Abstract
Purpose
The purpose of this study was to investigate the prognostic impact of pretreatment neutrophil to lymphocyte ratio (NLR) on breast cancer in view of disease-specific survival and the intrinsic subtype.
Methods
We retrospectively studied patients diagnosed with primary breast cancer that had completed all phases of primary treatment from 2000 to 2010. The association between pretreatment NLR and disease-specific survival was analyzed.
Results
A total of 442 patients were eligible for analysis. Patients with higher NLR (2.5 ≤NLR) showed significantly lower disease-specific survival rate than those with lower NLR (NLR <2.5). Higher NLR along with negative estrogen receptor status and positive nodal status were independently correlated with poor prognosis, with hazard ratio 4.08 (95% confidence interval [CI], 1.62-10.28), 9.93 (95% CI, 3.51-28.13), and 11.23 (95% CI, 3.34-37.83), respectively. Luminal A subtype was the only intrinsic subtype in which higher NLR patients showed significantly poor prognosis (87.7% vs. 96.7%, p=0.009).
Conclusion
Patients with an elevated pretreatment NLR showed poorer disease-specific survival than patients without elevated NLR, most evident in the luminal A subtype. Further validation and a feasibility study are required before it can be considered for clinical use.
Keywords: Breast neoplasms, Lymphocytes, Neutrophils
INTRODUCTION
Human breast cancers are diverse in their natural history and responsiveness to treatments [1]. While lymph node status is considered the most important prognosis marker, the behavior of breast cancer shows markedly different clinical outcomes among patients with the same lymph node status [2,3].
Several models including Oncotype DX® (Genomic Health Inc., Redwood City, USA) and MammaPrint® (Agendia BV, Amsterdam, The Netherlands) have been introduced recently, which are applied to the subgroup stratified by stage or hormonal receptor status. However the controversy remains on economic issue, as the Korean national insurance does not support these program as the routine evaluation for early breast cancer. Despite the assertions that the models have been ordered frequently and has improved clinical decision making, little is known on its adoption or impact on clinical practice [4]. These findings support the need for indicators that are not only accurate, standardized and reproducible but also cheap and easy to perform.
It has been reported that tumors are linked with systemic inflammation [5,6]. The combined index, using neutrophil and lymphocyte counts in the form of a neutrophil to lymphocyte ratio (NLR), has been used as a cost-effective and simple parameter of systemic inflammation or stress. The combined index may also be related to prognosis in many types of cancer, including gastrointestinal tract malignancies [7], hepatocellular carcinoma [8], pancreatic cancer [9], non-small cell lung cancer [10], and cervical cancer [11].
The purpose of this study was to investigate the prognostic impact of the pretreatment NLR in breast cancer, in view of disease-specific survival and also the prognostic impact of pretreatment NLR in the breast cancer stratified by the intrinsic subtype.
METHODS
Patients
We retrospectively identified patients who were diagnosed with primary breast cancer and completed all phases of their primary treatment for breast cancer at the Wonju Severance Christian Hospital from 2000 to 2010 with Institutional Review Board approval (2011-77).
Medical records were reviewed to find data on patient's medical history, age, sex, pathologic results such as tumor size, lymph node status (number of positive lymph nodes and all lymph nodes if axillary lymph nodes were dissected), hormonal status, human epidermal growth factor receptor 2 (HER2) receptor status, and laboratory data.
Patients with ductal carcinoma in situ with or without microinvasion and patients with lack of information on pathologic or laboratory results were excluded. We also excluded patients with stage IV breast cancer or inflammatory breast cancer, patients who were diagnosed preoperatively with systemic inflammatory or chronic disease such as Systemic Lupus Erythematosus (SLE), liver cirrhosis, end-stage renal disease, and patients with pregnancy-related breast cancer.
For the mortality data, we used survival information of patients from the database of the Wonju Severance Christian Hospital and the Korean National Cancer Center. For the breast cancer specific mortality, we included patients deceased from breast cancer and excluded patients who deceased from diseases other than breast cancer.
