Abstract
Asparagine synthetase (ASNS) catalyzes the conversion of aspartate and glutamine to asparagine and glutamate in an ATP-dependent reaction. The enzyme is ubiquitous in its organ distribution in mammals, but basal expression is relatively low in tissues other than the exocrine pancreas. Human ASNS activity is highly regulated in response to cell stress, primarily by increased transcription from a single gene located on chromosome 7. Among the genomic elements that control ASNS transcription is the C/EBP-ATF response element (CARE) within the promoter. Protein limitation or an imbalanced dietary amino acid composition activate the ASNS gene through the amino acid response (AAR), a process that is replicated in cell culture through limitation for any single essential amino acid. Endoplasmic reticulum stress also increases ASNS transcription through the PERK-eIF2-ATF4 arm of the unfolded protein response (UPR). Both the AAR and UPR lead to increased synthesis of ATF4, which binds to the CARE and induces ASNS transcription. Elevated expression of ASNS protein is associated with resistance to asparaginase therapy in childhood acute lymphoblastic leukemia and may be a predictive factor in drug sensitivity for certain solid tumors as well. Activation of the GCN2-eIF2-ATF4 signaling pathway, leading to increased ASNS expression appears to be a component of solid tumor adaptation to nutrient deprivation and/or hypoxia. Identifying the roles of ASNS in fetal development, tissue differentiation, and tumor growth may reveal that ASNS function extends beyond asparagine biosynthesis.
Keywords: amino acid metabolism, asparaginase, ATF4, nutrient sensing, unfolded protein response
Asparagine Synthetase Enzyme Function
the mammalian asparagine synthetase (ASNS) enzyme catalyzes the ATP-dependent conversion of l-aspartate and l-glutamine into l-asparagine and l-glutamate (reviewed in Ref. 73). The biochemical characterization of ASNS activity has been studied following purification from mammalian cells and recombinant systems of both bacterial and mammalian origin. Early investigation revealed that the reaction requires magnesium ions and ATP and involves formation of a β-aspartyladenylate intermediate (68). The β-aspartyladenylate intermediate is converted to l-asparagine by transfer of ammonia from l-glutamine to yield l-glutamate and AMP as the remaining products (74). There are two bacterial forms of ASNS, one that uses ammonia (AS-A) as the nitrogen donor and a second that uses glutamine as the nitrogen donor (AS-B) (73). The latter reaction is analogous to that performed by the mammalian enzyme, and structural studies on the Escherichia coli glutamine-dependent AS-B have revealed the presence of two distinct catalytic domains, an NH2-terminal amidotransferase domain and a COOH-terminal ATP-pyrophosphatase domain bridged by an intramolecular tunnel that allows for ammonia to shuttle between the two domains (56). Whereas the protein's name directs focus on its function in asparagine synthesis, the reaction it catalyzes may impact the cellular levels of the other three reactants as well. Given the critical function of glutamine as an oxidizable energy source, a key interorgan nitrogen carrier, and a mammalian target of rapamycin (mTOR) regulator, the possible impact of ASNS activity should also be considered when evaluating glutamine homeostasis.
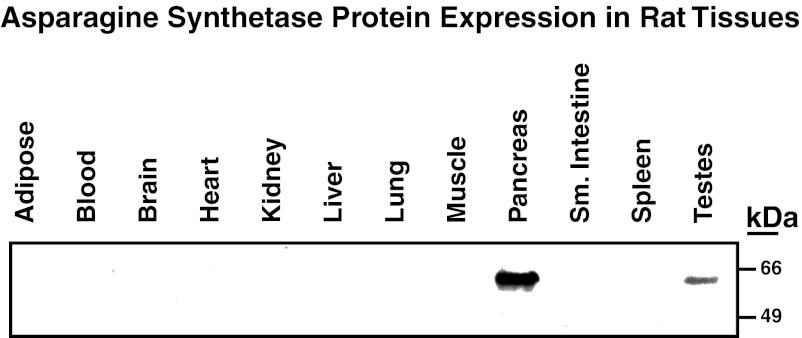
The level of ASNS expression among tissues in adult animals varies considerably. Based on a direct comparison of enzyme specific activity in many tissues, the pancreas was shown to exhibit much greater expression than any other tissue analyzed (63, 64). This distribution is consistent across many species, including humans, rodents, birds, and ox (63), and the prevalence of higher pancreatic ASNS expression has been confirmed at the protein level by immunoblotting using both polyclonal (47) and monoclonal antibodies (Fig. 1; R. Hutson and M. Kilberg, unpublished results). As illustrated by immunohistochemistry of human pancreatic tissue, pancreatic ASNS protein expression is largely associated with the exocrine cells (33). After fasting mice for 54 h or feeding them an asparagine-free diet for 10 days, their pancreatic ASNS activity was unaltered (64), in apparent contrast to the nutritional regulation of ASNS observed for other tissues, as discussed below. The pancreas does not release significant amounts of asparagine into the circulation, and radioactive incorporation studies have suggested that the bulk of newly synthesized asparagine is used for protein synthesis (64). It is tempting to speculate that serum ASNS activity may be a valuable marker for pancreatic exocrine cell lysis, as Cooney et al. observed release of ASNS protein from murine primary tumors into the serum at a rate proportional to tumor growth (27).
Fig. 1.
Expression of asparagine synthetase (ASNS) protein in rat tissues. The indicated tissues were harvested from rats fed a control chow, and immunoblotting of the resulting protein extracts was used to illustrate the basal expression of ASNS. These data are consistent with those of Milman et al. (63), who measured the tissue distribution of ASNS enzyme activity across many species, including humans.
The Mammalian Asparagine Synthetase Gene
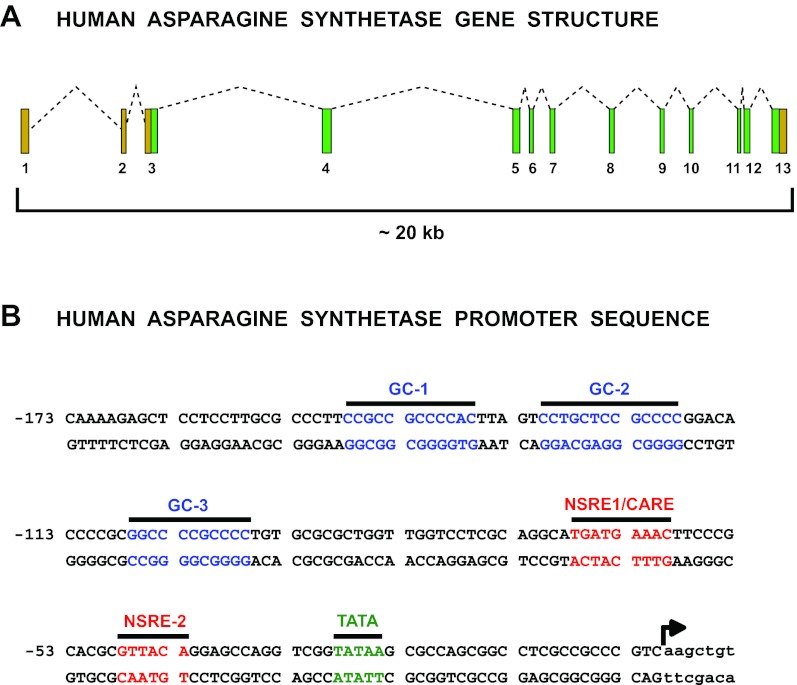
The human ASNS gene, a schema of which is illustrated in Fig. 2 A, was first assigned to chromosome 7 by analyses of somatic cell hybrids (9). Greco et al. (38) established that the ASNS locus is derived from the long arm of human chromosome 7, and in situ hybridization mapped the locus more precisely to chromosome region 7q21.3 (44). An ASNS cDNA was first cloned from Chinese hamster ovary (CHO) cells (71), and subsequently, human clones containing the complete ASNS coding sequence were obtained (6). Zhang et al. (100) determined that the human genomic sequence for ASNS was ∼35 kb long and organized into 13 exons. Those authors assigned the transcriptional start site of ASNS to be 203 nucleotides (nt) upstream of the translation start site. Greco et al. discovered that a temperature-sensitive hamster gene that controlled cell cycle progression, called ts11, was ASNS and characterized the human ASNS counterpart to show that the first two exons were noncoding, with translation starting in the third exon (38, 39). Both the Andrulis and Basilico groups determined that the region surrounding the transcription start site of the human ASNS gene has a high CG content, a circumstance that we now recognize as a promoter-associated CG island (38, 100). Greco et al. (38) discovered that the human ASNS gene exhibited multiple transcription start sites, with one major site at 117 nt upstream of the translation start site. Chen et al. further characterized the multiple transcription initiation sites for the human ASNS gene during amino acid (AA) deprivation of HepG2 human hepatoma cells (24). Those investigators used both 5′ rapid amplification of cDNA ends and a ribonuclease protection assay to establish that multiple transcription initiation sites were spread across a 70 nt region for cells cultured in AA complete medium. However, following AA limitation, which induces ASNS transcription, there was a shift toward a predominant utilization of the transcription initiation site 117 nt upstream of the translation start site originally identified by Greco et al. This transcription start site will be used as nt +1 to designate all genomic sequences in this review.
