Abstract
Recent advances in human brown adipose tissue (BAT) imaging technology have renewed interest in the identification of BAT activators for the treatment of obesity and diabetes. In uncontrolled diabetes (uDM), activation of BAT is implicated in glucose lowering mediated by intracerebroventricular (icv) administration of leptin, which normalizes blood glucose levels in streptozotocin (STZ)-induced diabetic rats. The potent effect of icv leptin to increase BAT glucose uptake in STZ-diabetes is accompanied by the return of reduced plasma thyroxine (T4) levels and BAT uncoupling protein-1 (Ucp1) mRNA levels to nondiabetic controls. We therefore sought to determine whether activation of thyroid hormone receptors is sufficient in and of itself to lower blood glucose levels in STZ-diabetes and whether this effect involves activation of BAT. We found that, although systemic administration of the thyroid hormone (TR)β-selective agonist GC-1 increases energy expenditure and induces further weight loss in STZ-diabetic rats, it neither increased BAT glucose uptake nor attenuated diabetic hyperglycemia. Even when GC-1 was administered in combination with a β3-adrenergic receptor agonist to mimic sympathetic nervous system activation, glucose uptake was not increased in STZ-diabetic rats, nor was blood glucose lowered, yet this intervention potently activated BAT. Similar results were observed in animals treated with active thyroid hormone (T3) instead of GC-1. Taken together, our data suggest that neither returning normal plasma thyroid hormone levels nor BAT activation has any impact on diabetic hyperglycemia, and that in BAT, increases of Ucp1 gene expression and glucose uptake are readily dissociated from one another in this setting.
Keywords: thyroid, diabetes, brown adipose tissue, sympathetic nervous system, glucose uptake
in rodents, brown adipose tissue (BAT) is implicated as a key mediator of both cold-induced (14) and diet-induced thermogenesis (38). Since BAT consumes energy to generate heat, several lines of evidence suggest that, despite its small size, BAT may play a role not only in the control of thermogenesis but in glucose metabolism as well (33). For example, in both cold-exposed humans (34) and rodents (4), BAT exhibits rates of uptake of glucose per gram of tissue that exceed those of insulin-stimulated skeletal muscle (34). Moreover, BAT transplantation improves glucose metabolism and insulin sensitivity in wild-type mice (46) and restores euglycemia to mice with streptozotocin-(STZ)-induced diabetes (20). We recently demonstrated that the effect of centrally administered leptin to normalize blood glucose levels in a rodent model of uncontrolled insulin-deficient diabetes (uDM) occurs via an insulin-independent mechanism characterized by increased rates of tissue glucose uptake in skeletal muscle and BAT (16). Our finding that the decline of circulating thyroxine (T4) hormone levels characteristic of STZ-induced uDM (35, 37) is prevented by intracerebroventricular (icv) leptin treatment suggests that the increase of BAT glucose uptake in this setting is linked to increased thyroid hormone receptor stimulation. This interpretation, in turn, suggests that thyroid hormone treatment should itself induce BAT activation and glucose uptake in STZ-diabetic rats, resulting in a decrease of blood glucose. This hypothesis is supported by evidence that thyroid hormone increases basal glucose uptake in various tissues including BAT (52) and that reversal of hypothyroidism dramatically improved glucose homeostasis in a patient with diabetes due to a mutation in the insulin receptor gene, an effect accompanied by enhanced PET-CT uptake in BAT (45).
In the current studies, we sought to determine whether thyroid hormone treatment 1) improves hyperglycemia in a rat model of uDM, and 2) if this effect is associated with BAT activation [as judged by induction of BAT markers of thermogenesis including uncoupling protein-1 (Ucp1) gene expression] or glucose uptake [measured by uptake of labeled 2-deoxy-d-glucose (2-DG)]. A third objective was to determine whether changes of BAT Ucp1 gene expression and glucose uptake parallel one another as measures of BAT activation state. To achieve these goals, STZ-induced diabetic rats were treated with either the synthetic thyroid hormone receptor β-selective (TRβ) agonist GC-1 or the active form of thyroid hormone (triiodothyronine, T3) and effects on glucose metabolism and energy homeostasis were assessed. In addition, since uDM is characterized by reduced levels of both sympathetic nervous system (SNS) outflow to BAT (55) and plasma thyroid hormone (35, 37), and since the SNS and thyroid hormone interact synergistically to activate BAT (42, 43), we also examined the effects of GC-1 on BAT activity in both the presence and absence of a specific β3-adrenergic receptor (β3-AR) agonist. Our findings demonstrate that, although GC-1 potently induced BAT Ucp1 mRNA when given in combination with a β3-AR agonist, it failed to either stimulate BAT glucose uptake or attenuate diabetic hyperglycemia in STZ-diabetic rats. Thus, activation of BAT appears to be dissociated from increased BAT glucose uptake in this setting. Furthermore, neither treatment with active thyroid hormone nor activating β3-ARs was sufficient to lower blood glucose levels in uDM. These findings in turn indicate that correcting defects in the thyroid axis and SNS outflow, either alone or in combination, do not mimic the effect of icv leptin to normalize blood glucose levels in uDM.
METHODS
Animals
Adult male Wistar rats (Harlan, IN) were housed in individual cages under specific-pathogen-free (SPF) conditions and maintained in a temperature-controlled room with a 12:12-h light-dark cycle. Animals were provided with ad libitum (AL) access to water and standard laboratory chow (PMI Nutrition International, St. Louis, MO) unless otherwise stated. All procedures were performed in accordance with NIH Guidelines for the Care and Use of Animals and were approved by the Animal Care Committee at the University of Washington.
Drugs
The synthetic thyroid hormone receptor-β-selective agonist GC-1 was kindly provided by Dr. Kevin Phillips (The Methodist Hospital Research Institute, Houston, TX) and dissolved in sterile water. The selective β3-AR agonist CL-316243 (Tocris Bioscience, Ellisville, MO) and T3 (Sigma, St. Louis, MO) were also dissolved in sterile water prior to injection.
Body Composition Analysis
Measurements of body lean and fat mass were determined in live, conscious animals by use of quantitative magnetic resonance spectroscopy (QMR; EchoMRI-700TM; Echo MRI, Houston, TX) the day prior to the completion of the study by the University of Washington Nutrition Obesity Research Center (NORC) Energy Balance and Glucose Metabolism (EBGM) Core.
Indirect Calorimetry and Ambulatory Activity
Rats were acclimated to calorimetry cages prior to the study and data collection. Energy expenditure measurements were obtained by a computer-controlled open-circuit indirect calorimeter using the Oxymax Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH) with support from the EBGM Core of the NORC at the University of Washington, as previously described (30). Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were measured for each rat at 20-min intervals for 2 min, and food and water intakes were measured using the feed-scale and volumetric drinking monitoring system, respectively (Columbus Instruments). V̇o2 was converted to total energy expenditure in kilocalories per hour by Columbus software, which uses the standard Lusk formula [TEE (kcal/h) = (3.815 + 1.232 × RQ) × V̇o2 (l/h)], where respiratory quotient (RQ) is the ratio of V̇o2 to V̇co2 (27). Ambulatory activity was determined simultaneously during all indirect calorimetry experiments. Locomotor activity was evaluated using an Opto-Varimetrix-3 sensor system (Columbus Instruments). Consecutive adjacent infrared beam breaks in the x-axis were scored as an activity count, and a tally was recorded every 20 min. Rats were evaluated over two consecutive 24-h periods, and we report the results for the dark (active) photoperiods.
Study Protocols
Effect of GC-1 and T3 on food intake, blood glucose levels, and body composition in STZ-diabetic rats.
