Abstract
Purpose
IBTR! 2.0 is a web-based nomogram that predicts the 10-year ipsilateral breast tumor recurrence (IBTR) rate after breast-conserving therapy. We validated this nomogram in Korean patients.
Methods
The nomogram was tested for 520 Korean patients, who underwent breast-conserving surgery followed by radiation therapy. Predicted and observed 10-year outcomes were compared for the entire cohort and for each group, predefined by nomogram-predicted risks: group 1, <3%; group 2, 3% to 5%; group 3, 5% to 10%; group 4, >10%.
Results
In overall patients, the overall 10 year predicted and observed estimates of IBTR were 5.22% and 5.70% (p=0.68). In group 1, (n=124), the predicted and observed estimates were 2.25% and 1.80% (p=0.73), in group 2 (n=177), 3.95% and 3.90% (p=0.97), in group 3 (n=181), 7.14% and 8.80% (p=0.42), and in group 4 (n=38), 11.66% and 14.90% (p=0.73), respectively.
Conclusion
In a previous validation of this nomogram based on American patients, nomogram-predicted IBTR rates were overestimated in the high-risk subgroup. However, our results based on Korean patients showed that the observed IBTR was higher than the predicted estimates in groups 3 and 4. This difference may arise from ethnic differences, as well as from the methods used to detect IBTR and the healthcare environment. IBTR! 2.0 may be considered as an acceptable nomogram in Korean patients with low- to moderate-risk of in-breast recurrence. Before widespread use of this nomogram, the IBTR! 2.0 needs a larger validation study and continuous modification.
Keywords: Breast neoplasms, Nomograms, Radiotherapy, Recurrence, Validation studies
INTRODUCTION
Through the past few decades, the numbers of diagnostic methods and treatment modalities for breast cancer have increased, and breast cancer survival and recurrence rates have improved. One of the treatment methods, breast-conserving surgery (BCS) with adjuvant radiation therapy (RTx) is accepted as a standard treatment modality for breast cancer patients [1]. Breast-conserving therapy shows no significant differences from mastectomy in overall survival and disease-free survival [2,3]. However, locoregional recurrence rates of breast cancer, 10 years after initial treatment are still noted to be between 6% and 20% [4,5].
The ability to predict the future recurrence of breast cancer can assist patients and physicians when making clinical decisions. Furthermore, the ability to accurately predict ipsilateral breast tumor recurrence (IBTR) based on individualized risk factors can provide additional information for adapting future treatment strategies. Some predictive tools such as ADJUVANT! Online [6], Nottingham Prognostic Index [7], and IBTR! 2.0 [8] have been created to assist in decision-making with regard to breast cancer treatment. Of these, the nomogram IBTR! 2.0 was developed according to the theory that local control is valuable for physicians and patients, because local recurrence is correlated with the risk of metastasis and mortality [8,9]. IBTR! 2.0 (http://www.tufts-nemc.org/ibtr) is a web-based tool that includes 7 prognostic factors (age, tumor size, tumor grade, margin status, lymphovascular invasion [LVI], use of chemotherapy [CTx], and use of hormone therapy [HTx]) to predict individualized risk of ipsilateral breast tumor recurrence after BCS with or without RTx [8]. The IBTR! 2.0 model was constructed using the British Columbia Cancer Agency (BCCA) Breast Cancer Outcomes Unit database and validated using Massachusetts General Hospital (MGH) patient data. The nomogram showed accurate results in low-risk patients, but overestimated the risk in high-risk patients [8]. One limitation of IBTR! 2.0 is that validation has occurred in United States patients only.
Asian patients, especially Korean patients, have different ethnic features and healthcare environments compared with United States patients. For example, the mean age at diagnosis of Korean breast cancer patients is lower than that of American patients [10] and the Korean government provides health insurance for all Koreans. Thus, postoperative examinations can be performed without a significant economic burden. These differences in ethnicity and heath care environment may result in the distinctive results observed and predicted IBTR rates between Korean and American patients.
