Abstract
Traumatic brain injury (TBI) is the leading cause of death in young adults and children. The treatment of TBI in the acute phase has improved substantially; however, the prevention and management of long-term complications remain a challenge. Blood–brain barrier (BBB) breakdown has often been documented in patients with TBI, but the role of such vascular pathology in neurological dysfunction has only recently been explored. Animal studies have demonstrated that BBB breakdown is involved in the initiation of transcriptional changes in the neurovascular network that ultimately lead to delayed neuronal dysfunction and degeneration. Brain imaging data have confirmed the high incidence of BBB breakdown in patients with TBI and suggest that such pathology could be used as a biomarker in the clinic and in drug trials. Here, we review the neurological consequences of TBI, focusing on the long-term complications of such injuries. We present the clinical evidence for involvement of BBB breakdown in TBI and examine the primary and secondary mechanisms that underlie such pathology. We go on to consider the consequences of BBB injury, before analyzing potential mechanisms linking vascular pathology to neuronal dysfunction and degeneration, and exploring possible targets for treatment. Finally, we highlight areas for future basic research and clinical studies into TBI.
Introduction
Traumatic brain injury (TBI) is the most frequent cause of deaths in young adults and children in the developed world. In the US alone, 1.4 million such injuries occur annually and account for over 50,000 deaths.1 Thus, TBI is a matter of major concern for clinicians, researchers, social workers and other health-care providers. Many individuals who survive TBI experience long-term or lifelong disabilities, which require daily medical or social attention. Indeed, over 2% of the US population is believed to experience TBI-associated disabilities.2 This figure equates to an annual expenditure of $60 billion on direct (medical services) and indirect (loss of productivity) costs relating to this condition.3
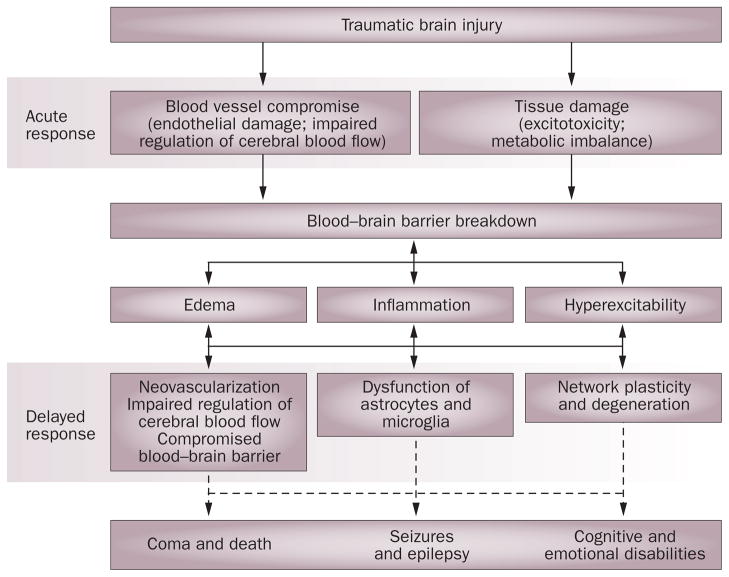
The pathology of TBI is highly heterogeneous, with diverse manifestations that result from both immediate and delayed mechanisms (Figure 1). The immediate primary injury is impact related and is considered to be untreatable but preventable, whereas the ‘rolling’ pathology of the delayed second phase of damage allows a time window for intervention and has, therefore, attracted a great deal of attention.
Figure 1.
Pathophysiological events in traumatic brain injury. Early symptoms of blood vessel and brain parenchyma compromise appear as blood flow irregularities and lead to metabolic imbalance, ischemia, hypoxia and excitotoxicity. Such processes, which are associated with breakdown of the blood–brain barrier, might lead directly to the induction of signaling cascades and complex interactions between pathological processes within the neurovascular unit. The result of these interactions is the formation of brain edema, a local inflammatory response and an increase in neuronal excitability. These early events might progress, interact and initiate acute complications, such as increased intracranial pressure, ischemic cell damage, seizures and death. In parallel, slower pathophysiological mechanisms, such as neovascularization, transformation and dysfunction of astrocytes, and changes in synaptic wiring, underlie the development of epilepsy, psychiatric and cognitive disabilities,165 and neurodegenerative pathologies such as Alzheimer disease.109,110
Patient management in TBI is complicated by the various temporal pathological phases. The mainstay of inflicted cranial-damage management in the immediate setting relies on strict adherence to prehospital guidelines.4 By contrast, the most appropriate therapeutic approach in the second injury phase, which develops within hours to days following the trauma, remains debatable and is subject to the clinical considerations of the individual trauma center,5 although evidence-based guidelines are issued periodically.6 In a similar fashion, current TBI classification methods and injury severity assessments mainly rely on the primary presentation and a patient’s symptoms (Box 1), and do not relate to ongoing pathophysiological processes. The situation surrounding care in the second injury phase might reflect the fact that, to date, clinical trials related to TBI management strategies have produced conflicting evidence-based data and, in many instances, the promising results obtained from animal models have not been replicated in phase III clinical studies.7,8 Thus, while outcomes of TBI in the acute setting have substantially improved in the past 20 years, the prevention, management and resolution of long-term complications still remain a challenge.9
Box 1. Classification of traumatic brain injury.
Traumatic brain injury (TBI) is a highly heterogeneous condition, with patients experiencing injuries that differ in etiology, anatomical location, clinical severity and pathophysiology. The course of TBI can also vary with age, temporal evolution of the insult, prior comorbidities, genetic background and other factors. The most commonly used TBI classification methods are based on injury mechanisms (for example, closed versus penetrating injuries). Further subclassification is usually made in accordance with injury severity and is often achieved by use of the 15-point Glasgow Coma Scale, which classifies patients into the broad categories of mild, moderate and severe injury.9 This symptomatic classification has been designed to allow rapid diagnosis, management, and assessment of prognosis; however, this scale possesses several shortcomings.9 Of note, no information is provided concerning the pathophysiological mechanisms that underlie the clinical presentation. Thus, this simplistic classification of patients might partly underlie the failure to translate favorable results from animal studies into successful clinical trials.
Improvements in classification systems will lead to a better understanding of the mechanisms of TBI and help to refine treatments and improve patient outcomes. Specifically, the need has been raised for a pathoanatomically based classification system for TBI to allow the successful translation of targeted therapies from the bench to the bedside.166
Advancement of the understanding of the mechanisms underlying the long-term complications of TBI is a prerequisite for the development of new management strategies to follow the initial life-saving treatments. A plethora of data has now accumulated indicating a central role for vascular integrity—specifically, the permeability of the blood–brain barrier (BBB)—in the mediation of brain damage, including the delayed appearance of neuronal dysfunction and death.10–17 BBB breakdown is frequently associated with a myriad of neurological pathologies (some of which can be long lasting14,18) and, hence, has attracted growing attention as a novel and eminent target for intervention in the setting of brain injury. An understanding of the mechanisms underlying BBB breakdown-induced brain damage might lead to novel targets for prospective treatment strategies in the management of patients with TBI. In this article, we review clinical and experimental data implicating the involvement of BBB breakdown in pathophysiological processes following TBI. We also outline potential intervention schemes for TBI that target BBB-related pathologies.
