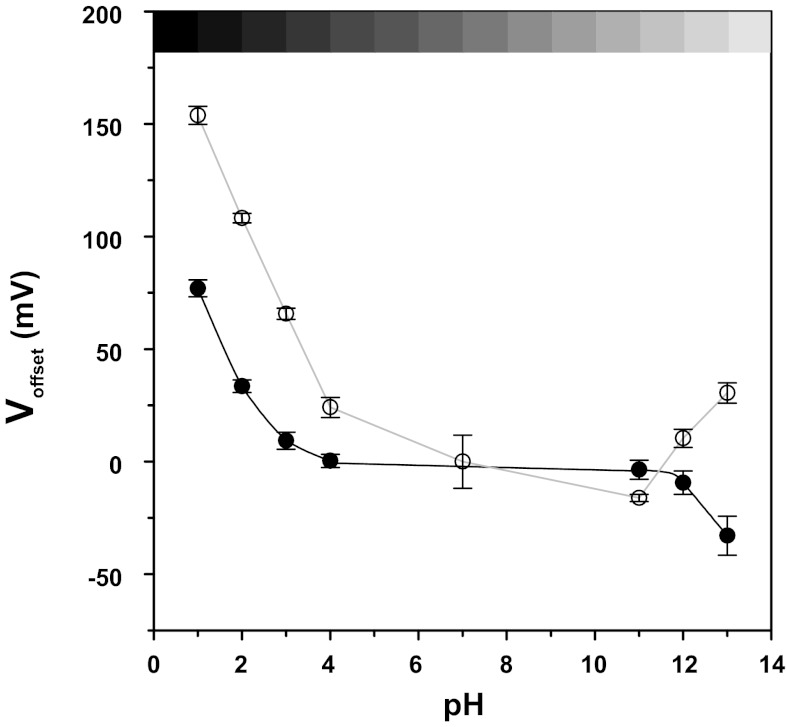
Fig. 5.
Voltage offsets produced by exposure of electrically chlorided Ag/AgCl electrodes (●) and bare silver wire (○) to solutions of various pH. Experiments were performed in unbuffered solution. All data points were corrected for the effects of Cl− concentration ([Cl−]) on Ag/AgCl electrodes as described in the text; n = 4 in each group.
As shown in Fig. 5, the offset due to pH on electrically chlorided wire was sigmoidal. In the range of pH 4–11, the voltage of the electrode was stable and exhibited a slope of 0.46 mV/pH unit between pH 4 and pH 11. Outside these values, pH <4.0 and >11.0 changes of pH caused marked effects on the Ag/AgCl electrodes with offsets up to 80 mV observed (Voffset = 76.94 ± 4.02 mV at pH 1). At the extreme end of the pH scale, the time constant of the exponential decay or rise of Voffset was 0.82.
The results for bare wire (Fig. 4) indicated a steeper slope between pH 4 and 11 (5.71 mV/pH unit). At pH <4 the offsets were larger, while at pH >11 Voffset was opposite in direction, possibly due to an effect of OH−. The maximum observed offset on bare wire at pH 1 was 153.77 ± 3.74 mV. Given these results, it is likely that most experiments modulating pH within physiological conditions are subject to minimal electrode artifacts; however, this only holds true in the case of very well-chlorided reference electrodes.
Effects of oxidizing agents.
We tested the effects of the oxidizing agent H2O2 (Fig. 6). Hydrogen peroxide is used in many experiments that examine the effects of biological oxidation and free radical formation (15, 16, 42, 43). Two concentrations were selected: 300 μM for its experimental and biological relevance (28, 43) and 10 mM to obtain better resolution when testing the effects of chloride deposition. Offsets at biologically relevant concentrations were small (−0.48 ± 0.06 mV; n = 4). The effect produced an offset of opposite polarity to that seen with the reducing reagents. Further, these effects were also sensitive to the quality of the chloride deposition where the offset voltage in bare wires was nearly threefold higher. These results are consistent with our results from the reducing reagents TCEP, DTT, and glutathione, indicating that the offsets observed are likely due to redox reactions with metallic silver.