The validation of predictive model is a vital step to determine the decrement in performance when applied to an external dataset. The validity of IBTR! 2.0 and its applicability to Asian women treated with breast-conserving therapy has not yet been evaluated. Therefore, we set out to assess the validity of the IBTR! 2.0 in Korean breast cancer patients.
METHODS
On a retrospective basis, we reviewed the data of 832 breast cancer patients who had undergone BCS at the Department of Surgery, Samsung Medical Center (SMC), Seoul, Korea between 1994 and 2002. The patients satisfying the study criteria were women with breast cancer who were treated with BCS followed by RTx. The exclusion criteria were as follows: the patients who were 1) treated with neoadjuvant CTx and/or RTx, and 2) diagnosed with distant metastasis on a preoperative basis. The end point in this study was IBTR, which was defined as any first recurrence involving the remaining ipsilateral breast without simultaneous regional or distant recurrence. Regional recurrence was defined as any tumor recurrence in the ipsilateral supraclavicular, infraclavicular, axillary, or internal mammary nodes. Recurrence at any other site was defined as a distant recurrence. The term "simultaneous" was defined as any subsequent recurrence within 4 months after the IBTR diagnosis. These definitions were guided by the published reports of the National Surgical Adjuvant Breast Projects (NSABP) [11], Eastern Cooperative Oncology Group (ECOG) studies [12], and the IBTR! 2.0 manuscript [8]. We identified 520 patients who were eligible for this study using our hospital's electronic database. The mean RTx dose for the breast was 5,040 cGy in 28 fractions, then a 1,000 cGy boost to the tumor bed in 5 fractions for a total dose of 6,040 cGy.
Age, tumor size, tumor grade, margin status, LVI, use of CTx, and use of HTx were entered into the IBTR! 2.0 nomogram for each patient and the expected IBTR rate was calculated. The observed 10-year IBTR values for each patient were determined from the SMC database using the Kaplan-Meier method. Competing events including regional recurrence, distant recurrence or death were treated as censored observations in the analysis. This statistics method was adopted to ensure consistency between the MGH and SMC datasets. The average predictive estimates of IBTR! 2.0 nomogram and observed IBTR estimates at SMC were compared. A standard error (SE) was calculated for the observed percentage, and a t-test was used for the entire cohort; for the 4 groups predefined by nomogram-predicted risk as in the MGH validation study [8]: group 1, less than 3%; group 2, 3% to 5%; group 3, 5% to 10%; and group 4, higher than 10%.
The statistical method for comparing predictive and observed recurrence rates was guided by previous publications validating Adjuvant! Online [13], IBTR! 2.0 [8] and neoadjuvant chemotherapy nomograms [14]. Discrimination for recurrent data was evaluated using Harrell's concordance statistics. The concordance index (C-index) was derived from the Wilcoxon two-sample test and provides the discrimination ability of binary data [15]. The receiver operating characteristic curve (ROC) and the area under the curve (AUC) were used to discriminate binary data. We used the bootstrapping resampling method (1,000 repetitions) to achieve unbiased estimates.
Data were analyzed using Microsoft Excel 2007® (Microsoft Corp., Redmond, USA). Statistical analyses were performed using PASW® Statistics version 18.0 (SPSS Inc., Chicago, USA) and R version 2.1. (The R Project for Statistical Computing, Vienna, Austria, available from http://www.r-project.org). Reported p-values are two-sided, and the statistical significance was set at p<0.05.
RESULTS
Clinicopathologic characteristics of the patients used for validation and hazard ratios (HRs) of each of the variables are shown in Table 1. The median follow-up time was 9.7 years for a total of 520 patients. The median age and tumor size at diagnosis were 45.0 years and 1.6 cm, respectively. All of the patients that had an unknown margin status experienced IBTR and the unknown margin status was the only statistically significant prognostic factor (HR, 67.05; 95% confidence interval [CI], 6.81-660.40) in this study.
Table 1.
