Abstract
Study Design:
Case Report.
Background and Purpose:
Myofascial trigger points (MTrPs) are widely accepted by clinicians and researchers as a primary source of regional neuromusculoskeletal pain. Trigger point dry needling (TrP‐DN) is an invasive procedure that involves stimulation of MTrPs using an monofilament needle. The purpose of this case report is to report the outcomes of TrP‐DN and intramuscular electrical stimulation (IES) as a primary treatment intervention in a subject with chronic low back pain.
Case Description:
The subject was a 30‐year‐old female, active duty military, who was referred to physical therapy for low back and right posterolateral hip pain. She noticed symptoms after suffering a lumbar flexion injury while picking up a barbell during weight training. Physical examination demonstrated findings that supported the diagnosis of lumbar segmental instability with a right hip stability dysfunction. Objective findings included a multi‐segmental flexion movement pattern dysfunction and MTrPs in the right gluteus maximus and gluteus medius muscles with deep palpation. The subject was treated with TrP‐DN and IES for a total of two visits. Bilateral L3 and L5 multifidus and right gluteus maximus and medius muscles were treated, along with implementing a home exercise program consisting of core stability exercises.
Outcomes:
The subject reported no existing pain and disability on the Numerical Pain Rating Scale and Oswestry Disability Questionnaire and a large perceived change in recovery on the Global Rating of Change at final follow‐up. Physical examination was normal, demonstrating no observed impairments or functional limitations, including normal multi‐segmental flexion and no MTrPs with deep palpation.
Discussion:
The subject was able to return to full military active duty without any physical limitations and resumed pre‐injury activity levels, including the ability to resume all activities without pain. There is much promise regarding the use of TrP‐DN with IES intervention for the treatment of lumbar and/or hip stability dysfunction. Future research is recommended to determine if TrP‐DN intervention, with and without IES, is effective for other body regions and long‐term subject outcomes.
Level of Evidence:
Level 4.
Keywords: Dry needling, intramuscular electrical stimulation, low back pain, myofasical trigger points
BACKGROUND AND PURPOSE
Myofascial trigger points (MTrPs) are widely accepted by clinicians and researchers as a primary source of regional neuromusculoskeletal pain.1,2 Travell and Simons define MTrPs as ‘hyperirritable points located in taut bands of skeletal muscle, which when compressed, produce a referred pain characteristic of that muscle and a pain that the subject recognizes.1,3 MTrPs are theorized to develop in muscle tissue in response to injury.
Several studies have shown that MTrPs are commonly seen in acute and chronic pain conditions, and in nearly all orthopaedic conditions.4,5 Vecchiet and colleagues demonstrated that acute pain following exercise or sports participation is often due to acutely painful MTrPs. MTrPs have been demonstrated to be responsible for complaints of pain in individuals with hip osteoarthritis,6 cervical disc lesions,7 temporomandibular dysfunction,8 pelvic pain,9 headaches,10 and epicondylitis.12 Hendler and Kozikowski concluded that myofascial pain syndrome (MPS) is the most commonly missed diagnosis in chronic pain subjects.12
Several dry needling approaches have been developed based on different individual theories, insights, and hypotheses. Two of the more popular conceptual models described in the literature include the myofasical trigger point model and the radiculopathy model.3,5,13,14 Janet Travell MD pioneered the use of MTrP injections that eventually led to the development of the trigger point model of dry needling. Together with Dr. David Simons, they wrote the 2‐volume Trigger Point Manual documenting the referred pain patterns of MTrPs in 147 muscles.3,12 The trigger point model specifically targets MTrPs as the clinical manifestation of MPS.3,14 Myofascial trigger points may consist of multiple contraction knots, which are theorized to be present, secondary to an excessive release of acetylcholine (ACh) from selective motor endplates and can be described as active and latent MTrPs.3,14‐16 The release of ACh has been associated with endplate noise, a characteristic electromyographic discharge at MTrP sites, consisting of low‐amplitude discharges (10‐50 μV) and intermittent high‐amplitude discharges (up to 500 μV) in painful MTrPs.17‐19 Active MTrPs can spontaneously trigger local pain in the area of the MTrP or refer pain or paraesthesia to distant locations.14 Additional symptoms of MTrPs include muscle weakness, limited range of motion, and autonomic symptoms. Latent MTrPs do not trigger local or referred pain without being stimulated, but may alter muscle activation patterns and contribute to limited range of motion.14,20
Gunn's model of radiculopathy is based on denervation super‐sensitivity and is due to the shortening of paraspinal (multifidus) muscles, which ultimately leads to peripheral neuropathy and compression of supersensitive nociceptors.14,21 Gunn stated that MPS is always the result of peripheral neuropathy or radiculopathy and defined (MPS) as “a condition that causes disordered function in the peripheral nerve”14,21 This radiculopathy model is based on Cannon and Rosenblueth's Law of Denervation, which states that the function and integrity of innervated structures is dependent upon the free flow of nerve impulses to provide a regulatory or trophic effect.14,22 Gunn's model states that when the flow of nerve impulses is restricted, super sensitivity is created in all innervated structures supplied by the pathological neural tissue.14,21
An adjunctive treatment known as trigger point dry needling (TrP‐DN), is increasingly being used by healthcare practitioners.23,24 TrP‐DN is an invasive procedure that involves stimulation of MTrPs using a monofilament needle.23 The exact mechanism of action of TrP‐DN is still largely unknown; however, TrP‐DN has been shown to alter the biochemical environment surrounding a MTrP.23,25 In addition, the elicitation of a local twitch response using TrP‐DN has been shown to reduce spontaneous electrical activity within the MTrP region of skeletal muscle in rabbits.23,26 Dry needling a MTrP is most effective when a local twitch responses (LTR) is elicited.26 A LTR has been shown to inhibit abnormal end plate noise.
A study by Shah and colleagues27 demonstrated that the increased levels of various biochemicals, such as bradykinin, calcitonin gene related peptide (CGRP), substance P, and others, at MTrPs are immediately corrected by eliciting a LTR with a monofilament needle. The phenomenon of the LTR is an involuntary spinal cord reflex contraction of the muscle fibers in a taut band following palpation or needling of the band or MTrP.28,29
From a mechanical standpoint, dry needling of an MTrP could mechanically disrupt the integrity of the dysfunctional motor end plates and be related to the extremely shortened sarcomeres.5 An accurately placed needle could provide a localized stretch to the contracted cytoskeletal structures, which may disentangle the myosin filaments from the titin gel at the Z‐band and allow the sarcomere to resume it's resting length by reducing the degree of overlap between actin and myosin filaments.5
There are also suggested neurophysiological explanations regarding the effects of dry needling. Baldry concluded that with superficial dry needling technique, A‐delta nerve fibers (group III) will be stimulated for as long as 72 hours after needle insertion.30 Prolonged stimulation of the sensory afferent A‐delta nerve fibers may activate the enkephalinergic inhibitory dorsal horn interneurons, which would imply that superficial dry needling causes opioid mediated pain suppression.30
Interventions, such as TrP‐DN and acupuncture, are commonly used in the treatment of neuromusculoskeletal pain conditions.31,32 Furthermore, these interventions can have a modulatory effect on hyperalgesia31,33 and they are effective in alleviating symptoms of fibromyalgia,31,32 a condition that also features characteristics of central hyperexcitability.31,34 Together, these findings suggest that TrP‐DN techniques may be effective in the management of chronic low back pain, but reports on the use of such interventions in this condition are limited in the literature. Overall, there is limited high‐quality literature that has evaluated the effectiveness of TrP‐DN, as well as the use of electrical stimulation with this intervention. The poor quality and heterogeneous nature of the current literature precludes definitive conclusions from being made.23 A recent Cochrane review concluded that TrP‐DN, added to other conventional therapies, such as exercise, is more effective at relieving neuromusculoskeletal pain than conventional therapies alone in non‐specific low back pain.31,35
The purpose of this case report was to report the outcomes of TrP‐DN and intramuscular electrical stimulation (IES) as a primary treatment intervention in a subject with chronic low back pain. An important feature of this case report is describing the use of TrP‐DN with IES which allows the delivery of electrotherapy deep within the targeted tissues, due to the utilized indwelling monofilament needles. Electrotherapy has been shown to elicit muscle relaxation and increase local blood circulation,36 so the decision to use TrP‐DN with IES was made to elicit further muscle relaxation. The subject featured in this case report gave informed consent to participate in the study and was informed that the data concerning the case report would be submitted for publication. This case report was not required to be reviewed or approved by a US Navy Institutional Review Board.
CASE DESCRIPTION
The subject was a 30‐year‐old female, active duty military, who was self‐referred to physical therapy for evaluation of low back and right posterolateral hip pain. She reported a history of low back and right posterior hip pain of an insidious nature, which has progressively worsened since she was 16 years old. She noticed an exacerbation of symptoms after suffering a lumbar flexion injury while performing a deadlift exercise during weight training. She could not recall the exact weight she was attempting to lift from the ground, but did attribute her injury to improper exercise technique. Her injury resulted in a confirmed right disc protrusion at L5‐S1 verified by Magnetic Resonance Imaging. Previous radiographs were normal without any evidence of serious lumbar spine pathology.