Events after traumatic brain injury
Blood–brain barrier breakdown
Results from animal model studies and substantial clinical data both suggest that BBB breakdown frequently follows head trauma and can last from several days to weeks, or even years after the acute event.14,19,20 In animal models of TBI, a focal impact delivered to the cranium has been shown to result in BBB breakdown,21–24 with the severity and extent of such breakdown being directly related to the method used to deliver the blow (these models have been comprehensively reviewed elsewhere25,26). Like blood–spinal cord barrier breakdown following spinal cord injury,27 BBB breakdown after experimental brain injury is typically biphasic in nature.24,28 Onset of the early phase is rapid: the permeability of the BBB typically reaches a maximum within a few hours and subsequently declines. The onset of the second phase is delayed, starting from 3–7 days following injury, and probably constitutes part of the brain’s response to the injury.
For most patients, clinical data indicate that BBB permeability returns to normal within days to weeks following TBI,29–31 although few quantitative data exist regarding the relationship between the extent of BBB damage and the mechanism and severity of TBI. Indeed, in some patients BBB disruption has been documented months or years after mild injuries.14,19 No firm evidence is available to indicate whether these long-lasting disruptions are primary events of TBI or secondary to ongoing pathological processes.
In some cases of TBI, BBB breakdown can be a direct result of the traumatic impact injury incurred (primary BBB damage). Following the infliction of a focal head insult, the endothelium of small blood vessels often incurs a concomitant shear injury,32 which leads to impairments in the regulation of the BBB, cerebral blood flow and metabolic processes. Metabolic imbalances can ultimately lead to various secondary complications, including the formation of an ischemic zone. Development of such a zone can result in tissue hypoxia and is associated with a poor long-term clinical outcome and an increase in the risk of mortality.33,34 Such a scenario might further facilitate localized BBB breakdown.35 Other mechanisms, such as vasospasm,36 cerebral blood flow autoregulatory failure,37 irregularities in nitric oxide secretion38 and coagulopathy,39 might coincide with damage to the structural integrity of the BBB40,41 and contribute to the resultant ischemic state. BBB breakdown might also contribute to the extracellular accumulation of excitatory amino acids, which results in excitotoxicity.42 The latter phenomen might occur indirectly through ischemia-mediated changes in neuronal membrane potential, or directly through loss of efflux transporters situated in the BBB.43 In addition, BBB breakdown leads to exposure of the brain tissue to serum-derived molecules, which serve as signaling mediators for brain repair mechanisms but also facilitate further BBB breakdown (see below). The nature of the reciprocal interactions between impact-related pathologies, innate defense and repair mechanisms, and BBB integrity determine the short-term and long-term consequences of the injury.
In addition to BBB dysfunction related to the initial injury, BBB damage can arise secondarily to the abnormal brain activity, astrocytic dysfunction, inflammation-related mechanisms and metabolic disturbances that typically comprise part of the brain’s response to injury (Box 2). Manifestations of secondary BBB breakdown, initiated within hours or days following TBI, are considered to be treatable and to have a considerable influence on the long-term clinical outcome of patients. This phase of damage has, therefore, occupied a central place in both clinical and basic research relating to TBI pathophysiology.
Box 2. The blood–brain barrier and the neurovascular unit.
The blood–brain barrier (BBB) is the regulated interface between the peripheral circulation and the CNS and was first observed by Paul Ehrlich in 1885,167 although the nature of the BBB was debated well into the 20th century. Anatomically, the BBB comprises the cerebral microvascular endothelium, which, together with astrocytes, pericytes, microglia, neurons and the extracellular matrix, constitute a ‘neurovascular unit’ that is essential for the normal function of the CNS. The various components of the neurovascular unit interact closely under physiological conditions. Following brain injury, the normal patterns of communication exhibited by these intercellular components can be markedly altered. Such changes lead to abnormalities in vascular responses to neuronal activity and metabolic demands (neurovascular coupling), tight junction protein expression and extracellular environment control (mediated by astrocytes), inflammatory responses (mediated by astrocytes and microglia), and synaptic activity (including the interactions between neurons, astrocytes and microglia). Development of our understanding of how the various components of the neurovascular unit interact with each other holds significant promise for the prevention and treatment of neurological diseases.
The underlying molecular changes leading to BBB breakdown following TBI are not completely clear, as data from animal models of isolated TBI are scarce and molecular data from patients are difficult to gather. In animal models, accumulated data suggest that hours to days after brain injury BBB breakdown is associated with an increase in the numbers of endothelial caveolae, which leads to transcytosis of plasma proteins44,45 and decreases in expression of junctional adhesion and tight junction proteins.46,47 Reactive cellular activity at the neurovascular junction has also been observed, including an increase in the migratory activity of pericytes,48 and proliferation of blood vessels due to upregulation of vascular endothelial growth factor (VEGF).49
Secondary brain damage
A number of mechanisms have been proposed for the emergence of secondary brain damage following TBI-associated BBB damage. These mechanisms are all a consequence of either the disruption of the intricate relationship between the cellular elements of the neurovascular unit10,12 (Box 2) or deficits in the structural integrity of the tight-junction complexes of the BBB. Processes such as edema, neuroinflammation and cell death might contribute to the pathophysiology of TBI independently or synergistically.
Edema
An inevitable consequence of BBB disruption is an increase in the permeability of the damaged endothelium, which can, in turn, lead to brain edema (characterized by a net gain of brain tissue volume). Edema that evolves after TBI has a substantial vasogenic component resulting from the entry of a protein-rich exudate through the widened endothelial tight junctions. The entry of this exudate disrupts the balance of hydrostatic–osmotic forces mediating parenchymal fluid homeostasis and can lead to fluid accumulation in the brain extracellular space. An increase in intracranial pressure can ensue from such fluid accumulation, thereby lowering the perfusion pressure—further compromising the already frail cerebral blood flow—and possibly initiating or exacerbating an ischemic state. Additional underlying extracerebral impediments to cerebral perfusion and oxygenation, such as massive peripheral bleeding or cardiovascular or pulmonary injury in the setting of trauma, might also prove detrimental to the patient’s condition.
Inflammation
The long-held view that the brain was an immune-privileged site has changed dramatically in the past two decades. Inflammatory processes are now known to accompany many brain pathologies, including multiple sclerosis, epilepsy, AIDS, stroke, Alzheimer disease (AD) and TBI.50–52 Furthermore, the BBB, which for years was regarded as the prime isolator of brain tissue from the immune system, is now recognized to be a mediator of neuroinflammatory processes.
The inflammatory response in patients with TBI begins within hours after injury and lasts up to several weeks.53 Animal models of TBI have shown that an influx of peripheral neutrophils occurs following injury, with a time course that correlates with BBB disruption.5,54 Macrophages, natural killer cells, T helper cells, and T cytotoxic–suppressor cells are also present in the brain following TBI.55 Migration of leukocytes into the damaged tissue is mediated, in part, by the expression of endothelial intercellular adhesion molecule (ICAM) 1, the upregulation of which has been described in a variety of experimental TBI models.56,57 Indeed, the level of soluble ICAM1 in the cerebrospinal fluid of patients with severe TBI is known to correlate with the extent of BBB breakdown.58
Following infiltration, leukocytes release pro-inflammatory cytokines, cytotoxic proteases and reactive oxygen species, thereby activating local microglia, which subsequently function in a similar way to infiltrating macrophages.59 Activated microglia can contribute to BBB opening.60 Chemokines, adhesion molecules, and pro-inflammatory cytokines released from activated microglia61 further mediate the recruitment of hematogenous cells from the periphery, perpetuate activation of resident CNS cells, and contribute to the overall increase in BBB permeability.62 In addition, activation and upregulation of matrix metalloproteinases (MMPs), which degrade the neurovascular basal lamina, lead to a further increase in blood vessel permeability63,64 and, as a result, contribute to the development of edema.