Adult male Wistar rats received either two consecutive daily subcutaneous (sc) injections of STZ (40 mg·kg−1·body wt−1) to induce uDM or vehicle (NaCit, pH 4.5) as previously described (16, 17). Four days following administration of STZ, nondiabetic or STZ-induced diabetic rats received daily intraperitoneal (ip) injections of either the synthetic thyroid hormone receptor-β-selective agonist GC-1 at a dose of either 100 or 250 μg/kg or its vehicle (sterile water) over 11 consecutive days. These doses of GC-1 were selected based on previous studies demonstrating its effect to reduce body weight in rats (18). In a separate cohort of animals, STZ-induced diabetic rats received daily ip injections of T3 at doses that were approximately equimolar to that of GC-1 (i.e., 200 μg/kg T3∼100 μg/kg GC-1) (36). Food intake, body weight, and blood glucose levels were measured daily. To determine the effect of GC-1 on energy expenditure, rats were placed into the indirect calorimeter system for measurement of energy expenditure on day 7 (i.e., on the 4th day of GC-1 administration) for a period of 40 h.
Effect of β3-AR agonist in combination with GC-1 or T3 on food intake, blood glucose levels, and body composition in STZ-diabetic rats.
Adult male Wistar rats received either STZ to induce uDM or vehicle as described above. Four days following administration of STZ, animals were implanted sc with an osmotic minipump (Alzet, DURECT, model 2002) containing either vehicle or the β3-AR agonist (1 mg·kg−1·day−1). This dose of β3-AR was selected based on previous literature (12). Animals also started receiving daily ip injections of either GC-1 at 2 μg/kg or 100 μg/kg or its vehicle at this time. These two doses of GC-1 were selected based from our initial study and the previous literature showing that GC-1 at 2 μg/kg corresponds to a dose that approximates physiological replacement of thyroid hormone (36). In total, therefore, five groups of animals were studied: 1) veh-veh-veh, 2) STZ-veh-veh, 3) STZ-β3-AR-veh, 4) STZ-β3-AR-GC-1 (2 μg/kg), and 5) STZ-β3-AR-GC-1 (100 μg/kg). In a separate cohort of animals, nondiabetic or STZ-diabetic rats followed the same protocol as described above except they received approximate equimolar doses of T3 (4 or 200 μg/kg) instead of GC-1 in combination with the β3-AR agonist. Food intake, body weight, and blood glucose levels were measured daily.
Effect of combined treatment with GC-1 and β3-AR agonist on rates of glucose appearance and tissue glucose uptake in STZ-diabetic rats.
A separate cohort of adult male Wistar rats bearing catheters implanted in the right jugular vein and left carotid artery were obtained from Harlan. Using the same paradigm as described above, STZ-induced diabetic animals or nondiabetic controls were implanted sc with an osmotic minipump containing either the β3-AR agonist or its vehicle and received daily ip injections of either GC-1 (100 μg/kg) or its vehicle. Ten days following STZ administration, animals were fasted for 4 h and received a 24 μCi prime of [3-3H]glucose at t = 0 min for 3 min followed by a continuous 0.2 μCi/min infusion for 90 min. At t = 60, 70, 80, and 90 min, blood samples were taken for determination of basal glucose turnover (as described below). A bolus of 12 μCi 2-deoxy-[14C]glucose (2-DG) was also given at t = 48 min. Blood samples were taken at 10-min intervals from 50–90 min and processed to determine plasma [3-3H]glucose and 2-DG (16). Briefly, plasma for [3-3H]glucose was deproteinized with ZnSO4 and Ba(OH)2 and dried overnight at 60°C. Plasma [3-3H]glucose radioactivity was determined by liquid scintillation on a Beckman Tri-Carb 2810 (16). Sample radioactivity was divided by plasma glucose concentration to give the plasma glucose specific activity. Glucose rate of appearance (Ra) and rate of disposal (Rd) were calculated using (Steele's) non-steady-state equations. At t = 90 min, animals were euthanized with ketamine-xylazine (62.5 mg/kg:7.5 mg/kg), and tissues (skeletal muscle, BAT, liver, and brain) were rapidly excised, snap-frozen, and stored at −80°C and subsequently processed for measurement of tissue glucose uptake as previously described (16).
Tissue Processing, Blood Collection, and Assay
At study completion, liver and BAT tissue samples were rapidly dissected, immediately frozen on dry ice, and stored at −80°C for subsequent RNA extraction. Plasma glucose levels were determined using a GM9D glucose direct analyzer (Analox Instruments, London, UK). Daily blood glucose levels were measured using a hand-held glucometer (Accu-Chek) on blood obtained from tail capillary samples. Trunk blood was collected into chilled EDTA-treated tubes and centrifuged at 1,500 rpm for 20 min; the plasma was removed, aliquoted, and stored at −20°C for subsequent assay. Plasma insulin and leptin levels were determined by ELISA (Crystal Chem, Downers Grove, IL). TSH levels were also determined using an ELISA (Alpco, Salem, NH), while free and total T4 levels and free and total T3 levels were measured using an enzyme immunosorbent assay (EIA; MP Biomedicals, Solon, OH).
RT-PCR
Total RNA was extracted from liver and BAT using TRIzol B according to manufacturer's instructions (MRC, Carrollton, OH). RNA was quantitated by spectrophotometry at 260 nm (Nanodrop 1000; Thermo Scientific, Rockford, IL) and reverse-transcribed with AMV reverse transcriptase (1 μg; Promega, Madison, WI). Real-time PCR was performed on an ABI Prism 7900 HT (Applied Biosystems Foster City, CA) for measurement of hepatic mRNA levels of glucose-6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (Pepck), and malic enzyme, as well as uncoupling protein-1 (Ucp1), peroxisome proliferator-activated receptor-γ coactivator-1α (Pgc-1α) and type II deiodinase (D2) in BAT. PCR data were analyzed using the Sequence Detection System software (SDS version 2.2; Applied Biosystems). Expression levels of each gene were normalized to a housekeeping gene (18S RNA) and expressed as a percentage of veh-veh controls. Nontemplate controls were incorporated into each PCR run.
Statistical Analyses
Results are expressed as means ± SE. Significance was established at P < 0.05, two tailed. Tests across more than two groups were performed using analysis of variance followed by a least significant difference post hoc test to assess pairwise differences. Comparisons between two groups were performed with Student's independent samples t-test. Analysis of variance and t-tests were performed using Statistica (version 7.1; StatSoft). Probability values of <0.05 were considered significant. To control for the influence of body size variation on total energy expenditure (TEE), group comparisons involving this outcome were adjusted for total or lean body mass using an analysis of covariance, as recommended (23, 24). Analyses involving repeated measurements of TEE across multiple dark cycles were performed using the linear mixed-model procedure in PASW Statistics (version 19; IBM, Somers, NY) to examine whether drug treatment modulates energy expenditure in diabetic rats after adjusting for body mass. Models included drug treatment [vehicle, GC-1 (100 μg/kg) and GC-1 (250 μg/kg)], individual cycle number, and total or lean body mass as fixed effects.