Fig. 2.
Gene structure and proximal promoter sequence for human ASNS. A: exon-intron structure and size of the human ASNS gene. The translation start site is within exon 3 and the stop site within exon 13, so tan exon boxes represent 3′- and 5′-untranslated regions of the mRNA, and protein coding exons are shown in green. B: sequence of the proximal 173 bp for the human ASNS promoter. Designated are a number of transcription factor binding sites that have been identified by in vivo footprinting and single nucleotide mutagenesis to contribute to either basal or stress-induced transcription (15, 57, 101). Details of the role for each of these sequences are discussed in the text.
Regulation of ASNS by Nutrient Limitation and Other Forms of Cell Stress
Protein or AA limitation: the AA response.
Mammals exhibit a wide spectrum of adaptive processes that serve to sense and respond to fluctuations in the diet, including dietary protein or AA imbalance. To illustrate the dynamic nature of this response, within 1 h after mice consumed a leucine-free diet there was increased expression of AA-responsive genes; conversely, within 2 h after refeeding a leucine-replete diet the changes were reversed (21). Expression array analysis in rats revealed that a diet deficient in total protein or limited in one or more of the essential AA, as many of the plant-derived proteins are, resulted in altered gene expression well beyond that for AA metabolism. For example, Endo et al. (34) discovered that, compared with casein, feeding rats a diet containing gluten, which is lysine and threonine deficient, resulted in significant changes in enzymes involved in cholesterol metabolism. Likewise, deprivation of cells in culture for a single essential AA (32) or feeding mice a leucine-free diet (41) results in a suppression of fatty acid synthase expression and changes in lipid metabolism.
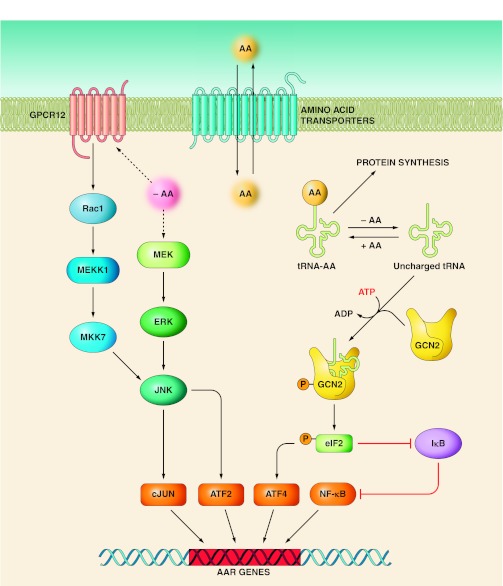
Genomic analysis in vivo is complicated because most tissues contain multiple cell types, which compromises the interpretation of mechanistic data with regard to cell-specific responses. Furthermore, in vivo studies do not allow for a direct test of AA regulation, because circulating AA levels will modulate the release and action of hormones and growth factors (76). Consequently, considerable work has been performed in cell culture model systems, which has allowed the identification of multiple signaling pathways, collectively referred to as the AA response (AAR) (Fig. 3). The AAR controls gene expression through changes in chromatin structure, transcription start site, transcription rates, mRNA splicing, RNA export, RNA turnover, and translation initiation (reviewed in Ref. 52). Among the AAR pathways, GCN2-eIF2-ATF4 (general control nonderepressible 2-eukaryotic initiation factor 2-activating transcription factor 4) appears to be the predominant signaling mechanism that activates transcription from the ASNS gene during the AAR, as induction of ASNS expression is blocked in ATF4-deficient cells (78, 81, 87). GCN2 is a well-characterized cellular AA monitoring mechanism that is conserved from yeast to humans. The GCN2 protein has an inherent kinase activity that is increased when the protein binds any one of the uncharged tRNA molecules; thus, GCN2 has the ability to sense the level of all 20 of the AA typically associated with protein synthesis. Activated GCN2 phosphorylates eIF2 on its α-subunit, which suppresses general protein synthesis (reviewed in Refs. 45, 53, 95, 99), but promotes a paradoxical increase in translation of selected mRNA species. As discussed in more detail below, in mammalian cells this translational control mechanism includes the transcription factors ATF4 and ATF5 (62, 91, 102).
Fig. 3.
Multiple signal pathways that make up the amino acid response (AAR) in mammals. The AAR is a collection of signaling pathways that result in an integrated transcriptional program. Whereas uncharged tRNA activation of the GCN2 kinase has been documented to be the AA sensor for the pathways leading to increased NF-κB activity (51) and ATF4 synthesis (42), the sensor that leads to MEK (90) and GPCR12 (22) activation has not been identified (indicated by dashed lines). AA transporters have been proposed as possible sensor molecules (89).
Long before molecular probes were available for ASNS analysis, Arfin et al. (10) demonstrated that CHO cells incubated in asparagine-free medium exhibit reduced aminoacylation of asparaginyl-tRNA and an increased level of ASNS enzymatic activity. Likewise, Andrulis (7) showed that ASNS activity was elevated when cells containing a temperature-sensitive asparaginyl-, leucyl-, methionyl-, or lysyl-tRNA synthetase mutant were transferred to the nonpermissive temperature. Those data are now understood to reflect the role of GCN2 as the sensor of AA availability by monitoring the level of each of the uncharged tRNAs. After discovering that the cell cycle defect of cells harboring the ts11/ASNS mutation could be reversed by asparagine supplementation, Basilico and colleagues [Greco et al. (37)] were the first to show that ASNS mRNA was induced by AA limitation of mammalian cells, not only for asparagine but also for leucine, isoleucine, or glutamine. Hutson and Kilberg (48) extended those studies by reporting that ASNS mRNA level increased following limitation for all AA or for a single essential AA. Consistent with the earlier reports that asparagine levels regulated ASNS enzymatic activity (10), after AA limitation there is increased association of ASNS mRNA with polysomes (50) and increased ASNS protein production, as revealed by pulse-chase labeling (49).
Endoplasmic reticulum stress: the unfolded protein response.