Alterations in systemic and intrathecal levels of cytokines—including interleukin (IL) 1, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor (TNF) and transforming growth factor β (TGF-β)—have been reported in patients following TBI. Regional messenger RNA levels and protein concentrations for several of these cytokines increase markedly in the acute post-traumatic period following experimental brain trauma.53,65 Post-traumatic activation of the complement system has also been well documented and shows a strong correlation with the level of BBB disruption in patients.31
Despite the evidence provided above, the true nature of the contribution of neuroinflammatory processes to the clinical outcome of TBI has not yet been clarified. Inflammation following BBB disruption in TBI might be viewed as simultaneously helpful and deleterious. Indeed, inflammation might be vital for the implementation of tissue repair and reorganization, but extended or excessive activation of inflammatory processes might lead to poor clinical outcomes by initiating processes such as cell death66 or epileptogenesis.67,68
Cell death
In TBI, a necrotic focus rapidly develops in the area that has sustained the blow, as a result of a loss of membrane integrity of vascular and parenchymal cells. This process is exacerbated by a decrease in metabolic supply (in the face of rising demand) and by excitotoxicity. TBI also induces the cell cycle re-entry of both mitotic cells (astroglia and microglia) and non-mitotic cells (neurons),69 with the former leading to glial scar formation and exacerbation of neuroinflammation, and the latter resulting in the induction of apoptotic signaling pathways and cell death. Indeed, a second, delayed wave of neuronal death mediated by apoptotic processes has been reported in both animal models and humans.70–74 The timescale for apoptosis correlates with the appearance of inflammation and edema formation. This finding suggests that the latter two processes—alongside increased intracranial pressure—are important initiators of apoptosis following TBI.75
The apoptosis of endothelial cells that occurs following injury is related to downregulation of constitutive anti-apoptotic growth factors and upregulation of pro-apoptotic factors, which are known to further promote BBB breakdown.76 The death of such cells can lead to augmentation of BBB permeability77 and, as a result, neuronal loss.17,78 In turn, this loss of neurons leads to a neuronal deficit and astroglial scar formation, which itself is associated with functional deficits and impairment of cognitive recovery following TBI.79 Termination of the apoptotic sequence following TBI, like the series of events that comprise BBB repair, can take a long time. This prolonged sequence of cell death might give rise to the chronic degenerative processes and cognitive decline that are often seen after the initial recovery period.80
Sequelae of blood–brain barrier damage
The pathological processes that develop following brain injury inevitably lead to neuronal death, which can be immediate or delayed. BBB disruption alone can initiate a transcriptional program that results in delayed neuronal loss.78,81 Such disruption might also influence the course of long-term TBI complications, such as cognitive and psychological impairments, AD and seizures.17,82 Of note, while these long-term complications are all characterized by neuronal death, loss of neurons might not be the sole or even the most important cause of these pathologies. Indeed, tissue remodeling and altered connectivity between neurons, scar formation, alterations in the extracellular ion composition, accumulation of toxic substances as a result of faulty influx–efflux mechanisms, and extravasation of plasma proteins might all contribute to aberrant neuronal function. Substantial evidence has now accumulated showing the direct influence of BBB disruption on the development of long-term brain pathologies. This evidence opens new vistas for neuroprotective intervention in patients with TBI.
Seizures and epilepsy
The fact that TBI can give rise to seizures and epilepsy has been accepted for several decades,83,84 although the pathophysiological mechanisms that underlie the development of these complications are still not properly understood. A relationship between BBB dysfunction and epilepsy has been well documented in both animal and clinical studies,51 with several groups providing evidence that BBB disruption is a fundamental catalyst for the initiation of hypersynchronized epileptic activity in the injured brain.
The probability of experiencing seizures increases directly with the severity of TBI,85 and several studies in patients have shown that cortical areas exhibiting BBB disruption can display spatial overlap with regions of abnormal neuronal activity (as measured by scalp EEG recordings).19,86 In line with these findings, a study in a rodent model of chronic epilepsy showed that seizure frequency correlated with the extent of BBB leakage.16
Studies in human patients have showed that a substantial proportion of post-traumatic seizures develop in the first 24 h after injury87 or in the subsequent week.84 These events can be suppressed by the prophylactic administration of antiepileptic drugs. By contrast, late-onset seizures, which appear in the weeks or months following trauma, are usually antiepileptic drug resistant and are viewed as a consequence of local circuit modulation by transcriptional changes and synaptic rewiring. Notably, in animal studies where the BBB has been disrupted to induce a chronic epileptic focus, early dysfunction of astrocytes was observed before neuronal hypersynchronization occurred.88,89 Changes in astrocyte morphology and functions following TBI have been well documented,90 and the potential role of astrocytes in seizure generation and epilepsy has been described in both experimental animals and patients with epilepsy.88,91–95 Recent studies have indicated novel physiological roles for glial cells in the CNS, including modulation of synaptic transmission and plasticity.96,97 Thus, glial cells—specifically, following their activation during injury—could have a notable role in the neuronal network reorganization, hypersynchronicity and hyperexcitability that leads to brain malfunction and seizures.89
Several functional changes in astrocyte properties could be involved in the alteration of neuronal functions. Reductions in the expression of inwardly rectifying potassium channels and aquaporin-4 water channels, which are considered to be crucial for the regulation of the brain’s extracellular potassium ion levels and water content, respectively, could influence neuronal function. In addition, a reduction in expression of gap junction proteins (connexins) might have a role, as these proteins form functional channels between cells that are important for the spatial buffering of small molecules and ions (for example, potassium ions). Astrocytes produce many pro-inflammatory and anti-inflammatory molecules that might be pro-epileptogenic or anti-epileptogenic. Thus, the observed increase in cytokine release from glial cells might affect the activity of neurons. Finally, as some glial cells have a crucial role in the uptake and metabolism of glutamate (with some glial cells expressing glutamatergic receptors and being able to release glutamate), impairments in these processes could affect neuronal function. Indeed, transformed (or activated) astrocytes seem to affect extracellular glutamate levels via decreases in their abilities to both take up and metabolize this neurotransmitter.98 The extent to which each of the potential mechanisms described above is critical to neuronal activity is still not known, although some experimental and theoretical models predict a critical role for the maintenance of potassium homeostasis.89
Abnormal focal rhythmogenesis and seizures in patients following TBI have been documented years after the primary injury and have been found to be associated with long-lasting focal increases in BBB permeability.14,19 Thus, in the clinical setting, a temporal overlap between BBB disruption and seizure activity has been revealed but a causal relationship has yet to be demonstrated. Experimental animal data, however, have consistently shown that seizures are readily initiated following BBB disruption.16,51,67,78,88,94,99 Furthermore, Marchi and colleagues100 reported focal motor seizures in some patients with primary CNS lymphoma who underwent chemotherapy that utilized mannitol, a well-known BBB opener, to maximize drug delivery. These researchers documented a strong correlation between serum S100β levels—a marker of BBB breakdown—and the appearance of immediate focal seizures. In addition, Ivens et al.99 documented prolonged seizures in BBB-compromised patients with hyperperfusion syndrome following carotid surgery.