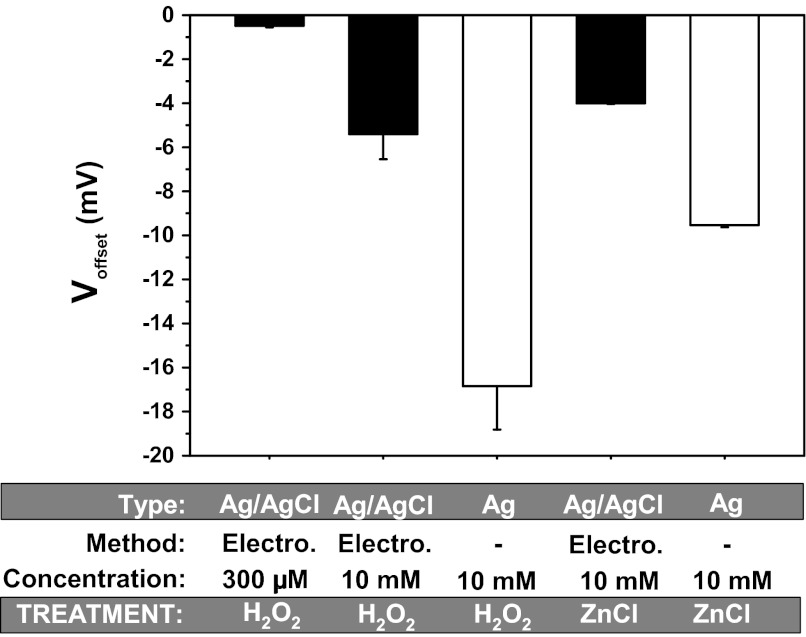
Fig. 6.
Voltage offsets produced by exposure of different electrodes to oxidizing agents. Two oxidizing agents were examined: H2O2 and ZnCl, and offsets were examined in electrically chlorided Ag/AgCl electrodes and bare wires. Although both reagents produced significant offsets, the effects were much smaller than those seen with the reducing agents. See text and discussion for additional details; n = 4–8 in each group.
In addition to H2O2, we also examined the effects of zinc, another established oxidizing agent. Zinc, because of its known interactions with histidines (30, 32, 44), is routinely used to examine the roles of these amino acids in ion channel function. We examined zinc chloride at physiologically relevant concentrations, adjusting for the offset due to chloride. As shown in Fig. 6, zinc produced offsets with the same polarity as H2O2 and opposite to those seen with the reducing agents. The effects with bare wire were also approximately threefold higher, indicating a reaction with metallic silver and a role of electrode aging in the development of larger offsets.
These data indicate a similar but opposite voltage offset produced by oxidizing agents compared with reducing agents. In all cases, the oxidizing agents produced a smaller offset than that observed with reducing agents. This is likely due to the immediate oxidation of the chlorided silver electrodes due to ambient oxygen, which would further underestimate the effect of any additional chemical oxidation (11).
Effects of ammonium chloride.
The ammonium ion is a high concentration endogenous biological molecule that can form salt complexes with chloride. High concentrations of ammonium are present in the kidneys, and ammonium-based buffers represent a major mechanism for H+ excretion by the kidneys (33, 46). Ammonium also shuttles between the kidneys and the liver as means of providing high concentrations of these ions to allow excretion of buffered excess H+ (2, 3, 37). Ammonium is also a critical component of the ammonium prepulse technique, a procedure used to allow intracellular pH changes (7). Therefore, ammonium represents a highly relevant biological molecule with special interest for its effects on ions channels and transporters.
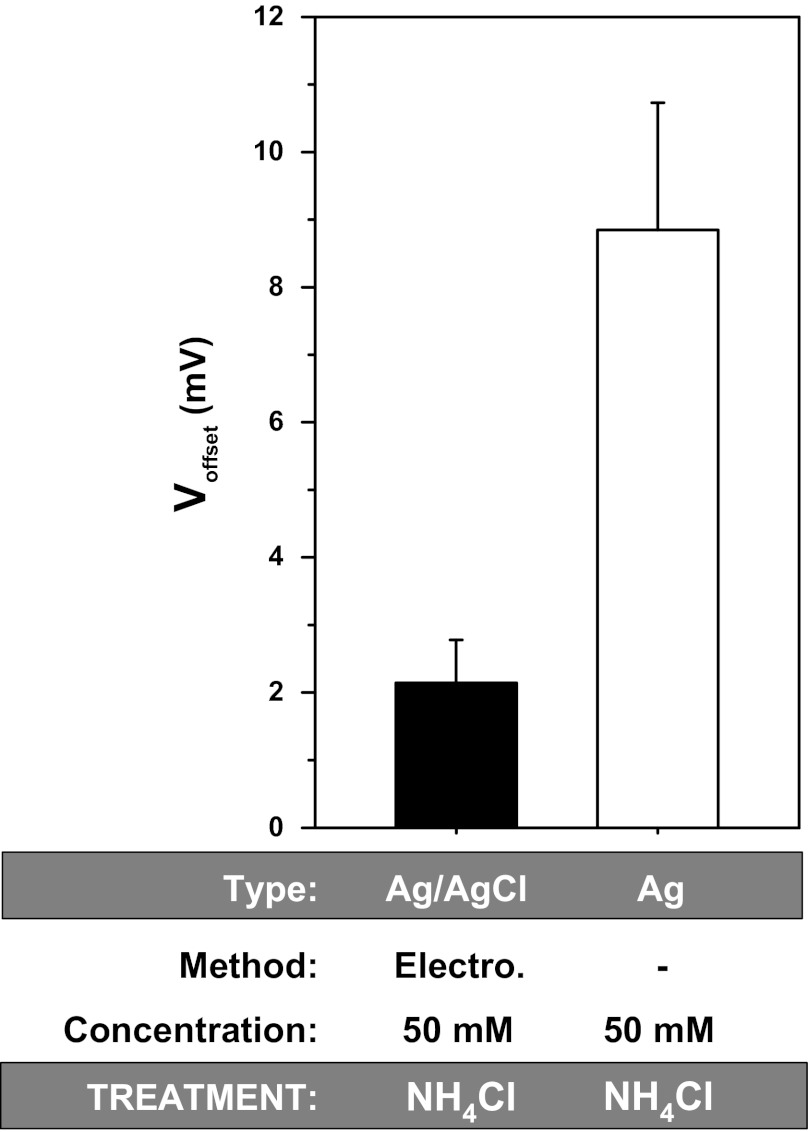
The effects of ammonium chloride are shown in Fig. 7. To separate the effects of ammonium from chloride, we first examined the effects of 50 mM NaCl followed by 50 mM NH4Cl. With electrically chlorided Ag/AgCl electrodes, the offset due to ammonium was 2.14 ± 0.63 mV (n = 4). With bare wire, the offset produced was 8.84 ± 1.88 mV (n = 4).
Fig. 7.
