Summary
This article introduces the special issue on “Blood–Brain Barrier and Epilepsy.” We review briefly current understanding of the structure and function of the blood–brain barrier (BBB), including its development and normal physiology, and ways in which it can be affected in pathology. The BBB formed by the endothelium of cerebral blood vessels is one of three main barrier sites protecting the central nervous system (CNS). The barrier is not a rigid structure, but a dynamic interface with a range of interrelated functions, resulting from extremely effective tight junctions, transendothelial transport systems, enzymes, and regulation of leukocyte permeation, which thereby generates the physical, transport, enzymatic, and immune regulatory functions of the BBB. The brain endothelial cells are important components of a “modular” structure, the neurovascular unit (NVU), with several associated cell types and extracellular matrix components. Modern methods have helped in identifying a range of proteins involved in barrier structure and function, and recent studies have revealed important stages, cell types, and signaling pathways important in BBB development. There is a growing list of CNS pathologies showing BBB dysfunction, with strong evidence that this can play a major role in certain disease etiologies. The articles that follow in this issue summarize in more detail reports and discussions of the recent international meeting on “BBB in Neurological Dysfunctions,” which took place recently at Ben-Gurion University of the Negev Desert Campus (Beer-Sheva, Israel), focusing on the link between experimental and clinical studies, and the ways in which these lead to improved drug treatments.
Keywords: Blood–brain barrier, Brain diseases, Neurovascular unit
The brain and spinal cord (central nervous system, CNS) are the control centers of the body, generating central programs and strategies, processing sensory input, regulating motor output, and coordinating many of the individual and concerted activities of tissues. Moreover, networks of CNS neurons use a combination of chemical and electrical signals, which involve precise ionic movements across their membranes. Hence, to work effectively it is crucial that the CNS maintains a stable internal microenvironment. The active “neural” cells of the CNS, including neurons, macroglia (astrocytes, oligodendrocytes), and microglia contribute to local “housekeeping” maintenance of their bathing medium, the interstitial (or extracellular) fluid (ISF, ECF), whereas cellular barriers at the interfaces between the CNS and the circulating blood act as key regulatory sites, by controlling molecular flux into and out of the CNS (Abbott et al., 2009). Thus essential nutrients are delivered, waste products removed, and entry of potentially toxic or neuroactive agents and pathogens is severely restricted. The barrier layers also form the interface between the central and peripheral immune systems and act as crucial “checkpoints” to regulate CNS access of leukocytes (Engelhardt & Coisne, 2011; Greenwood et al., 2011).
The following are the three main sites of CNS “interface” barriers: the endothelium of the brain microvessels (forming the blood–brain barrier, BBB); the epithelium of the choroid plexus (specialized ependyma), which secretes cerebrospinal fluid (CSF) into the cerebral ventricles; and the epithelium of the arachnoid mater covering the outer brain surface above the layer of subarachnoid CSF. The choroid plexus and arachnoid form the blood–CSF barrier (BCSFB) (Abbott et al., 2009). At each of these sites, tight junctions between adjacent cells restrict diffusion of polar solutes through the intercellular cleft (paracellular pathway), and solute carriers on the apical and basal membranes together with ectoenzymes and endoenzymes regulate small solute entry and efflux. In brain endothelium, mechanisms of adsorptive and receptor-mediated transcytosis allow restricted and regulated entry of certain large molecules (peptides, protein) that have particular growth factor and signaling roles within the CNS. Finally, the barriers help regulate the innate immune response as well as the recruitment and entry of leukocytes, and are hence involved in both the surveillance and the reactive functions of the central immune cell population. Thus the three interface layers function as physical, transport, enzymatic (metabolic), and immunologic barriers. The barrier functions are dynamic (not fixed), and they are able to respond to a variety of regulatory signals both from the blood and the brain side, and capable of being significantly disturbed in pathology.