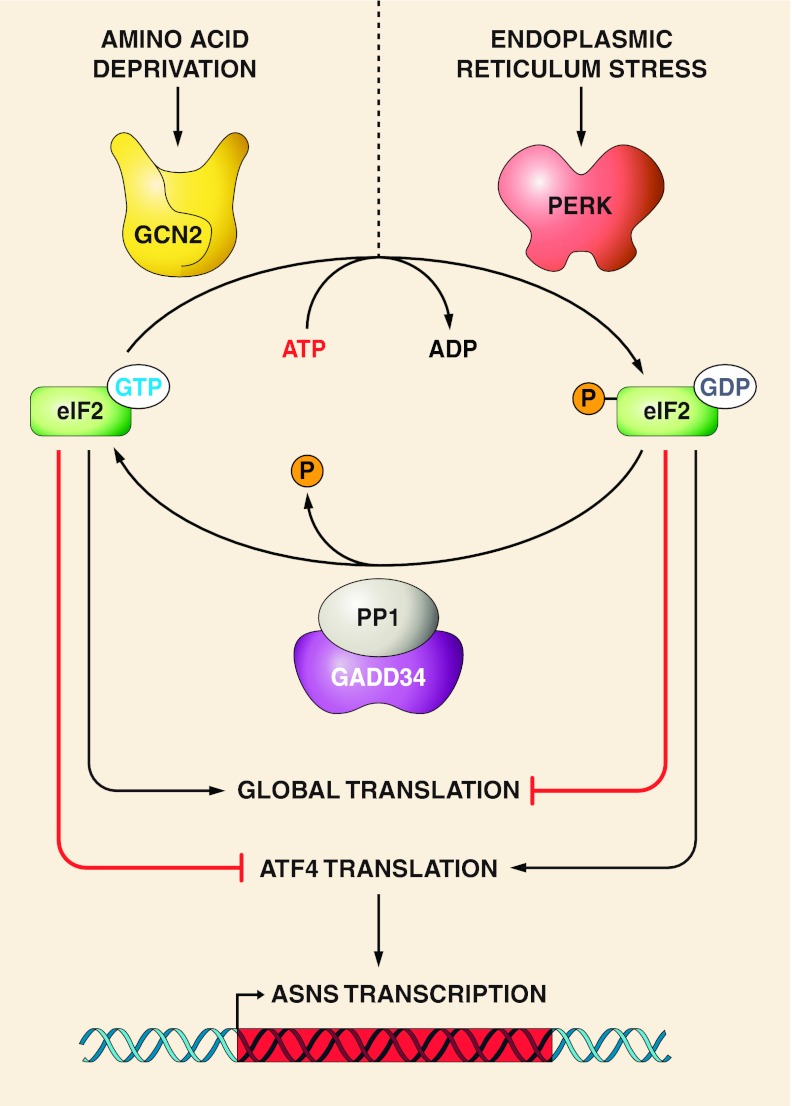
GCN2 is only one of four eIF2 kinases that mediate the response to a variety of cellular stresses (reviewed in Ref. 95). A second eIF2 kinase that impacts ASNS expression is the double-stranded RNA-activated protein kinase-like endoplasmic reticulum (ER) kinase (PERK) (Fig. 4). The PERK signaling pathway, together with the ATF6 and inositol-requiring enzyme (IRE)-1 pathways, make up what is referred to as the unfolded protein response (UPR), which is activated by cellular insults such as ER protein synthesis overload, oxidative stress, or other circumstances that perturb ER function. Like GCN2, activation of PERK leads to phosphorylation of eIF2, suppression of global translation, and increased ATF4 synthesis (42). Consequently, the ATF4-dependent downstream transcriptional programs for the AAR and UPR overlap. Thus, in many instances, either AA limitation or ER stress will lead to transcriptional activation of common ATF4-responsive target genes, including ASNS (14–16). However, there are also differences in the gene profile activated by GCN2 and PERK signaling, indicating that factors other than ATF4 add specificity to each pathway (29).
Fig. 4.
eIF2 kinase sensors for the AAR vs. the UPR. Limitation for one or more AA causes an increased abundance of the corresponding uncharged tRNA that in turn binds to and activates the GCN2 kinase. On the other hand, other cellular stress events including glucose starvation or tunicamycin treatment that leads to glycoprotein accumulation, thapsigargin-induced inhibition of calcium homeostasis, viral overload, oxidative stress, and a wide range of disease states or drug treatments trigger endoplasmic reticulum (ER) stress (43). The resulting ER stress causes stimulation of a collection of signaling pathways collectively referred to as the unfolded protein response (UPR). One of these pathways involves activation of the ER-bound kinase PERK. Both GCN2 and PERK are members of a family of kinases that phosphorylate the eukaryotic translation initiation factor eIF2 (95). Phospho-(p-)eIF2 leads to suppression of global protein synthesis, but enhanced translation of select mRNA species, such as transcription factors ATF4 and ATF5, that contain short upstream opening reading frames that serve as regulatory sequences. Among the hundreds of ATF4 target genes is GADD34, which directs protein phosphatase-1 (PP1) to p-eIF2 and thus returns the translation factor to its dephosphorylated state and the promotion of global translation. As an ATF4-responsive enhancer element, the CARE sequence in ASNS, and many other CARE-containing genes, results in activation of transcription during either the AAR or the UPR.
Genomic Sequences that Control ASNS Transcription During the AAR and UPR
C/EBP-ATF response element.
After discovering that the ASNS gene was regulated by AA availability, the Basilico group analyzed the promoter region to identify the essential cis-regulatory elements (40). A region around nt −68 was indispensible for the response and was designated an “amino acid response element” (AARE). This was the first description of an AA-responsive genomic element in a mammalian cell. To further dissect the ASNS promoter, Barbosa-Tessmann et al. (15) used a combination of in vivo footprinting, deletion analysis, and site-directed mutagenesis to identify the specific nucleotide sequences that mediated the AAR-induced transcription (Fig. 2 B). Those authors determined that two distinct regions within the human ASNS promoter were both necessary for AAR-sensitivity, one from nt −68 to −60 with the sequence 5′-TGATGAAAC-3′ (initially called “nutrient-sensing response element 1”, NSRE1) was the same as that identified by Basilico and colleagues, and a second previously undetected site from nt −48 to −43 with the sequence 5′-GTTACA-3′ (NSRE2). The NSRE1 sequence is now referred to as a CCAAT/enhancer-binding protein (C/EBP)-ATF response element (CARE). As is the hallmark of enhancer elements, the NSRE1/NSRE2 “unit” functions independently of location or orientation, and is also functional when linked to heterologous promoters. However, Zhong et al. (101) showed that the 5′ to 3′ orientation of NSRE1 relative to NSRE2 is not reversible and that the 11 nt spacer region between the NSRE1 and NSRE2 sequences is required for enhanced transcription. The requirement for ∼10 bp between the NSRE1 and NSRE2 suggests that it is functionally necessary for these sites to face the same side of the DNA helix.