Clinicopathologic characteristics of patients (n=520) and estimated Cox-regression hazard ratios of each prognostic factor
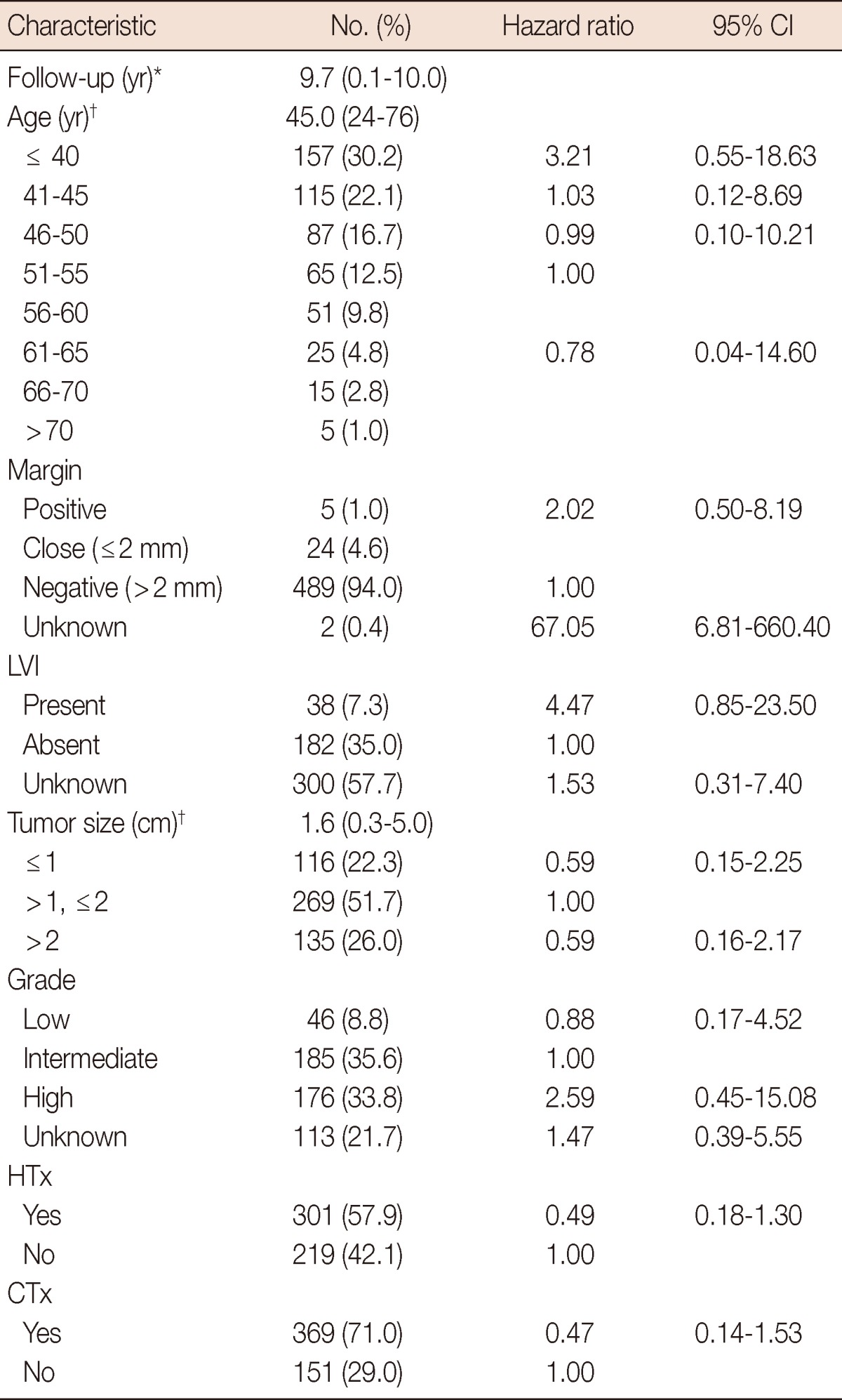
LVI=lymphovascular invasion; HTx=hormone therapy; CTx=chemotherapy; CI=confidence interval.