Additional mechanisms might contribute to the propensity of patients with hyperperfusion syndrome to generate seizures. The data described above, however, indicate the need for further research to elucidate the pathophysiological means by which injury to the BBB can lead to seizures and epilepsy. In support of this view, our group developed an animal model that showed that entry of serum constituents into the interstitial fluid through a disrupted BBB could initiate a cascade of events that led to the generation of focal paroxysmal hypersynchronous episodes.94 We demonstrated that the uptake of serum albumin by astrocytes was specifically regulated by TGF-β receptors and resulted in a detrimental effect on the capacity of such cells to maintain ionic equilibrium. The loss of ionic equilibrium heralded a transcription-mediated local synaptic reorganization, which underlied the focal seizures that appeared 4–7 days later.78,88,89,94,101 Our studies further demonstrated that blocking TGF-β receptors and, hence, astrocytic albumin uptake prevented the transcriptional changes and the generation of epileptiform activity.81 Thus, our model of focal BBB disruption extended previous work in a rodent TBI model, which showed that increased extracellular potassium was related to hyperexcitable activity.102 Contradictory results regarding this relationship have been published;103 however, we believe that albumin uptake could offer a novel target for intervention within a clinically relevant therapeutic time window.
Growing interest in local CNS inflammatory processes following TBI and their contribution to the evolution of secondary neuronal damage has revealed another potential mechanism for BBB-mediated epileptogenesis. Brain injury induces neuroinflammatory processes, including production and secretion of cytokines. In turn, cytokines contribute to the development of acute neurodegenerative processes, including seizure initiation.61 Since seizures themselves initiate a local inflammatory response, the issue of whether inflammation and the increased levels of cytokines observed in epileptic brains are a consequence of or prodromic to seizure appearance is not yet resolved. The roles of cytokines such as IL-1, IL-6 and TNF in the pathogenesis of seizures are rapidly being clarified through studies making use of advanced molecular and pharmacological techniques, as well as research involving genetically engineered mice that exhibit perturbed cytokine signaling. Over the past decade, Vezzani and colleagues68 and others have consistently produced evidence pertaining to the role of IL-1β in epileptogenesis. Following CNS damage, IL-1β is rapidly released from activated microglia and astrocytes. This cytokine directly promotes excito-toxicity104 and seizures105 through activation of neuronal IL-1 receptors and subsequent induction of Src kinase-mediated tyrosine phosphorylation of the NR2B subunit of the N-methyl-D-aspartic acid (NMDA) receptor. Such phosphorylation leads to an increase in NMDA receptor-mediated calcium influx into neurons and, hence, the promotion of epileptic activity and excitotoxicity.62
In addition to the role of IL-1β in the promotion of seizures and excitotoxicity, the BBB is rapidly disrupted after both acute and chronic exposure to this cytokine.106 Loss of BBB function is further increased by upregulation of adhesion molecules, activation of MMPs,107 and catabolism of arachidonic acid at the level of the brain microvasculature.108
Neurodegeneration and Alzheimer disease
TBI is known to be associated with an increased risk of developing AD;109,110 however, the role of the BBB in the pathogenesis of this disease is only just beginning to be revealed. Evidence suggests that the accumulation of amyloid-β (Aβ) in the brain is a primary process in the pathogenesis of AD.111 Alterations in BBB integrity might have an important role in the appearance of increased levels of Aβ in the cerebrospinal fluid after brain injury.112
The presence of the ε4 variant of apolipoprotein E (APOE) in the brain has been shown to be correlated with an increase in the risk of sporadic-type AD development.113 Interestingly, complexes of Aβ and APOE ε4 have been shown to more-readily cross the BBB than do complexes of this peptide with other variants of this lipid binding protein.114 A meta-analysis indicated that the presence of the APOE ε4 allele was associated with an increase in the risk of a poor long-term outcome following TBI but was not associated with the initial severity of brain injury.115 Another study, however, showed that the presence of APOE ε4 had no substantial effect on long-term outcomes following mild pediatric TBI.116
Activation of the BBB-derived low-density lipoprotein receptor related protein (LRP) 1 comprises one of the main routes of Aβ extrusion from the brain,117 while the receptor for advanced glycation end products (RAGE) controls an important mode of intrusion for this peptide into the brain.118 These receptors are both localized to the endothelial cells of the BBB. Under in vitro conditions mimicking brain ischemia119 and in brain tissue from patients with AD,120 the BBB exhibited a diminished ability to properly remove Aβ owing to changes in the levels of LRP1 and RAGE (with the former being downregulated and the latter being upregulated).119,121 An increase in Aβ secretion has been documented following TBI122 and during periods of elevated neuronal activity.123
As TBI and BBB disruption are both associated with increases in neuronal excitability and ischemia,12,14,19,33 it is tempting to assume that a link exists between parenchymal Aβ accumulation and TBI-induced BBB disruption. Whether the temporal dynamics of these processes overlap, however, is unclear, as is the extent to which the accumulation of Aβ following BBB disruption is associated with an increase in the probability of developing AD. Nevertheless, therapies that increase LRP1 expression in the BBB and/or enhance the peripheral Aβ binding activity of plasma soluble LRP have been suggested as means of controlling the accumulation of parenchymal Aβ.124 Such therapies might prove valuable in situations of BBB breakdown. Indeed, one study has demonstrated that in rabbits fed with a cholesterol-enriched diet—an animal model of BBB disruption that has been shown to exhibit the pathological features of AD—addition of low concentrations of caffeine to the diet prevented BBB disruption.125 These findings might confirm the loss of BBB integrity as an important mediator of the development of this neurodegenerative disease.
Targeting blood–brain barrier damage
As we have discussed, accumulating evidence is strengthening the hypothesis that BBB breakdown mediates and facilitates short-term and long-term secondary brain damage that follows TBI. Thus, maintenance of BBB integrity constitutes a potential target for brain protection in TBI. Reliable and reproducible quantitative methods for measuring BBB permeability (Box 3) will be essential for the successful development of drugs to achieve this goal.
Box 3. Measurement of blood–brain barrier permeability.
The routine use of an inexpensive, high-resolution and minimally invasive technique to measure vessel permeability and blood–brain barrier (BBB) integrity would improve the effectiveness of current treatment approaches for traumatic brain injury (TBI). Such a technique would give rise to prospective studies that are required to confirm the role of BBB breakdown in the mediation of TBI-related complications and facilitate the assessment of novel treatments.
Currently available clinical methods for the detection of BBB breakdown rely on qualitative evaluation of contrast-enhanced brain imaging methods, which include CT and MrI used in conjunction with contrast agents such as iohexol and gadolinium-labeled diethylenetriamine pentaacetic acid, respectively. A new class of MrI contrast agents—the superparamagnetic iron oxide nanoparticle compounds—is increasingly being used to image brain infiltration of macrophages.168
In parallel with largely qualitative techniques, several MrI-based approaches that aim for a more quantitative analysis of BBB integrity have been developed. These approaches include a method that compares precontrast and postcontrast images,14,18 and dynamic contrast-enhanced imaging.169,170
A number of non-imaging approaches have been used for rapid assessment of BBB integrity in the clinical setting, including measurement of plasma proteins (such as albumin) in the cerebrospinal fluid (CSF).22 This procedure is, however, invasive, and bleeding in the setting of brain trauma can lead to false-positive results. Measurement of CSF protein levels in blood might be an alternative method of monitoring the integrity of the blood–CNS barrier. In this regard, the protein S100β,171 which is synthesized primarily by astrocytes, has potential as a molecule to be measured. Basal plasma levels of S100β are extremely low, but markedly increase within minutes of BBB breakdown.12,172 The disadvantages of S100β as a possible marker are its short half-life in blood, and the possibility that its levels largely depend on the extent of injury to astrocytes and their state of activation.171
Increased permeability
Various endogenous mediators of the edematous process that affects tight-junction integrity are currently under study, with the goal of edema control following TBI. Efforts aimed at modification of the edema-promoting actions of bradykinin and VEGF have shown promising results.