Voltage offsets caused by exposure of electrically chlorided and bare wire to NH4Cl. Summarized offsets were compensated for the effects of [Cl−] as described in results; n = 4 in each group.
Effects of flow.
Silver electrodes, in the absence of AgCl or when poorly chlorided, are known to be sensitive to motion artifacts (29, 48). These effects of electrode or solution movement occur in polarizable electrodes due to a double layer of charged ions that accumulates at the interface of the electrode (Ag+ and Ag+Cl−) and the electrolyte solution (Cl−). Mechanical perturbations of this double layer can then lead to changes in the half-cell potential of the electrode and marked offsets (9). In these electrodes, it is expected that voltage offsets would be highly dependent on solution flow. Gone undetected, such offsets and the resulting currents may then be interpreted as flow-dependent effects on ion channels and/or mechanosensitivity.
To test the effects of mechanical perturbations on Ag/AgCl electrodes we examined the effects of flow on the voltages generated in these electrodes compared with those separated from solution by an agar bridge. Also, to determine the extent to which Ag/AgCl electrodes developed sensitivity to flow or motion artifacts over time or use, we examined the time course of these offsets in electrodes where the deposited AgCl was gradually depleted by an applied −80-μA current.
As shown in Fig. 8, freshly chlorided Ag/AgCl electrodes exhibited a consistent offset in response to flow that averaged 7.75 ± 1.63 mV. Partial depletion of AgCl caused an increase of this offset to 107.65 ± 30.30 mV, representing a >13-fold change in the effect of flow on these electrodes. By comparison, no significant changes were observed in agar electrodes. As shown in the discussion, these offsets and their linear dependence on membrane conductance can lead to currents that mimic those of biological origins leading to an erroneous interpretation, in this case an effect of flow on ion channels.
Fig. 8.
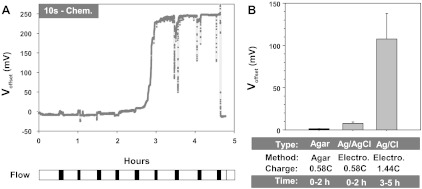
Time and use dependent increase in sensitivity of Ag/AgCl electrodes to motion artifacts. A: to simulate use and deplete the layer of deposited AgCl, a −80-μA current was applied to each electrode for the 4- to 5-h experimental duration. At ∼30-min intervals flow was stopped for ∼5 min as shown by the scale bar at the bottom. Data representative of n = 4. B: comparison of the average magnitude of offset in response to flow in the first and last 2 h of the experiment. Charge in Coulombs indicates the total charge passed to deplete the deposited AgCl under each experimental condition (calculated from current and time); n = 4 in each group.