RESULTS
CNS Leptin Normalizes Plasma Thyroid Levels in uDM
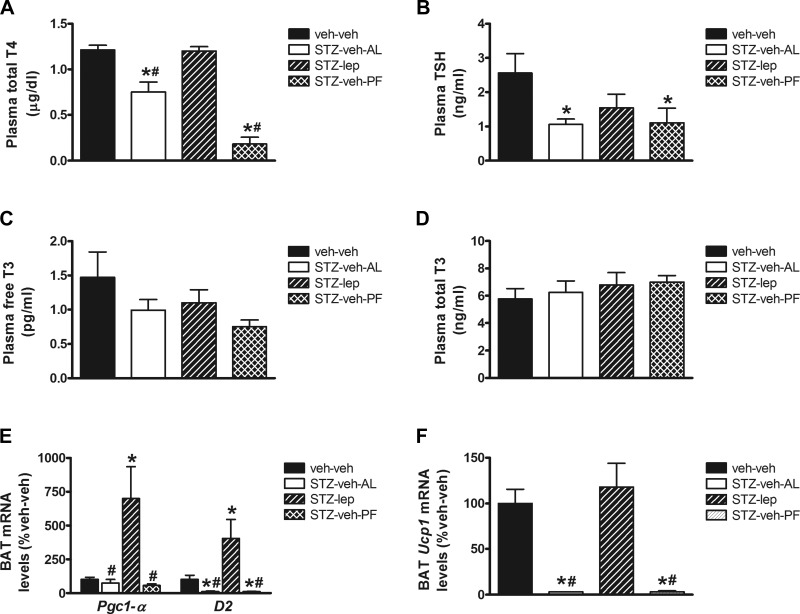
Our recently published data demonstrate that leptin action in the brain normalizes blood glucose levels in STZ-diabetic rats (16). This effect occurs, at least in part, via an insulin-independent mechanism characterized by increased glucose uptake in skeletal muscle and BAT (16). Here, we found that, similarly to fasting (1, 40), circulating plasma T4 and TSH levels were reduced in uDM, and this effect was either prevented or attenuated with continuous icv leptin infusion (Fig. 1, A and B). In contrast, there was no change in either free or total T3 levels (Fig. 1, C and D). These data suggest that, similarly to fasting, uDM induces a change of thyroid function better known as euthyroid sick syndrome, rather than causing frank hypothyroidism, and that this condition is reversed by icv leptin. In addition, we found that, in BAT, leptin treatment also increased expression of D2, an enzyme involved in the activation of thyroid hormones, as well as markers of thermogenesis including Pgc1-α and Ucp1 (Fig. 1, E and F). Thus, central leptin deficiency is implicated in the effect of uDM to lower T4 levels, and reversal of the euthyroid sick syndrome (a condition where thyroid levels are low, but the thyroid gland itself is not dysfunctional) could play a role in leptin-mediated glucose lowering and BAT activation. On the basis of these observations, we sought to determine in STZ-diabetic rats whether stimulating thyroid hormone receptors is itself sufficient to induce BAT activation and/or promote glucose uptake, thereby lowering blood glucose levels.
Fig. 1.
Central nervous system leptin normalizes plasma T4 levels and brown adipose tissue (BAT) markers of thermogenesis in uncontrolled diabetes (uDM). A: plasma thyroxine (T4). B: TSH. C: free triiodothyronine (T3). D: total T3 plasma levels and brown adipose tissue (BAT) expression of Pgc-1α and D2 (E) and Ucp1 (F) using real-time PCR in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving icv vehicle and fed ad libitum (STZ-veh-AL) or pair-fed (STZ-veh-PF) or icv leptin (n = 7–9 per group). Data represent means ± SE. *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-lep.
Effect of GC-1 on Food Intake, Blood Glucose Levels, and Body Composition in STZ-Diabetic Rats
As expected, plasma insulin and leptin levels were markedly reduced in all STZ-diabetic animals relative to nondiabetic controls (17, 44), irrespective of treatment group (data not shown), indicating that none of the treatments rescued severe insulin deficiency. Thus, although administration of thyroid hormone simultaneously with STZ has been reported to prevent STZ-induced destruction of β-cells and thereby attenuate diabetic hyperglycemia (49), this does not appear to have occurred in our studies, since thyroid analogs were administered only after diabetes was established and severe insulin deficiency was present in all STZ-treated groups.
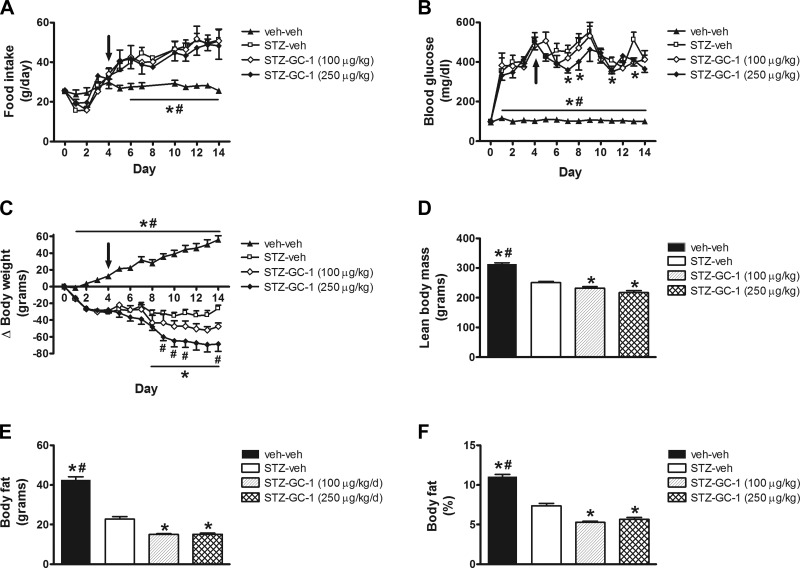
Consistent with previous studies (44), STZ-veh-treated animals were characterized by diabetic hyperphagia relative to nondiabetic controls, and this increase in food intake was not altered in STZ-diabetic animals that received the synthetic thyroid hormone receptor-β-selective agonist GC-1 at either dose (Fig. 2A). As expected, all animals that received STZ were hyperglycemic within 24 h and remained so throughout the duration of the experiment, and although the lower dose of GC-1 (100 μg/kg) did not lower blood glucose relative to STZ-diabetic animals that received vehicle, the higher dose of GC-1 (250 μg/kg) had a small but significant glucose-lowering effect on days 7, 8, 11, and 13 (Fig. 2B).
Fig. 2.
Effect of thyroid hormone (TR)β-selective agonist GC-1 on food intake, blood glucose levels, and body composition in uDM. A: food intake. B: blood glucose levels. C: body weight change. D: lean body mass. E: fat mass. and F: %body fat. A–F were compared in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving vehicle (STZ-veh) or GC-1 at either 100 or 250 μg/kg (n = 6–7 per group). Arrow indicates start of daily ip injections. Data represent means ± SE. *P < 0.05 vs. STZ-veh; #P < 0.05 vs. STZ-GC-1 (100 μg/kg).
Whereas nondiabetic animals gained weight during the study, STZ-diabetic rats displayed weight loss characteristic of insulin-deficient diabetes despite increased food intake (Fig. 2C). In STZ-diabetic animals receiving GC-1 at the highest dose, weight loss was increased owing to reductions of both lean and fat mass compared with STZ-veh controls (Fig. 2, D–F). As food intake did not differ between vehicle- and GC-1-treated groups, this outcome is suggestive of increased energy expenditure induced by GC-1, as previously reported in nondiabetic animals (50).
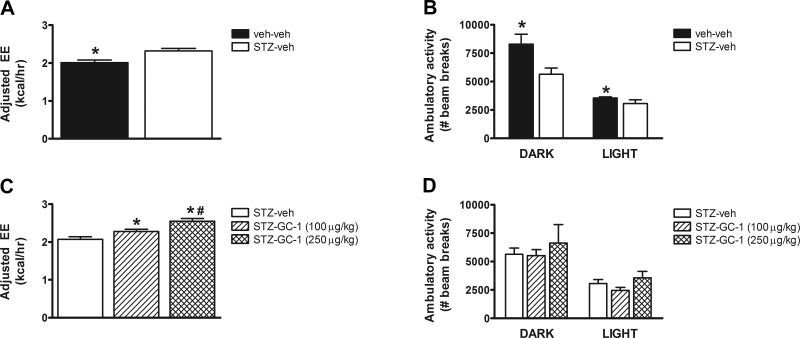
On the basis of this finding, we subjected STZ-diabetic animals to indirect calorimetry after receiving daily injections of either GC-1 or vehicle for 3 days. Given the marked differences in body weight and body composition between nondiabetic and STZ-diabetic animals, we used analysis of covariance to adjust energy expenditure for differences in either total body mass or lean body mass as previously recommended (23, 24). After adjustment for differences of total body mass, energy expenditure was significantly increased in rats with STZ-diabetes relative to nondiabetic controls (Fig. 3A), an effect that was even more significant following adjustment for lean body mass (data not shown). Moreover, this increase of energy expenditure was observed despite a reduction in ambulatory activity in STZ-diabetic rats (Fig. 3B). This finding is consistent with previous studies (17, 32) and likely results, at least in part, from the effect of severe insulin deficiency to reduce metabolic efficiency and increase futile cycling (32). In addition, we found that, relative to STZ-diabetic controls, adjusted energy expenditure was dose-dependently increased in STZ-diabetic rats treated with GC-1 (Fig. 3C), an effect that could not be attributed to changes in ambulatory activity (Fig. 3D).
