Abstract
Purpose
The objective of this study was to describe the evolution of health-related quality of life (HRQOL) in a cohort of breast cancer patients over 1 year after surgery and to analyse the predictive ability of HRQOL measurement instruments.
Methods
Observational, multicenter and prospective study of a cohort of breast cancer patients, assessing HRQOL at 1, 6, and 12 months after surgery using three questionnaires: EuroQol-5D-3L, EORTC QLQ-C30, and EORTC QLQ-BR23.
Results
A total of 364 women participated in the study. Visual Analogue Scale (VAS) scores from the EuroQol improved (1 month vs. 1 year: 70 vs. 80; p<0.0001); however, the EuroQol score showed no significant change (0.81 vs. 0.83; p=0.1323). In contrast, Global Health Status on the EORTC QLQ-C30 improved (66.67 vs. 100.00; p<0.0001), as did all of this instrument's scales and most of its independent items. The EORTC QLQ-BR23 dimensions showed improvement, except for sexual functioning (100.00 vs. 86.67; p=0.0030) and future perspective (33.33 vs. 66.67; p<0.0001). Patients with good HRQOL outcomes at 1 month showed improved levels of HRQOL at 1 year; HRQOL measured at 1 month was predictive of HRQOL at 1 year.
Conclusion
HRQOL improved during the follow-up period. Likewise, HRQOL measurement instruments can predict early HRQOL.
Keywords: Breast neoplasms, Quality of life, Questionnaires
INTRODUCTION
Due to screening programs and advances in oncologic treatment, it has been possible to achieve a survival rate of over 80% at 5-year follow-up for breast cancers [1]. Consequently, there may be changes that develop some time after initial diagnosis, but also chronic side effects that play out over the longer term [2,3]. Some prospective studies suggest that the recovery of breast cancer patients tends to occur relatively early, and that their health-related quality of life (HRQOL) 1 year after surgery is similar to that of the general population [4,5].
This study focuses on the transition period between the end of primary treatment and the beginning of the survival period, which is concurrent with patients making the shift from being sick and receiving treatment to becoming healthy and trying to re-establish a normal life. The study describes the evolution of HRQOL scores for a cohort of patients and analyses the changes these patients underwent during their first postoperative year.
To assess HRQOL several scales can be used. In oncology, the European Group of Quality of Life Questionnaire (EuroQol-5D-3L) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) are probably the most useful because they are reliable, simple, feasible, easy to answer and validated in several European languages. These HRQOL measurement instruments have been widely used in numerous global trials [6,7]. Furthermore, there are studies stating that HRQOL scales provide prognostic information in addition to that of sociodemographic and clinical measures and, moreover, may help to predict survival in patients with breast cancer [8,9].
This study also focuses on the predictive capacity of these instruments for HRQOL assessment, analysing whether the initial HRQOL of breast cancer patients could be a prognostic factor for forthcoming HRQOL. Having a better perspective of how these patients undergo this complex period of transition, as well as obtaining early detection of alterations in the HRQOL, may help to make specific interventions to improve HRQOL in the long run.
METHODS
Study design
This research was a multicenter, observational, prospective study of a scattered cohort of breast cancer patients who underwent oncological breast surgery consecutively in three institutions located in Valencia (Spain) between May 2003 and May 2007 and who were followed for 1 year to assess HRQOL. Only those patients with stage IV disease were excluded.
Study variables
The sociodemographic variables were age, marital status, level of education, and occupational status. The clinical variables were origin (i.e., from screening or outpatient clinic), disease stage, induction chemotherapy, sentinel lymph node biopsy (SLNB), axillary dissection, type of surgery, radiotherapy, and adjuvant chemotherapy.
HRQOL measurement instruments
The study assessment used a general HRQOL scale (EuroQol-5D-3L) [6], a cancer-specific HRQOL scale (EORTC QLQ-C30) [7] and the latter's module specific to breast cancer (EORTC QLQ-BR23). The required permission to implement these three questionnaires was obtained, as was ethics committee approval.