Apparent biological effects.
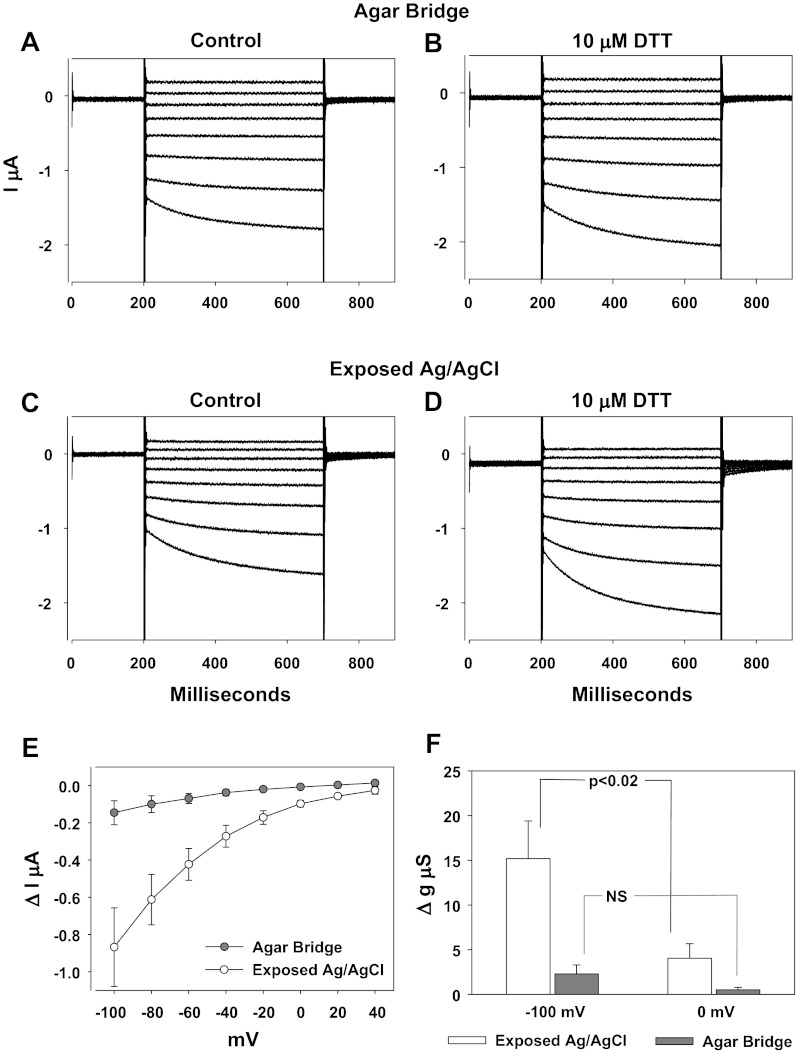
To test if the voltage offsets generated by redox reactions can alter the interpretation of biological data we examined the electrophysiological response of oocytes expressing the ENaC. Shown in Fig. 9 are the current traces in response to voltage pulses between −100 and +40 mV from a holding potential of 0 mV. Experiments tested the effects of 10 μM DTT. Two sets of experiments were carried out as follows: 1) those that utilized electrically chlorided Ag/AgCl electrodes separated from the bath by agar bridges (Fig. 9, A and B), and 2) those that utilized poorly chlorided Ag/AgCl electrodes directly exposed to the bath and DTT (Fig. 9, C and D).
Fig. 9.
Apparent stimulation of epithelial Na+ channel (ENaC) by DTT in poorly chlorided Ag/AgCl electrodes. Whole cell current in ENaC expressing oocytes were obtained between −100 and +40 mV as described in text. A and B: absence of an effect of 10 μM DTT on ENaC whole currents in an experiment which utilized agar bridge reference electrodes. C and D: apparent stimulation of whole cell currents in an experiment utilizing poorly chlorided Ag/AgCl reference electrodes (exposed electrodes). E: averaged differential I/V curves between control currents and those measured after 10 μM DTT. Note the much larger current differential at −100 mV in poorly chlorided electrodes. F: mean change of the slope conductance at 0 and −100 mV caused by 10 μM DTT; n = 4 in each group.
At 10 μM, DTT caused minimal to no effects on ENaC. This was evident from the raw data shown in Fig. 9, A and B, in cells clamped using an agar bridge. On the other hand, the currents in oocytes clamped with poorly chlorided Ag/AgCl electrodes were larger after DTT, leading to the erroneous interpretation of stimulation of ENaC.