The brain capillaries supply blood in proximity to neurons (maximum diffusion distances typically 8–25 μm), and the brain endothelium forms the largest interface for blood/CNS exchange; therefore, the activities of the BBB are key to brain homeostasis. It is now recognized that the brain endothelium of the BBB acts within a cellular complex, the “modular” neurovascular unit (NVU) (Fig. 1), which is composed of a capillary segment with its associated pericytes, basement membranes, perivascular astrocytes, and microglial cells (Mäe et al., 2011), subserving the needs of the small number (typically < 8) of “client” neurons within that particular NVU module (Iadecola & Nedergaard, 2007; Abbott et al., 2009). Several functions of the BBB can be listed and their role in CNS homeostasis highlighted (Abbott et al., 2009). The ability of the BBB to regulate molecular traffic and keep out toxins is essential to preserve longevity of neurons and the health and integrity of neural network connectivity; ion homeostasis is essential for normal neural signaling; restricting protein entry limits the brain’s innate immune response and the proliferative potential of the CNS microenvironment; separating CNS and peripheral nervous system (PNS) neurotransmitter pools controls “cross-talk” interference between signaling networks using the same transmitters, but also allows “nonsynaptic” specific signaling by agents capable of diffusing within the protected ISF compartment; regulating entry of leukocytes allows immune surveillance with minimal inflammation and cellular damage, and the system is well organized for self-repair involving minor maintenance and correction of defects (Liu et al., 2010; Tian et al., 2011). In many of these activities, the brain endothelium is supported by the other cells of the NVU, especially the astrocytes and pericytes, with the involvement of the components of the extracellular matrix (ECM), the “endothelial” basement membrane (BM) of the endothelium/pericyte layer and the “parenchymal” BM of the perivascular astrocyte end-feet. Given the key role of circulating cells in patroling, surveillance, and repair of the CNS, it has been proposed that these cells, plus the glycocalyx at the endothelial surface (Haqqani et al., 2011), should be included in an “extended NVU” (Neuwelt et al., 2011). This reflects our improved and growing understanding of the complexity of the system and the range of cell–cell interactions involved.
Figure 1.
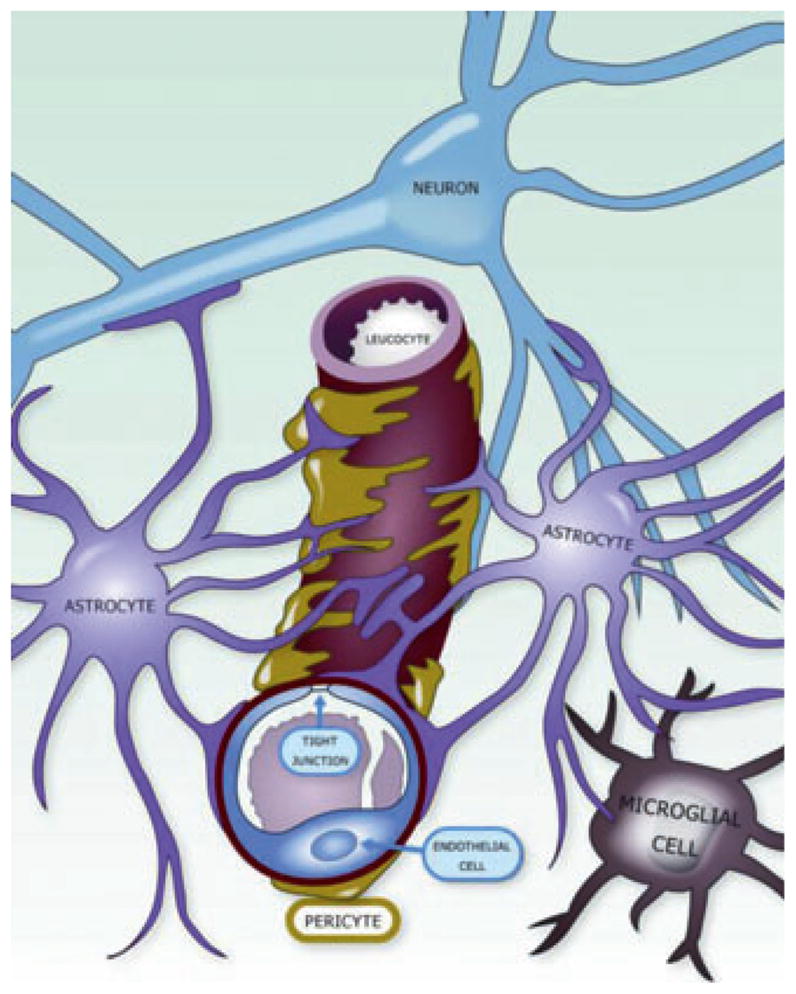
The neurovascular unit (NVU). Schematic drawing of the NVU, indicating the close spatial relationship and the complex physiologic interactions between endothelial cells and pericytes, astrocytes, microglia, and neurons.