Bradykinin, a proinflammatory peptide belonging to the kallikrein–kinin system, has been shown to cause vasodilatation and to increase vascular permeability following binding to endothelial bradykinin β2 receptors.126,127 Antagonism of bradykinin β2 receptors in post-traumatic animal models resulted in a reduction of brain edema,128,129 with similar results being reported in clinical trials involving patients with TBI.130 Large-scale clinical evidence for the beneficial action of bradykinin is, however, still lacking.131
VEGF is an important promoter of angiogenesis following head trauma.132 This growth factor has been shown to mediate BBB breakdown in vitro37 and in vivo,133 and to increase vascular permeability under hypoxic conditions through direct action on the tight junction proteins known to regulate paracellular permeability.41 Chronic states of BBB opening have been suggested to result from epilepsy-induced reactive angiogenesis, which is associated with high VEGF levels.134,135 Moreover, VEGF antagonism has been demonstrated to reduce BBB permeability in vivo,133,136,137 and corticosteroids, which are routinely used for acute therapy in cerebral edema, have been suggested to stabilize the BBB by regulating the expression of VEGF under conditions of BBB injury.138 Erythropoietin has been shown to confer protection against VEGF-mediated increased BBB permeability,139 although this hormone might also exert protection following TBI via non-VEGF-related mechanisms.140–142 Moreover, the human recombinant form of erythropoietin has shown promisie as a neuroprotective agent in mice,143 but has yet to be tested in patients with TBI.
Other prominent BBB breakdown-inducing candidates, including histamine144,145 and 5-hydroxytryptamine,13 have been proposed as potential targets for the prevention of edema. No therapies specifically aimed at modulating BBB permeability are routinely prescribed, however, and ongoing research and clinical trials are needed to explore both the effectiveness and safety of selected therapeutic candidates.
Neuroinflammation
Once initiated, neuroinflammatory processes are diverse and intertwining, and are induced by and lead to BBB breakdown. Thus, prevention of inflammation might represent a prudent therapeutic strategy. This goal might be achieved by controlling both cytokine-mediated recruitment of inflammatory cells and the migration of such cells into the injured brain. IL-1, the most widely studied pro-inflammatory cytokine,62 is rapidly released following injury and primarily acts on specific receptors that can be blocked endogenously by IL-1 receptor antagonist protein (IL-1ra). Endothelial cells of the BBB display IL-1 receptors and are, therefore, subject to the action of IL-1. Activation of these endothelial receptors results in BBB breakdown106 and increases in leukocyte recruitment, adhesion and infiltration.66 As shown previously in animal studies, data obtained from the microdialyzates of patients with TBI have indicated that IL-1ra has a neuroprotective role.146 Since the recombinant form of IL-1ra is in routine use for the treatment of rheumatoid arthritis and other immune-related pathologies, the potential use of this agent in the management of TBI should be investigated.
The control of neutrophil influx following TBI might attenuate secondary brain injury.67 At sites of inflammation, neutrophils adhere to endothelial cells by binding to adhesion molecules such as ICAM1, vascular cell adhesion molecule and platelet endothelial cell adhesion molecule 1 (the first two of these molecules are upregulated following brain trauma).147 Thus, targeting of leukocyte infiltration through use of inhibitors of such adhesion molecules125,148 might constitute a means of preventing or decreasing the neuroinflammatory response following TBI.
The central role of MMP-mediated pathological processes has been demonstrated in several models of neuroinflammation. Both systemic and brain MMPs are rapidly upregulated in patients with TBI,149 and contribute to BBB breakdown107,150 by degrading tight-junction proteins.151 The attenuation of MMP activity following head trauma64,152 might confer BBB protection, even though a marked decrease in MMP activity could hinder proper tissue recovery processes153 in much the same way as do decreases in the activities of other mediators of the inflammatory response. Activated protein C (APC), an endogenous anticoagulant that confers neuroprotection in stroke, has been proposed as a possible therapy for use following TBI,154 as this agent attenuates BBB breakdown by inhibiting the MMP-9 activation pathway.155 Until a modified APC with negligible anticoagulative activity is clinically available, further research into the beneficial properties of APC following brain injury would be prudent, as bleeding is a major concern in the setting of TBI.156
Neuronal hyperexcitability
Currently, anticonvulsant prophylaxis following TBI is not warranted, as clinical studies have failed to demonstrate long-term benefits of such a strategy.157 These clinical studies tested a variety of antiepileptic drugs and included a large-scale investigation of the ability of phenytoin to provide protection against the development of post-traumatic epilepsy.158 In spite of these results, the demonstration that BBB disruption has a key role in the evolution of post-traumatic seizures has opened up new and exciting avenues for intervention, and prevention of the network changes that lead to the hyperexcitable state.
BBB breakdown is associated with rapid activation of astrocytes, which occurs even before an increase in neuronal excitability.101 Indeed, alterations in astrocyte functionality are becoming recognized in an increasing number of neuropathologies.159 The results obtained from our studies showing the rapid uptake of serum albumin by astrocytic TGF-β receptor 2 not only further substantiate this view, but also offer a specific intervention target. These studies draw attention to the potential role of the cytokine TGF-β1 in BBB-mediated activation of astrocytes and the associated epileptogenesis, as well as to the potential use of TGF-β signaling pathway antagonists81,88 in the clinical setting. Indeed, specific antagonists of this pathway are currently being developed and might be tested in the near future in animal models. Efforts to prevent astrocytic dysfunction, increases in extracellular potassium levels and glutamate accumulation might prove to be of vital importance in combating TBI, as high levels of post-injury extracellular potassium are associated with a poor outcome in patients with such injuries.160
Conclusions and future directions
Optimization of the management and prevention of secondary damage following TBI poses a notable challenge to the medical community. Currently, no readily available neuroprotective agent exists that can effectively prevent or reverse the damage caused by secondary delayed pathologies following TBI. Novel therapeutic strategies that can meet this challenge might be found in the plethora of data generated in the past decade that reveal the extensive involvement of the BBB in mediating these injurious processes. Alternatively, the way forward for TBI drug development might lie in new genomic and proteomic technologies that will enable the discovery of BBB repair-related cytoprotective genes161 or proteins.162,163 Indeed, the use of molecular agents that increase the expression of transcription factors associated with regulating the expression of such cytoprotective proteins has been shown to confer neuroprotection and to decrease BBB breakdown in animal studies of brain vascular trauma.34,164
As the central role of BBB breakdown in the pathophysiology of TBI is rapidly becoming clear, a pressing need exists for further intense basic and clinical research. This research is expected to lead to the development of efficient diagnostic tools for the detection and monitoring of BBB breakdown and repair, and to agents that will control such breakdown or confer BBB protection in the relevant time window following TBI.
Key points.