This effect is also illustrated by the summary data in Fig. 9, E and F. Data in Fig. 9E were summarized as the mean change of current at each holding voltage, while those in Fig. 9F were summarized as the mean change of the slope conductance at 0 and −100 mV, two commonly used summary points for slope conductance measurement. As shown in Fig. 9, E and F, both methods indicate an erroneous and significant stimulation of ENaC by DTT in experiments that utilized exposed Ag/AgCl electrodes. Therefore, given the rectifying nature of the ENaC current-voltage relationship, both methods of examining ENaC activity (current and slope conductance) are sensitive to redox reaction-induced voltage shifts.
DISCUSSION
We examined effects of redox reagents on silver chloride electrodes. Experiments utilized chemical as well as biological redox reagents. In all cases, Ag/AgCl electrodes developed consistent voltage offsets that were opposite in magnitude for oxidation and reduction. These artifacts can be markedly, although not completely, attenuated by thorough chloride deposition at the start of each experiment. Further, the effects of fresh chloriding could be reversed by electrode use and were dependent on the amount of AgCl deposited. This resulted in a time- and use-dependent sensitivity of the electrodes to redox reagents. Electrodes also exhibited large offsets due to solution flow that were further increased by >13-fold when AgCl was depleted. In electrophysiological experiments with live cells, these offsets can lead to changes of whole cell currents and conductance, which can be erroneously interpreted as significant biological effects. Altogether, these results demonstrate the ineffectiveness of Ag/AgCl electrodes in experiments examining redox and flow effects and point to a source of artifacts that can under some circumstances be interpreted as biological in origin.
Interpretation as possible biological changes.
A hallmark of a specific biological response is its absence in null cells or its block by a specific channel blocker. However, as we demonstrate in Fig. 9, the redox reaction offsets can pass such criteria. In understanding this effect we used the example of a single cell, an ENaC-expressing oocyte. Similar rationale can be used for other cells expressing various ion channels measured by a variety of electrophysiological techniques that utilize Ag/AgCl electrodes.
In ENaC-expressing oocytes, the majority of membrane current (Im) and resistance (Rm) is blocked by amiloride (5). On average, ENaC expressing oocytes examined 2–3 days after injection exhibit an Rm of ∼10 kΩ. Following the addition of amiloride, Im and Rm approach values of those in control uninjected oocytes, which in the case of Rm is ∼1 MΩ. Assuming for the sake of simplicity a hypothetical Na+ reversal potential of 0 mV, a redox-induced electrode shift of ±20 mV would introduce an offset current (Ioffset) predicted by Ohm's law of 2 μA. If recordings of Im were carried out at −40 mV, the addition of the redox reagent would shift the driving voltage from −40 mV to either −60 or −20 mV resulting in a stimulation or inhibition of Im from 4 μA to either 6 or 2 μA. More importantly, this current, or at least 99% of it given the values of Rm, would appear to be sensitive to amiloride as this blocker would increase Rm from 10 KΩ to 1 MΩ and decrease Ioffset to 0.02 μA leading to a change of Im to 4.02 or 3.98 μA.
This predicted artifact was demonstrated experimentally in Fig. 9. In Fig. 9, we show the changes to Im with 10 μM DTT in electrodes exposed directly to the bath solution or separated by an agar bridge. In this case, this effect appeared as a stimulation of ENaC based on the polarity of this offset. These effects were also amiloride dependent, (not shown) and are therefore likely to be interpreted as biological in origin. Adding further confidence to the interpretation of a biological effect of DTT is that the changes of Im would also appear to be reversible and dependent on channel expression levels and essentially absent from control oocytes.
Further examination of these data would also lend credibility to the interpretation of a biologic effect of DTT. For example, a 20-mV shift of holding potential would modify the Na+ loading of oocytes during the course of an experiment and can subsequently modify the self and feedback inhibition of this channel by Na+ (1) leading to the interpretation of an effect of this reducing agent on an intrinsic channel property. This shift of Vm would also be expected to modify the kinetics of amiloride block as it is well established that it is the charged form of amiloride that blocks the channel and that blocking efficacy is voltage dependent (47) leading to an apparent shift in the Ki for amiloride with DTT.