Epilepsia © ILAE
The molecular basis underlying barrier functions are becoming clearer (Pottiez et al., 2011; Redzic, 2011), through application of many powerful techniques, including biophysical investigation of the lipid membranes, quantitative proteomics, imaging at close to the level of individual molecules, and use of genetic mutants and shRNAs to test the roles of individual components. The plasmalemma of the brain endothelium has a particularly tight packing of phospholipids and cholesterol (Seelig, 2007), permitting flux of small gaseous molecules (oxygen, CO2), but restricting permeation of certain hydrophobic molecules including many drugs, and regulating access to particular membrane proteins such as ABC (ATP-binding cassette) efflux transporters, P-glycoprotein (Pgp) (Aänismaa et al., 2008), and breast-cancer resistance protein (BCRP). The tight junctions involve interactions of cytoskeletal molecular complexes including the zonula occludens (ZO) proteins, coupled to transmembrane cleft-spanning proteins occludin and claudins 3 and 5; and junction-associated molecules (JAMs) (Paolinelli et al., 2011). The rapid turnover of many of the junctional proteins means that they show considerable dynamic activity (Shen et al., 2008), while maintaining overall junctional integrity and selectivity. Many modulators from both the blood and the brain sides can cause junctional opening (Abbott et al., 2006), perhaps to facilitate repair and removal of debris; however, in healthy conditions this is local and transient and does not significantly disturb the homeostatic function of the barrier.
The inventory of identified BBB solute carriers (SLCs) with relatively tight substrate specificities continues to grow (Abbott et al., 2009; Neuwelt et al., 2011; Parkinson et al., 2011; Redzic, 2011), mediating entry of major nutrients such as glucose, amino acids, nucleosides, monocarboxylates, and organic anions and cations, and efflux from the brain of some metabolites. Among the group of ABC transporters, Pgp (ABCB1) and/or BCRP (ABCG2) are the dominant players on the apical (blood-facing) membrane, especially Pgp in rodents and BCRP in primates, but the expression levels, localization, and roles of the multidrug-resistant associated proteins (MRPs, ABCC group) are less clear (Shawahna et al., 2011). These transporters have broader substrate specificity than the SLCs, making analysis of their structure-activity relationship (SAR) difficult (Demel et al., 2009). Synergistic activity between Pgp and BCRP has been observed (Kodaira et al., 2010), and ABC transporters and cytochrome P450 (CYP) enzymes together generate an active metabolic barrier within the NVU (Decleves et al., 2011). The array of transporters includes considerable overlap in function/apparent redundancy, possibly reflecting their evolutionary history and ensuring back-up provision in case of loss or defect of a single transporter.
Nonspecific fluid-phase endocytosis and transcytosis is downregulated in brain endothelium compared with non-brain endothelium, but for certain peptides and proteins, two main types of vesicle-mediated transfer have been documented in the BBB: receptor-mediated transcytosis (RMT) and adsorptive mediated transcytosis (AMT) (Abbott et al., 2009). There appears to be some overlap in function between caveolar- and clathrin-mediated vesicular routes, and likely involvement of other types of molecular entrapment, engulfment, and transendothelial movement that are less well characterized. Finally, although transendothelial traffic of leukocytes is restricted under normal conditions (Greenwood et al., 2011), their entry into the CNS can be facilitated in certain pathologies, both via paracellular and transcellular routes (Engelhardt & Wolburg, 2004; Muller, 2011).
Many of the routes across the endothelium described can be used for drug delivery; several classical CNS drugs including many antiepileptic agents are sufficiently lipid-soluble to diffuse through the endothelial cell membranes to reach the brain ISF, but entry for some can be significantly reduced by efflux on ABC transporters (Löscher et al., 2011). A complicating issue is that both barrier tightness and transporter expression may be altered in pathology (Potschka, 2010; Potschka et al., 2011), hence it is often difficult to predict pharmacokinetics of particular drugs in individual patients affected to different degrees by any specific CNS pathology.
Study of the development and maintenance of the NVU can give insights into changes in pathology. From the evolution of CNS barrier layers, traced back to their origin among invertebrate and lower vertebrate groups, it is clear that the first barriers were formed by specialized glial cells at the vascular-neural interface, later supplemented with pericytes and endothelium (Bundgaard & Abbott, 2008). In mammalian development, a basic barrier is formed in the endothelium of the ingrowing vessel sprouts, closely linking angiogenesis and barrier genesis with barrier induction signals likely originating from neural progenitor cells (NPCs) (Liebner et al., 2008; Daneman et al., 2009). Subsequently pericytes refine the barrier by downregulation of the “default” nonbrain endothelial features; then astrocytes upregulate the full differentiated barrier phenotype (Armulik et al., 2010; Daneman et al., 2010). Some of the signaling mechanisms involved in this induction are known including the Wnt/β-catenin (Liebner et al., 2008) and hedgehog pathways (Alvarez et al., 2011), and some of them may be involved in maintaining barrier integrity in the adult. In turn the endothelium “sculpts” the expression of channels, receptors, and transporters in the end-feet of the perivascular astrocytes to create the optimal differentiated phenotype for their role in the NVU (Abbott et al., 2006). It is likely that pericytes and microglia are also involved in this sculpting and maintenance of the NVU, but the details are still unclear.