Breakdown of the blood–brain barrier (BBB) follows traumatic brain injury (TBI) and can last from several days to years after the acute event
Secondary BBB breakdown—initiated within hours or days of injury—is associated with processes such as edema, neuroinflammation and cell death, and is considered to be potentially treatable
Experimental data indicate that BBB breakdown contributes to the clinical outcome of long-term TBI complications, such as Alzheimer disease, cognitive and psychological impairments, and epilepsy
A pressing need exists for new, practical and efficient diagnostic tools for the rapid detection and monitoring of BBB status
No accepted therapeutic protocols are available for the prevention or treatment of secondary damage resulting from BBB breakdown, although emergent treatment strategies aimed at modifying BBB-mediated injury are showing promise
Further basic and clinical research directed at the pathophysiology of BBB breakdown following TBI might provide novel targets for clinical intervention
Review criteria.
This review was based on searches of the PubMed database using each of the terms “traumatic brain injury”, “TBI”, “head trauma” and “trauma” in combination with the terms “blood–brain barrier” or “BBB”. No time limit was set with regard to publication date. Only English language articles were retrieved. Relevant articles were selected on the basis of abstract review. Full articles were subsequently obtained and their references were searched for further relevant material.
Acknowledgments
This work was supported by the Sonderforschungsbereich Tr3, the Israel Science Foundation (grant 566/07, AF), the Binational US–Israel Science Foundation (grant BSF 2007,185) and the National Institute for Neurological Disorders and Stroke (grant 1rO1N5066005). The authors thank Ms Inez Mureinik (research Authority, Ben-Gurion University of the Negev, Israel) for her useful comments and Dr Amihai Pima (Department of Urology, rabin Medical Center, Israel) for assistance with Figure 1. Charles P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Footnotes
Competing interests
The authors, the Journal Editor H. Wood and the CME questions author C. P. Vega declare no competing interests.
References
- 1.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention; 2006. [online], http://www.cdc.gov/ncipc/pub-res/TBI_in_US_04/TBI%20in%20the%20US_Jan_2006.pdf. [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Corso PS, Miller TR. Incidence and Economic Burden of Injuries in the United States. Oxford University Press; Oxford: 2006. [Google Scholar]
- 4.Badjatia N, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12 (Suppl 1):S1–S52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 5.Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- 6.Maas AI, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien) 1997;139:286–294. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- 7.Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- 8.Narayan RK, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 10.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 11.Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, Carlos TM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir Suppl. 1998;71:104–106. doi: 10.1007/978-3-7091-6475-4_31. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 13.Sharma HS, et al. Antibodies to serotonin attenuate closed head injury induced blood brain barrier disruption and brain pathology. Ann NY Acad Sci. 2007;1122:295–312. doi: 10.1196/annals.1403.022. [DOI] [PubMed] [Google Scholar]
- 14.Tomkins O, et al. Blood–brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- 15.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 16.van Vliet EA, et al. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 17.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Tomkins O, et al. Frequent blood–brain barrier disruption in the human cerebral cortex. Cell Mol Neurobiol. 2001;21:675–691. doi: 10.1023/A:1015147920283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood–brain barrier disruption in patients with postconcussion syndrome. J Clin Neurophysiol. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- 20.Strbian D, et al. The blood–brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153:175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Barzó P, Marmarou A, Fatouros P, Corwin F, Dunbar J. Magnetic resonance imaging-monitored acute blood–brain barrier changes in experimental traumatic brain injury. J Neurosurg. 1996;85:1113–1121. doi: 10.3171/jns.1996.85.6.1113. [DOI] [PubMed] [Google Scholar]
- 22.Csuka E, et al. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-α, TGF-β1 and blood–brain barrier function. J Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 23.Morganti-Kossmann MC, et al. TGF-β is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood–brain barrier function. J Neurotrauma. 1999;16:617–628. doi: 10.1089/neu.1999.16.617. [DOI] [PubMed] [Google Scholar]
- 24.Shapira Y, Setton D, Artru AA, Shohami E. Blood–brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Anesth Analg. 1993;77:141–148. doi: 10.1213/00000539-199307000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Cernak I. Animal models of head trauma. Neuro Rx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morales DM, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood–spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff C, et al. Intrathecal and systemic concentration of NT-proBNP in patients with severe traumatic brain injury. J Neurotrauma. 2006;23:943–949. doi: 10.1089/neu.2006.23.943. [DOI] [PubMed] [Google Scholar]
- 30.Lenzlinger PM, Marx A, Trentz O, Kossmann T, Morganti-Kossmann MC. Prolonged intrathecal release of soluble Fas following severe traumatic brain injury in humans. J Neuroimmunol. 2002;122:167–174. doi: 10.1016/s0165-5728(01)00466-0. [DOI] [PubMed] [Google Scholar]
- 31.Stahel PF, et al. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood–brain barrier dysfunction in patients with traumatic brain injury. J Neurotrauma. 2001;18:773–781. doi: 10.1089/089771501316919139. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Baeza A, Reina-de la Torre F, Poca A, Martí M, Garnacho A. Morphological features in human cortical brain microvessels after head injury: a three-dimensional and immunocytochemical study. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:583–593. doi: 10.1002/ar.a.10069. [DOI] [PubMed] [Google Scholar]
- 33.Bouma GJ, et al. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- 34.Robertson CS, Contant CF, Gokaslan ZL, Narayan RK, Grossman RG. Cerebral blood flow, arteriovenous oxygen difference, and outcome in head injured patients. J Neurol Neurosurg Psychiatry. 1992;55:594–603. doi: 10.1136/jnnp.55.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, et al. Hemodynamically significant cerebral vasospasm and outcome after head injury: a prospective study. J Neurosurg. 1997;87:221–233. doi: 10.3171/jns.1997.87.2.0221. [DOI] [PubMed] [Google Scholar]
- 37.Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus. 2008;25:E7. doi: 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]
- 38.Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nekludov M, Antovic J, Bredbacka S, Blombäck M. Coagulation abnormalities associated with severe isolated traumatic brain injury: cerebral arterio-venous differences in coagulation and inflammatory markers. J Neurotrauma. 2007;24:174–180. doi: 10.1089/neu.2006.0173. [DOI] [PubMed] [Google Scholar]
- 40.Muellner A, et al. Microvascular basal lamina antigen loss after traumatic brain injury in the rat. J Neurotrauma. 2003;20:745–754. doi: 10.1089/089771503767869971. [DOI] [PubMed] [Google Scholar]
- 41.Servadei F, et al. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002;50:261–267. doi: 10.1097/00006123-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 43.Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience. 2009;158:301–308. doi: 10.1016/j.neuroscience.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 44.Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood–brain barrier breakdown. Acta Neuropathol. 2007;114:459–469. doi: 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 45.Nag S, Manias JL, Stewart DJ. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol Appl Neurobiol. 2009;35:417–426. doi: 10.1111/j.1365-2990.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood–brain barrier breakdown. Acta Neuropathol. 2008;115:635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 48.Dore-Duffy P, et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 49.Nag S, Takahashi JL, Kilty D. W role of vascular endothelial growth factor in blood–brain barrier breakdown and angiogenesis in brain trauma. J Neuropathol Exp Neurol. 1997;56:912–921. doi: 10.1097/00005072-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Infante-Duarte C, Waiczies S, Wuerfel J, Zipp F. New developments in understanding and treating neuroinflammation. J Mol Med. 2008;86:975–985. doi: 10.1007/s00109-007-0292-0. [DOI] [PubMed] [Google Scholar]