The above calculations can yield larger or smaller changes of Ioffset depending on the ionic reversal potential of the channel being studied. Taking the reversal potential into account can further skew Ioffset, especially if accompanied by changes of ionic reversal. If we consider the relationship between driving force and current:
| (5A) |
The effect of a shift in Vm due to Voffset and the fractional change of I is Erev dependent and follows a modified version of the above equation:
| (5B) |
In this case a change from −40 to −20 mV represents a 50% change in I if Erev was 0 and would represent a 67% change of I if Erev was −10 mV because the net driving force would change from 30 to 10 mV. This effect would then be sensitive to any changes that modify Erev further ascertaining the biological origin of this response. Given this and other problems discussed above, measurements of current alone are highly sensitive to artifacts due to changes in reversal potential over the course of an experiment, or in between cells.
As observed in Fig. 9, measurement of conductance in this case does not entirely alleviate the problem. If the current-voltage relationship is linear, then it is expected that conductance would not be subject to these voltage artifacts. However, many ion channels exhibit a rectified current-voltage relationship either as an inherent channel property or following a Goldman type rectification due to the differences in the concentration of the permeant ions in the inner and outer mouths of the channel (21, 22, 49). In the example in Fig. 9, we show the ENaC currents in response to voltage trains between −100 and + 40 mV. It can be easily seen that a shift in holding potential due to the summation of Vm and Voffset can lead to apparent changes of gm in an ENaC specific manner. This effect is especially evident at −100 mV (Fig. 9, E and F). The artifact would be further exaggerated in voltage-gated channels in which a 20-mV offset can drive the channel to hyper or hypo excitability causing marked effects on channel kinetics. These offsets can also lead to the activation or inactivation of endogenous channels further confounding the interpretation of these results. For example, oocytes contain an endogenous cation channel that is activated by prolonged depolarizing holding voltage of >20 mV (25, 40).
The above examples hold true for any source of voltage offset. As demonstrated in Fig. 8, Ag/AgCl electrodes are sensitive to motions artifacts that can lead to changes of driving force as a result of flow. As predicted by Eq. 5, these effects of flow would then lead to effects on current and the interpretation of channel mechanosensitivity. These effects may even appear to be knocked out or modified by channel-specific mutations, if such mutations caused changes to channel expression and/or conductance.
Reaction chemistries.
Because the reaction of the redox reagents with the electrode is decreased by the presence of an AgCl coating, it is likely that a reaction is occurring between the Ag in the wire and the redox reagent. To illustrate how voltage offsets are generated by such reactions we examine established reactions between DTT and cysteines and extrapolate it to Ag and AgO as shown below:
| (6A) |
| (6B) |
| (6C) |
When DTT reduces AgO to Ag, it is itself oxidized to form a more stable ring structure that contributes to its strength as a reducing reagent (13). Each of the reactions we propose requires a change in the redox state of Ag, between Ag(I), and Ag(II), to Ag. These states exist as the oxides of silver, AgO, or Ag2O and likely exist in an equilibrium with Ag due to exposure to atmospheric oxygen or dissolved oxygen in experimental solutions (11). For simplicity, we delineate the reaction with Ag(I) or AgO rather than Ag2O, although both reactions are likely and follow similar chemistries.
Similarly, TCEP reduces disulfides by reaction with water and the disulfide, producing RSH groups and conjugating the oxygen to its phosphine group (38). We propose that in this case phosphine is reducing AgO directly to Ag in a reaction similar to DTT.
Glutathione can reduce or oxidize depending on the state of the thiol group in its central cysteine (34). When glutathione is in its oxidized state, it can form dimmers of glutathione disulfide and the equilibrium between glutathione and glutathione disulfide buffers redox reactions biologically (34). We propose that under ambient oxygen glutathione acts as a reducing agent and follows a similar reaction to the other reducing agents examined leading to reduction of silver oxide to metallic silver.
The oxidation reaction produced offsets of opposite direction to those observed with TCEP, DTT, and glutathione. The magnitudes of these offsets were also much lower than those observed with the reducing reagents. This is likely due to the fact that existing high levels of atmospheric oxygen is already driving the reaction in the direction of oxidation and therefore minimal further effects are observed with further oxidation. The reaction chemistries for the oxidizing agents are shown below.
Reaction chemistries of zinc with silver oxide are well established and are the main component of small “cell” batteries (18). AgO/Ag2O are reduced to Ag and generate a voltage. Reactions of H2O2 and H+ with silver are also established resulting in the production of water and reduction of silver oxide to Ag as follows:
| (7A) |
| (7B) |
| (7C) |
Conclusion.