The subject reported that sitting for an extended period of time and touching her toes aggravated her symptoms, while eliminating and avoiding lumbopelvic flexion‐based movements eased her symptoms. She reported no swelling, gait deviation, or numbness and tingling; however, she reported decreased stability when attempting to balance on her right extremity. The subject's general health was good and cleared for all red flags, including denying bowel or bladder dysfunction, saddle parasthesia, antalgic gait, unexplained weight loss, night pain, previous history of cancer, and the presence of fever or chills. She had attempted anti‐inflammatory medication in the past with no reported reduction in symptoms. The subject's goals for physical therapy included decreased low back and posterior hip pain and improved overall function.
The outcome measures utilized in this case report were the Numeric Pain Rating Scale (NPRS), Oswestry Disability Questionnaire (ODQ), and Global Rating of Change (GROC) scale. [Table 1] On an 11‐point Numerical Pain Rating Scale, the subject reported a 4/10 pain level at initial evaluation. The NPRS was designed to measure a subject's perceived pain level on an 11‐point scale (0 indicating “no pain” and 10 the “worst pain imaginable”). The subject rated her current level of pain, as well as her least and worst amount of pain in the last 24 hours. The average of the 3 ratings was used to represent the subject's level of pain. The NPRS has been shown to have adequate reliability and validity in populations with musculoskeletal disorders and require a 2‐point change to be clinically meaningful.37,38 The modified ODQ was used to measure disability and consists of 10 questions, each scored from 0 to 5, with higher scores indicating greater disability. Scores were then converted to a percentage score. The subject scored a 20% on the QDQ, which falls into the minimal disability classification. This perceived level of disability was not a surprise to the author secondary to the subject's occupation and current activity level. The minimal clinically important difference (MCID) for the modified ODQ has been reported as 6% in a sample of subjects with low back pain undergoing physical therapy.39 The ODQ was utilized as the primary outcome measure. The GROC was completed by the subject during the follow‐up periods. The 15‐point GROC scale described by Jaeschke et al,40 ranges from −7 (“a very great deal better”) to 0 (“about the same”) to +7 (“a very great deal better”). It has been reported in the literature that scores of +4 to +5 are indicative of moderate changes in subject‐perceived status and scores of +6 and +7 indicate large changes in subject status.41 MCID for the GROC has been reported as a 3‐point change from baseline.40
Table 1.
Outcome Measures.
| Initial Exam | FU#1 | FU#2 | FU#3 | |
|---|---|---|---|---|
| NPRS | 4/10 | 2/10 | 0/10 | 0/10 |
| GROC | +3 | +6 | +6 | |
| ODQ | 20% | 0% | 0% |
FU= Follow up; NPRS= Numerical Pain Rating Scale; GROC= Global Rating of Change; ODQ=Oswestry Disability Questionnaire
CLINICAL IMPRESSION
This subject complained that sitting and forward bending aggravates her symptoms, which led to the suspicion that her low back pain was of lumbopelvic or hip origin. The subject also reported decreased stability when attempting to balance on her right extremity, which supported the diagnosis that a lumbar and/or hip stability dysfunction existed. Van Dillen et al42 reported certain history examination findings, including pain with sitting (k=0.99, 1.0) and bending (k=0.98, 0.99), from subjects with low back pain were suggestive that the dysfunction was of lumbar spinal origin.42 Fritz et al41 also determined that certain findings from subjects' history examination were predictive of lumbar instability. According to Fritz et al,41 subjects aged less than 37 years old who experience back pain (Sp=0.81, Sn=0.57, +LR=3.0, –LR=0.53) is suggestive of lumbar segmental instability. This subject fit the age classification according to Fritz et al41 to support this diagnosis. The selected objective tests that were used to test the diagnosis of lumbar and/or hip stability dysfunction included single leg stance assessment, hip manual muscle testing, prone instability test, lumbar segmental joint mobility testing, and pain provocation testing utilizing a posterior to anterior directed force on targeted segments.
This subject reported a previous history of a lumbar disc herniation, so in order to rule out a diagnosis of lumbar radiculopathy, a lower quarter neurological screen was performed. To be as thorough as possible, the neurological screen included dermatomal, myotomal, deep tendon reflex, and neurodynamic testing. The neurodynamic test utilized was the passive straight leg raise test, since it has been reported to help rule out a lumbar radiculopathy (Sp=0.44, Sn=0.98, +LR=1.75, –LR=0.5)43.
As previously stated, the subject complained of pain with sitting, therefore sacroiliac dysfunction was also part of the differential diagnosis. Dreyfuss et al44 determined the diagnostic utility of history and physical examination in determining pain of sacroiliac origin. Pain with sitting (Sp=0.90, Sn=0.03, +LR=0.30, –LR= 1.07) and buttock pain (Sp=0.14, Sn=0.80, +LR=0.90, –LR=1.42) were suggestive of sacroiliac origin.44
Due to the subject's reported complaints of posterior hip pain, the FABER (Flexion‐Abduction‐External Rotation) and FADIR (Flexion‐Adduction‐Internal Rotation) tests were performed to determine if an intra‐articular hip pathology existed. Postural assessment in relaxed standing, lumbar and hip active range of motion in all planes, and lower extremity muscle length testing, including hamstrings, quadriceps, iliopsoas, and iliotibial band, was performed in order to quantify gross mobility limitations throughout the lumbopelvic and hip regions.
Based upon the subject's history finding, the differential diagnoses for this subject included pain of lumbopelvic and/or hip origin, including lumbar segmental instability and lumbar radiculopathy, as well as possible hip stability, sacroiliac, and hip intra‐articular dysfunction.
EXAMINATION
A postural examination was performed in relaxed standing by the physical therapist, which included assessment of head position, shoulder and thoracolumbar deviations, pelvic height and rotation, lower extremity alignment, and symmetrical weight bearing.45 Postural gait assessment was conducted while watching the subject ambulate to and from the treatment area. Physical examination revealed a non‐antalgic gait, normal standing posture, and symmetrical lower extremity alignment. She presented with negative crepitus, apprehension, effusion, erythema, and ecchymosis through visual inspection of the trunk and lower quarter.
A lower quarter neurological examination was performed to screen for symptoms of spinal origin,46 which included dermatomal, myotomal, deep tendon reflex, and neurodynamic testing. Dermatomal testing was performed by accessing the L2 to S2 nerve roots through light touch and pin prink. Myotomal testing was performed by manual muscle testing the representative muscles for the L2 to S2 levels bilaterally. Deep tendon reflex testing was performed by assessing the patellar tendon (L2‐L4) and Achilles tendon (S1‐S2) reflexes. Neurodynamic testing was assessed with a passive straight‐leg raise to determine the presence of neural tension.47 Babinski and clonus testing was performed and was absent bilaterally. All components of the neurological screen were normal and did not reveal any abnormalities, nor reproduce the subject's symptoms.
The Selective Functional Movement Assessment (SFMA) was used as the primary functional screen to assess movement patterns and identify primary areas of dysfunction.48 Multi‐segmental flexion (toe touch or forward flexion), single leg stance, and overhead squat were found to be dysfunctional patterns (with multi‐segmental flexion painful) and were used as test‐retest movements in order to determine objective subject outcomes. See Figure 1 and 2 for forward flexion movement dysfunction displayed at initial exam.
Figure 1.

Multi‐segmental flexion (side view).
Figure 2.
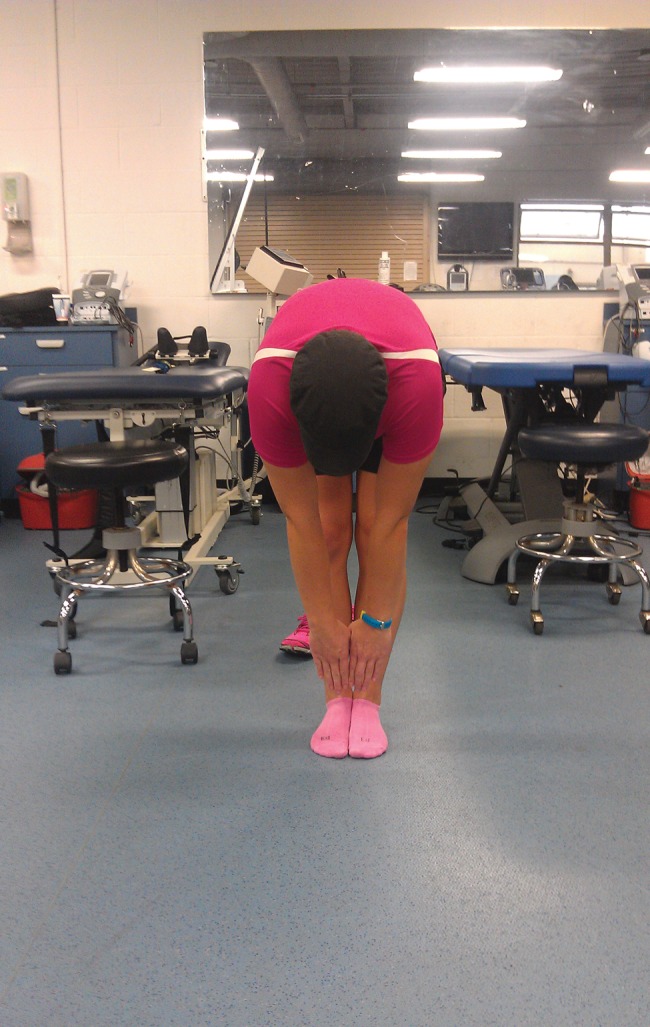
Multi‐segmental flexion (front view).