Pathological characteristics
Estrogen receptor (ER) and progesterone receptor (PR) status were assessed by immunohistochemistry. HER2 status was assessed by immunohistochemistry or fluorescent in situ hybridization (FISH). It was considered positive in the following: if the score was 3 with immunohistochemistry; or at least 2.2 times more HER2 signals than CEP 17 signals in the tumor cells was considered as the criterion for HER2 amplification with FISH.
Intrinsic breast cancer subtypes were determined according to the following criteria: luminal A subtype, ER positive and/or PR positive and HER2 negative; luminal B subtype, ER positive and/or PR positive and HER2 positive; HER2 enriched subtype, ER and PR negative with positive HER2; triple negative tumors, ER negative, PR negative and HER2 negative.
Laboratory data
NLR taken as a baseline sample immediately after breast cancer diagnosis was confirmed and before the initiation of any treatment modality (pretreatment NLR). NLR is calculated as neutrophil count divided by lymphocyte count. The cutoff value of 2.5 was decided as the maximum (sensitivity+specificity) point according to receiver operating characteristics curve.
Statistical analysis
Frequency distributions between categorical variables among the groups were compared using the chi-square test. The Fisher's exact test was used if the expected frequency was <5. The disease-specific survival curves were calculated according to the Kaplan-Meier method with the log-rank test. The Cox proportional hazards model was used for the multivariable analyses. Statistical analyses were performed by SPSS software version 18.0 (SPSS Inc., Chicago, USA). A p-value <0.05 was considered statistically significant.
RESULTS
Patients demographics, tumor characteristics
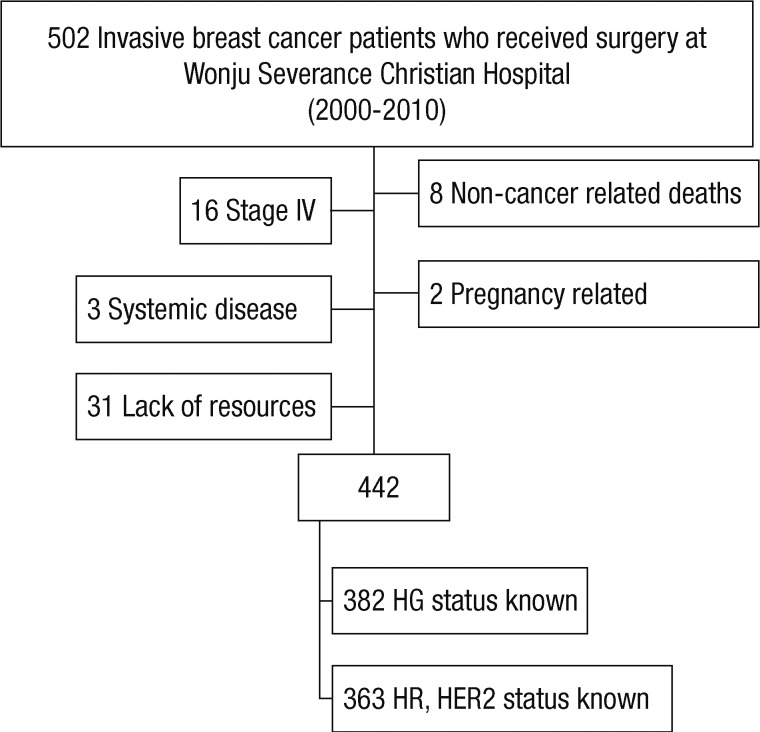
We identified 502 patients who were diagnosed and completed the treatment of breast cancer; and 442 patients were eligible for analysis. The reasons for the excluded patients are summarized in Figure 1. The baseline characteristics of the study subjects are summarized in Table 1.
Figure 1.
Enrollment and outcomes. We identified 502 patients who were diagnosed and completed the treatment of breast cancer; and 442 patients were eligible for analysis.
HG=histologic grade; HR=hormonal receptor; HER2=human epidermal growth factor receptor 2.
Table 1.