Fig. 3.
Effect of GC-1 on energy expenditure in uDM. A and C: dark cycle energy expenditure (EE) adjusted for total body mass. B and D: ambulatory activity levels in nondiabetic controls (veh-veh) relative to STZ-induced diabetic animals receiving vehicle (STZ-veh) and those treated with GC-1 at either 100 or 250 μg/kg (n = 6–7 per group). Data represent means ± SE. *P < 0.05 vs. STZ-veh; #P < 0.05 vs. STZ-GC-1 (100 μg/kg).
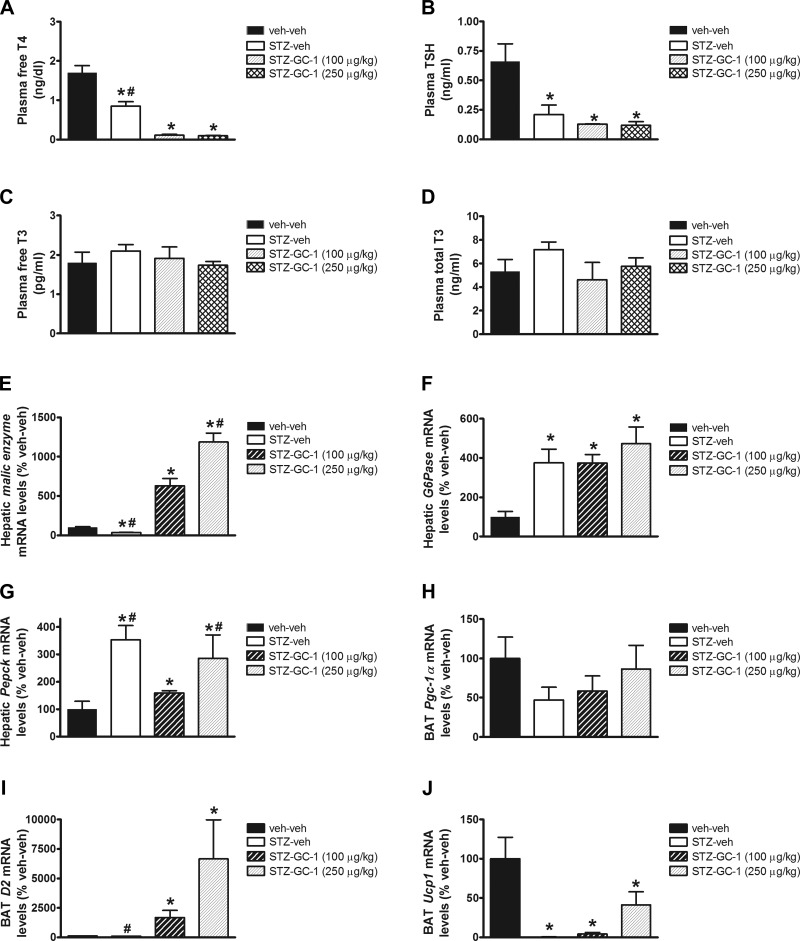
Consistent with our earlier observations, plasma free T4 and TSH levels were reduced in uDM, and these either remained low or were further reduced with GC-1 treatment, whereas there were no differences in plasma free or total T3 levels (Fig. 4, A–D). The concomitant fall of both free T4 and TSH levels while maintaining T3 levels in the normal range suggests that, similar to fasting, uDM induces the euthyroid sick syndrome but not frank hypothyroidism. To confirm that GC-1 exerted its intended effects, we first measured tissue markers of thyroid hormone action. Consistent with previous reports (36), hepatic mRNA levels of malic enzyme were reduced with uDM relative to nondiabetic controls, and these were dose-dependently increased with increasing doses of GC-1 (Fig. 4E). In a similar manner, GC-1 dose-dependently increased D2 mRNA levels in BAT, an enzyme involved in the activation of thyroid hormones (Fig. 4I). Not surprisingly however, since GC-1 failed to attenuate diabetic hyperglycemia, hepatic expression of the gluconeogenic genes G6Pase and Pepck were markedly elevated in STZ-diabetic animals relative to nondiabetic controls irrespectively of whether they received GC-1 (Fig. 4, F and G).
Fig. 4.
Effect of GC-1 actions in BAT and liver in uDM. Plasma free T4 (A), TSH (B), free T3 (C), and total T3 plasma levels (D); hepatic expression of malic enzyme (E), G6Pase (F), and Pepck (G); and BAT expression of Pgc1-α (H), D2 (I), and Ucp1 (J), using real-time PCR in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving vehicle (STZ-veh) or GC-1 at 100 or 250 μg/kg (n = 6–7 per group). Data represent means ± SE. *P < 0.05 vs. veh-veh; #P < 0.05 vs. STZ-GC-1 (100 μg/kg).
We next determined whether the increase of energy expenditure observed in STZ-diabetic rats treated with GC-1 involves activation of BAT by measuring BAT Ucp1 gene expression. As previously reported (7, 15), BAT Ucp1 mRNA levels were dramatically reduced in all STZ-diabetic animals relative to nondiabetic controls (4J). This effect of uDM is comparable to that observed during fasting, another condition characterized by weight loss and associated responses that inhibit BAT activity, including reduced SNS outflow and low levels of insulin, leptin, and thyroid hormone (1). uDM is therefore characterized by the unique combination of increased energy expenditure (due to metabolic inefficiency) despite profound inhibition of BAT (55). Somewhat surprisingly, GC-1 treatment did not significantly increase BAT Ucp1 mRNA levels (Fig. 4J), even though this drug increased energy expenditure (Fig. 3C) and despite clear evidence that thyroid hormone receptors were activated in the liver, raising the possibility that skeletal muscle thermogenesis may contribute to the increase in energy expenditure.
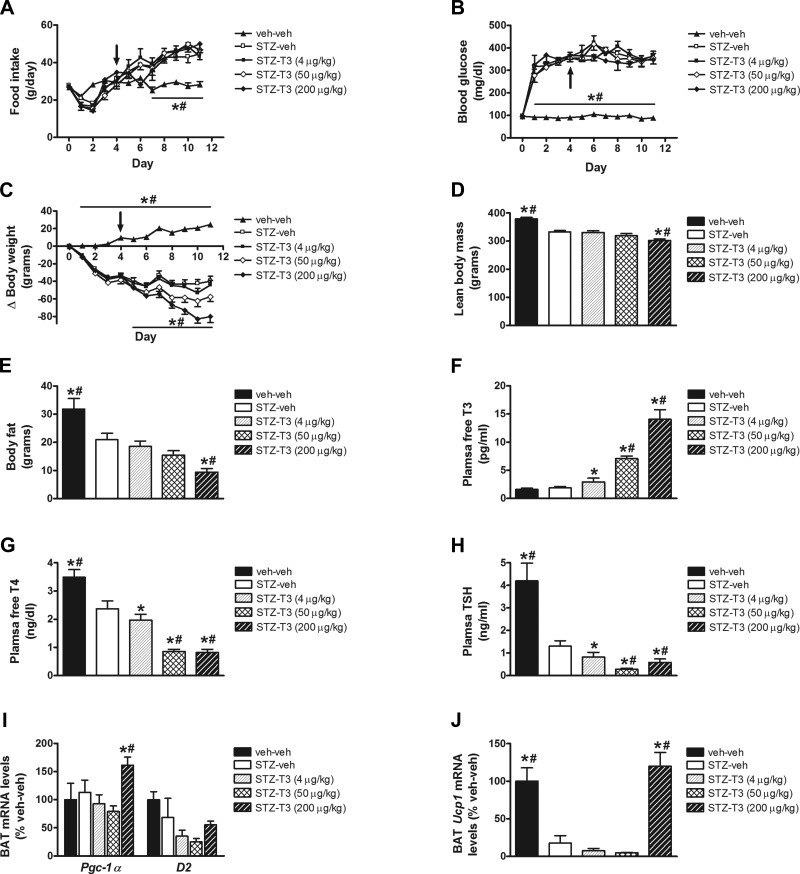
Although activation of the TRβ receptor by GC-1 was predicted to activate BAT based on studies in nondiabetic animals (36), its failure to do so in the current study prompted us to substitute administration of the active form of thyroid hormone, T3 (which activates both TRα and TRβ) for GC-1 using approximately equimolar doses (36). As was observed with GC-1, T3 administration did not attenuate the effect of STZ-diabetes to induce either hyperphagia or hyperglycemia (Fig. 5, A and B), and the highest dose of T3 caused greater losses of body weight, body adiposity, and lean body mass than vehicle in rats with STZ-diabetic controls (Fig. 5, C–E). Again, plasma free T4 and TSH levels were reduced in uDM, and these either remained low or were further reduced by T3 treatment, while as expected, free T3 levels increased dose-dependently with escalating doses of T3 (Fig. 5, F–H). Unlike the response to GC-1, however, T3 administration had no effect on BAT D2 mRNA levels, although the highest dose increased BAT mRNA levels of Pgc1-α and Ucp1 (Fig. 5, I and J).