- 51.Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 52.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann NY Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 53.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Holmin S, Mathiesen T. Intracerebral administration of interleukin-1β and induction of inflammation, apoptosis, and vasogenic edema. J Neurosurg. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- 55.Holmin S, Söderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298. doi: 10.1097/00006123-199802000-00047. [DOI] [PubMed] [Google Scholar]
- 56.Balabanov R, et al. Endothelial cell activation following moderate traumatic brain injury. Neuro Res. 2001;23:175–182. doi: 10.1179/016164101101198514. [DOI] [PubMed] [Google Scholar]
- 57.Chen G, Shi J, Hu Z, Hang C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pleines UE, Stover JF, Kossmann T, Trentz O, Morganti-Kossman MC. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J Neurotrauma. 1998;15:399–409. doi: 10.1089/neu.1998.15.399. [DOI] [PubMed] [Google Scholar]
- 59.Aihara N, Hall JJ, Pitts LH, Fukuda K, Noble LJ. Altered immunoexpression of microglia and macrophages after mild head injury. J Neurotrauma. 1995;12:53–63. doi: 10.1089/neu.1995.12.53. [DOI] [PubMed] [Google Scholar]
- 60.Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir Suppl. 2006;96:444–450. doi: 10.1007/3-211-30714-1_91. [DOI] [PubMed] [Google Scholar]
- 61.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 62.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 (Suppl 1):S232–S340. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harkness KA, et al. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123:698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- 64.Suehiro E, et al. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma. 2004;21:1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- 65.Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- 66.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 67.Fabene PF, et al. A role for leukocyte–endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Cernak I, Stoica B, Byrnes KR, Di Giovanni S, Faden AI. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle. 2005;4:1286–1293. doi: 10.4161/cc.4.9.1996. [DOI] [PubMed] [Google Scholar]
- 70.Clark RS, et al. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 1999;13:813–821. doi: 10.1096/fasebj.13.8.813. [DOI] [PubMed] [Google Scholar]
- 71.Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood–brain barrier breakdown following traumatic brain injury. Brain Res. 2006;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 72.Rink A, et al. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- 73.Uzan M, et al. Evaluation of apoptosis in cerebrospinal fluid of patients with severe head injury. Acta Neurochir (Wien) 2006;148:1157–1164. doi: 10.1007/s00701-006-0887-1. [DOI] [PubMed] [Google Scholar]
- 74.Yakovlev AG, et al. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J Neurosci. 1997;17:7415–7424. doi: 10.1523/JNEUROSCI.17-19-07415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Chen Y, Jenkins LW, Kochanek PM, Clark RS. Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Crit Care. 2005;9:66–75. doi: 10.1186/cc2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nag S, Papneja T, Venugopalan R, Stewart DJ. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood–brain barrier breakdown. Lab Invest. 2005;85:1189–1198. doi: 10.1038/labinvest.3700325. [DOI] [PubMed] [Google Scholar]
- 77.Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS. Endothelial apoptosis initiates acute blood–brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950–5956. [PubMed] [Google Scholar]
- 78.Tomkins O, et al. Blood–brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Di Giovanni S, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams S, et al. In situ DNA fragmentation occurs in white matter up to 12 months after head injury in man. Acta Neuropathol. 2001;102:581–590. doi: 10.1007/s004010100410. [DOI] [PubMed] [Google Scholar]
- 81.Cacheaux LP, et al. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruttan L, Martin K, Liu A, Colella B, Green RE. Long-term cognitive outcome in moderate to severe traumatic brain injury: a meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch Phys Med Rehabil. 2008;89 (12 Suppl):S69–S76. doi: 10.1016/j.apmr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 84.Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 85.Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9:453–457. doi: 10.1053/seiz.2000.0458. [DOI] [PubMed] [Google Scholar]
- 86.Pavlovsky L, et al. Persistent BBB disruption may underlie alpha interferon-induced seizures. J Neurol. 2005;252:42–46. doi: 10.1007/s00415-005-0596-3. [DOI] [PubMed] [Google Scholar]
- 87.Wang HC, et al. Factors predictive of outcome in posttraumatic seizures. J Trauma. 2008;64:883–888. doi: 10.1097/TA.0b013e31804a7fa4. [DOI] [PubMed] [Google Scholar]
- 88.Ivens S, et al. TGF-β receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 89.David Y, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Floyd CL, Lyeth BG. In: Progress in Brain Research Neurotrauma: New Insights into Pathology and Treatment. Weber JT, Maas AIR, editors. Elsevier; Amsterdam: 2007. pp. 61–79. [Google Scholar]
- 91.Heinemann U, et al. Alterations of glial cell function in temporal lobe epilepsy. Epilepsia. 2000;41 (Suppl 6):S185–S189. doi: 10.1111/j.1528-1157.2000.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 92.Jabs R, Seifert G, Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;49(Suppl 2):S3–S12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- 93.Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 94.Seiffert E, et al. Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian GF, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Filosa A, et al. Neuron–glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12:1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ivens S, et al. Blood–brain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol. 2010;257:615–620. doi: 10.1007/s00415-009-5384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marchi N, et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman A, Kaufer D, Heinemann U. Blood–brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K+ homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santhakumar V, Voipio J, Kaila K, Soltesz I. Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium. J Neurosci. 2003;23:5865–5876. doi: 10.1523/JNEUROSCI.23-13-05865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Viviani B, et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrari CC, et al. Reversible demyelination, blood–brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165:1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gursoy-Ozdemir Y, et al. Cortical spreading depression activates and upregulates MMP-9. J Clin Invest. 2004;113:1447–1455. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 109.Guo Z, et al. Head injury and the risk of AD in the MIrAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 110.Jellinger K, Paulus W, Wrocklage C, Litvan I. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 2001;1:3. doi: 10.1186/1471-2377-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer-Luehmann M, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emmerling MR, et al. Traumatic brain injury elevates the Alzheimer’s amyloid peptide Aβ42 in human CSF. A possible role for nerve cell injury. Ann NY Acad Sci. 2000;903:118–122. doi: 10.1111/j.1749-6632.2000.tb06357.x. [DOI] [PubMed] [Google Scholar]
- 113.Saunders AM, et al. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 114.Martel CL, et al. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood–brain barrier transport of circulating Alzheimer’s amyloid β. J Neurochem. 1997;69:1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhou W, et al. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma. 2008;25:279–290. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]