Patients' characteristics (n=442)
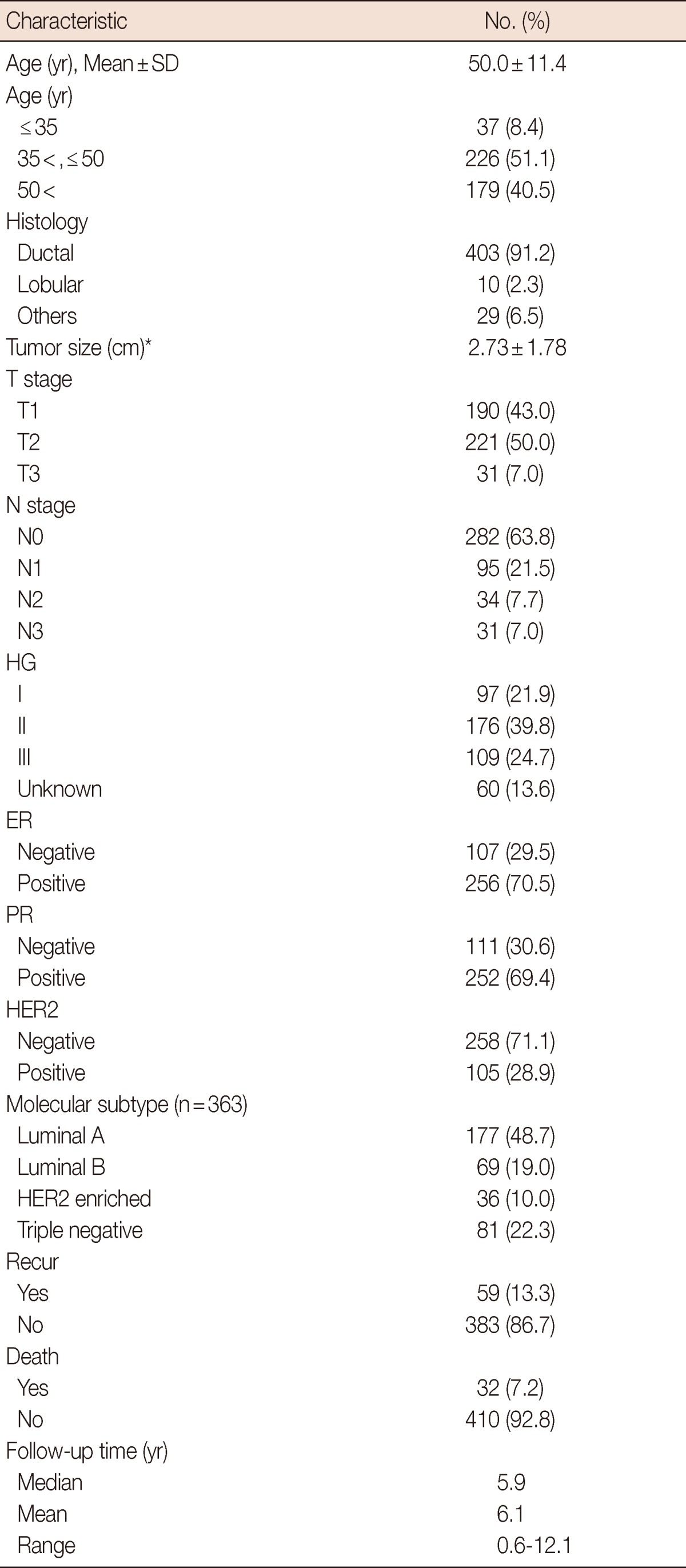
HG=histologic grade; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Pattern of NLR distribution and disease-specific survival by NLR status
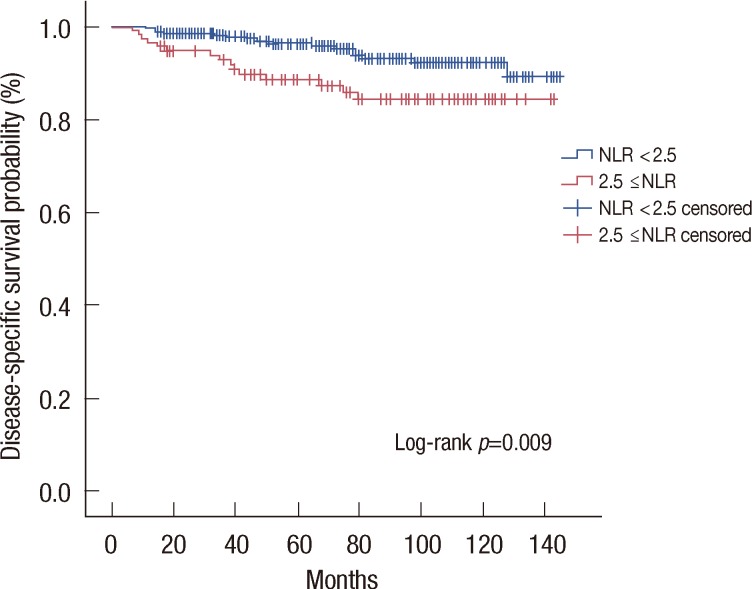
The median value of NLR was 1.85 (range, 0.63-7.79). In total 442 patients, 327 patients had NLR less than 2.5, and 115 patients had NLR equal to or higher than 2.5. Patients with NLR equal to or higher than 2.5 showed significantly lower 5-year and 10-year disease-specific survival rate than patients with NLR lower than 2.5 (5-year survival, 88.6% vs. 96.4%; 10-year survival, 84.3% vs. 92.2%; p=0.009) (Figure 2).