Fig. 5.
Effect of T3 on food intake, blood glucose levels, and body composition in uDM. Food intake (A), blood glucose levels (B), body weight change (C), lean body mass (D), fat mass (E), and plasma free T3 (F), free T4 (G), and TSH levels (H), and BAT expression of Pgc1-α and D2 (I) and Ucp1 (J) in nondiabetic controls (veh-veh) or in STZ-induced diabetic animals receiving vehicle (STZ-veh) or T3 at 4, 50, or 200 μg/kg (n = 6–7 per group). Arrow indicates start of daily ip injections. Data represent means ± SE. *P < 0.05 vs. STZ-veh, #P < 0.05 vs. STZ-T3 (4 μg/kg).
Combined Effects of GC-1 and β3-AR Agonist on Food Intake, Blood Glucose Levels, and Body Composition in STZ-Diabetic Rats
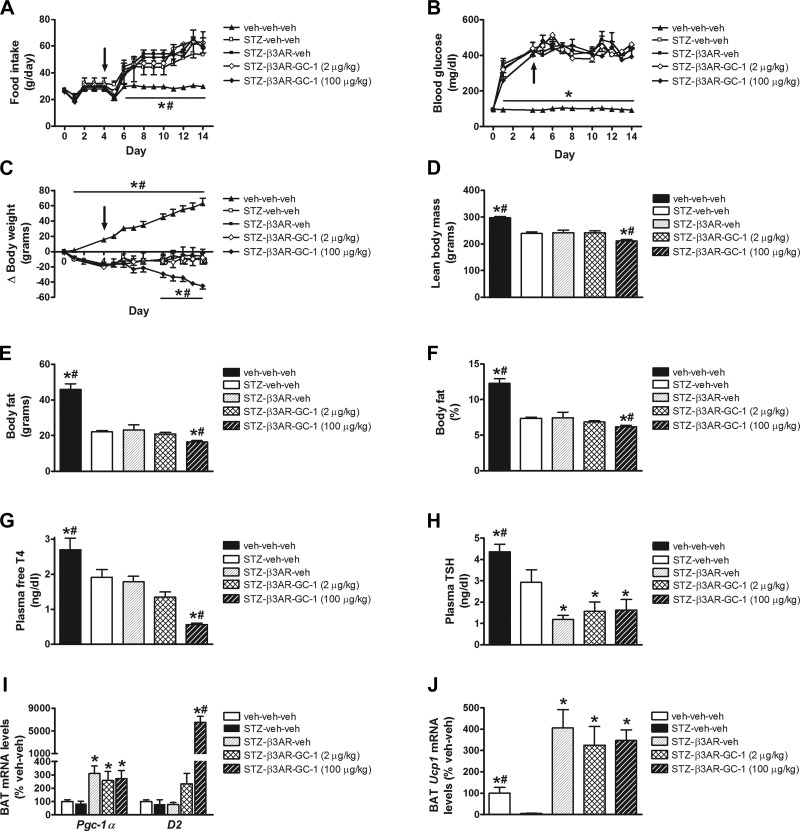
Since the full thermogenic response of BAT to cold exposure requires synergistic interactions between the SNS and thyroid hormone (42), we hypothesized that failure of GC-1 to activate BAT in uDM might reflect persistent inhibition of SNS outflow to BAT, even after thyroid status was normalized. To test this hypothesis, we treated STZ-diabetic animals with a combination of GC-1 (100 μg/kg) and the β3-AR agonist CL-316243. Consistent with our earlier observations, food intake was significantly increased in STZ-diabetic animals receiving vehicle relative to nondiabetic controls, and this diabetic hyperphagia remained unchanged in STZ-diabetic animals that received GC-1 whether or not they received the β3-AR agonist (Fig. 6A). In a similar manner, all animals that received STZ were characterized by a diabetic hyperglycemia, and there was no effect of either GC-1 or the β3-AR agonist or the combination of both to lower blood glucose levels in STZ-diabetic rats (Fig. 6B).
Fig. 6.
Effect of GC-1 and β3-AR agonist on food intake, blood glucose levels and body composition in uDM. Food intake (A), blood glucose levels (B), body weight change (C), lean body mass (D), fat mass (E), and %body fat (F), plasma free T4 (G), and TSH levels (H), and BAT expression of Pgc1-α and D2 (I) and Ucp1 (J) using real-time PCR in nondiabetic controls (veh-veh-veh) or in STZ-induced diabetic animals receiving vehicle (STZ-veh-veh) or in STZ-induced diabetic animals receiving β3-AR agonist alone (STZ-β3AR-veh) or in combination with GC-1 at 2 or 100 μg/kg (n = 6–7 per group). Arrow indicates start of daily ip injections. Data represent means ± SE. *P < 0.05 vs. STZ-veh-veh; #P < 0.05 vs. STZ-β3AR-veh.