Although the trans-acting factors that bind to the NSRE2 site have not been identified, some of those that bind to the NSRE1 sequence have been established, and they include several members of the ATF and C/EBP subfamilies of the basic-leucine zipper (bZIP) superfamily of transcription factors (81, 82). The ASNS NSRE1 sequence, composed of a half-site for the C/EBP family and a half-site for the ATF family of transcription factors, is just one example of a family of related sequences with the consensus sequence of 5′-TGATGXAAX-3′ that are present in a wide spectrum of genes activated by the AAR and/or the UPR (52). This type of “C/EBP-ATF composite site” element was first identified by Wolfgang et al. within the C/EBP-homology protein (CHOP) gene (96), and the same laboratory showed that, following arsenite treatment, ATF4 binds to the element as an activator but is later displaced by ATF3, which represses transcription (35). To continue the use of the “C/EBP-ATF” nomenclature, we refer to this family of sequences, including the ASNS NSRE1 site, as C/EBP-ATF response elements (CARE). Following the determination that ER stress, initiated by either carbohydrate deprivation or disruption of protein folding, leads to induction of ASNS transcription (14, 16), analysis of the promoter established that the same two elements that were responsible for the response to AA limitation, NSRE1 and NSRE2, were also necessary for the transcriptional response to the UPR (15). The sensitivity of the gene to limitation of either AA or glucose is the reason that those investigators coined the term NSRE. Although these genomic sequences exhibit “AARE activity”, their responsiveness to a range of other cellular stress signals is also clear.
The in vivo footprinting studies of the human ASNS gene also revealed constitutive protein binding to three GC boxes located upstream of the promoter-localized CARE (15). These sites are spread across the region covered by nt −153 to −93 and were discovered to be critical for maintenance of basal transcription (Fig. 2 B), but were also required for a maximal response to AA deprivation. Upon further characterization of the three GC boxes, it was established that exogenous expression of Sp1 supported basal ASNS promoter activity only, whereas expression of Sp3 enhanced the basal activity and permitted AAR-induced ASNS transcription (57).
The network of transcription factors that control the ASNS gene.
Chen et al. employed chromatin immunoprecipitation (ChIP) assays to show that within 30–45 min after initiating AA limitation in HepG2 hepatoma cells, newly synthesized ATF4 rapidly translocated to the nucleus and was associated with the ASNS proximal promoter region (25). The amount of ATF4 binding continuously increased up to 4 h, coinciding with increased histone acetylation, recruitment of the general transcription machinery, and the peak of ASNS transcription. The authors observed that a subsequent decline in ASNS transcription between 4 and 24 h of continued AA limitation is mirrored by a gradual decline in ATF4 binding and a parallel increase in ATF3, C/EBPβ, and CHOP recruitment to the CARE site (25, 85). This auto-regulatory feedback cycle results from ATF4-dependent induction of the C/EBPβ, ATF3, and CHOP transcription followed by a subsequent recruitment of the corresponding proteins to the ASNS promoter (23, 66, 85). Subsequently, this self-limiting cycle for ATF4-dependent transcriptional activation was also observed for numerous CARE-containing genes (58, 66). While the transcriptional induction of other AA-responsive genes, such as CHOP, requires the histone acetyltransferase ATF2, ASNS exhibits less dependence on this ATF family member (18, 36). Therefore, although histone acetylation is an important component of the transcriptional activation of the ASNS gene following AA limitation, additional acetyltransferases appear to be involved (13, 25).
The ATF5 protein is homologous to ATF4 (3), and its synthesis is also subject to AA-dependent control through the GCN2-eIF2 translation mechanism (93, 102). Overexpression of ATF5 activates transcription driven by the ASNS promoter through the CARE site, and, like CHOP's antagonism of ASNS induction by ATF4 (85), CHOP also counteracts ATF5 with regard to ASNS regulation (3). Interestingly, Al Sarraj et al. (3) showed that ATF4 was required to induce ATF5 expression and to support the ATF5-dependent transcription of ASNS. Although the ATF4 dependence for induction of ATF5 expression was confirmed by Su et al. (87), they did not observe a decrease in ASNS induction by AA limitation following ATF5 knockdown. Thus, the exact role of ATF5 in regulating the ASNS gene remains unresolved. However, an additional link between ASNS expression and ATF5 arises from the observation that ATF5 polymorphisms have been linked to an altered response to childhood acute lymphoblastic leukemia (ALL) therapy. During treatment for childhood ALL, patients are given asparaginase (ASNase), a component of combination chemotherapy (see below). ASNase therapy causes depletion of plasma asparagine followed by the loss of intracellular asparagine (26). Due to the lack of a rapid upregulation of ASNS protein content in ALL cells, they are preferentially sensitive to ASNase (11, 86). Rousseau et al. (77) observed that ALL patients with a T1562C polymorphism within the ATF5 gene have decreased event-free survival times after ASNase therapy. The T1562C polymorphism results in higher ATF5 promoter activity, and the investigators proposed that elevated ATF5-driven expression of ASNS within the leukemia cells causes decreased sensitivity to ASNase therapy.
Clinical Relevance of ASNS
ASNS and ASNase treatment of childhood ALL.
Asparagine metabolism in transformed cells has received considerable attention after it was determined that certain types of tumors are susceptible to ASNase treatment (17). Primary ALL cells and many ALL cell lines exhibit a particularly low level of ASNS expression (12, 75) and are therefore unusually sensitive to asparagine depletion. Conversely, drug-selected ASNase-resistant ALL cell lines exhibit elevated expression of ASNS (11, 30), and overexpression of exogenous ASNS protein alone results in an ASNase-resistant phenotype in the absence of drug selection (11). Clinically, the relationship is less clear. In a survey of 173 patients, Holleman et al. observed a correlation between in vitro ASNase sensitivity and ASNS mRNA abundance measured by oligonucleotide arrays (46). Although ASNS was not one of the top genes associated with ASNase resistance, ASNS mRNA levels were about threefold higher in the resistant patients. However, several clinical studies have shown a lack of correlation between ASNS mRNA levels and ASNase sensitivity in ALL patients (8, 46, 55, 83, 84). One feature of all of these investigations was that ASNS mRNA was analyzed, rather than ASNS protein content or enzymatic activity. Su et al. (86) addressed this apparent conflict by demonstrating that ASNS protein level, not mRNA content, serves as a prognostic indicator of ASNase sensitivity in ALL cells. The molecular basis for this observation appears to be a significant time delay between upregulation of ASNS mRNA following ASNase treatment and the translation of that mRNA into ASNS protein. Interestingly, the correlation of ASNase sensitivity to ASNS protein, rather than mRNA, was later observed for several human ovarian tumor lines as well (59). The molecular mechanism for the delay between mRNA synthesis and protein expression has not been established, nor is there any published evidence for posttranslational modification of the ASNS protein.
One aspect of ASNase treatment that has not been fully explored is the effect of the drug on normal tissues, despite the fact that ASNase therapy is associated with toxicity. Bunpo et al. (20) demonstrated that ASNase treatment leads to the induction of the AAR in the liver, as illustrated by induction of hepatic ASNS and downregulation of mTOR. Using a Gcn2 knockout mouse model, the authors showed that the ASNase action was mediated through a Gcn2-dependent mechanism. In a subsequent study, they also documented that the Gcn2 dependence extended to the immunosuppressive action of ASNase on the thymus and spleen (19). The studies in Gcn2 knockout mice suggest that if there are humans with a deficiency in GCN2 activity they may be even more susceptible to ASNase toxicity. Collectively, these results provide mechanistic insight into the consequences of ASNase antitumor therapy on normal tissues and suggest that cotreatment with ASNase and GCN2-specific inhibitors may be even more effective than ASNase alone.
Methylation of the ASNS gene.