Figure 2.
Disease-specific survival curves stratified by neutrophil to lymphocyte ratio (NLR). Kaplan-Meier estimates for disease-specific survival stratified by NLR.
The patients with NLR equal to or higher than 2.5 were associated with increased T stage, younger age, positive HER2 status, and higher disease-specific mortality (Table 2). The Cox proportional multivariate hazard model for disease-specific mortality revealed that higher NLR along with negative ER status and positive nodal status were independently correlated with poor prognosis, with hazard ratio 4.08 (95% confidence interval [CI], 1.62-10.28), 9.93 (95% CI, 3.51-28.13), and 11.23 (95% CI, 3.34-37.83), respectively (Table 3).
Table 2.
Baseline characteristics by NLR
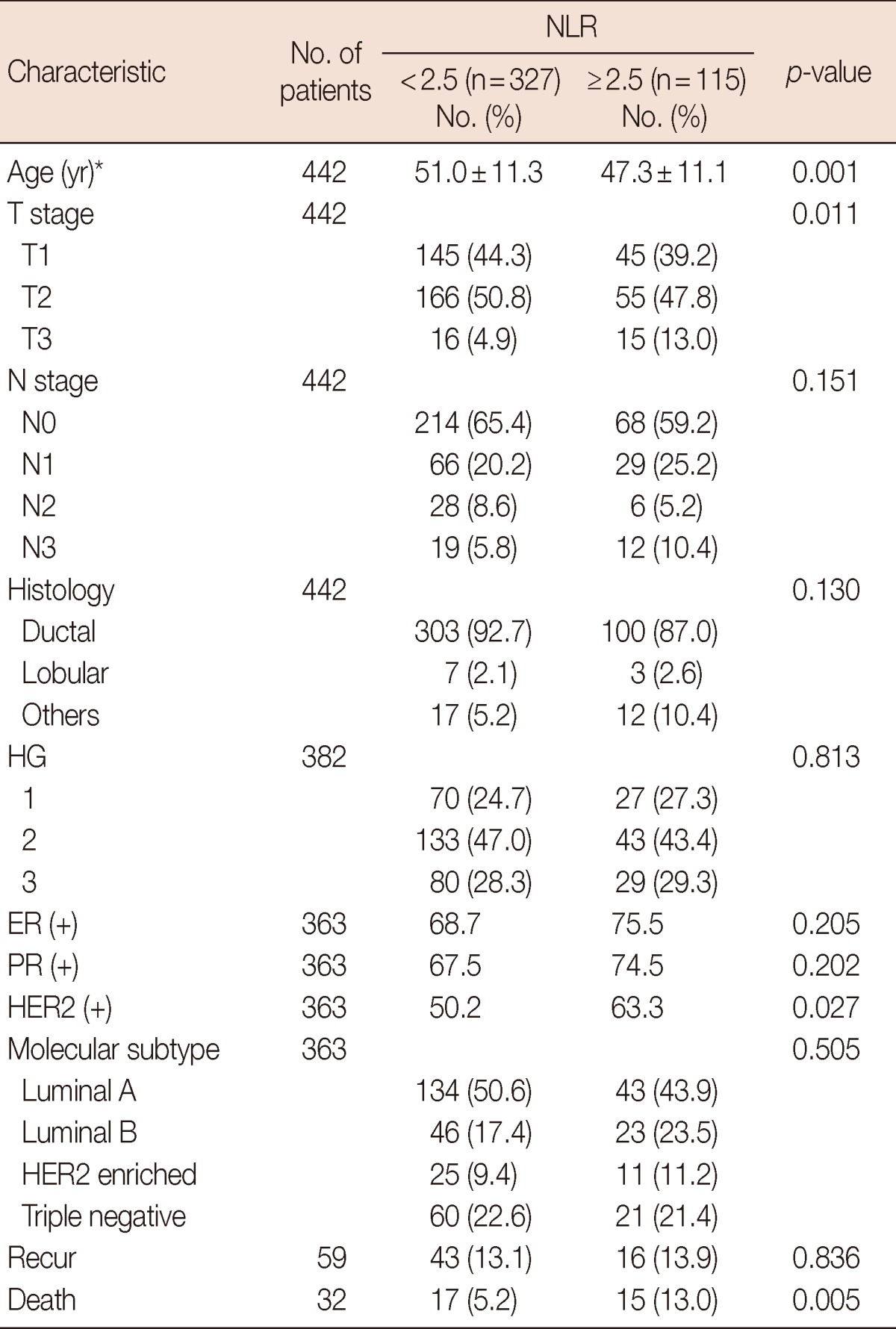
NLR=neutrophil to lymphocyte ratio; HG=histologic grade; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Mean±SD.
Table 3.
Cox proportional multivariate hazard model for disease-specific mortality
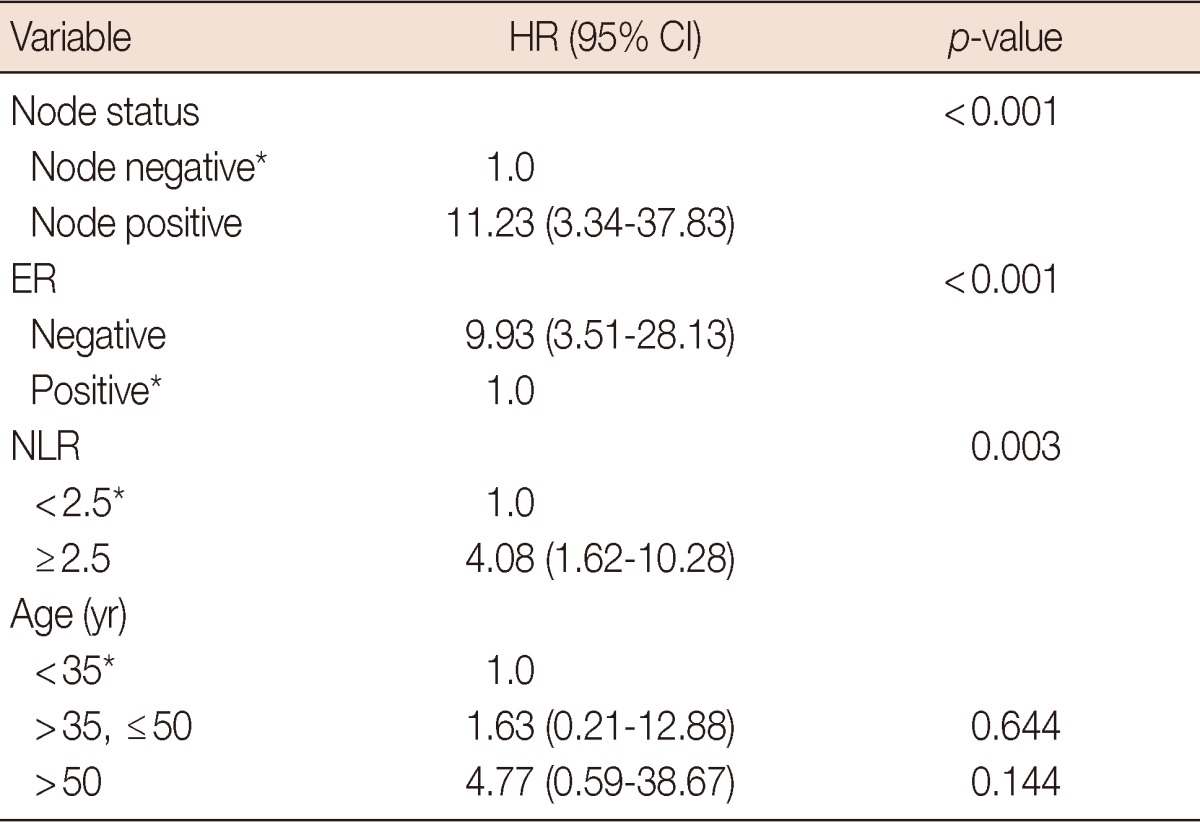
HR=hazard ratio; CI=confidence interval; ER=estrogen receptor; NLR=neutrophil to lymphocyte ratio.
*Reference.
Disease-specific survival stratified by intrinsic subtype according to NLR
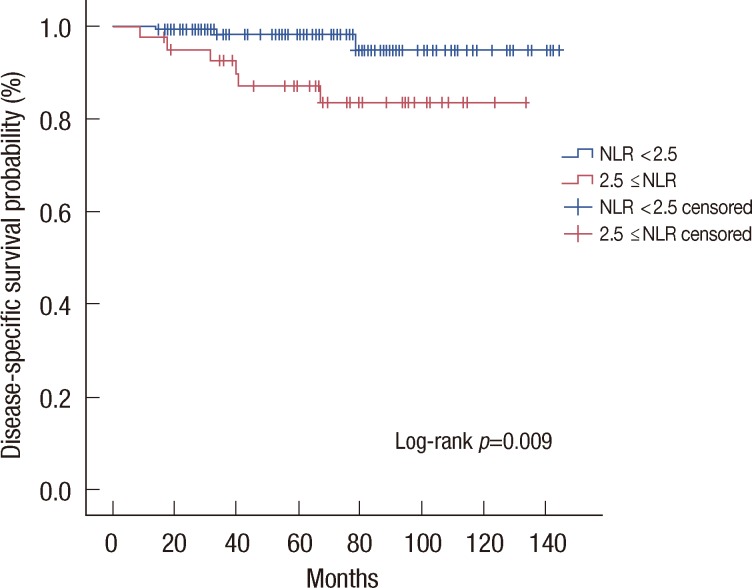
Luminal A subtype was the only intrinsic subtype in which NLR equal to or higher than 2.5 showed significantly lower 5-year survival rate than NLR lower than 2.5 (87.7% vs. 96.7%, p=0.009) (Figure 3). There was no significant survival difference according to NLR in other subtypes such as luminal B (95.0% vs. 97.7%, p=0.838), HER2 enriched (70.1% vs. 95.8%, p=0.076), and triple-negative (90.5% vs. 92.9%, p=0.769).
Figure 3.
Disease-specific survival curves stratified by neutrophil to lymphocyte ratio (NLR) of luminal A subtype. Kaplan-Meier estimates for disease-specific survival stratified by NLR of luminal A subtype.