Our data support the effects of flow on Ag/AgCl electrodes and reactions of electrophysiological bath solutions with Ag and Ag oxides, which result in offsets in the measured voltage. These offsets may appear to be channel specific and accompanied by changes of channel current. These offsets can also exhibit diverse effects on channel activation, blocker kinetics, rundown, and conductance further lending credibility to their biological nature. In most cases, these electrode artifacts can be eliminated by the use of an agar salt bridge to isolate the Ag/AgCl electrodes from the bath solution.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-55626 and the John R. Oishei Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.B. and M.S.A. conception and design of research; J.M.B. performed experiments; J.M.B. and M.S.A. analyzed data; J.M.B. and M.S.A. interpreted results of experiments; J.M.B. and M.S.A. prepared figures; J.M.B. and M.S.A. drafted manuscript; J.M.B. and M.S.A. edited and revised manuscript; J.M.B. and M.S.A. approved final version of manuscript.
REFERENCES
- 1. Abriel H, Horisberger JD. Feedback inhibition of rat amiloride-sensitive epithelial sodium channels expressed in Xenopus laevis oocytes. J Physiol 516: 31–43, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adeva MM, Souto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metabolism 61: 1495–1511, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Alesutan I, Daryadel A, Mohebbi N, Pelzl L, Leibrock C, Voelkl J, Bourgeois S, Dossena S, Nofziger C, Paulmichl M, Wagner CA, Lang F. Impact of bicarbonate, ammonium chloride, and acetazolamide on hepatic and renal SLC26A4 expression. Cell Physiol Biochem 28: 553–558, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Asami M, Kosaka K, Kunikane S. Bromate, chlorate, chlorite and perchlorate in sodium hypochlorite solution used in water supply. J Water Supply 58: 107–115, 2009 [Google Scholar]
- 5. Awayda MS. Specific and nonspecific effects of protein kinase C on the epithelial Na (+) channel. J Gen Physiol 115: 559–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernard CL, Hirsch JC, Khazipov R, Ben-Ari Y, Gozlan H. Redox modulation of synaptic responses and plasticity in rat CA1 hippocampal neurons. Exp Brain Res 113: 343–352, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Bonnet U, Wiemann M. Ammonium prepulse: effects on intracellular pH and bioelectric activity of CA3-neurones in guinea pig hippocampal slices. Brain Res 840: 16–22, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Bretschneider F, Weille JR. Introduction to Electrophysiological Methods and Instrumentation. Boston, MA: Elsevier/Academic, 2006 [Google Scholar]
- 9. Bronzino JD. The Biomedical Engineering Handbook. Boca Raton, FL: CRC: IEEE, 1995 [Google Scholar]
- 10. Buchbinder G, Verblyudov N, Clavering A. Analysis of impurities in silver by spark ablation sampling, combined with ICP-OES. Spectroscopy 24: 13–13, 2009 [Google Scholar]
- 11. Bukhtiyarov VI, Boronin AI, Savchenko VI. Stages in the modification of a silver surface for catalysis of the partial oxidation of ethylene: I. Action of oxygen. J Catalysis 150: 262–267, 1994 [Google Scholar]
- 12. Caputo C, Bolanos P, Gonzalez A. Effects of sulfhydryl inhibitors on depolarizations-contraction coupling in frog skeletal muscle fibers. J Gen Physiol 101: 411–424, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cleland WW. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 3: 480–482, 1964 [DOI] [PubMed] [Google Scholar]
- 14. Cuozzo JW, Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol 1: 130–135, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflügers Arch 459: 143–150, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species and inflammatory mediators enhance muscle spindles mechanosensitivity in rats. Pflügers Arch 457: 877–884, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Ferreira HG, Marshall MW. The Biophysical Basis of Excitability. Cambridge, UK: Cambridge Univ. Press, 1985 [Google Scholar]
- 18. Fleischer A, Lander JJ, Electrochemical Society, Battery Division, and Air Force Aero Propulsion Laboratory Zinc-Silver Oxide Batteries. New York: J. Wiley, 1971 [Google Scholar]
- 19. Getz EB, Xiao M, Chakrabarty T, Cooke R, Selvin PR. A comparison between the sulfhydryl reductants Tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal Biochem 273: 73–80, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8: 722–728, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol 27: 37–60, 1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gordon D, Chung SH. Permeation and block of the Kv1.2 channel examined using brownian and molecular dynamics. Biophys J 101: 2671–2678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrmann JM, Jakob U. Special issue: redox regulation of protein folding. Preface. Biochim Biophys Acta 1783: 519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kellenberger S, Gautschi I, Pfister Y, Schild L. Intracellular thiol-mediated modulation of epithelial sodium channel activity. J Biol Chem 280: 7739–7747, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kuruma A, Hirayama Y, Hartzell HC. A hyperpolarization- and acid-activated nonselective cation current in Xenopus oocytes. Am J Physiol Cell Physiol 279: C1401–C1413, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Lange NA. Handbook of Chemistry. New York: McGraw-Hill Book, 1934, p. 10 [Google Scholar]