A number of investigations have focused on a possible correlation between ASNase sensitivity and the DNA methylation status of the ASNS gene. Sugiyama et al. (88) proposed that the ASNS gene in asparagine-auxotrophic Jensen rat sarcoma cells was silenced by DNA hypermethylation because ASNS expression was detectable after 5-azacytidine (5-Aza-C) treatment, which leads to hypomethylation of the genome. Subsequently, several studies in asparagine-dependent and asparagine-independent cell lines have revealed a correlation between the DNA methylation within the ASNS gene locus and ASNS expression (5, 70, 97). In the context of human leukemic cell lines, Ren et al. (72) also observed that a high degree of ASNS promoter methylation correlated with lack of ASNS expression and that 5-Aza-dC treatment enhanced ASNS expression. Furthermore, by electromobility shift analysis with nuclear extracts from the leukemia cells, the authors detected the presence of a “methyl-binding protein” that was bound to an oligonucleotide that contained a methylated CpG site near the ASNS CARE sequence. Interestingly, those authors observed that binding of ATF4 and several C/EBP members was significantly diminished when the methylated oligonucleotide was compared with the same sequence in the unmethylated state (72). They postulated that in vivo the methyl-binding protein might compete with transcriptional activators of ASNS expression, including ATF4. Ding et al. (31) cocultivated asparagine-dependent human leukemia cells with mouse peritoneal macrophages and observed subsequent demethylation of the ASNS promoter, enhanced ASNS expression, and acquisition of asparagine-independence. In ALL bone marrow samples, 74% of B cells and 83% of T cells displayed methylation of the ASNS promoter, in contrast with a lack of methylation observed in brain and breast tumors (2). On the basis of these studies, Akagi et al. (2) hypothesized that ASNS methylation may underlie the susceptibility of ALL cells to ASNase chemotherapy.
ASNS and solid tumor growth.
In contrast to ALL, the potential relationship between ASNS expression and solid tumor initiation/promotion has not been as extensively studied. Koumenis and colleagues investigated the role of ATF4 in tumor cell survival and proliferation using an ATF4 shRNA knockdown strategy (98). They observed that, after ATF4 knockdown in HT1080 fibrosarcoma and DLD1 colorectal adenocarcinoma cells, survival was reduced in the absence of nonessential AA. The authors showed that reduced proliferative capacity and increased apoptosis were correlated with decreased ASNS expression in the ATF4-deficient cells. Supplementation of shATF4-expressing tumor cells with asparagine, but no other individual AA, reversed the increased apoptosis and autophagy, leading to increased cell survival. Ye et al. (98) sought to further define the mechanism of ASNS activation in transformed cells and discovered that GCN2 was activated in response to shATF4 knockdown, presumably due to lower ASNS expression and therefore, asparagine deficiency. Once again, supplementation of the cells with asparagine repressed the activation of GCN2. When the authors probed for the ability of transformed GCN2+/+ and GCN2−/− cells to form tumors in a xenograft model, they observed that tumor growth was impeded in the GCN2−/− cells. As eIF2 is the sole known substrate for GCN2, the researchers concluded that activation of the GCN2-eIF2-ATF4 pathway 1) increases the survival and proliferative capabilities of tumor cells undergoing nutrient limitation, 2) is required for starvation-activated autophagy in transformed cells, and 3) induces ASNS as a key factor in tumor initiation and growth under AA limiting conditions (98). The exact role of ASNS and its product asparagine in modulating tumor growth are unknown. The most obvious explanation, protein synthesis, seems too simplified, as other AA synthesis pathways are not as highly regulated and other AA do not appear to play as critical a role.
Dufour et al. (33) used immunohistochemistry to screen 98 human pancreatic ductal carcinomas and determined that ASNS expression was low or below detection in about 70% of the patients. These results suggest that some pancreatic tumors may be susceptible to ASNase therapy, although clinical trials using ASNase were disappointing because of toxicity. Cui et al. (28) used expression array analysis to identify genes that were induced in pancreatic tumor cells exposed to low glucose, and one of those identified was ASNS. As mentioned above, ASNS transcription is induced via the UPR pathway in response to glucose deprivation (14–16). Cui et al. proposed that the function of ASNS upregulation in response to low glucose is to protect the pancreatic cancer cells from apoptosis, based on the observation that ASNS overexpression suppressed JUN NH2-terminal kinase (JNK) activation and reduced apoptosis, whereas ATF4 knockdown increased the susceptibility of the cells to apoptosis. In addition to greater tolerance for glucose limitation, pancreatic cancer cells overexpressing ASNS exhibited increased resistance to apoptosis induced by cis-diamine-dichloroplatinum (CDDP), a result also linked to the suppression of JNK activation by ASNS (28).
In addition to JNK, extracellular signal-regulated kinase (ERK) is activated by AA limitation and influences downstream signaling in the AAR (1, 22, 36, 65, 69, 90). Among the AAR-associated targets of JNK and ERK are ATF2 (22) and cJUN (36). The AP-1 family of transcription factors can form complexes composed of homo- or heterodimers of cJUN, JUN-B, JUN-D, cFOS, FOS-B, Fra1, and Fra2 (80), and of these, the cJUN, cFOS, JUN-B, and FOS-B genes are activated by the AAR in HepG2 human hepatoma cells, whereas those for JUN-D, FRA-1, and FRA-2 are not (36, 79). Fu et al. (36) showed that for some, but not all, cell lines from several human tissues, the relative induction of cJUN expression was greater in transformed cells compared than in nontransformed cells, independent of cell growth rate. Those authors also showed that overexpression of cJUN exhibited a concentration-dependent activation of both the basal and AAR- or ATF4-induced ASNS-driven transcription, whereas a dominant negative cJUN form suppressed the increased ASNS transcription. The results of Fu et al. also revealed that existing cJUN protein is phosphorylated through a cascade that involves both ERK and JNK, and subsequently, cJUN-ATF2 dimers induce transcription from the cJUN gene itself. Presumably, homo- or heterodimers containing cJUN then activate additional downstream genes. Given that cJUN promotes cell growth by increasing cyclin D expression (92), induction of cJUN by the AAR may contribute to tumor cell survival in the presence of a limited AA supply.
Lorenzi et al. (60) screened the NCI-60 human cancer cell panel using microarray assays and noted a negative correlation between ASNS mRNA expression and susceptibility to ASNase treatment in several ovarian cancer cells. They also observed increased ASNase sensitivity after siRNA knockdown of ASNS expression. In a second study, with a larger number of ovarian cell lines, the same group determined that, whereas the correlation between ASNase efficacy and ASNS mRNA expression was weak, there was a stronger correlation between ASNase efficacy and ASNS protein levels (59). As mentioned above, this result confirmed previous observations in human MOLT4 leukemia cells (86). These ovarian cell culture studies may lead to the use of ASNS as a biomarker in ovarian cancer screening (61).
ASNS and tumor metastasis.
Metastasis is a complicated and incompletely understood process, but some evidence suggests that ASNS may be important in certain metastatic mechanisms. For cancer to spread from the primary tumor to distant sites, a cell or group of cells must detach from the primary tumor and enter the bloodstream, where they exist in suspension until they reach the metastatic site. Patrikainen et al. (67) mimicked this transition by adapting PC-3 prostate cancer cells from adherent to suspension culture and then examined changes in gene expression concomitant with suspension-adaption. They discovered that the ASNS expression was sixfold greater in the suspension-adapted PC-3 cells than in the adherent cells. Subsequently, Ameri et al. (4) created orthotopic xenografts by using human MDA-MB-231 breast cancer cells in an established metastatic mouse model. The authors observed that the protein abundance for ATF4 and ATF3, as well as their target gene ASNS, were elevated in circulating tumor cells isolated from mouse blood compared with the parental MDA-MB-231 cell line. When returned to in vitro culture and exposed to hypoxia, the circulating tumor cells showed higher basal expression and greater induction of ATF4 and ASNS than the parental MDA-MB-231 cell line. Furthermore, the circulating tumor cells had an increased capacity for colony formation in a soft agar assay under hypoxic conditions and grew faster when reimplanted as xenografts. The authors speculated that circulating tumor cells may represent a subpopulation that is selected for its ability to survive insults such as hypoxia and nutrient deprivation as the result of upregulation of factors such as ATF4, ATF3, and ASNS. With specific regard to ASNS, its increased abundance in metastasizing cells suggests that ASNS activity is beneficial for cancer cell survival once they detach from the primary tumor and enter the bloodstream.