*Mean (range); †Median (range).
An IBTR occurred in 25 cases of the entire cohort. The IBTR! 2.0 nomogram predicted an overall 10-year recurrence rate of 5.22% (95% CI, 4.97-5.47), while the observed estimate was 5.70% (95% CI, 3.43-7.97). This difference was not statistically significant (p=0.68).
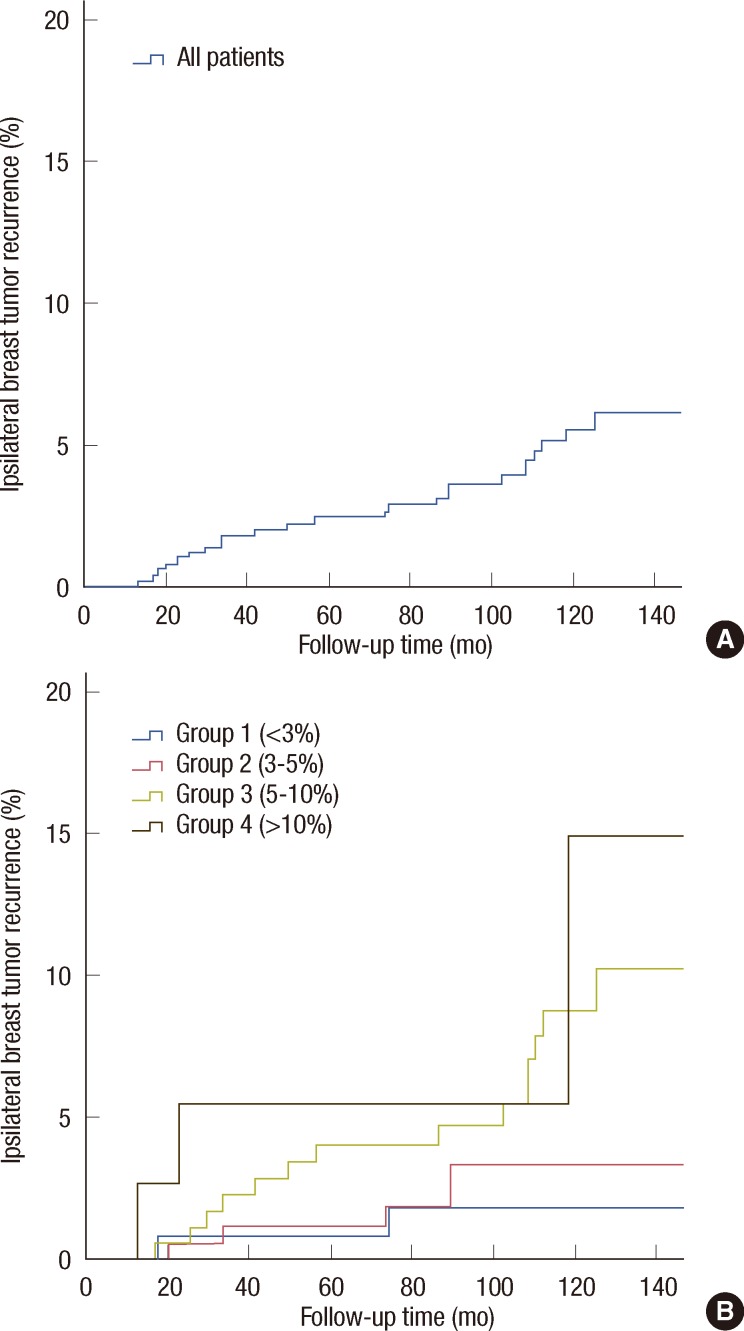
The Kaplan-Meier IBTR values in the entire cohort and the 4 groups are shown in Figure 1. The predicted and observed IBTR estimates for the cohort overall and for each group are listed in Table 2. In group 1, the predicted IBTR risk was less than 3%, there were two cases of IBTR in 124 patients, and the predicted versus observed IBTR values were 2.25% and 1.80% (p=0.73), respectively. In group 2, there were 6 cases of IBTR in 177 total patients, with predicted and observed IBTR values of 3.95% and 3.90% (p=0.97), respectively. In group 3, 14 cases of IBTR were found in 181 patients, with predicted and observed IBTR values of 7.14% and 8.80% (p=0.42), respectively. In group 4, 3 cases of IBTR occurred in 38 patients, with predicted versus observed values of 11.66% and 14.90% (p=0.73), respectively. No statistically significant differences were demonstrated in the cohort overall or in each group between the predicted and observed IBTR estimates.