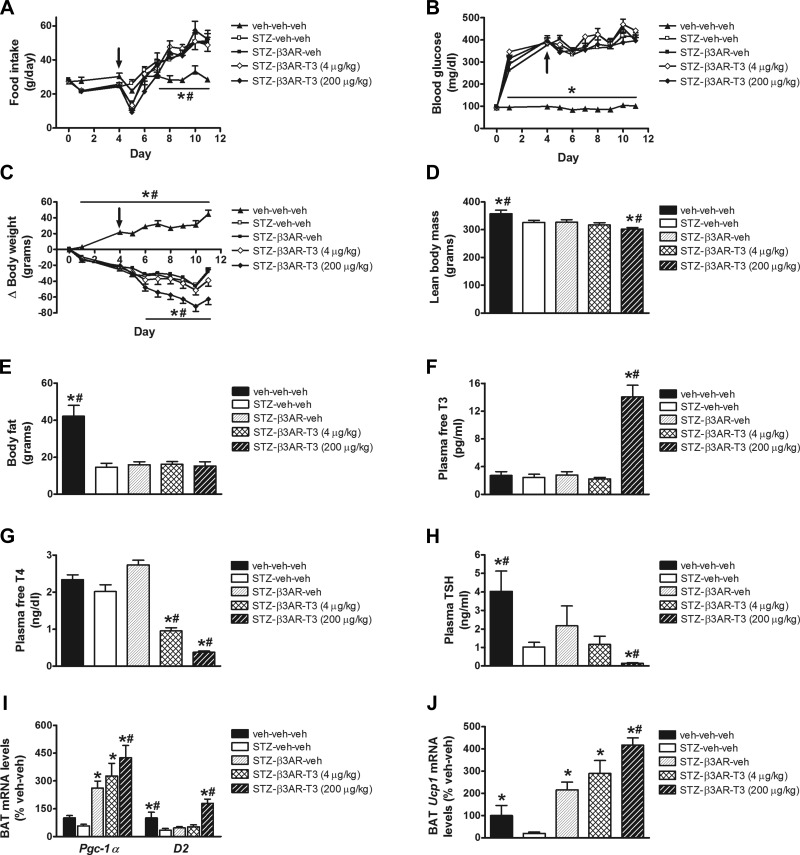
Similar to our earlier observations, there was a significant reduction of body weight in STZ-diabetic animals receiving GC-1 (relative to nondiabetic controls), and this effect was unaltered by the β3-AR agonist (Fig. 6C). Similarly, the effect of uDM or GC-1 on plasma free T4, TSH, or free or total T3 levels was not affected by the β3-AR agonist (Fig. 6, G and H). In contrast, however, combined treatment with GC-1 and the β3-AR agonist induced a further weight loss beginning on day 10 (Fig. 6C) that was accompanied by significant reductions of both lean and fat mass (Fig. 6, D–F). Moreover, although BAT Ucp1 mRNA levels were markedly reduced in STZ-diabetic relative to nondiabetic controls (irrespective of whether they received GC-1), both Ucp1 and Pgc1-α mRNA levels were elevated more than threefold in STZ-diabetic animals that received the β3-AR agonist (relative to nondiabetic controls) regardless of whether GC-1 was also given, whereas GC-1 dose-dependently increased D2 mRNA levels (Fig. 6, I and J). The use of T3 in combination with the β3-AR agonist yielded similar outcomes to that of T3 alone (Fig. 7). Thus, there was no effect to lower food intake or blood glucose levels in STZ-diabetic rats despite a marked increase in BAT Ucp1 and Pgc1-α mRNA levels (Fig. 7, I and J). These data suggest that, although diabetes-related inhibition of BAT (as measured by Ucp1 gene expression) is potently reversed by activation of β3-ARs (or the highest dose of T3), these interventions do not attenuate diabetic hyperglycemia. One potential explanation for this outcome is that, in uDM, induction of UCP1 can be dissociated from increased glucose uptake in BAT.
Fig. 7.
Effect of T3 and β3-AR agonist on food intake, blood glucose levels and body composition in uDM. Food intake (A), blood glucose levels (B), body weight change (C), lean body mass (D), fat mass (E), and plasma free T3 (F), free T4 (G), and TSH levels (H), and BAT expression of Pgc1-α and D2 (I) and Ucp1 (J) using real-time PCR in nondiabetic controls (veh-veh-veh) or in STZ-induced diabetic animals receiving vehicle (STZ-veh-veh) or in STZ-induced diabetic animals receiving β3-AR agonist alone (STZ-β3AR-veh) or in combination with T3 at either 4 or 200 μg/kg (n = 6–7 per group). Arrow indicates start of daily ip injections. Data represent means ± s.e.m. * P < 0.05 vs. STZ-veh-veh; # P < 0.05 vs. STZ-β3AR-veh.
Effects of GC-1 and β3-AR Agonist on Glucose Appearance and Tissue Glucose Uptake in STZ-Diabetic Rats
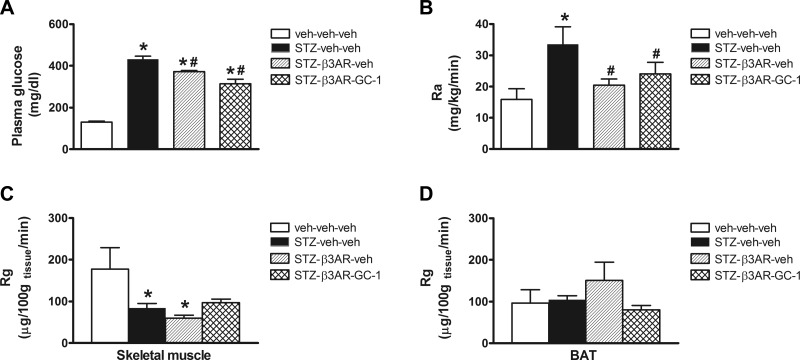
To investigate this hypothesis, we utilized tracer dilution techniques during a basal clamp to measure glucose Ra and tissue glucose uptake (Rg). Following a short-term fast, there was a modest effect of the β3-AR agonist either alone or in combination with GC-1 to lower plasma glucose levels in STZ-diabetic animals, although these animals still remained markedly hyperglycemic (Fig. 8A). As expected, Ra was markedly elevated in STZ-diabetic animals relative to nondiabetic controls, and this effect was significantly attenuated by systemic administration of the β3-AR agonist whether given alone or in combination with GC-1 (Fig. 8B). This reduction in Ra likely contributes to the slight fall in plasma glucose levels. Because of the confounding effect of glycosuria on measurement of the rate of Rd, we used labeled 2-DG to measure tissue Rg in a manner that is not affected by urinary glucose losses. We found that there was no effect of the β3-AR agonist whether given alone or in combination with GC-1 to increase glucose uptake in skeletal muscle relative to STZ-veh diabetic animals (Fig. 8C). Somewhat surprisingly, however, the β3-AR agonist also did not increase Rg in BAT relative to controls that were either STZ-diabetic or nondiabetic, and whether the drug was given alone or in combination with GC-1 (Fig. 8D). This outcome was particularly unexpected given the clear effect of the β3-AR agonist to markedly increase BAT Ucp1 mRNA (Fig. 6J) and indicates that Ucp1 gene expression and Rg in BAT can be dissociated from one another in the setting of uDM.
Fig. 8.
Effect of GC-1 and β3-AR agonist on glucose appearance and tissue glucose uptake in uDM. Five-hour-fasted plasma glucose levels (A), rate of glucose appearance (Ra; B) determined from [3-3H]glucose tracer studies and tissue glucose uptake (Rg) determined from 2-deoxy-[14C]glucose (2-DG) studies in skeletal muscle (C) and BAT (D) in nondiabetic controls (veh-veh-veh) or in STZ-induced diabetic animals receiving either vehicle (STZ-veh-veh) or in STZ-induced diabetic animals receiving β3-AR agonist alone (STZ-β3AR-veh) or in combination with GC-1 (100 μg/kg; STZ-β3AR-GC1; n = 5–6 per group). Data represent means ± SE. *P < 0.05 vs. veh-veh-veh; #P < 0.05 vs. STZ-veh-veh.
DISCUSSION
Recent advances in human BAT imaging, based on detection of glucose uptake in this tissue (11, 48, 51), have sparked renewed interest in identifying potential activators of BAT for the treatment of obesity and diabetes (47). That BAT activation might contribute to glucose lowering in diabetes is suggested by our recent demonstration that in STZ-diabetic rats reversal of hyperglycemia by icv leptin is accompanied by a marked increase of glucose uptake in this tissue despite persistent, severe insulin deficiency (16). The current studies were undertaken in part to determine if BAT activation is itself sufficient to lower blood glucose in this setting. Similarly, our observation that the reduced circulating plasma T4 levels and expression of Ucp1 in BAT characteristic of STZ-diabetes is reversed by icv leptin treatment prompted us to ask two related questions. Does thyroid hormone treatment attenuate hyperglycemia in STZ-diabetic rats? And might this effect be mediated in part by activating BAT and promoting glucose uptake in this tissue? Here, we report that, whereas systemic administration of the TRβ-selective agonist GC-1 increased energy expenditure adjusted for total body mass and induced further weight loss in STZ-diabetic rats, it failed to lower blood glucose levels. Moreover, when used either alone or in combination with the β3-AR agonist CL-316243 to mimic SNS activation of BAT, GC-1 treatment failed to increase BAT glucose uptake in STZ-diabetic rats, despite activating BAT, as measured by Ucp1 gene expression. Collectively, these data suggest that, in uDM, 1) Ucp1 can be strongly induced in BAT without an associated increase of BAT glucose uptake, and 2) neither treatment with thyroid hormone nor activation of BAT is sufficient in and of itself (or even when used in combination) to increase glucose uptake and thereby reverse hyperglycemia.