- 116.Moran LM, et al. Apolipoprotein E4 as a predictor of outcomes in pediatric mild traumatic brain injury. J Neurotrauma. 2009;26:1489–1495. doi: 10.1089/neu.2008.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamada K, et al. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid β peptides in an in vitro model of the blood–brain barrier cells. J Biol Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deane R, et al. RAGE mediates amyloid-β peptide transport across the blood–brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 119.Yan FL, Zheng Y, Zhao FD. Effects of ginkgo biloba extract EGb761 on expression of rAGE and LrP-1 in cerebral microvascular endothelial cells under chronic hypoxia and hypoglycemia. Acta Neuropathol. 2008;116:529–535. doi: 10.1007/s00401-008-0435-6. [DOI] [PubMed] [Google Scholar]
- 120.Donahue J, et al. RAGE, LrP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 121.Jeynes B, Provias J. Evidence for altered LrP/rAGE expression in Alzheimer lesion pathogenesis. Curr Alzheimer Res. 2008;5:432–437. doi: 10.2174/156720508785908937. [DOI] [PubMed] [Google Scholar]
- 122.Ikonomovic MD, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 123.Cirrito JR, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sagare A, et al. Clearance of amyloid-β by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen X, Gawryluk J, Wagener J, Ghribi O, Geiger J. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Unterberg A, Wahl M, Baethmann A. Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab. 1984;4:574–585. doi: 10.1038/jcbfm.1984.82. [DOI] [PubMed] [Google Scholar]
- 127.Plesnila N, et al. Role of bradykinin B2 receptors in the formation of vasogenic brain edema in rats. J Neurotrauma. 2001;18:1049–1058. doi: 10.1089/08977150152693746. [DOI] [PubMed] [Google Scholar]
- 128.Ivashkova Y, et al. Bradykinin B2 receptor antagonism with LF 18–1505T reduces brain edema and improves neurological outcome after closed head trauma in rats. J Trauma. 2006;61:879–885. doi: 10.1097/01.ta.0000234722.98537.01. [DOI] [PubMed] [Google Scholar]
- 129.Stover JF, Dohse NK, Unterberg AW. Bradykinin 2 receptor antagonist LF 16–0687Ms reduces posttraumatic brain edema. Acta Neurochir Suppl. 2000;76:171–175. doi: 10.1007/978-3-7091-6346-7_34. [DOI] [PubMed] [Google Scholar]
- 130.Narotam PK, et al. Traumatic brain contusions: a clinical role for the kinin antagonist CP-0127. Acta Neurochir (Wien) 1998;140:793–803. doi: 10.1007/s007010050181. [DOI] [PubMed] [Google Scholar]
- 131.Ker K, Blackhall K. Bradykinin beta-2 receptor antagonists for acute traumatic brain injury. Cochrane Database of Systematic Reviews. 2008;(1):Art. No.:CD006686. doi: 10.1002/14651858.CD006686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nag S. The blood–brain barrier and cerebral angiogenesis: lessons from the cold-injury model. Trends Mol Med. 2002;8:38–44. doi: 10.1016/s1471-4914(01)02221-3. [DOI] [PubMed] [Google Scholar]
- 133.Chi OZ, Hunter C, Liu X, Weiss HR. Effects of anti-VEGF antibody on blood–brain barrier disruption in focal cerebral ischemia. Exp Neurol. 2007;204:283–287. doi: 10.1016/j.expneurol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 134.Nicoletti JN, et al. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151:232–241. doi: 10.1061/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rigau V, et al. Angiogenesis is associated with blood–brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 136.van Bruggen N, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood–brain barrier permeability by YC-1 through the antagonism of HIF-1α accumulation and VEGF expression. Mol Pharmacol. 2007;72:440–449. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- 138.Kim H, et al. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood–brain barrier. Biochem Biophys Res Commun. 2008;372:243–248. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 139.Martínez-Estrada OM, et al. Erythropoietin protects the in vitro blood–brain barrier against VEGF-induced permeability. Eur J Neurosci. 2003;18:2538–2544. doi: 10.1046/j.1460-9568.2003.02987.x. [DOI] [PubMed] [Google Scholar]
- 140.Chen G, et al. Inhibitory effect on cerebral inflammatory agents that accompany traumatic brain injury in a rat model: a potential neuroprotective mechanism of recombinant human erythropoietin (rhEPO) Neurosci Lett. 2007;425:177–182. doi: 10.1016/j.neulet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 141.Grasso G, et al. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 142.Liao ZB, Zhi XG, Shi QH, He Z. H recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur J Neurol. 2008;15:140–149. doi: 10.1111/j.1468-1331.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- 143.Xiong Y, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stokely ME, Orr EL. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- 145.Tósaki A, Szerdahelyi P, Joó F. Treatment with ranitidine of ischemic brain edema. Eur J Pharmacol. 1994;264:455–458. doi: 10.1016/0014-2999(94)00546-x. [DOI] [PubMed] [Google Scholar]
- 146.Hutchinson PJ, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1α, IL-1β, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 147.Clausen F, Lorant T, Lewén A, Hillered L. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- 148.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 149.Vilalta A, et al. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med. 2008;34:1384–1392. doi: 10.1007/s00134-008-1056-1. [DOI] [PubMed] [Google Scholar]
- 150.Petty MA, Lo EH. Junctional complexes of the blood–brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 151.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2006;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 152.Truettner JS, Alonso OF, Dalton Dietrich W. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:1505–1516. doi: 10.1038/sj.jcbfm.9600150. [DOI] [PubMed] [Google Scholar]
- 153.Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 154.Petraglia AL, Marky AH, Walker C, Thiyagarajan M, Zlokovic BV. Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery. 2010;66:165–171. doi: 10.1227/01.NEU.0000363148.49779.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cheng T, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, et al. Differential neuroprotection and risk for bleeding from activated protein C with varying degrees of anticoagulant activity. Stroke. 2009;40:1864–1869. doi: 10.1161/STROKEAHA.108.536680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database of Systematic Reviews. 2001;4:Art. No.:CD000173. doi: 10.1002/14651858. CD000173. [DOI] [PubMed] [Google Scholar]
- 158.Temkin NR, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 159.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 160.Reinert M, et al. High level of extracellular potassium and its correlates after severe human head injury: relationship to high intracranial pressure. J Neurosurg. 2000;93:800–807. doi: 10.3171/jns.2000.93.5.0800. [DOI] [PubMed] [Google Scholar]
- 161.Pardridge WM. Blood–brain barrier genomics. Stroke. 2007;38 (2 Suppl):686–690. doi: 10.1161/01.STR.0000247887.61831.74. [DOI] [PubMed] [Google Scholar]
- 162.Cucullo L, et al. Blood–brain barrier damage induces release of α2-macroglobulin. Mol Cell Proteomics. 2003;2:234–241. doi: 10.1074/mcp.M200077-MCP200. [DOI] [PubMed] [Google Scholar]
- 163.Redell JB, Zhao J, Dash PK. Acutely increased cyclophilin a expression after brain injury: a role in blood–brain barrier function and tissue preservation. J Neurosci Res. 2007;85:1980–1988. doi: 10.1002/jnr.21324. [DOI] [PubMed] [Google Scholar]
- 164.Zhao X, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 165.Luukinen H, Viramo P, Koski K, Laippala P, Kivela SL. Head injuries and cognitive decline among older adults: a population-based study. Neurology. 1999;52:557–562. doi: 10.1212/wnl.52.3.557. [DOI] [PubMed] [Google Scholar]
- 166.Saatman KE, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ehrlich P. Oxygen need by the organism: analytical study using color [German] Hirschwald. 1885;8:167. [Google Scholar]
- 168.Jander S, Schroeter M, Saleh A. Imaging inflammation in acute brain ischemia. Stroke. 2007;38:642–645. doi: 10.1161/01.STR.0000250048.42916.ad. [DOI] [PubMed] [Google Scholar]
- 169.Tofts PS, Kermode AG. Measurement of the blood–brain barrier permeability and leakage space using dynamic Mr imaging. 1 Fundamental concepts. Magn Reson Med. 1991;17:357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 170.Zaharchuk G. Theoretical basis of hemodynamic Mr imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR Am J Neuroradiol. 2007;28:1850–1858. doi: 10.3174/ajnr.A0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kanner AA, et al. Serum S100β: a noninvasive marker of blood–brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kapural M, et al. Serum S-100β as a possible marker of blood–brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]