These studies documenting the upregulation of ATF4 and its target genes ATF3 and ASNS in metastasis reinforce studies revealing the importance of the GCN2-ATF4 (98) and PERK-ATF4 pathways, as well as their downstream target ASNS, in transformed cells. As illustrated in Fig. 4, activation of either eIF2 kinase GCN2 or PERK will lead to ATF4 production and the subsequent downstream transcriptional programs. However, two points are noteworthy and indicate that further investigation is required to fully understand the roles of these kinases in tumor biology. First, while some of the cell stress circumstances that trigger each of these kinases are known, such as AA limitation for GCN2 and ER stress/hypoxia for PERK, others may yet to be discovered (54, 94). Second, while both PERK and GCN2 catalyze the phosphorylation of eIF2, which leads to ATF4 synthesis, the translational and transcriptional output for the two kinases differs, suggesting that additional modulating signals have yet to be discovered. (29).
Examples of the Many Remaining Questions
Although many facets of ASNS regulation and function have been characterized over the past several decades, there remain major gaps in our knowledge. The exact roles and physiological impact that this enzyme activity plays in maintaining homeostasis of two substrates and two products remain largely unexplored. For example, the name implies a focus on asparagine biosynthesis, but the activity could have significant effects on cellular glutamine content, especially during periods of long-term upregulation. Knockout animals have not been investigated to address the in vivo impact during embryonic development or physiological stress states during adulthood. The striking level of ASNS expression in the exocrine pancreas is an interesting opportunity for further exploration. The high ASNS content may simply be related to the need for asparagine availability for glycoprotein synthesis and secretion, but the liver also has high rates of glycoprotein secretion with what appears to be much less ASNS activity. The importance of increased ASNS expression in tumor proliferation in solid tumors and development of resistance to ASNase chemotherapy in leukemia illustrates that a better comprehension of ASNS regulation, as well as the AAR in general, is needed in transformed cells. Additional investigation of the impact of tumor-associated changes in ASNS regulation, such as the polymorphisms in the ATF5 gene, are likely to contribute to our understanding of the interesting links between aberrant ASNS expression and transformed cell growth that have been observed for many tumors.
GRANTS
This research was supported by grants to M. S. Kilberg from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK-92062 and DK-94729).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.N.B., E.A.B., and M.S.K. conception and design of research; M.N.B., E.A.B., and M.S.K. drafted manuscript; M.N.B., E.A.B., and M.S.K. edited and revised manuscript; M.N.B., E.A.B., and M.S.K. approved final version of manuscript; E.A.B. and M.S.K. prepared figures.
ACKNOWLEDGMENTS
We thank Tracy Anthony, Rutgers University, for helpful discussion about asparaginase toxicity. We acknowledge the contribution of Dr. Richard Hutson, who generated the data shown in Fig. 1 while a member of the Kilberg laboratory. We also thank other members of the laboratory for helpful discussion.
REFERENCES
- 1. Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W, Baccarini M. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem 275: 22300–22304, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Akagi T, Yin D, Kawamata N, Bartram CR, Hofmann WK, Wolf I, Miller CW, Koeffler HP. Methylation analysis of asparagine synthetase gene in acute lymphoblastic leukemia cells. Leukemia 20: 1303–1306, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Al Sarraj J, Vinson C, Thiel G. Regulation of asparagine synthetase gene transcription by the basic region leucine zipper transcription factors ATF5 and CHOP. Biol Chem 386: 873–879, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Ameri K, Luong R, Zhang H, Powell AA, Montgomery KD, Espinosa I, Bouley DM, Harris AL, Jeffrey SS. Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. Br J Cancer 102: 561–569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrulis IL, Barrett MT. DNA methylation patterns associated with asparagine synthetase expression in asparagine-overproducing and -auxotrophic cells. Mol Cell Biol 9: 2922–2927, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrulis IL, Chen J, Ray PN. Isolation of human cDNAs for asparagine synthetase and expression in Jensen rat sarcoma cells. Mol Cell Biol 7: 2435–2443, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrulis IL, Hatfield GW, Arfin SM. Asparaginyl-tRNA aminoacylation levels and asparagine synthetase expression in cultured Chinese hamster ovary cells. J Biol Chem 254: 10629–10633, 1979 [PMC free article] [PubMed] [Google Scholar]
- 8. Appel IM, den Boer ML, Meijerink JP, Veerman AJ, Reniers NC, Pieters R. Upregulation of asparagine synthetase expression is not linked to the clinical response to l-asparaginase in pediatric acute lymphoblastic leukemia. Blood 107: 4244–4249, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Arfin SM, Cirullo RE, Arredondo-Vega FX, Smith M. Assignment of structural gene for asparagine synthetase to human chromosome 7. Somatic Cell Genet 9: 517–531, 1983 [DOI] [PubMed] [Google Scholar]
- 10. Arfin SM, Simpson DR, Chiang CS, Andrulis IL, Hatfield GW. A role for asparaginyl-tRNA in the regulation of asparagine synthetase in a mammalian cell line. Proc Natl Acad Sci USA 74: 2367–2369, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aslanian AM, Fletcher BS, Kilberg MS. Asparagine synthetase expression alone is sufficient to induce l-asparaginase resistance in MOLT-4 human leukaemia cells. Biochem J 357: 321–328, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asselin BL, Kurtzburg J. Asparaginase. In: Treatment of Acute Leukemias, edited by Pui CH. Totawa, NJ: Humana, 2003, p. 365–379 [Google Scholar]
- 13. Balasubramanian MN, Shan J, Kilberg MS. Dynamic changes in genomic histone association and modification during activation of the ASNS and ATF3 genes by amino acid limitation. Biochem J 449: 219–229, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barbosa-Tessmann IP, Chen C, Zhong C, Schuster SM, Nick HS, Kilberg MS. Activation of the unfolded protein response pathway induces human asparagine synthetase gene expression. J Biol Chem 274: 31139–31144, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Barbosa-Tessmann IP, Chen C, Zhong C, Siu F, Schuster SM, Nick HS, Kilberg MS. Activation of the human asparagine synthetase gene by the amino acid response and the endoplasmic reticulum stress response pathways occurs by common genomic elements. J Biol Chem 275: 26976–26985, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Barbosa-Tessmann IP, Pineda VL, Nick HS, Schuster SM, Kilberg MS. Transcriptional regulation of the human asparagine synthetase gene by carbohydrate availability. Biochem J 339: 151–158, 1999 [PMC free article] [PubMed] [Google Scholar]