Several lines of evidence support the hypothesis that changes of BAT thermogenesis can substantially affect systemic glucose uptake and thereby contribute to the regulation of blood glucose levels. For one, glucose uptake is markedly stimulated by cold exposure in both rodents (9, 26) and humans (11, 48, 51), and norepinephrine (NE)-induced glucose utilization in BAT requires UCP-1-mediated uncoupling, which underlies thermogenesis in this tissue (22). In addition, administration of selective TRβ agonists dose-dependently increases energy expenditure and improves glucose tolerance and insulin sensitivity in ob/ob mice (6) and lowers blood glucose levels in diet-induced obese rodents (2, 13). Our data suggest that glucose lowering associated with BAT activation in these models does not occur in uDM. Although GC-1 induced the expected increase of energy expenditure and weight loss (18, 50), neither GC-1 nor T3 was sufficient to lower blood glucose levels in STZ-diabetic rats (a small but significant effect was detected in fasted animals with STZ-diabetes, but not in animals fed ad libitum). Since increasing thyroid hormone action does not lower blood glucose levels in this setting, we infer that, in uDM, 1) reduced T4 levels are a relatively unimportant mediator of hyperglycemia arising from insulin deficiency, and 2) the effect of icv leptin to normalize T4 levels does not make a major contribution to its glucose-lowering effect (although it may still play a role). This being said, it remains possible that leptin-induced activation of TRH neurons in the hypothalamic paraventricular nucleus contributes to its antidiabetic effects independently of increased thyroid hormone levels per se (25). Consistent with this hypothesis, TRH-deficient mice are characterized by hyperglycemia as well as hypothyroidism, and treatment of these mice with thyroid hormone for their hypothyroidism is insufficient to ameliorate their hyperglycemia (54).
Since uDM is characterized by both reduced SNS outflow to BAT, as measured by reduced NE turnover (55), and low thyroid hormone levels (35, 37), we asked whether a selective β3-AR agonist is capable of stimulating BAT in this setting, since BAT expresses the β3-AR and is strongly activated by agonists of this receptor (31). Related to this, we also asked whether BAT activation by a β3-AR agonist is sufficient to increase BAT glucose uptake and/or lower blood glucose levels in uDM. Previous studies have demonstrated that intact SNS function is required for the ability of leptin action in the brain to increase Ucp1 gene expression (19) and stimulate glucose uptake in BAT (21, 29). Moreover, treatment with a β3-AR agonist has antidiabetic effects in rodent models of obesity and diabetes (3, 53, 56), and this effect is accompanied by increases of both Ucp1 gene expression and glucose uptake in BAT. Indeed, despite the fact that glucose is not the primary substrate that fuels BAT uncoupling and heat production, BAT imaging by PET is based on increased glucose uptake into this tissue (11, 48, 51). Although there are conflicting reports regarding the antidiabetic effects of different β3-AR agonists in chemically induced rodent models of uDM (12, 28), a consistent finding in our studies was that, despite a pronounced increase of Ucp1 gene expression, BAT glucose uptake was not increased, and blood glucose was not lowered, by β3-AR stimulation. Thus, although the SNS is clearly important in the control of BAT activity, our data demonstrate that in uDM increased expression of Ucp1 can be dissociated from increased glucose uptake in BAT. It is noteworthy in this context that, although chronic treatment of STZ-diabetic rats with a β3-AR agonist had no effect on fed blood glucose levels, both plasma glucose levels and the basal rate of glucose appearance were modestly lowered following a short fast. This observation is consistent with evidence that β3-AR agonists reduce glucose output from the liver of animals with uDM (28).
Since SNS outflow and thyroid hormone act synergistically to produce heat and maintain body temperature (43), we also considered the possibility that stimulation by both inputs is required to increase BAT tissue glucose uptake and thereby attenuate diabetic hyperglycemia. According to this hypothesis, hypothyroidism blunts the response to adrenergic stimulation in uDM, thereby impairing thermogenic activation (39, 43). Conversely, the ability of thyroid hormone to induce BAT thermogenesis in uDM may also be limited by reduced SNS activity. Although our data reveal interactions between thyroid hormone and β3-AR stimulation in the control of energy balance, these did not translate into measurable changes of glucose metabolism. Thus, although giving the β3-AR agonist and GC-1 in combination caused expected increases of weight loss, energy expenditure, and induction of Ucp1 mRNA levels in BAT, there was no increase of glucose lowering or stimulation of glucose uptake in BAT or other tissues. The mechanism underlying increased energy expenditure in this setting may involve increases of both skeletal muscle thermogenesis and oxidation of free fatty acids by BAT (8), but does not appear to have involved increased BAT glucose oxidation. In uDM, therefore, activation of BAT Ucp1 mRNA levels can be dissociated from increased BAT glucose uptake.
Given that cold exposure in rodents and humans activates both thermogenesis and glucose uptake in BAT (9, 11, 48, 51), two possible interpretations of our findings can be considered. The first is that the mechanism(s) mediating the response to cold is not mimicked using a pharmacological approach that combines thyroid hormone with a β3-AR agonist. Consistent with this, although both cold exposure and treatment of a sympathomimetic drug, ephedrine, raises blood pressure and increases energy expenditure in humans, only cold exposure increases BAT glucose uptake as measured by 18FDG PET-CT (10), and cold exposure significantly increases glucose uptake in BAT of fasted rats (41). Alternatively, it is possible that persistent severe deficiency of insulin, leptin, or both hormones (as is characteristic of uDM) undermines the stimulatory effects of SNS and thyroid hormone on BAT glucose uptake. This possibility is consistent with evidence that insulin treatment (which increases both insulin and leptin levels) reverses the reduction of NE turnover and thermogenesis in BAT of rats with uDM (57). Furthermore, although acute cold exposure activates NE turnover in BAT of STZ-diabetic rats, the thermogenic response of BAT to acute cold exposure is absent (57). Correction of insulin and/or leptin deficiency may therefore be required for thermogenic activation of BAT to stimulate glucose uptake in this tissue.
Because of the generally negative data we observed with GC-1, which selectively activates TRβ, we considered the possibility that activation of BAT by thyroid hormone requires TRα signaling as well. This notion is consistent with evidence that, relative to T3, GC-1 treatment in hypothyroid mice does not remedy impaired NE-induced BAT thermogenesis, nor does it restore the ability of hypothyroid mice to either maintain core body temperature during cold exposure or to induce Ucp1 mRNA expression in BAT (36). It is therefore possible that thyroid stimulation can be mediated via different TR isoforms in the same tissue (36) or that stimulation by both TR isoforms is needed for BAT activation. Yet we found that, similar to GC-1 when given alone or in combination with the β3-AR agonist, T3 (which activates both TR isoforms) also failed to lower blood glucose levels despite causing weight loss and markedly increasing Ucp1 gene expression in BAT. It remains possible, however, that T3 administration increased BAT glucose uptake, as we did not directly address this possibility, and thyroid administration does increase rates of glucose uptake in peripheral tissues of nondiabetic animals (5).
In summary, our results support several conclusions. First, the effect of icv leptin to normalize thyroid hormone levels in uDM is unlikely to explain either its antidiabetic effects or its ability to increase BAT glucose uptake. Second, although systemic administration of the TRβ agonist GC-1 is sufficient to increase energy expenditure and induce weight loss in STZ-diabetic rats, it does not ameliorate hyperglycemia or induce BAT glucose uptake in this setting despite potently inducing Ucp1 gene expression. Taken together, these data suggest that, in uDM, combined pharmacological activation of adrenergic and thyroid hormone receptors cannot mimic the potent increase of BAT glucose uptake observed in response to cold. Last, our results indicate that, in uDM, stimulation of uncoupling can be dissociated from increased glucose uptake in BAT. Whether this effect results from deficiency of insulin, leptin, or both hormones awaits further study.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-089056 (G. J. Morton) and DK-083042 (M. W. Schwartz), by the Nutrition Obesity Research Center (NORC, DK-035816) at the University of Washington, and an American Heart Association Scientist Development Grant (G. J. Morton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.E.M., K.O., A.C., J.D.F., and G.J.M. performed experiments; M.E.M., K.O., A.C., J.D.F., K.J.K., and G.J.M. analyzed data; M.E.M., J.P.T., B.E.W., S.J.G., T.H.M., K.O., K.J.K., M.W.S., and G.J.M. edited and revised manuscript; M.E.M., J.P.T., B.E.W., S.J.G., T.H.M., K.O., A.C., J.D.F., K.J.K., M.W.S., and G.J.M. approved final version of manuscript; J.P.T., B.E.W., M.W.S., and G.J.M. conception and design of research; J.P.T., B.E.W., S.J.G., T.H.M., K.O., K.J.K., M.W.S., and G.J.M. interpreted results of experiments; G.J.M. prepared figures; G.J.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kevin Phillips from The Methodist Hospital Research Institute, Houston, TX, for providing the TRβ-selective agonist GC-1 synthetic thyroid. We also acknowledge the excellent technical assistance provided by Jarrell Nelson for performing body composition and indirect calorimetry measures as part of the University of Washington Nutrition Obesity Research Center Energy Balance and Glucose Metabolism Core.