Designed by a group of European researchers, the EuroQol-5D-3L is a generic, standardised questionnaire that has been validated in Spanish for the measurement of HRQOL. The scale consists of two parts. The first is a Visual Analogue Scale (VAS), which records respondents' self-rated health on a vertical scale, the endpoints of which are labelled "Best imaginable health state" (100) and "Worst imaginable health state" (0). There is also a score that captures five dimensions of respondents' state of health (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Each of these five dimensions is divided into three levels of perceived problems (1: no problem, 2: some problems, and 3: extreme problems). A total of 243 possible health states are defined in this way, each of which is referred to using a five-digit code, where the numerals 1 to 5 have no arithmetic properties. For example, state 11111 indicates no problems on any of the five dimensions. Once obtained, the five-digit code may then be converted into a single summary index by applying a formula that essentially attaches values to each of the levels in each dimension. The Spanish value set supplied by the EuroQol Group was used to obtain this index or score, which ranges from -1 (worst state of health) to +1 (best state of health). The results were analysed according to the EuroQol Group guidelines [10].
The EORTC QLQ-C30 is a questionnaire from the European Organisation for Research and Treatment of Cancer consisting of 30 items structured in terms of a global health status scale, a functional area (physical, role, emotional, cognitive, and social functioning areas) and a symptoms area (fatigue, pain, nausea and vomiting, dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties). The EORTC QLQ-BR23 measures aspects more specific to breast cancer and is comprised of 23 items distributed over one functional area (body image, sexual functioning, sexual enjoyment, and future perspective) and a symptoms area (systemic therapy side-effects, breast symptoms, arm symptoms, and upset by hair loss). According to the EORTC guidelines, scores range from 0 to 100, where higher scores for the functioning scales represent a higher level of functioning and, higher scores for the symptoms represent a greater extent of symptoms [11].
All patients received the three surveys by mail at 1 month, 6 months, and 1 year after surgery. Those who did not respond received a phone call, and the questionnaires were sent again.
The patients gave written informed permission to use the three questionnaires, and the local ethics committees approved the study (CI.004.2006).
Analysis
A descriptive study of participants and of those who dropped out was carried out, assessing the differences between the two groups with the Fisher exact test. Afterwards, the results of the three questionnaires were described. Given that the majority of the variables did not follow a normal distribution (significant Kolmogorov-Smirnov test), the median was chosen as the descriptive parameter. Paired Wilcoxon test was used to look for the existence of possible differences in the HQROL between the first and sixth month, the sixth and twelfth month, and the first and twelfth month of the follow-up. The baseline values for HRQOL (1 month after surgery) were then grouped into tertiles because these variables did not follow a normal distribution and this was as such an illustrative way to represent the data distribution. The Kruskal-Wallis test was used to analyze the relationship between a subject belonging to one of these tertiles at 1 month and her final values at 1 year for the HRQOL instruments that were used, thus asses whether or not the initial HRQOL was a prognostic factor for subsequent HRQOL. The analysis was carried out using the SPSS version 15.0 (SPSS Inc., Chicago, USA) and STATA version 9 (Stata-Corp., College Station, USA) statistical software packs.
RESULTS
Of the 551 patients invited to participate in the study, 105 (19.06%) did not respond to the first survey, 76 (17.04%) did not respond at 6 months, and 40 patients (10.81%) declined to participate in the third survey. Finally, after recovering some patients who did not respond at 6 months, but did so at 1 and 12 months, 364 patients were included in the study.
Of this total, 37.20% attended at hospital A, 25.40% at hospital B, and 37.40% at hospital C. There was a homogeneous distribution among these hospitals, with no differences in the sociodemographic or clinical variables of their respective patients.
Upon comparison, no differences were found between the patients that participated in the study and those who did not, except for their hospital of origin. Hospital C had a significantly lower rate than did the other two. The mean age of patients was 59.09±13 years (range, 20-91 years). The most frequent age group was 60 to 69 years (29.40%). Most of the patients were married (63.70%), had finished their primary studies (52.20%), and the most common occupational status was housewife (36.30%). The clinical characteristics of the patients are shown in Table 1.
Table 1.