- 17. Broome JD. Studies on the mechanism of tumor inhibition by l-asparaginase. J Exp Med 127: 1055–1072, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruhat A, Cherasse Y, Maurin AC, Breitwieser W, Parry L, Deval C, Jones N, Jousse C, Fafournoux P. ATF2 is required for amino acid-regulated transcription by orchestrating specific histone acetylation. Nucleic Acids Res 35: 1312–1321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bunpo P, Cundiff JK, Reinert RB, Wek RC, Aldrich CJ, Anthony TG. The eIF2 kinase GCN2 is essential for the murine immune system to adapt to amino acid deprivation by asparaginase. J Nutr 140: 2020–2027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. The GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent, l-asparaginase. J Biol Chem 284: 32742–32749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carraro V, Maurin AC, Lambert-Langlais S, Averous J, Chaveroux C, Parry L, Jousse C, Ord D, Ord T, Fafournoux P, Bruhat A. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2alpha/ATF4 pathway. PLoS One 5: e15716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaveroux C, Jousse C, Cherasse Y, Maurin AC, Parry L, Carraro V, Derijard B, Bruhat A, Fafournoux P. Identification of a novel amino acid response pathway triggering ATF2 phosphorylation in mammals. Mol Cell Biol 29: 6515–6526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen C, Dudenhausen E, Chen H, Pan YX, Gjymishka A, Kilberg MS. Amino-acid limitation induces transcription from the human C/EBPbeta gene via an enhancer activity located downstream of the protein coding sequence. J Biol Chem 391: 649–658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen H, Kilberg MS. Alignment of the transcription start site coincides with increased transcriptional activity from the human asparagine synthetase gene following amino acid deprivation of HepG2 cells. J Nutr 136: 2463–2467, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive bZIP transcription factors as well as localized histone acetylation. J Biol Chem 279: 50829–50839, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Cooney DA, Capizzi RL, Handshumacher RE. Evaluation of l-asparagine metabolism in animals and man. Cancer Res 30: 929–935, 1970 [PubMed] [Google Scholar]
- 27. Cooney DA, King VD, Cable RG, Taylor JB, Wodinsky I. l-Asparagine synthetase in serum as a marker for neoplasia. Cancer Res 36: 3238–3245, 1976 [PubMed] [Google Scholar]
- 28. Cui H, Darmanin S, Natsuisaka M, Kondo T, Asaka M, Shindoh M, Higashino F, Hamuro J, Okada F, Kobayashi M, Nakagawa K, Koide H. Enhanced expression of asparagine synthetase under glucose-deprived conditions protects pancreatic cancer cells from apoptosis induced by glucose deprivation and cisplatin. Cancer Res 67: 3345–3355, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Dang Do AN, Kimball SR, Cavener DR, Jefferson LS. eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiol Genomics 38: 328–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. den Boer ML, Pieters R, Kazemier KM, Rottier MMA, Zwaan CM, Kaspers GJL, Janka-Schaub G, Henze G, Creutzig U, Scheper RJ, Veerman AJP. Relationship between major vault protein/lung resistance protein, multidrug resistance-associated protein, P-glycoprotein expression, and drug resistance in childhood leukemia. Blood 91: 2092–2098, 1998 [PubMed] [Google Scholar]
- 31. Ding Y, Li Z, Broome JD. Epigenetic changes in the repression and induction of asparagine synthetase in human leukemic cell lines. Leukemia 19: 420–426, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Dudek SM, Semenkovich CF. Essential amino acids regulate fatty acid synthase expression through an uncharged transfer RNA-dependent mechanism. J Biol Chem 270: 29323–29329, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Dufour E, Gay F, Aguera K, Scoazec JY, Horand F, Lorenzi PL, Godfrin Y. Pancreatic tumor sensitivity to plasma l-asparagine starvation. Pancreas 41: 940–948, 2012 [DOI] [PubMed] [Google Scholar]
- 34. Endo Y, Fu Z, Abe K, Arai S, Kato H. Dietary protein quantity and quality affect rat hepatic gene expression. J Nutr 132: 3632–3637, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339: 135–141, 1999 [PMC free article] [PubMed] [Google Scholar]
- 36. Fu L, Balasubramanian M, Shan J, Dudenhausen EE, Kilberg MS. Auto-activation of c-JUN gene by amino acid deprivation of hepatocellular carcinoma cells reveals a novel c-JUN-mediated signaling pathway. J Biol Chem 286: 36724–36738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong SS, Guerrini L, Basilico C. Regulation of asparagine synthetase gene expression by amino acid starvation. Mol Cell Biol 11: 6059–6066, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greco A, Gong SS, Ittmann M, Basilico C. Organization and expression of the cell cycle gene, ts 11, that encodes asparagine synthetase. Mol Cell Biol 9: 2350–2359, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greco A, Ittmann M, Basilico C. Molecular cloning of a gene that is necessary for G 1 progression in mammalian cells. Proc Natl Acad Sci USA 84: 1565–1569, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guerrini L, Gong SS, Mangasarian K, Basilico C. Cis- and trans-acting elements involved in amino acid regulation of asparagine synthetase gene expression. Mol Cell Biol 13: 3202–3212, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 5: 103–114, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Heng HHQ, Shi XM, Scherer SW, Andrulis IL, Sui LCT. Refined localization of the asparagine synthetase gene ASNA) to chromosome 7, region q 21.3, and characterization of the somatic cell hybrid line 4AF/106/KO15. Cytogenet Cell Genet 66: 135–138, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Hinnebusch AG. Translational regulation of yeast GCN4. J Biol Chem 272: 21661–21664, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV, Janka-Schaub GE, Pieters R, Evans WE. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med 351: 533–542, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Hongo S, Toshiaky C, Sato T. Induction of asparagine synthetase by follicle-stimulating hormone in primary cultures of rat sertoli cells. Arch Biochem Biophys 313: 222–228, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Hutson RG, Kilberg MS. Cloning of rat asparagine synthetase and specificity of the amino acid-dependent control of its mRNA content. Biochem J 303: 745–750, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hutson RG, Kitoh T, Amador DAM, Cosic S, Schuster SM, Kilberg MS. Amino acid control of asparagine synthetase: relation to asparaginase resistance in human leukemia cells. Am J Physiol Cell Physiol 272: C1691–C1699, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Hutson RG, Warskulat U, Kilberg MS. An example of nutrient control of gene expression: amino acid-dependent regulation of asparagine synthetase. Clin Nutr 15: 327–331, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Jiang HY, Wek RC. GCN2 phosphorylation of eIF2alpha activates NF-kappaB in response to UV irradiation. Biochem J 385: 371–380, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kimball SR, Anthony TG, Cavener DR, Jefferson LS. Nutrient Signaling Through Mammalian GCN2. Berlin: Springer -Verlag, 2005, p. 113–129 [Google Scholar]
- 54. Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22: 7405–7416, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krejci O, Starkova J, Otova B, Madzo J, Kalinova M, Hrusak O, Trka J. Upregulation of asparagine synthetase fails to avert cell cycle arrest induced by l-asparaginase in TEL/AML1-positive leukaemic cells. Leukemia 18: 434–441, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Larsen TM, Boehlein SK, Schuster SM. Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product. Biochemistry (Mosc) 38: 16146–16157, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Leung-Pineda V, Kilberg MS. Role of Sp1 and Sp3 in the nutrient-regulated expression of the human asparagine synthetase gene. J Biol Chem 277: 16585–16591, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Lopez AB, Wang C, Huang CC, Yaman I, Li Y, Chakravarty K, Johnson PF, Chiang CM, Snider MD, Wek RC, Hatzoglou M. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J 402: 163–173, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lorenzi PL, Llamas J, Gunsior M, Ozbun L, Reinhold WC, Varma S, Ji H, Kim H, Hutchinson AA, Kohn EC, Goldsmith PK, Birrer MJ, Weinstein JN. Asparagine synthetase is a predictive biomarker of l-asparaginase activity in ovarian cancer cell lines. Mol Cancer Ther 7: 3123–3128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lorenzi PL, Reinhold WC, Rudelius M, Gunsior M, Shankavaram U, Bussey KJ, Scherf U, Eichler GS, Martin SE, Chin K, Gray JW, Kohn EC, Horak ID, Von Hoff DD, Raffeld M, Goldsmith PK, Caplen NJ, Weinstein JN. Asparagine synthetase as a causal, predictive biomarker for l-asparaginase activity in ovarian cancer cells. Mol Cancer Ther 5: 2613–2623, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Lorenzi PL, Weinstein JN. Asparagine synthetase: a new potential biomarker in ovarian cancer. Drug News Perspect 22: 61–64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 167: 27–33, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Milman HA, Cooney DA. The distribution of l-asparagine synthetase in the principal organs of several mammalian and avian species. Biochem J 142: 27–35, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Milman HA, Cooney DA, Young DM. Role of pancreatic l-asparagine synthetase in homeotasis of l-asparagine. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E746–E753, 1979 [DOI] [PubMed] [Google Scholar]