The BBB is altered in many CNS pathologies including epilepsy (Abbott et al., 2006; van Vliet et al., 2007; Abbott et al., 2010; Marchi et al., 2012; Raabe et al., 2012). Changes can include upregulation of luminal adhesion molecules, increased adhesion and transmigration of leukocytes, increased leakiness of tight junctions and extravasation of plasma proteins, and altered expression of drug transporters and channels on endothelial cells and glia (Potschka, 2010; and see Kovacs et al., 2012, in this special issue). Given the importance of the BBB in CNS homeostasis, it is clear that gross barrier dysfunction is likely to be associated with disturbance of neural signalling. Rapid changes in neural functions appear to result from equilibrium between serum and brain electrolyte levels, leading to increased K+ and decreased Ca2+ and Mg2+ in the brain ISF. Delayed and long-lasting alterations are due to activation of the brain immune system once serum proteins leak into the brain ISF (Heinemann et al., 2012). In many pathologic states, a combination or sequence of events may make the barrier vulnerable, including trauma, hypoxia, infection, and activation of the clotting system and inflammation, but also components of the diet and environmental toxins, and perhaps genetic factors as well (Shlosberg et al., 2010). Inflammation and involvement of free radicals are proving to play major roles in many or even most of the pathologies with BBB disturbance, but the etiology and sequence of changes is generally unclear, and in many cases it is not known whether changes occur simultaneously or as part of an inflammatory cascade (Friedman, 2011; and see Kim et al., 2012, in this special issue). Certain brain regions are more often affected, including the hippocampus and cerebral cortex gray matter, but again the reasons are uncertain.
For minor damage, the cells of the NVU aided by recruitment of leukocytes may affect a repair, and short-and long-term changes in protective mechanisms including upregulation of efflux transporters and enzymes may be involved. Certainly several types of altered cell–cell interaction can be detected in pathology, particularly between endothelium and astrocytes, but also with powerful roles played by microglia, changing from a relatively quiescent and static process-bearing morphology to a more ameboid and migratory form, secreting a different repertoire of cytokines and chemokines (Saijo & Glass, 2011; Smith et al., 2012). Agents released from most of the cells of the NVU in pathology can modulate brain endothelial tight junctions, with several inflammatory mediators increasing barrier permeability, and a few agents able to counter or reverse this (Abbott et al., 2006). Potentiating effects of several cytokines including inter-leukin (IL)-1β and tumor necrosis factor α (TNFα) on the “first line” of inflammatory mediators (e.g., bradykinin) have been documented (Fraser, 2011), and there may be a system of global control of the permeability of the cerebral microvasculature via the cholinergic nervous system (Meshorer et al., 2005). Evidence suggests tight direct interactions between the cholinergic and immune systems in both the peripheral and the central nervous systems (Tracey, 2009; Gnatek et al., 2012). At the molecular level, a great many signaling pathways can be identified, regulating both the expression and activity of barrier features, particularly well documented for the effects of xenobiotics, neurotransmitters, and inflammation on Pgp (Miller, 2010). Recent identification of a number of microRNAs (miRNAs) shown to influence angiogenesis (Caporali & Emanueli, 2011) and vascular functions (Hartmann & Thum, 2011) adds a further level of complexity, and new information on a whole family of secreted and information-carrying extracellular vesicles including exosomes (György et al., 2011) adds to the repertoire of ways in which a cell or group of cells can influence other cells nearby or further away. Indeed the flow pathways allowing circulation of the brain ISF have suitable properties for this kind of nonneural communication (Abbott, 2004), and could play an important part in the dissemination of CNS pathologies that start at a relatively restricted locus.