Clinical characteristics of breast cancer patients comparing nonrespondents and respondents (n=551)
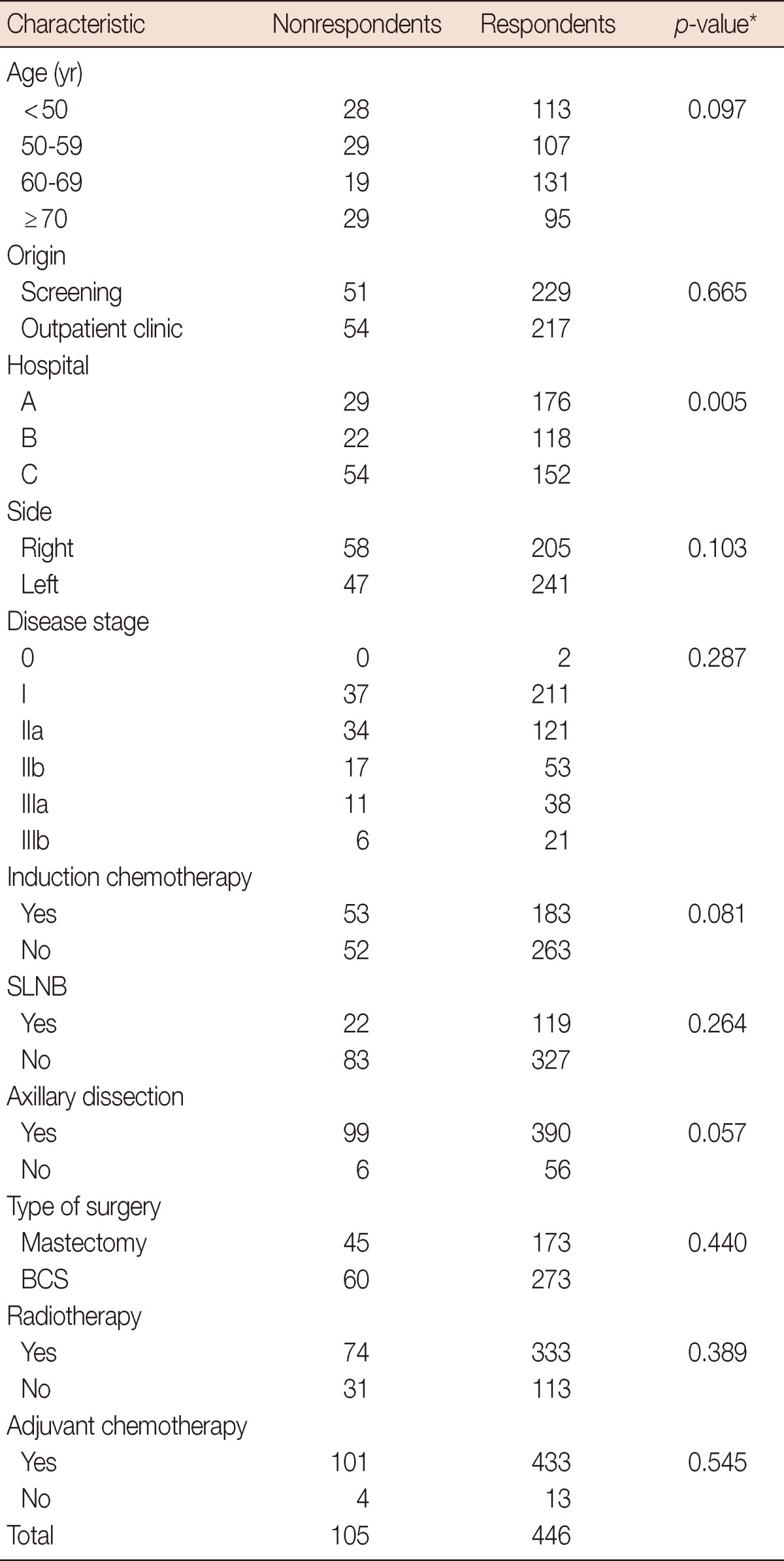
Values are presented as number.
SLNB=sentinel lymph node biopsy; BCS=breast-conserving surgery.
*p<0.005 is considered to indicate statistical significance from Fisher's exact test.