The overhead squat pattern demonstrated visually perceived excessive bodyweight shifting toward the left lower extremity and lack of right lower extremity weight acceptance, which may occur with a stability dysfunction. Single leg stance assessment demonstrated moderate hip and trunk compensatory corrections and instability bilaterally with greater difficulty perceived during right stance leg compared to the left, indicating a possible asymmetrical core and/or hip stability dysfunction.
Lumbar and hip active range of motion (AROM) assessment was performed and recorded using an inclinometer and standard goniometer. A goniometer was used to assess hip flexion and extension AROM and has been reported to have moderate reliability (ICC = 0.58‐0.79).49 Hip internal and external rotation was assessed using an inclinometer. An inclinometer was used to measure lumbar motions. Fritz et al41 found moderate reliability when accessing lumbar flexion and extension AROM with an inclinometer (ICC = 0.60‐0.61). Active lumbar ROM assessment revealed 65° of lumbar forward flexion (finger tips to mid‐tibia; Figure 1 and 2); 25° of lumbar extension (finger tips to popliteal space); 34° of left lumbar side bending (finger tips to fibula head); 32° of right lumbar side bending (finger tips to fibula head); 55° of left lumbar rotation; and 55° of right lumbar rotation. Active Hip AROM assessment revealed 124° of left hip flexion; 122° of right hip flexion; 20° of left hip extension; 22° of right hip extension; 37° of left hip internal rotation; 34° of right hip internal rotation; 48° of left hip external rotation; and 50° of right external rotation. Hip abduction and adduction was visually assessed and determined to be equal bilaterally and within normal limits. Lumbar AROM assessment revealed all planes were within normal limits. Hip AROM revealed no limitations in all planes and were within normal limits. The lumbar locked rotation test (ICC = 0.87‐0.90)50 was performed and ROM was WNL bilateral. The thoracic spine involvement was eliminated as a primary source of the subject's complaint.
Manual muscle testing (MMT) of the hip musculature was performed described by Kendall,51 including hip flexion, extension, abduction, adduction, internal rotation, and external rotation. Hip MMT assessment revealed a 5/5 hip flexion bilaterally; 5/5 hip extension on left versus 4/5 on right; 5/5 hip abduction on left versus 4/5 on right; 5/5 hip adduction bilaterally; 5/5 hip internal rotation bilaterally; 5/5 hip external rotation on left versus 4/5 on right. In summary, strength assessment of the hip musculature revealed weakness in hip extension, abduction, and external rotation on the right. All other hip planes assessed using manual muscle testing was symmetrical with no functional deficits.
Muscle length testing was performed by assessing the flexibility of the hamstrings, quadriceps, iliopsoas, and iliotibial band. The passive straight leg raise52 was utilized to assess hamstring flexibility and is considered positive for hamstring muscle tightness when measured <70° hip flexion.53 The passive straight leg raise was objectively measured using an inclinometer, in which Piva et al,54 showed excellent reliability when utilizing an inclinometer for hamstring flexibility (ICC = 0.91‐0.92). Passive straight leg raise measured 75° hip flexion bilaterally. The modified Thomas test, described by Bullock‐Saxton et al,55 was utilized to assess the muscle length of the quadriceps and iliopsoas, and both were deemed to be normal bilaterally. Bullock‐Saxton et al55 found excellent reliability with accessing iliopsoas muscle length (ICC = 0.98). The Ober test was utilized to assess the muscle length of the iliotibial band.56 Reese and Brandy,56 describe the reliability of the Ober test to be excellent (ICC = 0.90). No limitations were noted with lower extremity muscle length tests, including hamstrings, quadriceps, iliopsoas, and iliotibial band.
Provocative special testing was performed to access a patho‐anatomical reason for the subject's symptoms. The FABER (Flexion‐Abduction‐External Rotation), FADIR (Flexion‐Adduction‐Internal Rotation), and Log Roll tests were utilized to determine if a hip intra‐articular pathology was present. The FABER (Sn=0.89)57, FADIR (ICC=0.87, Sp=0.43, Sn=0.75, +LR=1.32, –LR=0.58)45, and Log Roll (ICC=0.61)58 tests were negative. Manual overpressure was added to both the FABER and FADIR end ROM to further stress the hip joint. The overpressure was intended to “scour” the joint and clear the hip joint for the presence of any intra‐articular derangement. The sacroiliac joint was screened by utilizing the distraction (k=0.69, Sp=0.81, Sn=0.60), compression (k=0.73, Sp=0.69, Sn=0.69), gaenslen (k=0.76, Sp=0.71‐0.73, Sn=0.50‐0.53), thigh thrust (k=0.88, Sp=0.69, Sn=0.88), and sacral thrust (k=0.56, Sp=0.75, Sn=0.63) tests.59,60 All sacroiliac provocation tests performed were negative. The prone instability test was performed to access lumbar segmental instability. The prone instability test (k= 0.87, ICC= 0.87)61 was positive, due to the subject experiencing painful symptoms with feet on floor that disappeared when feet were lifted off floor when a posterior to anterior (P‐A) directed force was applied to the target spinal segment.
Lumbar spinal accessory motion testing was performed in the prone position to assess joint mobility and pain reproduction by performing a P‐A directed force over the spinous process of T10 to L5 segments and judged to be either normal, hypomobile, or hypermoble. Pain provocation was positive over the L3 to L5 segments with normal pain‐free joint mobility over the remaining tested segments. Superficial to deep palpation was performed throughout the thoracolumbar and bilateral gluteal regions using flat and pincer palpatory techniques. There was tenderness to palpation present along the right gluteus maximus and gluteus medius muscles with a palpable taut band within the skeletal muscle. There was a presence of a hypersensitive tender spot within this taut band indicating suspected active MTrPs.
Motor‐Autonomic‐Sensory‐Trophic (MAST) inspection according to Gunn13 was performed to determine the likelihood of the presence of a radiculopathy. There was no observed muscle spasm or shorting present upon palpatory examination; however, taut bands were discovered in the gluteus maximum and gluteus medius muscles (as previously described). No autonomic responses were observed, such as vasomotor changes (sweating, coldness, etc.), and sensory responses were normal with no signs of supersensitivity. Lastly, no trophic changes, including dry skin, redness, trophedema, or dermatomal hair loss were present during the examination.
EVALUATION
Following the subject's history and physical examination, lumbar segmental instability was suspected with a presence of a hip stability dysfunction on the right during weight bearing activities. The subject's physical examination demonstrated findings that supported the diagnosis of lumbar segmental instability with a right hip stability dysfunction. The prone instability test was positive, which has an excellent intra‐ and inter‐reliability for lumbar segmental instability.61 Pain provocation was positive over the L3 to L5 segments when a posterior to anterior directed force was applied over the target segment. Hicks et al61 reported a poor to moderate reliability depending on the segmental level tested when accessing for lumbar instability (k=0.25‐0.55).
Fritz et al41 determined that certain findings from subjects' history and physical examination were predictive of radiographic lumbar instability. The two most predictive factors were lumbar flexion ROM >53° (Sp=0.86, Sn=0.68, +LR=4.8, –LR=0.38) and a lack of hypomobility during lumbar ‐intervertebral motion testing (Sp=0.95, Sn=0.46, +LR=8.6, –LR=0.60). The presence of both findings demonstrated a +LR of 12.8 and increased the probability of lumbar instability from 50 to 93%.41
A normal multi‐segmental flexion movement pattern is the ability of the subject to touch fingertips to toes without bending the knees. During the physical examination, the subject's multi‐segmental flexion movement pattern was reduced (inability to touch fingers to toes), however, the lumbar spinal flexion ROM was within normal limits when measured by an inclinometer. This normalization of lumbar spinal flexion ROM, despite a limited forward flexion movement pattern may be indicative of lumbar segmental instability.41
A right hip stability dysfunction was noted during many tests during the physical examination. The subject was observed shifting toward the left lower extremity while performing a bodyweight overhead squat, as well as instability with visual compensatory corrections bilaterally during a single leg stance assessment, with greater difficulty on the right compared to the left extremity. She demonstrated weakness in hip extension, abduction, and external rotation on the right with manual muscle testing, which is indicative of hip instability during resistive activities. Hyperirritable, taut bands were discovered in the right gluteus maximus and gluteus medius with deep palpation. These tender to palpation findings were suggestive of MTrPs within the gluteal musculature, which is a common finding of hip stability dysfunction. The subject reported discomfort level as “moderate to severe” when questioned on rating her discomfort level from “mild”, “moderate”, or severe”.