Figure 1.
Observed ipsilateral breast tumor recurrence (IBTR) estimates using the Kaplan-Meier method in four predefined groups. (A) Observed IBTR estimates in entire cohort. (B) Observed IBTR estimates in four risk groups.
Table 2.
10-Year comparison of predicted and observed IBTR estimates
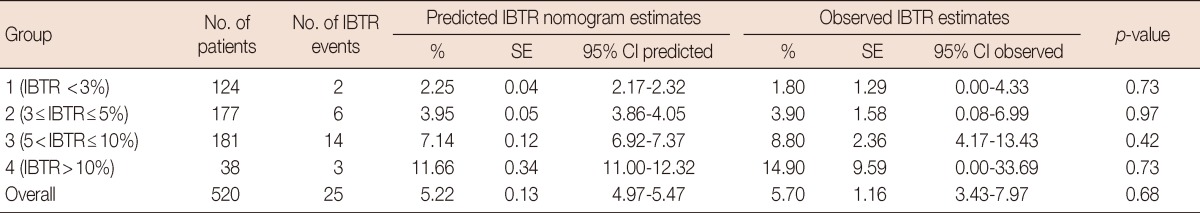
IBTR=ipsilateral breast tumor recurrence; SE=standard error; CI=confidence interval.
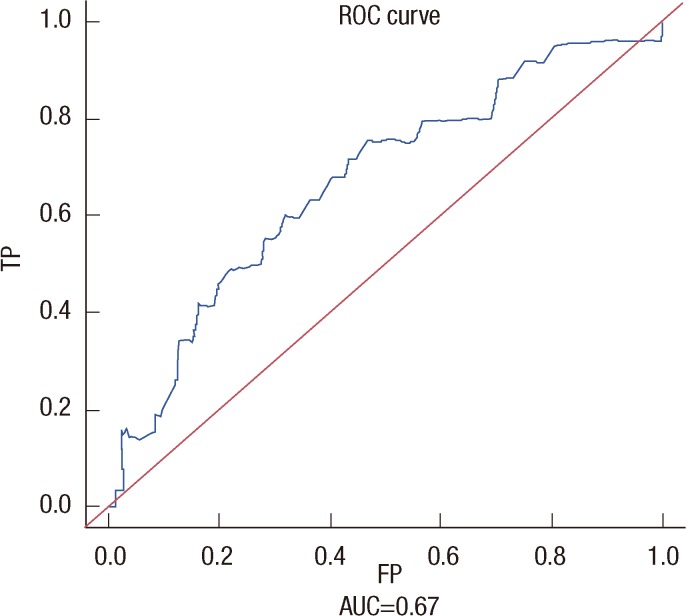
The ROC curve and calibration plot are shown in Figure 2. The area under the ROC curve of the nomogram was 0.670 (95% CI, 0.569-0.781), showing a reasonable agreement between the predicted and observed estimates for the overall cohort.
Figure 2.
A receiver operating characteristic (ROC) curve. Area under the ROC (AUC) is 0.670 (95% confidence interval, 0.569-0.781).
TP=true positive; FP=false positive.