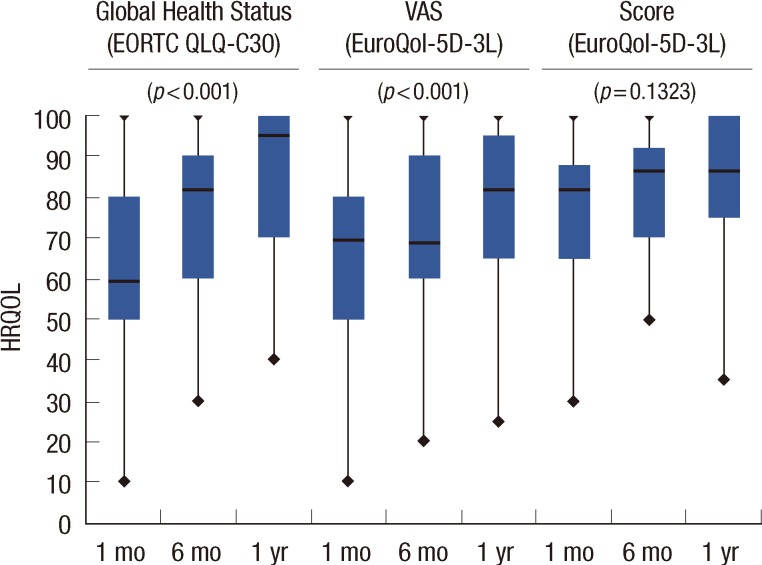
The changes in HRQOL from 1 month to 1 year after surgery are reported in Table 2. With respect to the outcomes of the EuroQol-5D-3L, VAS scores improved for the patients as a whole (from a median of 70 at first month to 80 at 1 year, p<0.0001). Despite this, the EuroQol-5D-3L score did not show significant changes over the period (0.81 vs. 0.83, p=0.1323). Global health status on the EORTC QLQ-C30 improved during follow-up (66.67 vs. 100.00, p<0.0001) (Figure 1). All of the functional dimension scores for the EORTC QLQ-C30 also showed significant improvements between the 1 month and 1 year measurements. The majority of the symptom scales showed little patient impairment and scores improved throughout the entire period.
Table 2.
Changes in HRQOL from 1 month to 1 year after surgery

HRQOL=health-related quality of life; EuroQol-5D-3L=European Group of Quality of Life Questionnaire; VAS=Visual Analogue Scale; EORTC QLQ-C30=European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 items; EORTC QLQ-BR23=Quality of life Questionnaire Breast 23 items.
*n=364 (n corresponds to the comparison between 1 month vs. 1 year; values lower than 364 are due to no answer for some items); †p<0.005 is considered to indicate statistical significance from Wilcoxon matched pairs test.
Figure 1.
Evolution of health-related quality of life (HRQOL) from 1 month to 1 year after surgery.
VAS=Visual Analogue Scale; EuroQol-5D-3L=European Group of Quality of Life Questionnaire; EORTC QLQ-C30=European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 items.
Regarding the EORTC QLQ-BR23, scores were initially very high for body image, and these also underwent improvement throughout the entire follow-up period. Sexual functioning showed high scores across the three measurements, but worsened slightly as the study progressed. The dimension of sexual enjoyment showed intermediate scores with no significant changes over time. Anxiety about the future worsened significantly throughout the follow-up. Finally, the side effects dimension of the EORTC QLQ-BR23 revealed low scores that decreased throughout the follow-up period, with the exception of the upset by hair loss item, for which there were no significant differences between follow-up points.