The use of TrP‐DN was performed to the lumbar spine and right hip musculature. Clinical reasoning determined that TrP‐DN may be the intervention of choice, secondary to the subject's suspected lumbar and/or hip stability dysfunction findings, as well as the palpable trigger points in the right gluteus maximus and gluteus medius muscles. It is the author's opinion that TrP‐DN may evoke a ‐“neurophysiological reset,” allowing motor learning to occur and significantly improving motor control and stability of the lumbar spine and hip regions.
INTERVENTION
Risks and potential complications were discussed with the subject and a verbal consent was given to proceed with the intervention of TrP‐DN. Complications that may occur post needling include muscle soreness, fatigue, bruising, and vasovagal reaction. Potential but rare complications post needling include infection, a stuck or broken needle, and pneumothorax (when technique is performed about the chest wall). There were no subject contraindications to TrP‐DN, such as local infection, history of immune suppression (e.g., cancer) or bleeding disorders (e.g., haemophilia), high anti‐coagulant use, pregnancy (especially first trimester), compromised or questionable equipment sterility, denied subject consent, and inadequate practitioner practical knowledge.
The subject was treated with TrP‐DN for a total of two subject visits with two days (48 hours later) between each treatment session. The following muscles were treated: Bilateral L3 and L5 multifidus and the right gluteus maximus and medius. All muscles were treated during both treatment sessions. The monofilament needle used was 60 mm in length, 0.25 mm in diameter and was held by the therapist's dominant hand. After skin inspection and disinfecting with 70% isopropyl alcohol, the needle was inserted utilizing a clean technique. The needle was inserted into the skin just above the taut band over the palpable TrP. After the needle was inserted into the skin tissue, it was directed into the muscle TrP until reaching the target muscle. The needle was manipulated up and down (inserted and withdrawn repeatedly from the TrP) in a rapid frequency at a rate of approximately 1‐2 strokes per second without fully withdrawing the needle from the skin. This “pistoning” movement of the needle within a TrP is intended to provoke a local twitch response (LTR), as well as an attempt to reproduce the subject's symptoms.
The needle insertions were repeated to elicit as many LTRs local twitch responses as possible until the therapist perceived tissue changes. As soon as the needle was pulled out of the skin, the needle insertion site was compressed firmly for a minimum of three seconds and the needle discarded into a sharps container.
For the multifidus muscles, the subject was positioned prone over a pillow, and the needle was placed just lateral to spinous process within one finger breath from the subject's midline. The needle was angled just medial to the vertical axis and perpendicular to the lamina and inserted until it touched the lamina, which was used as a “bony backdrop” to ensure that the needle reached the multifidus muscle. The index and middle fingers of the non‐dominant hand of the therapist was splayed to tension the skin. The splaying of the tissue allowed for specific placement of the needle tip and a smoother needle insertion. Bilateral L3 and L5 multifidus muscles were treated in the above manner [Figure 3]. For the gluteus maximus muscle, the subject was positioned prone with a pillow under her pelvis and abdomen. The needle was inserted into the gluteus maximus at a vertical orientation [Figure 4] and the technique manner previous described above was performed. Adequate needle depth in gluteus maximus was ensured by staying within the superficial gluteal musculature using the needle to feel for tissue texture changes. For the gluteus medius muscle, the technique is identical as the gluteus maximus except that the needle insertion angle is perpendicular to the ilium [Figure 4]. The ilium was contacted by the needle, due to the depth of the gluteus medius muscle and the technique previously described above was performed. The ilium was used as a “bony backdrop” to ensure adequate needle depth to reach the gluteus medius muscle.
Figure 3.
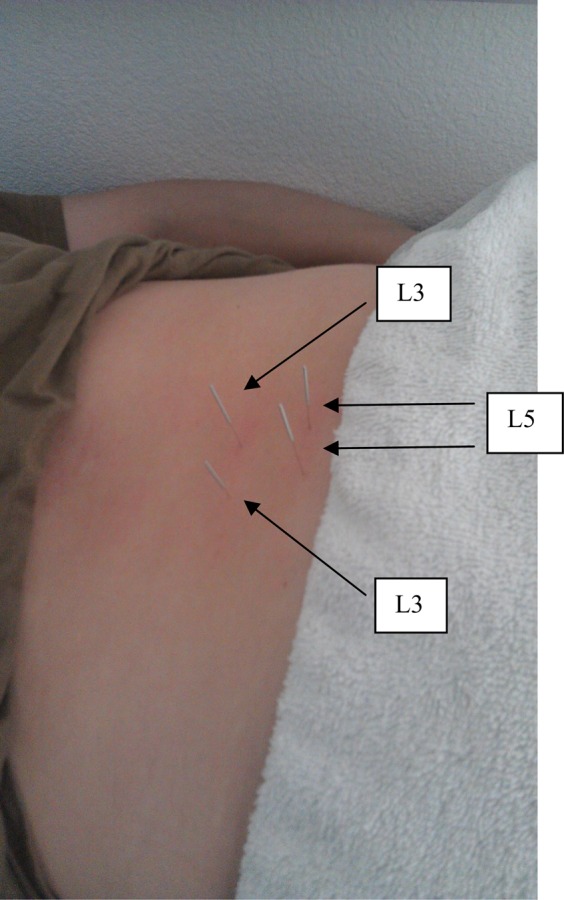
Lumbar Multifidus needle placements (L3 and L5).
Figure 4.
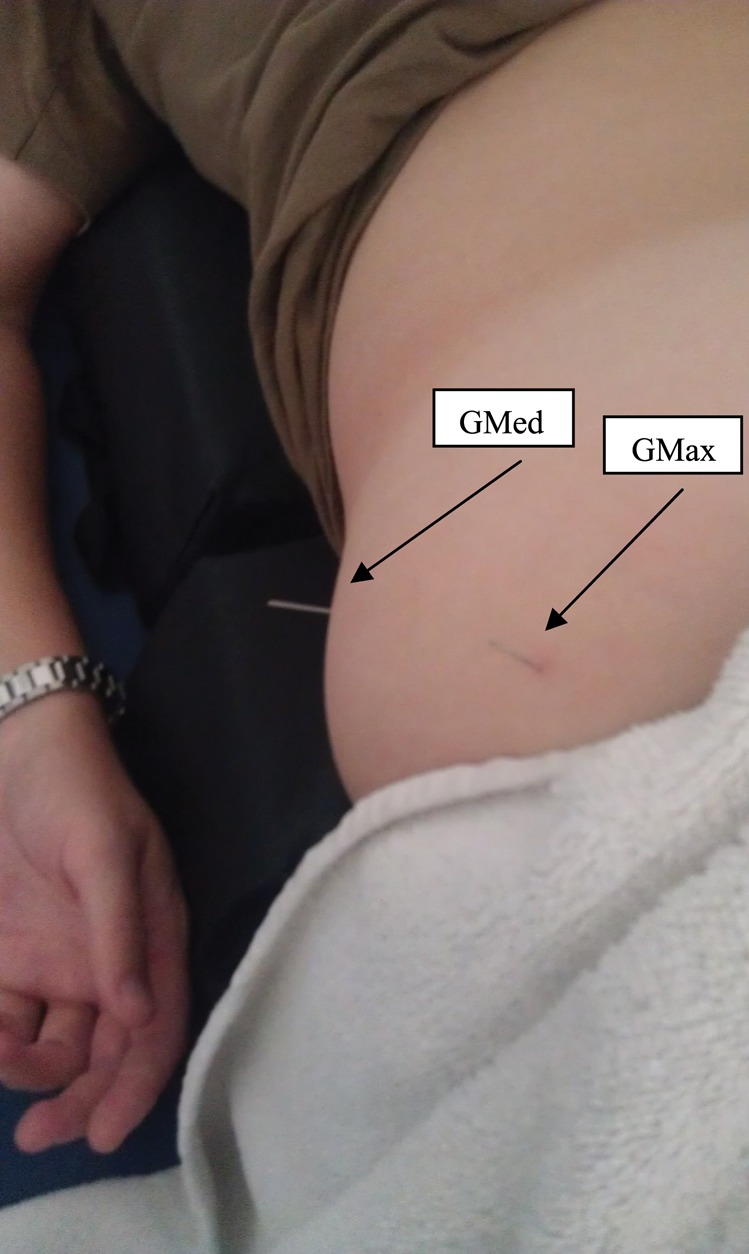
Needle placements for Gluteus Medius Gluteus Maximus.