DISCUSSION
In this study, we found that elevated NLR at initial clinical presentation of breast cancer was an independent factor for poor survival rate in breast cancer patients. This finding is consistent with previous reports for several other cancer as well breast cancer [7-12].
The association between high NLR and poor prognosis is probably complex and largely unclear, but there are several possible explanations.
High neutrophil count has been associated with poor survival in patients with many types of cancer [7-12], and although the cause is not completely understood, a multifactorial process has been hypothesized [9]. First, neutrophils may inhibit the immune system. In support of this notion, neutrophils suppress the cytolytic activity of lymphocytes, natural killer cells, and activated T-cells upon coculture of neutrophils and lymphocytes form normal healthy donor [12]. The tumor-associated neutrophils, via their enzymatic action, also promote remodeling of the extracellular matrix, which results in the release of basic fibroblast growth factor, migration of endothelial cells, and the dissociation or tumor cells. In addition, neutrophil-derived reactive oxygen species further decrease the adhesion-promoting properties of the extracellular matrix and, via activation of nuclear factor (NF)-κB, inhibit apoptosis of the tumor cells. These events finally result in enhanced angiogenesis, tumor growth, and progression to a metastatic phenotype. In breast cancer, it has been show that neutrophil-derived oncostatin M signals human breast cancer cells to secret VEGF and increases breast cancer cells detachment and invasiveness [13].
On the other hand, a low lymphocyte count has been associated with poor outcomes in patients with advanced cancer attributed immunity, with destruction of host cancer cells [14]. It is plausible that host cell-mediated immunity continues to exert important effects on destruction of any residual tumor cells and micrometastases [15]. Tumor infiltration by lymphocytes has been reported to indicate the generation of an effective antitumor cellular immune response, and increased lymphocyte infiltration correlated with a better prognosis [12].
Based on these findings it is likely that a high NLR correlates to poor prognosis and further investigation in its role is warranted.
Our study also focused on NLR and its prognostic implication on intrinsic subtype. Our results show elevated NLR to be significantly associated with poorer prognosis for the luminal A subtype. The HER2 enriched, triple-negative breast cancer (TNBC) tumors are known to have poor tumor biology compared with the luminal A subtype [16-18]. Thus, luminal A subtype may be more influenced by its microenvironment than tumors with HER2 enriched, TNBC which may be less susceptible to its environment. There are studies on ER positive breast cancer and the effects of microenvironment supporting this theory [19]. A large prospective study on hormonal therapy and its influence on breast cancer incidence also suggests that luminal A subtypes are more influenced by microenvironment than other types [20-22]. Further studies into the precise mechanism in which NLR exerts its effect, especially in luminal A subtypes, are needed.
In conclusion, patients with an elevated pretreatment NLR showed poorer disease-specific survival than patients without elevated NLR, especially in luminal A subtype. The routine preoperative workup is available nearly universally and adds no additional costs, in comparison to the more sophisticated and costly technologies. Further validation work and feasibility study are required before the results of this study can be considered for clinical use.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Saez RA, McGuire WL, Clark GM. Prognostic factors in breast cancer. Semin Surg Oncol. 1989;5:102–110. doi: 10.1002/ssu.2980050206. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Aizawa M, Gotohda N, Takahashi S, Konishi M, Kinoshita T. Predictive value of baseline neutrophil/lymphocyte ratio for T4 disease in wall-penetrating gastric cancer. World J Surg. 2011;35:2717–2722. doi: 10.1007/s00268-011-1269-2. [DOI] [PubMed] [Google Scholar]
- 8.Halazun KJ, Hardy MA, Rana AA, Woodland DC, 4th, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 9.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31:2995–2998. [PubMed] [Google Scholar]
- 11.Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32:1555–1561. [PubMed] [Google Scholar]
- 12.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 13.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 14.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 15.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 19.Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 22.Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE. Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2004;13:220–224. doi: 10.1158/1055-9965.epi-03-0301. [DOI] [PubMed] [Google Scholar]