Table 3 shows the association between the HRQOL outcomes at 1 year and the results obtained at 1 month for the EuroQol-5D-3L (for both VAS and score), global health status on the EORTC QLQ-C30, some further selected dimensions of the latter instrument, and finally the EORTC QLQ-BR23. The analysis was performed using Kruskal-Wallis test, comparing each tertile at 1 month with the corresponding tertile at 1 year in every case; at 1 year only the median was represented to simplify the data comprehension.
Table 3.
Association between HRQOL at 1 month and EuroQol-5D-3L (score and VAS) and Global Health Status of EORTC QLQ-C30 at 1 year after surgery
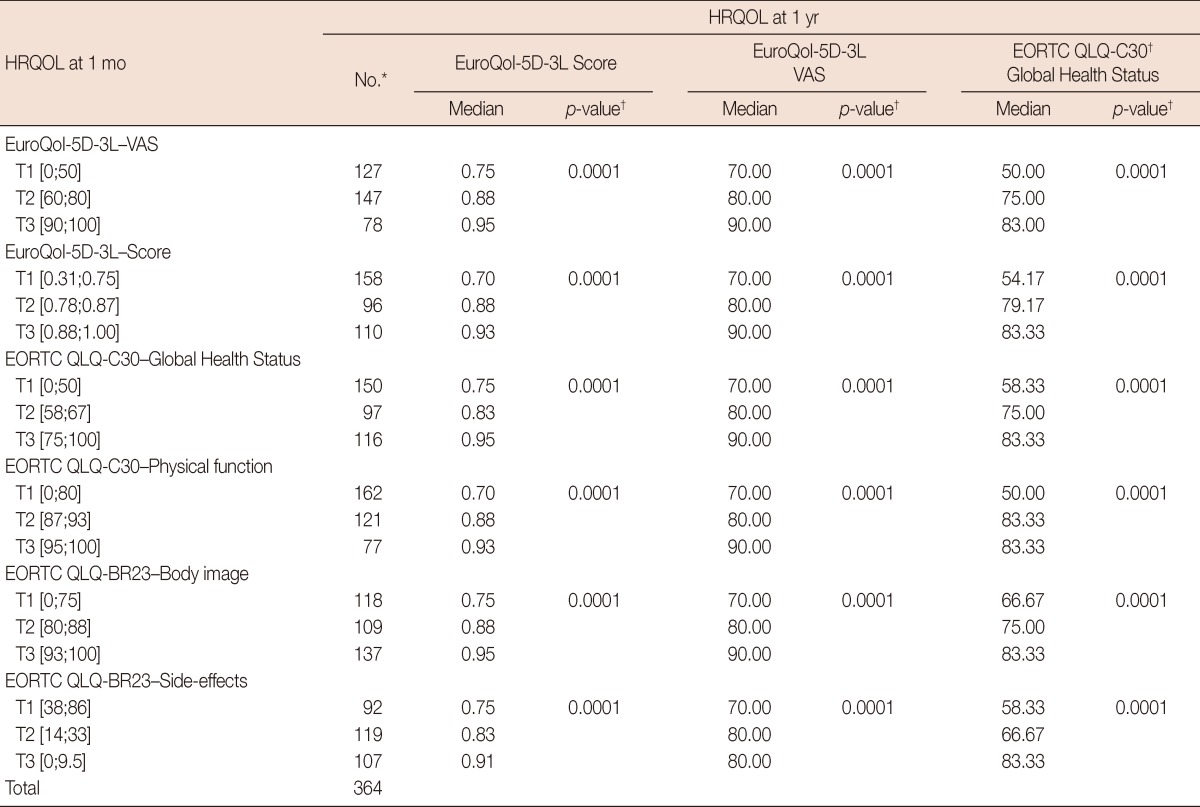
HRQOL=health-related quality of life; EuroQol-5D-3L=European Group of Quality of Life Questionnaire; VAS=Visual Analogue Scale; EORTC QLQ-C30=European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 items; T=tertiles of scores at 1 month after surgery in the corresponding scale.
Values between brackets are initial and final scores of the tertile; EORTC QLQ-BR23=Quality of life Questionnaire Breast 23 items.
*n=364; †p<0.005 is considered to indicate statistical significance from Kruskal-Wallis test.
Regarding the associations between HRQOL at 1 month and EuroQol-5D-3L score at 1 year, those patients with the lowest tertile values for VAS at 1 month had a median score of 0.75 on the EuroQol-5D-3L at 1 year. Those in the second tertile had a score of 0.88, while those in the highest tertile scored 0.95 at 1 year (p=0.0001). The tertiles for the EuroQol-5D-3L score (0.70, 0.88, and 0.93) and global health status on the EORTC QLQ-C30 (0.75, 0.83, and 0.95) showed improvement during follow-up (p=0.0001). The median scores for the physical function dimension of the EORTC QLQ-C30 at 1 year were 0.70, 0.88, and 0.93 for the respective tertiles (p=0.0001). With respect to the body image dimension of the EORTC QLQ-BR23, the EuroQol-5D-3L at 1 year varied between 0.75 for the women in the lowest tertile to 0.88 and 0.95 for those in the tertiles above (p=0.0001). For the systemic therapy side effects dimension, the scores at 1 year for the EuroQol-5D-3L were 0.75 and 0.83 for the lowest two tertiles of women at 1 month, and 0.91 for the upper tertile, with the latter group showing the fewest adverse side effects (p=0.0001).
In relation to the associations between HRQOL at 1 month and the VAS of the EuroQol-5D-3L at 1 year, those patients with good results at 1 month for VAS, EuroQol-5D-3L score, global health status, physical functioning, body image, and side effects from systemic treatment on the EORTC QLQ-C30 and BR23, showed improved levels on the VAS at 1 year (p=0.0001).