Due to the chronic nature of the subject's symptoms, the decision was to leave the needles within the ‐target muscles [Figure 5] and use an IES unit in order to conduct an electrical impulse deep within the muscles and produce repeated muscular contractions. TrP‐DN with IES was selected in order to evoke a longer duration of muscular contraction that can be produced with traditional TrP‐DN and elicit further muscular relaxation.
Figure 5.
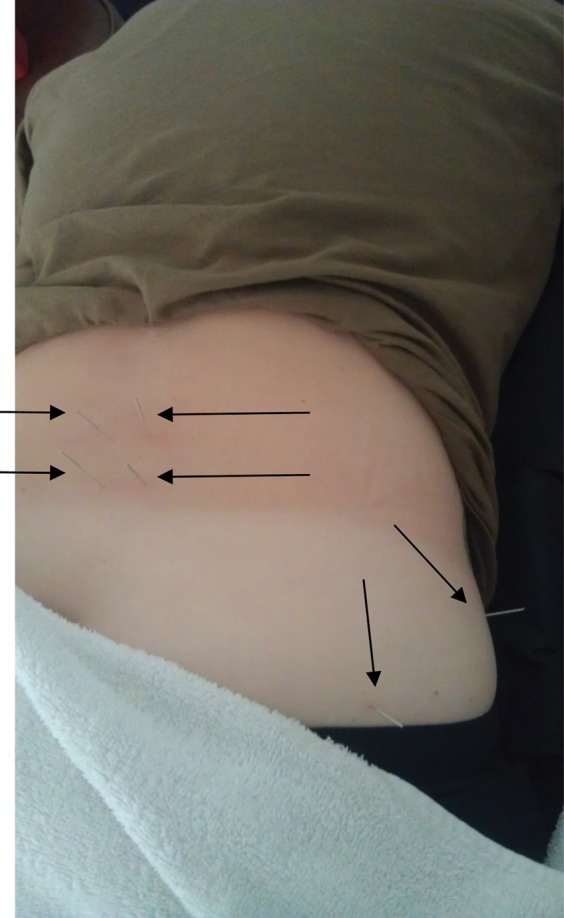
Needle placement for the Multifidi and the Gluteus Maximus/Medius.
The IES unit selected was an ES‐130 by ITO® (Japan), which is a palm‐sized three channel unit that utilizes a DC 9V battery and produces an asymmetric biphasic square waveform. This unit has six (6) leads available and uses electrode clips that attach directly onto the needle shafts. Four (4) leads were attached to the needles that were inserted into bilateral lumbar multifidus muscles at the L3 and L5 levels. The remaining two (2) leads were attached to the needles inserted into the right gluteus maximus and gluteus medius muscles. The exact unit set‐up is shown in Figure 6. The intensity was set at a level 4 and remained there throughout the treatment session. The treatment duration utilized was 20 minutes. The frequency level was set on low at level 4 (1.5 Hz) and was used throughout the treatment session. The IES parameters were at the motor level and were selected to elicit repeated muscular contraction. Identical unit set‐up and parameters, including intensity, duration, and frequency, were used during both treatment sessions.
Figure 6.
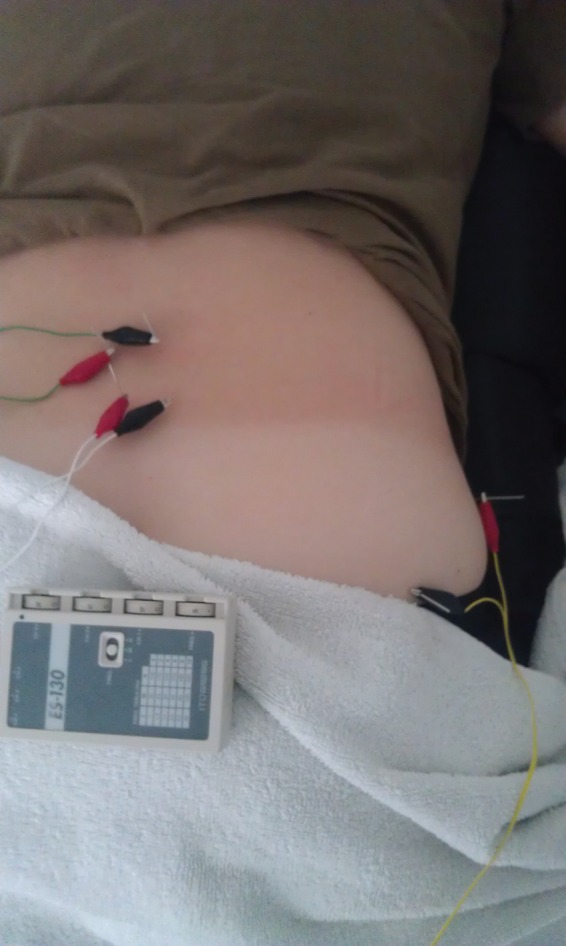
Intramuscular Electrical Stimulation Set‐up.
Using the needles as electrodes offer many advantages over more traditional transcutaneous nerve stimulation (TENS).14 Not only is the resistance of the skin to electrical currents eliminated, but several studies have demonstrated more pain relief and improved functionality than TENS in subjects with sciatica and chronic low back pain.14,62,63 N‐methyl‐D‐aspartate (NMDA) receptor on the central terminals of the dorsal root ganglion appears to be play an important role in the development of central sensitization related to persistent inflammatory pain.64 Animal experiments have shown that electrical activity can modulate the expression of NMDA in primary sensory neurons, resulting in an analgesic effect.14,64,65
Following the TrP‐DN treatment interventions, the subject was given follow‐up instructions in order to minimize complications post needling. She was instructed to rest and hydrate throughout the next 24 hours and apply ice as needed if muscle soreness or bruising occured. The subject was instructed to perform core stability corrective exercises daily (1‐2 times per day) for her home exercise program (HEP). The exercises chosen for her HEP focused on reflexive core stabilization predominately for a flexion‐based movement pattern [Figures 7‐10]. Exercise methodology followed a neurodevelopmental sequence in which lower levels of posture (e.g., supine or prone and quadruped) and core stability initiation (acting to assist the movement pattern) were advocated first until exercise competency occurred.48 Once competency occurred, exercise progression entailed utilizing higher levels of postures (half or tall kneeling and standing) and eliminating the pattern assistance.48 The corrective exercises chosen include half kneeling in‐line balance, quadruped diagonals (i.e., bird dogs), active straight leg raise with core activation, and standing single leg deadlift with neutral spine. Each exercise was performed for a 1‐minute duration and repeated for two sets or until the subject's exercise technique was unable to be maintained. Subject instruction was kept at a minimal in order to improve motor learning by reinforcing reflexive core stability corrections. See Appendix for specific descriptions of all corrective exercises.
Figure 7.
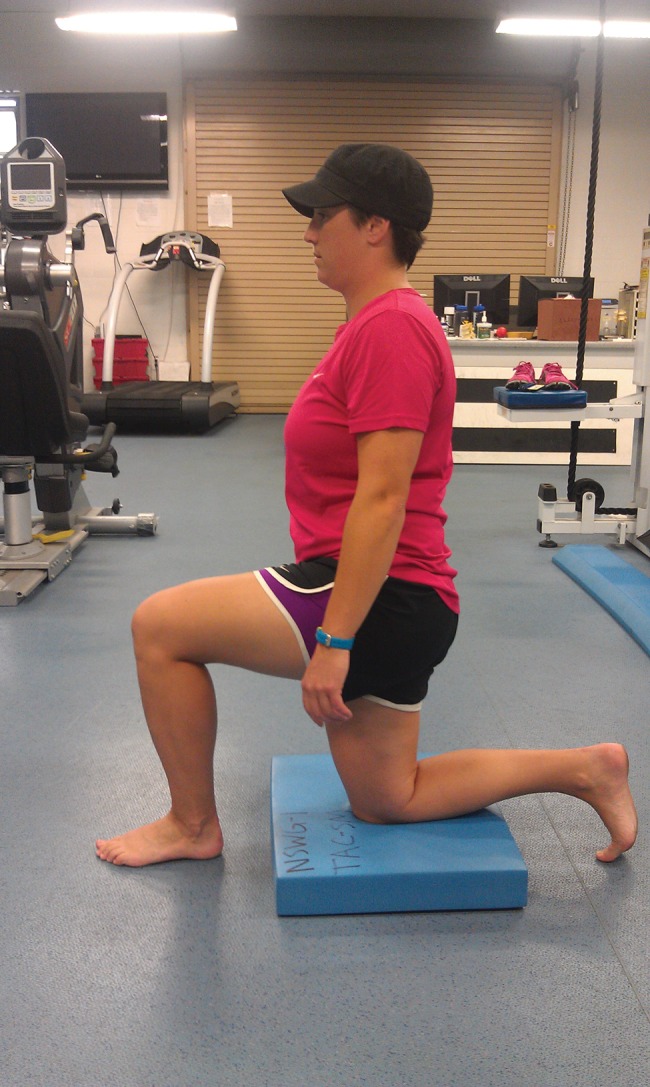
Half Kneeling In‐line Balance.