- 27. Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, Dong Y. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol 299: R590–R595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Markadieu N, Crutzen R, Blero D, Erneux C, Beauwens R. Hydrogen peroxide and epidermal growth factor activate phosphatidylinositol 3-kinase and increase sodium transport in A6 cell monolayers. Am J Physiol Renal Physiol 288: F1201–F1212, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Miller HA, Harrison DC. Biomedical Electrode Technology: Theory and Practice. New York: Academic, 1974 [Google Scholar]
- 30. Musset B, Smith SM, Rajan S, Cherny VV, Sujai S, Morgan D, DeCoursey TE. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J Physiol 588: 1435–1449, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura T, Teshima M, Kitahara T, Sasaki H, Uematsu M, Kitaoka T, Nakashima M, Nishida K, Nakamura J, Higuchi S. Sensitive and real-time method for evaluating corneal barrier considering tear flow. Biol Pharm Bull 33: 107–110, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Nevin ST, Cromer BA, Haddrill JL, Morton CJ, Parker MW, Lynch JW. Insights into the structural basis for zinc inhibition of the glycine receptor. J Biol Chem 278: 28985–28992, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Owen EE, Robinson RR. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest 42: 263–276, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G, Federici G. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin Chem 47: 1467–1469, 2001 [PubMed] [Google Scholar]
- 35. Preocanin T, Supljika F, Kallay N. Evaluation of interfacial equilibrium constants from surface potential data: silver chloride aqueous interface. J Colloid Interface Sci 337: 501–507, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Ratner MA, Decker SE, Aller SG, Weber G, Forrest JN., Jr Mercury toxicity in the shark (Squalus acanthias) rectal gland: apical CFTR chloride channels are inhibited by mercuric chloride. J Exp Zool A Comp Exp Biol 305: 259–267, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Rudman D, DiFulco TJ, Galambos JT, Smith RB, Salam AA, 3rd, Warren WD. Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. J Clin Invest 52: 2241–2249, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rüegg UT, Rudinger J. Reductive cleavage of cystine disulfides with tributylphosphine. In: Methods in Enzymology, edited by Hirs CH, Serge NT: Academic, 1977, p. 111–116 [DOI] [PubMed] [Google Scholar]
- 39. Schmalwasser H, Neef A, Elliott AA, Heinemann SH. Two-electrode voltage clamp of Xenopus oocytes under high hydrostatic pressure. J Neurosci Methods 81: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Shao W, Orlando RC, Awayda MS. Bisphosphonates stimulate an endogenous nonselective cation channel in Xenopus oocytes: potential mechanism of action. Am J Physiol Cell Physiol 289: C248–C256, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Shao XM, Feldman JL. Micro-agar salt bridge in patch-clamp electrode holder stabilizes electrode potentials. J Neurosci Methods 159: 108–115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soliman D, Hamming KS, Matemisz LC, Light PE. Reactive oxygen species directly modify sodium-calcium exchanger activity in a splice variant-dependent manner. J Mol Cell Cardiol 47: 595–602, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Soodvilai S, Jia Z, Yang T. Hydrogen peroxide stimulates chloride secretion in primary inner medullary collecting duct cells via mPGES-1-derived PGE2. Am J Physiol Renal Physiol 293: F1571–F1576, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sundberg RJ, Martin RB. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chemical Reviews 74: 471–517, 1974 [Google Scholar]
- 45. Thio LL, Zhang HX. Modulation of inhibitory glycine receptors in cultured embryonic mouse hippocampal neurons by zinc, thiol containing redox agents and carnosine. Neuroscience 139: 1315–1327, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Arch 458: 137–156, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Warncke J, Lindemann B. Voltage dependence of Na channel blockage by amiloride: relaxation effects in admittance spectra. J Membr Biol 86: 255–265, 1985 [DOI] [PubMed] [Google Scholar]
- 48. Webster JG. Reducing motion artifacts and interference in biopotential recording. IEEE Trans Biomed Eng 31: 823–826, 1984 [DOI] [PubMed] [Google Scholar]
- 49. Yang L, Edvinsson J, Sackin H, Palmer LG. Ion selectivity and current saturation in inward-rectifier K+ channels. J Gen Physiol 139: 145–157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]