Regarding the associations between HRQOL at 1 month and global health status on the EORTC QLQ-C30 at 1 year, patients in the highest VAS tertile at 1 month had a median 1-year score for global health status on the EORTC QLQ-C30 of 83.00, compared to 75.00 and only 50.00 for those in the second and third tertiles, respectively (p=0.0001). The 1-month tertiles for the EuroQol-5D-3L score (83.33, 79.17, and 54.17) and for global health status on the EORTC QLQ-C30 (83.33, 75.00, and 58.33) showed 1-year medians for the EORTC QLQ-C30 that were very similar to those for the VAS at 1 year (p=0.0001). In relation to the physical function dimension at 1 month, the medians for the EORTC QLQ-C30 at 1 year were 50.00 for the worst tertile and 83.33 for the upper two tertiles; however, the interquartile amplitude was lower in the last of these (p=0.0001). Compared to the body image dimension of the EORTC QLQ-BR23, the overall scores for the EORTC QLQ-C30 at 1 year varied from 66.67 for the women in the lowest tertile to 83.33 for those in the upper tertile (p=0.0001). For the systemic therapy side effects dimension, the 1-year global health status scores on the EORTC QLQ-C30 were 58.33 and 66.67 for the two tertiles of women who had the worst overall health at 1 month, and 83.33 for the tertile of women with the fewest adverse effects (p=0.0001).
DISCUSSION
This study analysed how the HRQOL of breast cancer patients evolved over 1-year follow-up, using questionnaires that were simple, standardised and had been widely internationally validated. The rate of participation was good, and the characteristics of the cohort were representative of the patients operated on in the three hospitals involved; moreover, there were no differences found between the study participants and those who did not respond.
According to the VAS of the EuroQol-5D-3L, patients showed good baseline levels of HRQOL, and they underwent improvement during the year after surgery. Nevertheless, the EuroQol-5D-3L scores confirmed high HRQOL values even though there were no changes during the follow-up. This disparity may be related to the large range between the VAS readings compared to the very narrow one between the scores, making the latter much more sensitive to small changes that the VAS would not detect.
Global health status on the EORTC QLQ-C30 showed high scores, and improvement could be noted after 1 year of follow-up. In the majority of studies, breast cancer survivors show a good global health status that is, similar, if not superior, to the healthy population for this age group [4,5,12]. Nevertheless, studies with longer follow-up periods of 5 to 10 years only report subtle changes, probably because symptoms decrease over a short period of time, despite the fact that some emotional, cognitive, and social alterations may last for a longer time [2,3,13].
In relation to the functional dimension of the EORTC QLQ-C30, physical and role functioning showed very high scores that improved over time, which was to be expected in that nonmetastatic breast cancer does not produce severe physical symptoms that could cause significant functional deterioration. Most published studies confirm these findings [4]. However, in Bloom's systematic revision covering 20 articles [14], as well as in Klein's population-based controlled study [15], there was found to be a worse physical functioning in breast cancer survivors than in the general population. Emotional and cognitive functioning had improved 1 year after surgery. Amongst others, Ganz et al. [5] affirms that improvement in emotional functioning occurs mainly in the first year of follow-up, and that differences for years 2 and 3 are less notable. The cognitive alterations involved in breast cancer comprise deficits in attention, concentration and memory, and are commonly related to chemotherapy [4]. Yet in relation to social functioning, the present results contrast with those of other authors, who have reported poor functioning initially and the need for a long period of time in order to return to predisease levels. Given that the majority of patients in our sample were housewives and were married, it may be that their social and family life changed little due to the disease. This may be the explanation for why there were no changes reflected in the questionnaire results for this dimension [16,17].
In the symptoms dimension of the EORTC QLQ-C30, fatigue, pain and insomnia showed little impact and, moreover, their scores decreased during follow-up. These three symptoms are frequent in cancer patients, and although they may improve initially, as seen in short-term studies such as the present one, it may be that patients never recover completely, with these symptoms persisting for many years after surgery, as has been reflected in long-term studies [3,13].
The remaining symptoms (nausea/vomiting, dyspnoea, appetite loss, constipation, diarrhoea, and financial difficulties) were scarce with median scores of 0. This is probably because the study was carried out in nonmetastatic breast cancer patients, and these symptoms are usually side effects of chemotherapy and have a transient effect that does not change HRQOL in the long run.
Standing out in the functional area of the EORTC QLQ-BR23 is body image, which was good initially and became notably better after 1 year. According to our experience, the main objective of these patients initially was to overcome the surgery and chemotherapy treatment, with body image being of secondary importance. Nevertheless, with the passage of time and with the perception that they were overcoming the disease, body image began to take on more relevance. For this reason, aesthetic changes may manifest themselves in later HRQOL scores, which also explains why these were not reflected in the present results [18,19]. Sexual functioning and sexual enjoyment in the present patients were reported as good, however deteriorated over the follow-up. This probably resulted from not only psychological factors such as changing feelings and a perceived diminution of physical attractiveness, but also due to somatic and physical factors such as secondary vaginal dryness from the menopause induced by the treatments administered, which produces a prolonged and progressive deterioration in this dimension, especially among younger women [2,20,21]. Regarding the future perspectives of the patients, it is noticeable that although symptoms improved, some physical and psychological alterations may persist, and these, although small, may well sustain stress and anxiety pertaining to possible recurrence. In a majority of papers, as well as in the present results, worries about the future occur frequently in this study group and can increase during follow-up [22-24].