Figure 9.
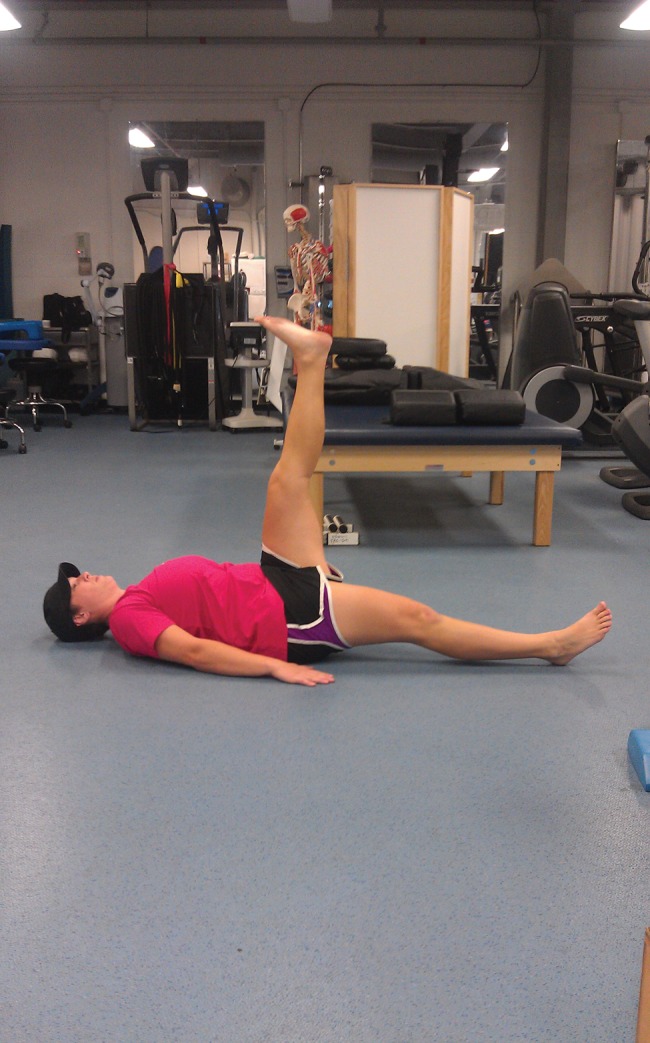
Straight Leg Raise with Core Activation.
Figure 10.

Standing Single Leg Deadlift.
OUTCOMES
Effectiveness of treatment was measured by reduction in pain and disability levels, objective range‐of‐motion measurements and provocation tests, and improvements in the subject's impressions of overall recovery and quality of life. The subject was treated twice over a 48‐hour period (with a day in between) and was followed up two days (48 hours later) ‐following treatment #1 and #2 to determine if subject outcomes were successful. A total of three (3) follow‐ups were performed with follow‐up #2 and #3 performed 14 days and 12 weeks following treatment #2 to determine if outcomes would be sustained past initial short‐term treatment periods. A 48‐hour follow‐up period was selected to evaluate the spontaneous effectiveness of TrP‐DN. Two week and 12‐week follow‐up periods were selected to determine the maintenance of short‐term subject outcomes. All assessments and treatment interventions were standardized to allow for easy replication and enhance internal validity. During follow‐up #1, the subject was assessed using the NPRS and GROC outcome measures and reexamined using the identical physical examination procedures as the initial evaluation. The subject reported a 2/10 on the NPRS, which was successful in obtaining the required 2‐point change to be clinically meaningful. The subject rated her perceived recovery on the GROC scale as “somewhat better” with exhibited score of +3.
At follow‐up #1, physical examination demonstrated the subject improving her lumbar forward flexion ROM from fingertips to mid‐tibia to fingertips to ankle joint line [Figures 11 and 12]. Functional testing was assessed and showed improvement in her overhead squat and single leg stance. Overhead squat functional testing demonstrated a bilaterally equal squatting pattern with no visual bodyweight shifting toward the left lower extremity. Single leg stance assessment demonstrated improved stability bilaterally with no asymmetrical instability noted. The single leg stance assessment was symmetrical and improved; however, mild compensatory trunk corrections were observed indicating a small, yet remaining lumbar and/or hip stability dysfunction.
Figure 11.

Multi‐segmental flexion, post‐treatment (side view).
Figure 12.
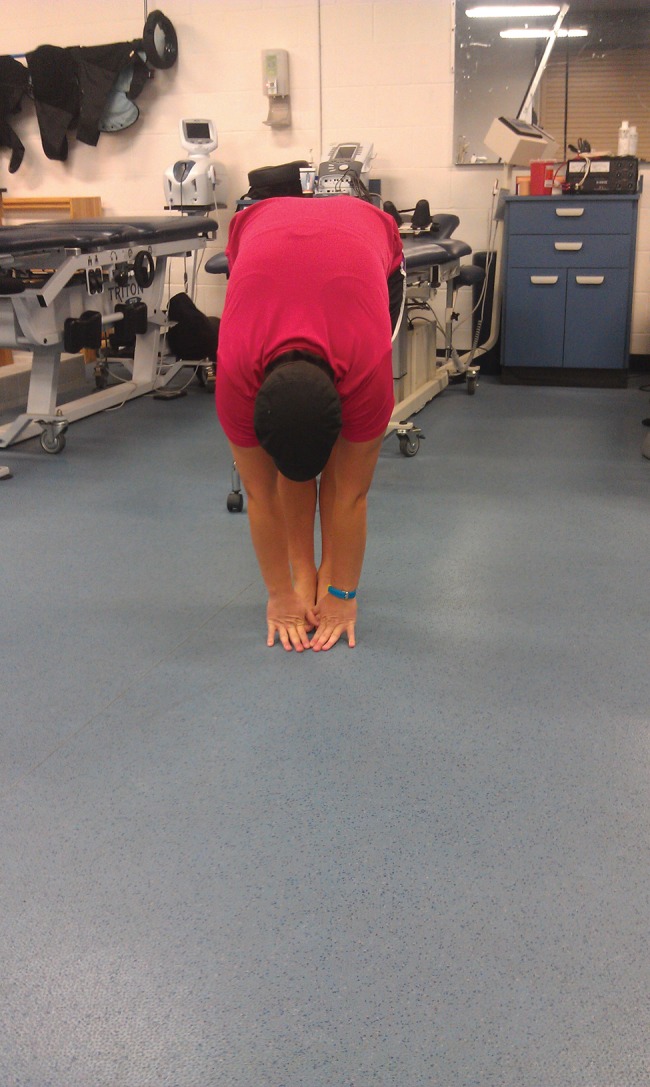
Multi‐segmental flexion, post‐treatment (front view).
Manual muscle testing of the hip musculature was improved to 5/5 hip extension on left versus 4+/5 on right; 5/5 hip abduction on left versus 4+/5 on left; and 5/5 hip external rotation bilaterally. Strength of the right hip musculature improved ½ to 1 muscle grades in all hip planes that demonstrated strength deficits at initial examination.
Provocative special testing was performed to re‐access if the subject's symptoms could be reproduced. The prone instability test was negative and lumbar spinal accessory testing utilizing a P‐A force was positive for pain provocation over the L5 segment only. The negative prone instability test and L3 to L4 segments being negative for pain provocation demonstrated that lumbar segmental stability may have improved as a result of the treatment intervention. Tenderness to palpation was significantly reduced from initial evaluation when deep manual pressure was applied to the gluteus maximus and gluteus medius muscles. The subject reported discomfort level as “mild” when questioned on rating her discomfort level from “mild”, “moderate”, or severe”. [Table 2]
Table 2.
Objective Findings.
| Initial Exam | FU#1 | FU#2 | FU#3 | |
|---|---|---|---|---|
| Toe Touch | Finger tips to mid‐tibia | Finger tips to ankle joint line | Palms to floor | Palms to floor |
| Overhead Squat | Dysfunctional lower extremity weight acceptance | Normal, functional | Normal, functional | Normal, functional |
| Single Leg Stance | Moderate asymmetrical dysfunction | Mild symmetrical dysfunction | Normal, functional | Normal, functional |
| Manual Muscle Testing |
|
|
|
|
| Prone Instability Test | Positive | Negative | Negative | Negative |
| P‐A Pain Provocation | Pain over L3‐L5 | Pain over L5 | Normal and painless | Normal and painless |
| Deep Palpation | Mod‐to‐severe with MTrPs in gluteals | Mild with MTrPs in gluteals | Normal and no MTrPs | Normal and no MTrPs |
During the final two follow‐up sessions (14 days and 12 weeks post treatment #2), the subject was re‐assessed in order to determine if subject outcomes would continue past initial short‐term treatment periods.