REFERENCES
- 1. Ahima RS, Prabakaran D, Mantxoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrince response to fasting. Nature 382: 250–252, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Amorim BS, Ueta CB, Freitas BC, Nassif RJ, Gouveia CH, Christoffolete MA, Moriscot AS, Lancelloti CL, Llimona F, Barbeiro HV, de Souza HP, Catanozi S, Passarelli M, Aoki MS, Bianco AC, Ribeiro MO. A TRbeta-selective agonist confers resistance to diet-induced obesity. J Endocrinol 203: 291–299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arbeeny CM, Meyers DS, Hillyer DE, Bergquist KE. Metabolic alterations associated with the antidiabetic effect of beta 3-adrenergic receptor agonists in obese mice. Am J Physiol Endocrinol Metab 268: E678–E684, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Brenta G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J Thyroid Res 2011: 152850, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, Dong G, Singh R, Shapses S, Malm J, Webb P, Baxter JD, Grover GJ. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem Mol Biol 111: 262–267, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Burcelin R, Kande J, Ricquier D, Girard J. Changes in uncoupling protein and GLUT4 glucose transporter expressions in interscapular brown adipose tissue of diabetic rats: relative roles of hyperglycaemia and hypoinsulinaemia. Biochem J 291: 109–113, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cooney GJ, Caterson ID, Newsholme EA. The effect of insulin and noradrenaline on the uptake of 2-[1–14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett 188: 257–261, 1985 [DOI] [PubMed] [Google Scholar]
- 10. Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, Kahn CR. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci USA 109: 10001–10005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiol Regul Integr Comp Physiol 291: R1275–R1282, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 104: 15490–15495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57: 257–270, 1979 [DOI] [PubMed] [Google Scholar]
- 15. Geloen A, Trayhurn P. Regulation of the level of uncoupling protein in brown adipose tissue by insulin. Am J Physiol Regul Integr Comp Physiol 258: R418–R424, 1990 [DOI] [PubMed] [Google Scholar]
- 16. German JP, Thaler JP, Wisse BE, Oh -IS, Sarruf DA, Matsen ME, Fischer JD, Taborsky GJ, Jr, Schwartz MW, Morton GJ. Leptin action in the brain normalizes diabetic hyperglycemia via insulin-independent mechanisms. Endocrinology 152: 394–404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. German JP, Wisse BE, Thaler JP, Oh -IS, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ, Jr, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes 59: 1626–1634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-l-thyronine. Endocrinology 145: 1656–1661, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Gullicksen PS, Flatt WP, Dean RG, Hartzell DL, Baile CA. Energy metabolism and expression of uncoupling proteins 1, 2, and 3 after 21 days of recovery from intracerebroventricular mouse leptin in rats. Physiol Behav 75: 473–482, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61: 674–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48: 1706–1712., 1999 [DOI] [PubMed] [Google Scholar]
- 22. Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 54: 1385–1391, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60: 17–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138: 2569–2576, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Ma SW, Foster DO. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Canadian Can J Physiol Pharmacol 64: 609–614, 1986 [DOI] [PubMed] [Google Scholar]
- 27. McLean J, Tobin G. Animal and Human Calorimetry. Cambridge, UK: Cambridge Univ. Press, 1987 [Google Scholar]
- 28. Milagro FI, Gomez-Ambrosi J, Forga L, Martinez JA. A beta3-adrenergic agonist increases muscle GLUT1/GLUT4 ratio, and regulates liver glucose utilization in diabetic rats. Diabetes Obes Metab 1: 97–104, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab 300: E392–E401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem 266: 24053–24058, 1991 [PubMed] [Google Scholar]
- 32. Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia 27: 13–16, 1984 [DOI] [PubMed] [Google Scholar]
- 33. Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 11: 268–272, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Ortiz-Caro J, Gonzalez C, Jolin T. Diurnal variations of plasma growth hormone, thyrotropin, thyroxine, and triiodothyronine in streptozotocin-diabetic and food-restricted rats. Endocrinology 115: 2227–2232, 1984 [DOI] [PubMed] [Google Scholar]
- 36. Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone–sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest 108: 97–105, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rondeel JM, de Greef WJ, Heide R, Visser TJ. Hypothalamo-hypophysial-thyroid axis in streptozotocin-induced diabetes. Endocrinology 130: 216–220, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979 [DOI] [PubMed] [Google Scholar]
- 39. Sellers EA, You SS. Role of the thyroid in metabolic responses to a cold environment. Am J Physiol 163: 81–91, 1950 [DOI] [PubMed] [Google Scholar]
- 40. Seoane LM, Carro E, Tovar S, Casanueva FF, Dieguez C. Regulation of in vivo TSH secretion by leptin. Regul Pept 92: 25–29, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Shibata H, Perusse F, Vallerand A, Bukowiecki LJ. Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. Am J Physiol Regul Integr Comp Physiol 257: R96–R101, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86: 435–464, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18: 157–165, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes 48: 1275–1280, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Skarulis MC, Celi FS, Mueller E, Zemskova M, Malek R, Hugendubler L, Cochran C, Solomon J, Chen C, Gorden P. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab 95: 256–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Disc 9: 465–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Verga Falzacappa C, Mangialardo C, Madaro L, Ranieri D, Lupoi L, Stigliano A, Torrisi MR, Bouche M, Toscano V, Misiti S. Thyroid hormone T3 counteracts STZ induced diabetes in mouse. PLos One 6: e19839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Villicev CM, Freitas FR, Aoki MS, Taffarel C, Scanlan TS, Moriscot AS, Ribeiro MO, Bianco AC, Gouveia CH. Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol 193: 21–29, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Weinstein SP, O'Boyle E, Haber RS. Thyroid hormone increases basal and insulin-stimulated glucose transport in skeletal muscle. The role of GLUT4 glucose transporter expression. Diabetes 43: 1185–1189, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Williams CA, Shih MF, Taberner PV. Sustained improvement in glucose homeostasis in lean and obese mice following chronic administration of the beta 3 agonist SR 58611A. Br J Pharmacol 128: 1586–1592, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamada M, Saga Y, Shibusawa N, Hirato J, Murakami M, Iwasaki T, Hashimoto K, Satoh T, Wakabayashi K, Taketo MM, Mori M. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci USA 94: 10862–10867, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoshida T, Nishioka H, Nakamura Y, Kondo M. Reduced noradrenaline turnover in streptozotocin-induced diabetic rats. Diabetologia 28: 692–696, 1985 [DOI] [PubMed] [Google Scholar]
- 56. Yoshida T, Sakane N, Wakabayashi Y, Umekawa T, Kondo M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in yellow KK mice. Life Sci 54: 491–498, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Yoshioka K, Yoshida T, Wakabayashi Y, Nishioka H, Kondo M. The role of insulin in norepinephrine turnover and thermogenesis in brown adipose tissue after acute cold-exposure. Endocrinol Japon 36: 491–499, 1989 [DOI] [PubMed] [Google Scholar]