Both the systemic therapy side effects as well as upset by hair loss showed declining scores. Most studies carried out in this area indicate that the changes caused by chemotherapy occur primarily in the first year and then diminish considerably. Breast cancer patients pay little attention to these alterations in terms of long-term HRQOL. Some authors, however, affirm that the adverse effects of chemotherapy on physical functioning and symptoms related to menopause can persist for 5 to 10 years after diagnosis [25,26].
In the present study, local symptoms in the operated breast were scarce and diminished over time; arm symptoms, however, improved only slightly during follow-up. Lymphedema is a frequent occurrence, which is slow to heal and in many cases can last for up to 20 years after the intervention and in some cases may be incapacitating [16,27]. SLNB implies a lesser aggression in the axillary region and a diminishing number of axillary lymphadenectomies carried out, thus minimising the prevalence of arm symptomatology. SLNB is associated with reduced arm morbidity and better HRQOL than is standard axillary dissection; nevertheless this finding requires confirmation in long-term randomized studies. In the present paper, SLNB showed no differences in this aspect. This is probably because of the low percentage of cases in which it had been applied, given that this technique was in the validation period at the time of study [28,29].
Practical this study offers the implication that the EuroQol-5D-3L, EORTC QLQ-C30, and EORTC QLQ-BR23 questionnaires can serve as predictors for HRQOL. Schou et al. [4] argues that those patients at risk for presenting worsened social and emotional functioning at 1 year after surgery can be identified at the moment of the diagnosis. Other authors have also noted that baseline functioning and initial HRQOL are among the variables that best predict functioning and HRQOL 1 or 2 years postsurgery [12].
Furthermore, several studies state that HRQOL scales may help, in addition to sociodemographic and clinical measures, to predict survival in patients with breast cancer [8,9].
Our results indicate that patients who presented good results in terms of VAS, EuroQol-5D-3L score, global health status, physical function, body image, and systemic therapy side effects as rated by the EORTC QLQ-C30, showed improved levels of HRQOL at 1 year as measured by the three instruments employed. Therefore, the use of these questionnaires for screening purposes could help in the early detection of those patients who later on will show worse HRQOL and hence determine which areas could need support or treatment by a psycho-oncologist. Knowing the HRQOL of patients diagnosed with breast neoplasms and the alterations that take place in the different related dimensions could be essential in the correct selection of the best treatment.
The study has several limitations due to its observational character and its lack of baseline HRQOL measurements prior to treatment, which could otherwise have been compared with the follow-up measurements. There is also the issue of the absence of a HRQOL study in a healthy population. Moreover, patients with physical or psychological disorders at baseline could show altered HRQOL outcomes; however this group was not excluded because the focus of the study was to describe the evolution of a cohort of patients and to analyze the predictive ability of HRQOL measurement instruments, meaning that patients with poor HRQOL secondary to physical or psychological impairments at baseline would maintain this condition at the end of the follow-up.
In our study, all patients had sufficient cognitive capacity to respond to the HRQOL surveys.
Furthermore, the high participation rate in a large sample, the use of internationally validated questionnaires, as well as the elevated proportion of completed surveys in several postoperative measurements and their longitudinal analysis contribute to the value of the results shown in this study.
In conclusion, most of the HRQOL features of breast cancer patients recovered within 1 year of surgery. Those patients with good HRQOL outcomes at 1 month showed improved levels of HRQOL at 1 year. This suggests that early HRQOL scores can predict longer-term HRQOL.