DISCUSSION
IBTR! 2.0 was created to predict local recurrence after breast-conserving therapy based on individualized risk factors. As local recurrence is correlated significantly with an increased risk of metastasis and decreased survival [9], predicting local recurrence can assist patients and physicians in making clinical decisions. Because breast-conserving therapy is the standard management for Korean patients with early stage breast cancer, the IBTR! 2.0 nomogram could be helpful for predicting ipsilateral breast tumor recurrence in Korean patients. However, the nomogram has not been validated for Korean patients who have features that differ from the original Uinted States study cohort, such as ethnicity and healthcare environment. As such, the application of the nomogram to Korean people required validation. In this manuscript, we validated the IBTR! 2.0 to the Korean population, and the results showed that expected and observed estimates were not significantly different.
The seven prognostic factors in IBTR! were initially selected based on prognostic relevance for local control from published clinical studies [16]. Then, the authors modified the nomogram based on data from 7,811 patients in the BCCA dataset and developed IBTR! 2.0. The prognostic factors and their HR were calculated using Cox-regression modeling, and the revised HRs formed the basis of IBTR! 2.0 [8]. We calculated HRs using 520 patients in our dataset and compared them with those of the BCCA dataset. Although most of the factors did not show statistical significance because of the small numbers of patients and events in our cohort, the hazard ratios showed similar tendencies with those of BCCA.
Patient age is a well-known important factor in predicting local recurrence, and the benefit of RTx varies significantly according to age group [3,4]. HRs in our study decreased with age, from 3.12 to 0.78, which was similar to the HRs in the BCCA dataset (range, 2.03-0.53). There have been numerous reports that positive or close margins are significantly correlated to increased local recurrence [4,17]. The HR of positive/close margin status was calculated as 2.02 using our dataset. All two patients with an unknown margin status experienced local recurrence; therefore, the HR of unknown margin status was much higher than those of the other subgroups. The local recurrence rate of patients with LVI was reported from 11% to 35% compared to the patients without LVI (range, 3%-21%) [17,18]. The HR of LVI in the BCCA dataset was 1.12, while the HR in our study was calculated to be 4.47. A high tumor grade was reported to have a higher relative risk of local recurrence and ranged from 1.44 to 2.51 [4,19]. The HR of a higher tumor grade calculated from the BCCA was 1.55, which was similar to the HR of this study, 1.47. The effect of tumor size on local recurrence is contentious. Some studies reported that tumor size was associated with local recurrence (LR), while Arriagada et al. [20] and Veronesi et al. [3] reported a smaller effect of tumor size on LR. In the IBTR! 2.0, a tumor size ≤1 cm had a higher HR (1.40) of LR as compared with size 1.1 to 2 cm (HR, 1.00) and >2 cm (HR, 1.07). In this study, sizes ≤1 cm and >2 cm had lower HRs than size 1.1 to 2 cm (HRs were 0.59 in both subgroups), although the difference was not significant. We could not explain the exact reasons for the different HRs between BCCA and SMC. We could only speculate that these differences were caused by statistical factors or ethnic distinctions. Because our dataset was much smaller than the BCCA, the HRs in this data set may not have shown statistical significance. Regarding ethnic distinction, most Korean studies reported that tumor size was not associated with LRs [21,22].
The use of hormonal therapy or chemotherapy has been found consistently to decrease LR [23]. Because the effect of hormonal therapy and chemotherapy could not be separated from the impact of nodal status and hormone receptor status, these factors were not incorporated into the IBTR! nomogram [16].