This special issue summarizes new insights and discussions evolved during a workshop on “Blood–Brain Barrier Dysfunction in Neurological Disorders: Clinical Studies, Underlying Mechanisms and Therapeutic Implications” held in January 2012 at Ben-Gurion University Campus for Desert Research. We present summaries of clinical evidence for BBB involvement in epilepsy and other brain disorders, and cellular mechanisms critical to BBB function and dysfunction. Advances in understanding BBB function in pathology coming from a variety of experimental models, both in vivo and in vitro, help to identify the cell–cell interactions and the molecules involved, and progress is being made to link these with clinical observations. Understanding the clinical course of patients with BBB dysfunction is essential (see Shocknecht & Shalev, 2012, in this special issue); a key requirement for progress is identification and quantification of biomarkers, which will help to identify at-risk individuals, give further insights into disease etiology, and serve as indicators of disease progression and response to therapy. Part of this effort is the development of new imaging methods for analyzing vessel permeability in the human brain (Wunder et al., 2012, in this special issue). Better understanding of the roles of cells of the NVU in CNS homeostasis, and their distinct and collaborative contributions in repair, will reveal additional potential targets for therapy (see Kim et al., 2012, and de Vries et al., 2012, in this special issue). This should make it possible to design staged “cocktails” of several drugs with particular cellular and molecular targets, each given at a low dose to minimize side effects, but together providing effective therapy at critical points in the disease process.
The Workshop gave many striking examples of similar mechanisms acting across a range of CNS disorders, as well as individual features that act as hallmarks for particular pathologies, and emphasized the tremendous advantages and insights to be gained from bringing together scientists and clinicians of different disciplines.
Acknowledgments
The workshop on “Blood–Brain Barrier Dysfunction in Neurological Disorders: Clinical Studies, Underlying Mechanisms and Therapeutic Implications” was supported by the Israel Science Foundation (ISF), The Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, The International League Against Epilepsy (ILAE) – the Israeli Chapter and the Commission on European Affairs (CEA), the Edmond & Lily Safra Center for Brain Sciences, Hebrew University, and the German Science Foundation (Transregional Collaborative Research Centre, SFB/TR 3). We would like to thank Boaz Frimmerman for helping with the illustration.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Aänismaa P, Gatlik-Landwojtowicz E, Seelig A. P-glycoprotein senses its substrates and the lateral membrane packing density: consequences for the catalytic cycle. Biochemistry. 2008;47:10197–10207. doi: 10.1021/bi800209h. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2009;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bundgaard M, Abbott NJ. All vertebrates started out with a glial blood-brainbarrier 4-500 million years ago. Glia. 2008;56:699–708. doi: 10.1002/glia.20642. [DOI] [PubMed] [Google Scholar]
- Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascul Pharmacol. 2011;55:79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decleves X, Jacob A, Yousif S, Shawahna R, Potin S, Scherrmann JM. Interplay of drug metabolizing CYP450 enzymes and ABC transporters in the blood-brain barrier. Curr Drug Metab. 2011;12:732–741. doi: 10.2174/138920011798357024. [DOI] [PubMed] [Google Scholar]
- Demel MA, Krämer O, Ettmayer P, Haaksma EE, Ecker GF. Predicting ligand interactions with ABC transporters in ADME. Chem Biodivers. 2009;6:1960–1969. doi: 10.1002/cbdv.200900138. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D. Inflammatory events at blood–brain barrier in neuroinflammatory and neurodegenerative disorders: Implications for clinical disease. Epilepsia. 2012;53(Suppl 6):45–52. doi: 10.1111/j.1528-1167.2012.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immuneprivilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg H. Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med. 2011;51:967–977. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Friedman A. Blood-brain barrier dysfunction, status epilepticus, seizures, and epilepsy: a puzzle of a chicken and egg? Epilepsia. 2011;52(Suppl 8):19–20. doi: 10.1111/j.1528-1167.2011.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatek Y, Zimmerman G, Goll Y, Najami N, Soreq H, Friedman A. Acetylcholinesterase loosens the brain’s cholinergic anti-inflammatory response and promotes epileptogenesis. Front Mol Neurosci. 2012;5:66. doi: 10.3389/fnmol.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqani AS, Hill JJ, Mullen J, Stanimirovic DB. Methods to study glycoproteins at the blood-brain barrier using mass spectrometry. Methods Mol Biol. 2011;686:337–353. doi: 10.1007/978-1-60761-938-3_16. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011;55:92–105. doi: 10.1016/j.vph.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Kaufer D, Friedman A. Blood-brain barrier dysfunction, TGFbeta signaling, and astrocyte dysfunction in epilepsy. Glia. 2012;60:1251–1257. doi: 10.1002/glia.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Kim SY, Buckwalter M, Soreq H, Vezzani A, Kaufer D. Blood–brain barrier dysfunction–induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia. 2012;53(Suppl 6):37–44. doi: 10.1111/j.1528-1167.2012.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333:788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- Kovács R, Heinemann U, Steinhäuser C. Mechanisms underlying blood–brain barrier dysfunction in brain pathology and epileptogenesis: Role of astroglia. Epilepsia. 2012;53(Suppl 6):53–59. doi: 10.1111/j.1528-1167.2012.03703.x. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, Sharp FR. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol. 2010;67:526–533. doi: 10.1002/ana.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Luna-Tortós C, Römermann K, Fedrowitz M. Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? Problems and approaches in determining which antiepileptic drugs are affected. Curr Pharm Des. 2011;17:2808–2828. doi: 10.2174/138161211797440212. [DOI] [PubMed] [Google Scholar]
- Mäe M, Armulik A, Betsholtz C. Getting to know the cast – cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17:2750–2754. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- Marchi N, Granata T, Alexopoulos A, Janigro D. The blood-brain barrier hypothesis in drug resistant epilepsy. Brain. 2012;135(Pt 4):e211. doi: 10.1093/brain/awr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E, Biton IE, Ben-Shaul Y, Ben-Ari S, Assaf Y, Soreq H, Cohen Y. Chronic cholinergic imbalances promote brain diffusion and transport abnormalities. FASEB J. 2005;19:910–922. doi: 10.1096/fj.04-2957com. [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnár Z, O’Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolinelli R, Corada M, Orsenigo F, Dejana E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol Res. 2011;63:165–171. doi: 10.1016/j.phrs.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Damaraju VL, Graham K, Yao SY, Baldwin SA, Cass CE, Young JD. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr Top Med Chem. 2011;11:948–972. doi: 10.2174/156802611795347582. [DOI] [PubMed] [Google Scholar]
- Potschka H. Transporter hypothesis of drug-resistant epilepsy: challenges for pharmacogenetic approaches. Pharmacogenomics. 2010;11:1427–1438. doi: 10.2217/pgs.10.126. [DOI] [PubMed] [Google Scholar]
- Potschka H, Baltes S, Fedrowitz M, Löscher W. Impact of seizure activity on free extracellular phenytoin concentrations in amygdala-kindled rats. Neuropharmacology. 2011;61:909–917. doi: 10.1016/j.neuropharm.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Pottiez G, Duban-Deweer S, Deracinois B, Gosselet F, Camoin L, Hachani J, Couraud PO, Cecchelli R, Dehouck MP, Fenart L, Karamanos Y, Flahaut C. A differential proteomic approach identifies structural and functional components that contribute to the differentiation of brain capillary endothelial cells. J Proteomics. 2011;75:628–641. doi: 10.1016/j.jprot.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Raabe A, Schmitz AK, Pernhost K, Grote A, von der Brelie C, Urbach H, Friedman A, Becker AJ, Elger CE, Niehusmann P. Cliniconeuropathologic correlations show astroglial albumin storage as a common factor in epileptogenic vascular lesions. Epilepsia. 2012;53:539–548. doi: 10.1111/j.1528-1167.2012.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;18:8. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Schoknecht K, Shalev H. Blood–brain barrier dysfunction in brain diseases: Clinical experience. Epilepsia. 2012;53(Suppl 6):7–13. doi: 10.1111/j.1528-1167.2012.03697.x. [DOI] [PubMed] [Google Scholar]
- Seelig A. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J Mol Neurosci. 2007;33:32–41. doi: 10.1007/s12031-007-0055-y. [DOI] [PubMed] [Google Scholar]
- Shawahna R, Uchida Y, Declèves X, Ohtsuki S, Yousif S, Dauchy S, Jacob A, Chassoux F, Daumas-Duport C, Couraud PO, Terasaki T, Scherrmann JM. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8:1332–1341. doi: 10.1021/mp200129p. [DOI] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Sawyer A, Kocaoglu FB, Kyriakides TR. Astrocyte-derived thrombospondin-2 is critical for the repair of the blood-brain barrier. Am J Pathol. 2011;179:860–868. doi: 10.1016/j.ajpath.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Wunder A, Schoknecht K, Stanimirovic DB, Prager O, Chassidim Y. Imaging blood–brain barrier dysfunction in animal disease models. Epilepsia. 2012;53(Suppl 6):14–21. doi: 10.1111/j.1528-1167.2012.03698.x. [DOI] [PubMed] [Google Scholar]