Footnotes
The authors declare that they have no competing interests.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Pumo V, Milone G, Iacono M, Giuliano SR, Di Mari A, Lopiano C, et al. Psychological and sexual disorders in long-term breast cancer survivors. Cancer Manag Res. 2012;4:61–65. doi: 10.2147/CMAR.S28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee ES, Lee MK, Kim SH, Ro JS, Kang HS, Kim SW, et al. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: a prospective cohort study. Ann Surg. 2011;253:101–108. doi: 10.1097/sla.0b013e3181f662ce. [DOI] [PubMed] [Google Scholar]
- 4.Schou I, Ekeberg Ø, Sandvik L, Hjermstad MJ, Ruland CM. Multiple predictors of health-related quality of life in early stage breast cancer. Data from a year follow-up study compared with the general population. Qual Life Res. 2005;14:1813–1823. doi: 10.1007/s11136-005-4344-z. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 6.Szende A, Williams A EuroQol Group. Measuring self-reported population health: an international perspective based on EQ-5D. Budapest: SpringMed Publishing; 2004. [PubMed] [Google Scholar]
- 7.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 8.Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 9.Staren ED, Gupta D, Braun DP. The prognostic role of quality of life assessment in breast cancer. Breast J. 2011;17:571–578. doi: 10.1111/j.1524-4741.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 10.Rabin R, Oemar M, Oppe M. EQ-5D-3L User Guide Basic Information on How to Use the EQ-5D-3L Instrument. Rotterdam: EuroQol Group; 2011. [Google Scholar]
- 11.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 12.Härtl K, Engel J, Herschbach P, Reinecker H, Sommer H, Friese K. Personality traits and psychosocial stress: quality of life over 2 years following breast cancer diagnosis and psychological impact factors. Psychooncology. 2010;19:160–169. doi: 10.1002/pon.1536. [DOI] [PubMed] [Google Scholar]
- 13.Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Møller S, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology. 2007;16:691–706. doi: 10.1002/pon.1208. [DOI] [PubMed] [Google Scholar]
- 15.Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, et al. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat. 2011;129:125–134. doi: 10.1007/s10549-011-1408-3. [DOI] [PubMed] [Google Scholar]
- 16.Kornblith AB, Herndon JE, 2nd, Weiss RB, Zhang C, Zuckerman EL, Rosenberg S, et al. Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer. 2003;98:679–689. doi: 10.1002/cncr.11531. [DOI] [PubMed] [Google Scholar]
- 17.Cui Y, Shu XO, Gao Y, Cai H, Wen W, Ruan ZX, et al. The long-term impact of medical and socio-demographic factors on the quality of life of breast cancer survivors among Chinese women. Breast Cancer Res Treat. 2004;87:135–147. doi: 10.1023/B:BREA.0000041620.76871.97. [DOI] [PubMed] [Google Scholar]
- 18.Fobair P, Stewart SL, Chang S, D'Onofrio C, Banks PJ, Bloom JR. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 19.Collins KK, Liu Y, Schootman M, Aft R, Yan Y, Dean G, et al. Effects of breast cancer surgery and surgical side effects on body image over time. Breast Cancer Res Treat. 2011;126:167–176. doi: 10.1007/s10549-010-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8:294–302. doi: 10.1111/j.1743-6109.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- 21.Bredart A, Dolbeault S, Savignoni A, Besancenet C, This P, Giami A, et al. Prevalence and associated factors of sexual problems after early-stage breast cancer treatment: results of a French exploratory survey. Psychooncology. 2011;20:841–850. doi: 10.1002/pon.1789. [DOI] [PubMed] [Google Scholar]
- 22.Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (>/=5 years) cancer survivors: a systematic review of quantitative studies. Psychooncology. 2013;22:1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 23.Mehnert A, Berg P, Henrich G, Herschbach P. Fear of cancer progression and cancer-related intrusive cognitions in breast cancer survivors. Psychooncology. 2009;18:1273–1280. doi: 10.1002/pon.1481. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Perez M, Schootman M, Aft RL, Gillanders WE, Jeffe DB. Correlates of fear of cancer recurrence in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2011;130:165–173. doi: 10.1007/s10549-011-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King MT, Kenny P, Shiell A, Hall J, Boyages J. Quality of life three months and one year after first treatment for early stage breast cancer: influence of treatment and patient characteristics. Qual Life Res. 2000;9:789–800. doi: 10.1023/a:1008936830764. [DOI] [PubMed] [Google Scholar]
- 26.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17:317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 27.Nesvold IL, Reinertsen KV, Fosså SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv. 2011;5:62–72. doi: 10.1007/s11764-010-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Wu LC, Chen JQ. Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: a meta-analysis. Breast Cancer Res Treat. 2011;129:675–689. doi: 10.1007/s10549-011-1665-1. [DOI] [PubMed] [Google Scholar]
- 29.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]