In the validation process, we found results that differed between American patients and Korean patients. Previous validation using the MGH data set based on American patients showed high correlations between the expected and observed recurrence in groups 1 and 2 (expected vs. observed estimates were 2.2% vs. 1.3% in group 1, 3.8% vs. 3.5% in group 2), while in groups 3 and 4, the predicted IBTR rates were overestimated compared to the observed rates (6.7% vs. 3.2% in group 3, 12.5% vs. 8.7% in group 4). The absolute differences in the nomogram were 3.5% and 3.8% in groups 3 and 4, respectively, and these differences were not statistically significant. The results of our study were similar with the MGH data, showing high correlations between expected and observed estimates in low-risk patients (expected vs. observed estimates were 2.25% vs. 1.80% in group 1, 3.95% vs. 3.90% in group 2). However, there were significant differences between MGH and our results in the high-risk group (i.e., predicted IBTR estimates >5%). In our study, the observed IBTR estimates were higher than predicted (8.80% vs. 7.14% in group 3 and 14.90% vs. 11.66% in group 4), and there was a smaller absolute difference between the observed and predicted estimates, as compared to the MGH data set (1.7% vs. 3.5% in group 3 and 3.2% vs. 3.8% in group 4). Sanghani et al. [8] explained that the reason for overestimation in the high-risk group was a relatively low IBTR event rate in these groups. Compared to the MGH data, the IBTR event rates were also low, and there were more patients in groups 3 and 4 in our data set than in the MGH data set (111 vs. 181 patients in group 3 and 33 vs. 38 patients in group 4). However, our results showed smaller absolute differences and did not show overestimation compared to the MGH validation. The reasons for these differences may be multifactorial, including, not only population origin, but also healthcare environments such as public health insurance and a method used to detect IBTR.
Asian breast cancer patients, especially Korean patients, have different characteristics from American patients. Patient age has been identified as an important prognostic factor [1] and age is already included in IBTR! 2.0 nomogram as a risk factor. Recent statistics have shown that the highest peak age group for Korean breast cancer patients is 45 to 49 years [10]. Patients with breast cancer at MGH had a median age of 58 years, the median age of our patients was 45.0 years, and more than 68% of our cases were under 50 years old. There is another interesting factor characteristic to young Korean women. Recent studies have shown that young Korean women had denser breasts than American women of the same age. In Korean women, the frequency of dense mammograms was about 61% to 78% in 40 to 49 year olds and 30% to 35% in women in their 50s, which is much higher than American women [24]. High breast density has been suggested as a risk factor for the occurrence of breast cancer [25] and this extensive mammographic density could mask present breast cancer. Other differences in Korean women include later age at first full-term pregnancy and a lower birth rate than American women [26,27]. These factors change breast tissue susceptibility to hormonal stimulus and increase the risk of cancer development and progression.
The patients in the BCCA and the MGH database received RTx, with or without a boost. While, all patients in our database received adjuvant RTx with a 1,000 cGy boost in 5 fractions. From the published studies [28], a boost led to improved local control in all age groups. Although we do not know the exact number or proportion of patients who received RTx with a boost in the BCCA and the MGH cohorts, this difference in additional boost could affect the IBTR rate. These differences in patient characteristics, capturing and treatment methods may account for the different results in the high-risk group between the SMC and MGH cohort.
One of the limitations in this study is a wide confidence interval which could leads to imprecise validation. In our opinion, there may be two reasons for wide confidence interval. First, our data set showed high proportion of unknown results in LVI status (57.7%) and tumor grade (21.7%). Second, the validation method used in IBTR! 2.0 manuscript might be somewhat inappropriate. However, we followed the same method discussed in the IBTR! 2.0 manuscript to compare the validation result of United States and Korean patients. The relatively small number of patients and events in this study are limitations of this study. And this limitation could make this validation under-powered to detect significant differences. MGH validation had 22 IBTR cases in 664 patients. Our data set was smaller than MGH cohort, 25 IBTR cases in 520 patients. However, the high-risk group (greater than 5% risk of IBTR) in our study had more patients than the MGH dataset. Although this validation test has some limitations, our manuscript could show some different results between Korean and United States patients. Therefore, we were able to describe the differences of population origin and a treatment method. Furthermore, this study is the first validation of independent clinical data outside of the North America. The IBTR! 2.0 nomogram has some limitations due to its simplicity. Molecular and genetic analyses have developed in recent periods, and these new biologic parameters have been reported as important markers for recurrence and mortality [29]. In addition, the biologic subtype of breast cancer may affect local recurrence. Because of the short follow-up time and lack of consistency of the available data [8], the IBTR! 2.0 nomogram does not yet incorporate these factors. We hope the IBTR! 2.0 will be further modified to incorporate genetic and other biologic factors, and we also hope that a large independent Korean cohort will be studied for further validation.