All outcome measures were again re‐assessed at follow‐up #2 and #3. The subject reported a 0/10 on the NPRS and A 0 score on the ODQ, indicating no existing or perceived pain and disability. GROC scale exhibited a score of +6 indicating a large perceived change in recovery. Subjects who rated their perceived recovery on the GROC scale as “a very great deal better”, “a great deal better”, or “quite a bit better” (a score of +5 or greater) at any follow‐up treatment session were considered to have experienced dramatic improvements.66
At follow‐up #2 and #3, the subject made significant progress by increasing her lumbar forward flexion ROM to being able to touch palms of her hands to the floor [Figure 7 and 8]. Overhead squat and single leg stance assessment demonstrated bilateral equality with no weight shifting or compensatory corrections noted during the assessment, suggesting normalized lumbar and/or hip stability. Manual muscle testing of the hip musculature revealed improved and normal (5/5) strength bilaterally for hip extension, abduction, and external rotation. Strength of the right hip musculature ultimately improved one full muscle grade in all hip planes that demonstrated strength deficits at initial examination. Provocative special testing was performed and compared to the last follow‐up as a re‐test to gauge continued improvements in lumbar and/or hip stability. The prone instability test and lumbar spinal accessory testing were negative for pain provocation over T10 to L5 segments. No tenderness to palpation existed when deep manual pressure was applied to the gluteus maximus and gluteus medius muscles, suggesting the elimination of what appeared to be active MTrPs previously. The subject reported adhering to her HEP throughout all reported follow‐up periods. The subject maintained all subjective and objective outcomes at the 12 week follow‐up as she did from the 14 day follow‐up. This showed that significant subject outcomes were maintained even 12 weeks post treatment intervention.
Figure 8.

Quadruped Diagonals (i.e., Bird Dogs).
DISCUSSION
The subject reported no further lumbar and/or posterior hip pain during daily activities and during functional tasks, such as prolonged sitting, bending over, or lifting objects from ground level. The subject was able to return to all her recreational activities and allow her to perform her occupational duties and responsibilities. At the two week follow‐up, the subject returned to full military active duty without any physical limitations and resumed pre‐injury activity levels, including the ability to resume all pain‐free activities.
There is not much known about the ideal treatment parameters for IES, which leads to a limitation of this case report. While IES units offer various amplitude and frequencies settings, there is limited research linking specific IES settings to the management of pain. Frequencies between 2 and 4 Hz with as high intensity as tolerable are commonly used in nociceptive pain conditions and may result in the release of endorphins and enkephalins.5 This is a single subject design, which in itself is a limitation. Studies conducted in the future should use higher sample sizes and rigorous randomized controlled methodology with measures such as blinding to reduce bias.
The subject tolerated the TrP‐DN intervention very well with no observed side effects following treatment. The subject reported a hypoalgesic effect following the TrP‐DN intervention and only complained of minimal muscle soreness that lasted approximately two (2) hours following treatment at the local site of needle penetration. Thus, there is promise regarding the use of TrP‐DN intervention with IES for the treatment of lumbar and/or hip stability dysfunction. Future research is recommended to determine if TrP‐DN intervention, with and without IES, is effective for other body regions or muscles and if long‐term subject outcomes are demonstrated.
APPENDIX
Half Kneeling In‐Line Balance – the stance or “down” thigh remains vertical. The “forward” heel was in line with the down knee. The patient held this tall posture position without too much sway.
Quadruped Diagonals (i.e., Bird Dogs) – a foam roller was placed along the curve of the lumbar spine, in order to cue patient to maintain a neutral spine during the movement. The opposite arm and leg extended outward while staying in the sagittal plane and without deviation of the trunk.
Straight Leg Raise with Core Activation – the palms of the hands were pressed into the floor to activate the core prior to initiating the movement. Both legs were kept perfectly straight and ankles dorsiflexed throughout the movement. The leg was raised as far as possible until the knee began to bend, or the “down” leg began to bend or roll out.
Standing Single Leg Deadlift – a dowel was placed along the back touching the head, thoracic spine, and sacrum. The stance leg was flexed slightly throughout the entire movement. The swing leg was hinged at the hips pushing it back as far as possible while keeping the dowel contacting all points.
References
- 1.Tough EA, White AR. Effectiveness of acupuncture/dry needling for myofascial trigger point pain. Physical Therapy Reviews. 2011;16(2):147 [Google Scholar]
- 2.Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome–evidence from a review of the literature. Clin J Pain. 2007;23:278–6 [DOI] [PubMed] [Google Scholar]
- 3.Simons DG, Travell JG, Simons LS. Travell and Simons' myofascial pain and dysfunction: the trigger point manual. Vol.1 Upper half of the body. Baltimore: MD: Lippincott Williams & Wilkins; 1999 [Google Scholar]
- 4.Dommerholt J. Muscle pain syndromes. In: Myofascial Manipulation. Cantu RI, Grodin AJ, ed. Gaithersburg, Md:Aspen; 2001:93–140 [Google Scholar]
- 5.Dommerholt J. Dry needling in orthopedic physical therapy practice. Orthop Phys Ther Pract. 2004;16(3):15–20 [Google Scholar]
- 6.Bajaj P, et al. Trigger points in patients with lower limb osteoarthritis. J Musculoskeletal Pain. 2001;9(3):17–33 [Google Scholar]
- 7.Hsueh TC, Yu S, Kuan TS, Hong CZ. Association of active myofascial trigger points and cervical disc lesions. J Formos Med Assoc. 1998;97(3):174–180 [PubMed] [Google Scholar]
- 8.Kleier DJ. Referred pain from a myofascial trigger point mimicking pain of endodontic origin. J Endod. 1985;11(9):408–411 [DOI] [PubMed] [Google Scholar]
- 9.Ling FW, Slocumb JC. Use of trigger point injections in chronic pelvic pain. Obstet Gynecol Clin North Am. 1993;20(4):809–815 [PubMed] [Google Scholar]
- 10.Mennell J. Myofascial trigger points as a cause of headaches. J Manipulative Physiol Ther. 1989;12(4):308–313 [PubMed] [Google Scholar]
- 11.Simunovic Z. Low level laser therapy with trigger points technique: a clinical study on 243 patients. J Clin Laser Med Surg. 1996;14(4):163–167 [DOI] [PubMed] [Google Scholar]
- 12.Hendler NH, Kozikowski JG.Overlooked physical diagnoses in chronic pain patients involved in litigation. Psychosomatics. 1993;34(6):494–501 [DOI] [PubMed] [Google Scholar]
- 13.Gunn CC. The Gunn Approach to the Treatment of Chronic Pain. 2nd ed New York, NY: Churchill Livingstone, 1997 [Google Scholar]
- 14.Dommerholt J, Bron C, Franssen J. Myofascial trigger points: an evidence-informed review. Journal of Manual & Manipulative Therapy. 2006;14(4):203–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons DG. Do endplate noise and spikes arise from normalvmotor endplates? Am J Phys Med Rehabil. 2001;80:134–140 [DOI] [PubMed] [Google Scholar]
- 16.Simons DG, Hong C-Z, Simons LS. Endplate potentials are common to midfiber myofascial trigger points. Am J Phys Med Rehabil. 2002;81:212–222 [DOI] [PubMed] [Google Scholar]
- 17.Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18:1803–1807 [DOI] [PubMed] [Google Scholar]
- 18.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107 [DOI] [PubMed] [Google Scholar]
- 19.Weeks VD, Travell J. How to give painless injections. In: AMA Scientific Exhibits New York, NY: Grune & Stratton, 1957:318–322 [Google Scholar]
- 20.Lucas KR, Polus BI, Rich PS. Latent myofascial trigger points: Their effect on muscle activation and movement efficiency. J Bodywork Mov Ther. 2004;8:160–166 [Google Scholar]
- 21.Gunn CC. Radiculopathic pain: Diagnosis, treatment of segmental irritation or sensitization. J Musculoskeletal Pain. 1997;5(4):119–134 [Google Scholar]
- 22.Cannon WB, Rosenblueth A. The Supersensitivity of Denervated Structures: A Law of Denervation. New York, NY: MacMillan, 1949 [Google Scholar]