- 65. Ogier-Denis E, Pattingre S, El Benna J, Codogno P. Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem 275: 39090–39095, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Pan YX, Chen H, Thiaville MM, Kilberg MS. Activation of the ATF3 gene through a co-ordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J 401: 299–307, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Patrikainen L, Porvari K, Kurkela R, Hirvikoski P, Soini Y, Vihko P. Expression profiling of PC-3 cell line variants and comparison of MIC-1 transcript levels in benign and malignant prostate. Eur J Clin Invest 37: 126–133, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Patterson MK, Jr, Orr GR. Asparagine biosynthesis by the Novikoff Hepatoma isolation, purification, property, and mechanism studies of the enzyme system. J Biol Chem 243: 376–380, 1968 [PubMed] [Google Scholar]
- 69. Pattingre S, Bauvy C, Codogno P. Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem 278: 16667–16674, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Peng H, Shen N, Qian L, Sun XL, Koduru P, Goodwin LO, Issa JP, Broome JD. Hypermethylation of CpG islands in the mouse asparagine synthetase gene: relationship to asparaginase sensitivity in lymphoma cells. Partial methylation in normal cells. Br J Cancer 85: 930–935, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ray PN, Siminovitch L, Andrulis IL. Molecular cloning of a cDNA for Chinese hamster ovary asparagine synthetase. Gene 30: 1–9, 1984 [DOI] [PubMed] [Google Scholar]
- 72. Ren Y, Roy S, Ding Y, Iqbal J, Broome JD. Methylation of the asparagine synthetase promoter in human leukemic cell lines is associated with a specific methyl binding protein. Oncogene 23: 3953–3961, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Richards NG, Kilberg MS. Asparagine synthetase chemotherapy. Annu Rev Biochem 75: 629–654, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Richards NGJ, Schuster SM. Mechanistic issues in asparagine synthetase catalysis. Adv Enzymol 72: 145–198, 1998 [DOI] [PubMed] [Google Scholar]
- 75. Rizzari C. Asparaginase treatment. In: Treatment of Acute Leukemias, edited by Pui CH. Totawa, NJ: Humana, 2003, p. 381–391 [Google Scholar]
- 76. Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest 51: 2346–2351, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rousseau J, Gagne V, Labuda M, Beaubois C, Sinnett D, Laverdiere C, Moghrabi A, Sallan SE, Silverman LB, Neuberg D, Kutok JL, Krajinovic M. ATF5 polymorphisms influence ATF function and response to treatment in children with childhood acute lymphoblastic leukemia. Blood 118: 5883–5890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shan J, Fu L, Balasubramanian MN, Anthony T, Kilberg MS. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J Biol Chem 287: 36393–36403, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shan J, Lopez MC, Baker HV, Kilberg MS. Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics 41: 315–327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shaulian E. AP-1—the Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal 22: 894–899, 2010 [DOI] [PubMed] [Google Scholar]
- 81. Siu F, Bain PJ, LeBlanc-Chaffin R, Chen H, Kilberg MS. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem 277: 24120–24127, 2002 [DOI] [PubMed] [Google Scholar]
- 82. Siu FY, Chen C, Zhong C, Kilberg MS. CCAAT/enhancer-binding protein beta (C/EBPb) is a mediator of the nutrient sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem 276: 48100–48107, 2001 [DOI] [PubMed] [Google Scholar]
- 83. Stams WA, den Boer ML, Beverloo HB, Meijerink JP, Stigter RL, Van Wering ER, Janka-Schaub GE, Slater R, Pieters R. Sensitivity to l-asparaginase is not associated with expression levels of asparagine synthetase in t(12;21)+ pediatric ALL. Blood 101: 2743–2747, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Stams WA, den Boer ML, Holleman A, Appel IM, Beverloo HB, Van Wering ER, Janka-Schaub GE, Evans WE, Pieters R. Asparagine synthetase expression is linked with l-asparaginase resistance in TEL-AML1 negative, but not in TEL-AML1 positive pediatric acute lymphoblastic leukemia. Blood 11: 4223–4225, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem 283: 35106–35117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Su N, Pan YX, Zhou M, Harvey RC, Hunger SP, Kilberg MS. Correlation between asparaginase sensitivity and asparagine synthetase protein content, but not mRNA, in acute lymphoblastic leukemia cell lines. Pediatr Blood Cancer 50: 274–279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Su N, Thiaville MM, Awad K, Gjymishka A, Brant JO, Yang TP, Kilberg MS. Protein or amino acid deprivation differentially regulates the hepatic forkhead box protein A (FOXA) genes through an activating transcription factor-4-independent pathway. Hepatology 50: 282–290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sugiyama RH, Arfin SM, Harris M. Properties of asparagine synthetase in asparagine-independent variants of Jensen rat sarcoma cells induced by 5-azacytidine. Mol Cell Biol 3: 1937–1942, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Taylor PM. Amino acid transporters: eminences grises of nutrient signalling mechanisms? Biochem Soc Trans 37: 237–241, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Thiaville MM, Pan YX, Gjymishka A, Zhong C, Kaufman RJ, Kilberg MS. MEK signaling is required for phosphorylation of eIF2alpha following amino acid limitation of HepG2 human hepatoma cells. J Biol Chem 283: 10848–10857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101: 11269–11274, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Verde P, Casalino L, Talotta F, Yaniv M, Weitzman JB. Deciphering AP-1 function in tumorigenesis: fra-ternizing on target promoters. Cell Cycle 6: 2633–2639, 2007 [DOI] [PubMed] [Google Scholar]
- 93. Watatani Y, Ichikawa K, Nakanishi N, Fujimoto M, Takeda H, Kimura N, Hirose H, Takahashi S, Takahashi Y. Stress-induced translation of ATF5 mRNA is regulated by the 5′-untranslated region. J Biol Chem 283: 2543–2553, 2008 [DOI] [PubMed] [Google Scholar]
- 94. Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal 9: 2357–2371, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11, 2006 [DOI] [PubMed] [Google Scholar]
- 96. Wolfgang CD, Chen BP, Martindale JL, Holbrook NJ, Hai T. Gadd153/Chop10, a potential target gene of the transcriptional repressor ATF3. Mol Cell Biol 17: 6700–6707, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Worton KS, Kerbel RS, Andrulis IL. Hypomethylation and reactivation of the asparagine synthetase gene induced by l-asparaginase and ethyl methanesulfonate. Cancer Res 51: 985–989, 1991 [PubMed] [Google Scholar]
- 98. Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, DePanis DN, Bobrovnikova-Marjon E, Diehl A, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumor cell survival and proliferation in response to nutrient deprivation. EMBO J 29: 2082–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang YP, Lambert MA, Cairney AEL, Wills D, Ray PN, Andrulis IL. Molecular structure of the human asparagine synthetase gene. Gene 4: 259–265, 1989 [DOI] [PubMed] [Google Scholar]
- 101. Zhong C, Chen C, Kilberg MS. Characterization of the nutrient sensing response unit in the human asparagine synthetase promoter. Biochem J 372: 603–609, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem 283: 7064–7073, 2008 [DOI] [PubMed] [Google Scholar]