In conclusion, this validation study for Korean patients showed that the IBTR! 2.0 model has an acceptably accurate predictive ability in low- to moderate-risk patients. In its current state, this model underestimates the expected IBTR rate in high-risk Korean patients. As progress in genetic and molecular techniques continues, new tools will be designed to predict cancer development and systemic or locoregional recurrence with new prognostic factors. Until that time, an easily accessible nomogram is warranted, and IBTR! 2.0 may be one reasonable predictive tool for application in low to moderate-risk patients.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Marubini E, Mariani L, Galimberti V, Luini A, Veronesi P, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 4.Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabár L, Nordgren H, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 5.Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 7.Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–219. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- 8.Sanghani M, Truong PT, Raad RA, Niemierko A, Lesperance M, Olivotto IA, et al. Validation of a web-based predictive nomogram for ipsilateral breast tumor recurrence after breast conserving therapy. J Clin Oncol. 2010;28:718–722. doi: 10.1200/JCO.2009.22.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 13.Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 14.Keam B, Im SA, Park S, Nam BH, Han SW, Oh DY, et al. Nomogram predicting clinical outcomes in breast cancer patients treated with neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2011;137:1301–1308. doi: 10.1007/s00432-011-0991-3. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Sanghani M, Balk E, Cady B, Wazer D. Predicting the risk of local recurrence in patients with breast cancer: an approach to a new computer-based predictive tool. Am J Clin Oncol. 2007;30:473–480. doi: 10.1097/COC.0b013e31805c13d9. [DOI] [PubMed] [Google Scholar]
- 17.Kunos C, Latson L, Overmoyer B, Silverman P, Shenk R, Kinsella T, et al. Breast conservation surgery achieving>or=2 mm tumor-free margins results in decreased local-regional recurrence rates. Breast J. 2006;12:28–36. doi: 10.1111/j.1075-122X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanna WM, Kahn HJ, Chapman JA, Fish EB, Lickley HL, McCready DR. Pathologic characteristics of breast cancer that predict for local recurrence after lumpectomy alone. Breast J. 1999;5:105–111. doi: 10.1046/j.1524-4741.1999.00133.x. [DOI] [PubMed] [Google Scholar]
- 19.Clark RM, Whelan T, Levine M, Roberts R, Willan A, McCulloch P, et al. Ontario Clinical Oncology Group. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 20.Arriagada R, Lê MG, Contesso G, Guinebretière JM, Rochard F, Spielmann M. Predictive factors for local recurrence in 2006 patients with surgically resected small breast cancer. Ann Oncol. 2002;13:1404–1413. doi: 10.1093/annonc/mdf227. [DOI] [PubMed] [Google Scholar]
- 21.Kang SH, Chung KY, Kim YS, Kim JH. Disease free survival and prognostic factors for patients with breast conserving surgery. J Korean Surg Soc. 2004;67:274–278. [Google Scholar]
- 22.Kim JH, Han W, Moon HG, Ko E, Lee JW, Kim EK, et al. Factors affecting the ipsilateral breast tumor recurrence after breast conserving therapy in patients with T1 and T2 tumors. J Breast Cancer. 2009;12:324–330. [Google Scholar]
- 23.Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kim MH, Oh KK. Analysis and comparison of breast density according to age on mammogram between Korean and Western women. J Korean Radiol Soc. 2000;42:1009–1014. [Google Scholar]
- 25.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistics Korea. Birth statics 2010. [Accessed October 1st, 2012]. http://kostat.go.kr.
- 27.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 28.Poortmans PM, Collette L, Horiot JC, Van den Bogaert WF, Fourquet A, Kuten A, et al. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol. 2009;90:80–85. doi: 10.1016/j.radonc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Cheng SH, Horng CF, West M, Huang E, Pittman J, Tsou MH, et al. Genomic prediction of locoregional recurrence after mastectomy in breast cancer. J Clin Oncol. 2006;24:4594–4602. doi: 10.1200/JCO.2005.02.5676. [DOI] [PubMed] [Google Scholar]