- 23.Cotchett MP, Landorf KB, Munteanu SE, Raspovic AM. Consensus for dry needling for plantar heel pain (plantar fasciitis): a modified Delphi study. Acupunct Med. 2011;29:193–202 [DOI] [PubMed] [Google Scholar]
- 24.Dommerholt J, Mayoral del Moral O, Grobli C. Trigger point dry needling. In: Dommerholt J, Huijbregts P, eds. Myofascial Trigger Points: Pathophysiology and Evidence-Informed Diagnosis and Management. Sudbury, MA: Jones and Bartlett; 2010:159–90 [Google Scholar]
- 25.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16–23 [DOI] [PubMed] [Google Scholar]
- 26.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil 1994;73:256–63 [DOI] [PubMed] [Google Scholar]
- 27.Shah J, et al. A novel microanalytical technique for assaying soft tissue demonstrates significant quantitative biomechanical differences in 3 clinically distinct groups: normal, latent and active. Arch Phys Med Rehabil. 2003;84:A4 [Google Scholar]
- 28.Hong CZ. Persistence of local twitch response with loss of conduction to and from the spinal cord. Arch Phys Med Rehabil. 1994;75:12–16 [PubMed] [Google Scholar]
- 29.Hong CZ, Torigoe Y. Electrophysiological characteristics of localized twitch responses in responsive taut bands of rabbit skeletal muscle. J Musculoskeletal Pain. 1994;2:17–43 [Google Scholar]
- 30.Baldry PE. Myofascial Pain and Fibromyalgia Syndromes. Edinburgh: Churchill Livingstone; 2001 [Google Scholar]
- 31.Ernst E. Musculoskeletal conditions and complementary alternative medicine. Best practice and research clinical rheumatology. 2004;18(4):539–556 [DOI] [PubMed] [Google Scholar]
- 32.Sterling M, Valentin S, Vicenzino B, Souvlis T, Connelly LB. Dry needling and exercise for chronic whiplash: a randomized controlled trial. BMC Musculoskeletal Disorders. 2009;10:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong J, Fufa D, Gerber A, Rosman I, Vangel M, Graceley R, Gollub R: Psychophysical outcomes from a randomized pilot study of manual, electro and sham acupuncture treatment on experimentally induced thermal pain. Journal of Pain. 2005;6(1):55–64 [DOI] [PubMed] [Google Scholar]
- 34.Mclean S, Clauw D, Abelson J, Liberzon I: The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosomatic Medicine. 2005;67:783–790 [DOI] [PubMed] [Google Scholar]
- 35.Furlan A, van Tulder M, Cherkin D, Tsukayama H, Lao L, Koes B, Berman B: Acupuncture and dry needling for low back pain: an updated systematic review within the framework of the Cochrane Collaboration. Spine. 2005;30(8):944–963 [DOI] [PubMed] [Google Scholar]
- 36.Cameron MH. Physical Agents in Rehabilitation: From Research to Practice. 3rd ed St. Louis, MO: Elsevier Saunders; 2008 [Google Scholar]
- 37.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334 [DOI] [PubMed] [Google Scholar]
- 38.Farrar JT, Berling JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Syndrom Manag. 2003;25:406–411 [DOI] [PubMed] [Google Scholar]
- 39.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry disability questionnaire and the Quebec back pain disability scale. Phys Ther. 2001;81:776–88 [DOI] [PubMed] [Google Scholar]
- 40.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10: 407–415 [DOI] [PubMed] [Google Scholar]
- 41.Fritz JM, Piva S, Childs JD. Accuracy of the clinical examination to radiographic instability of the lumbar spine. Euro Spine J. (2005).14:743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dillen L, Sahrmann S, Norton B, et al. Reliability of physical examination items used for classification of patients with low back pain. Phys Ther. 1998;78:979–988 [DOI] [PubMed] [Google Scholar]
- 43.Kerr R., Cadoux-Hudson T, Adams C. The value of accurate clinical assessment in the surgical management of lumbar disc protusion. Neurol Neurosurg Psychiatry. 1988;51:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreyfuss P, Michaelsen M, Pauza K, McLarty J, Bogduk N. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594–2602 [DOI] [PubMed] [Google Scholar]
- 45.Narvani A, Tsirdis E, Kendall S, Chaudhuri R, Thomas P. A preliminary report on prevalence of acetabular labral tears in sports patients with groin pain. Knee Surg Sports Traumatol Arthrosc. 2003;11:403–408 [DOI] [PubMed] [Google Scholar]
- 46.Hoppenfeld S. Physical Examination of the Spine and Extremities. Norwalk, CT: Appleton & Lange; 1976 [Google Scholar]
- 47.Vroomen PC, de Krom MC, Knottnerus JA, Consistency of history taking and physical examination in patients with suspected lumbar nerve root involvement. Spine. 2000;25:91–96;discussion 97 [DOI] [PubMed] [Google Scholar]
- 48.Cook G. Movement: Functional Movement Systems: Screening, Assessment and Corrective Strategies. 2010, Aptos, CA: Target Publications [Google Scholar]
- 49.Browder D, Enseki K, Fritz J. Intertester reliability of hip range of motion measurements and special tests. J Orthop Sports Phys Ther. 2004;34:A115844280 [Google Scholar]
- 50.Johnson KD, Kim KM, Yu BK, Saliba SA, Grindstaff TL. Reliability of Thoracic Spine Rotation Range-of-Motion Measurements in Healthy Adults. J Athl Train. 2012. Jan-Feb; 47(1): 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kendall F, McCreary EK, Provance PG. Muscles: Testing and Function. 4th ed Baltimore MD: Lippincott, Williams & Wilkins; 1993 [Google Scholar]
- 52.Magee D. Orthopedic Physical Assessment. Philadelphia, PA: W.B. Saunders Co; 1997 [Google Scholar]
- 53.Sutlive TG, Mitchell SD, Maxfield SN, et al. Identification of individuals with patellofemoral pain whose symptoms improved after a combined program of foot orthosis use and modified activity: a preliminary investigation. Phys Ther. 2004;84:49–61 [PubMed] [Google Scholar]
- 54.Piva SR, Fitzgerald K, Irrgang JJ, et al. Reliability of measures of impairments associated with patellofemoral pain syndrome. BMC Musculoskeletal Disord. 2006;7:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bullock-Saxon J, Bullock M. Repeatability of muscle length measures around the hip. Physiother Can. 1994;46:105–109 [Google Scholar]
- 56.Reese NB, Bandy WD. Use of an inclinometer to measure flexibility of the iliotibial band using the Ober test and the modified Ober test: differences in magnitude and reliability of measurements. J Orthop Sports Phys Ther. 2003;33:326–330 [DOI] [PubMed] [Google Scholar]
- 57.Mitchell B, McCroy B, Brukner P, O'Donnell J, Colson E, Howells R. Hip joint pathology: Clinical presentation and correlation between magnetic resonance arthrography, ultrasound, and arthroscopic findings in 25 consecutive cases. Clin J Sports Med. 2003;(13):152–156 [DOI] [PubMed] [Google Scholar]
- 58.Martin RL, Sekiya JK. The interrater reliability of 4 clinical tests used to assess individuals with musculoskeletal hip pain. J Orthop Sports Phys Ther. 2008;38:71–77 [DOI] [PubMed] [Google Scholar]
- 59.Laslett M, Williams M. The reliability of selected pain provocation tests for sacroiliac pain pathology. Spine. 1994;19:1243–1249 [DOI] [PubMed] [Google Scholar]
- 60.Lastell M, Aprill CH, McDonald B, Young SB. Diagnosis of Sacroiliac Joint Pain: Validity of Individual Provocation. Manual Therapy. 2005;10:207–218 [DOI] [PubMed] [Google Scholar]
- 61.Hicks G, Fritz J, Delitto A, Mishock J, Interrater reliability of clinical examination measures for identification of lumbar segmental instability. Arch Phys Med Rehabil. 2003;84:1858–1864 [DOI] [PubMed] [Google Scholar]
- 62.Ghoname EA, Craig WF, White PF, Ahmed HE, Hamza MA, Henderson BN, Gajraj NM, Huber PJ, Gatchel RJ. Percutaneous electrical nerve stimulation for low back pain: A randomized crossover study. JAMA. 1999;281:818–823 [DOI] [PubMed] [Google Scholar]
- 63.Ghoname EA, White PF, Ahmed HE, Hamza MA, Craig WF, Noe CE. Percutaneous electrical nerve stimulation: An alternative to TENS in the management of sciatica. Pain. 1999;83:193–199 [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Zhang Y, Dai J, Yang J, Gang S. Electroacupuncture Trigger Point Dry Needling / E85 (EA) modulates the expression of NMDA receptors in primary sensory neurons in relation to hyperalgesia in rats. Brain Res. 2006;1120:46–53 [DOI] [PubMed] [Google Scholar]
- 65.Choi BT, Lee JH, Wan Y, Han JS: Involvement of ionotropic glutamate receptors in low-frequency electroacupuncture analgesia in rats. Neurosci Lett. 2005;377(3):185–188 [DOI] [PubMed] [Google Scholar]
- 66.Puentedura EJ, Landers MR, Cleland JA, Mintken P, Huijbregts P, Fernandez-De-Las-Penas C. Thoracic spine thrust manipulation versus cervical spine thrust manipulation in patients with acute neck pain: a randomized clinical trial. J Ortho Sports Phys Ther. 2011;41(4):208–220 [DOI] [PubMed] [Google Scholar]


