Abstract
Recently, it has been demonstrated that uncoupling protein-1 (UCP1)-expressing white adipocytes (brown-like adipocytes) are important for energy expenditure in white adipose tissue (WAT), in which energy expenditure decreases under obese conditions. However, the relationship between the induction of brown-like adipocytes and the decrease in energy expenditure in obese WAT remains to be elucidated. Here, we show that proinflammatory cytokines derived from activated macrophages suppress the induction of UCP1 promoter activity and mRNA expression via an extracellular signal-related kinase (ERK) in white adipocytes. The coculture with RAW264.7 (RAW) macrophages suppressed the induction of UCP1 mRNA expression by isoproterenol (ISO), a typical β-adrenergic receptor agonist, in C3H10T1/2 (10T1/2) adipocytes. A conditioned medium derived from lipopolysaccharide (LPS)-activated macrophages and tumor necrosis factor-α (TNF-α) also suppressed the induction of UCP1 mRNA but did not affect its mRNA stability. By using a luciferase reporter assay system, the conditioned medium and TNF-α also suppressed the activity of the UCP1 promoter and transcriptional factors binding to the cAMP response element (CRE). Importantly, PD98059, an ERK inhibitor, partially abrogated the suppression of UCP1 promoter activation and mRNA induction. These results indicate that ERK is an important factor in the suppression of UCP1 transcriptional activation in the interaction between white adipocytes and activated macrophages. This report suggests a possible mechanism of the UCP1 transcriptional suppression in white adipocytes associated with obese and diabetic conditions.
Keywords: TNF-α, CRE, promoter activity, ERK, inflammation
adaptive thermogenesis is a physiological process whereby energy is dissipated in the form of heat in response to external stimuli such as cold (29). It occurs primarily in brown adipose tissue (BAT). Active BAT consumes lipids to dissipate chemical energy for protection against hypothermia. This physiological response is mediated by mitochondrial uncoupling protein-1 (UCP1) in BAT. UCP1 generates heat by the uncoupling of oxidative phosphorylation, that is, by leaking protons across the mitochondrial inner membrane without ATP production, resulting in the release of chemical energy as heat (30).
Recent data have indicated that adult humans contain significant deposits of UCP1-expressing adipocytes that can be detected by positron emission tomography (PET)-scanning methods, particularly in the supraclavicular and neck region (10, 39, 40) and that the suppression of their activation is associated with the accumulation of body fat with age (31, 44). BAT activation in animals is associated with the suppression of weight gain and a healthy phenotype (3, 6, 8, 13, 18). In contrast, the loss of BAT function leads to obesity and metabolic diseases (11, 20). Many studies have demonstrated that changes in BAT activity can profoundly affect glucose and lipid metabolism and body weight.
There are two distinct types of brown adipocytes: classical brown adipocytes that arise from muscle-like type progenitors (33), and UCP1-expressing, brown-like adipocytes that emerge in most white adipose tissue (WAT) under certain physiological and pharmacological conditions (2, 9, 12, 24). Notably, brown-like adipocytes increase oxygen consumption rate (OCR) in response to norepinephrine stimulation (26) and are abundant in an obesity-resistant strain of mice (1, 13). In addition, the emergence of this type of cell in WAT correlates with improved insulin sensitivity (6, 34). These reports suggest a crucial role of brown-like adipocytes in the regulation of energy metabolism.
Energy expenditure decreases in obese WAT (7, 41, 42). However, there is little knowledge about the relationship between the reduction in energy expenditure and the induction of brown-like adipocytes in WAT. In obese WAT, chronic inflammation occurs, leading to metabolic disorders (14, 16). Macrophages infiltrate WAT via monocyte chemoattractant protein-1 (MCP-1), which is released from hypertrophied adipocytes in obese WAT. The infiltrated macrophages are activated by free fatty acid (FFA) released by the hypertrophied adipocytes, resulting in the secretion of a variety of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α; 36, 43). We postulated that these proinflammatory cytokines derived from the activated macrophages are one of the inhibitory factors for the induction of brown-like adipocytes, particularly the induction of UCP1 expression in WAT, leading to a decrease in energy expenditure in obese WAT.
In this study, we investigated whether proinflammatory cytokines derived from activated-macrophages can suppress UCP1 mRNA induction in C3H10T1/2 (10T1/2) adipocytes.1 10T1/2 adipocytes derived from mouse mesenchymal stem cells were used as a model of brown-like adipocytes, in which UCP1 mRNA is induced by isoproterenol (ISO), a typical β-adrenergic receptor (β-AR) agonist. The stimulation of coculture with RAW264.7 (RAW) macrophages or treatment with a conditioned medium from lipopolysaccharide (LPS)-activated RAW macrophages suppressed UCP1 promoter activation and mRNA induction but did not affect its mRNA stability in 10T1/2 adipocytes. In addition, treatment with conditioned medium and TNF-α, a proinflammatory cytokine, partially suppressed the activation and induction via an extracellular signal-related kinase (ERK)-mediated pathway. These results suggest that the suppression of UCP1 expression in obese WAT is due to inflammation and partially to TNF-α derived from activated macrophages.
MATERIALS AND METHODS
Chemicals and cell culture.
(−)−Isoproterenol hydrochloride (ISO) and LPS were purchased from Sigma (St. Louis, MO). TNF-α was purchased from Peprotech (Rocky Hill, NJ). PD98059 was purchased from Calbiochem (San Diego, CA). Forskolin from Coleus forskohlii (FSK) and actinomycin D were purchased from Nacalai Tesque (Kyoto, Japan).
C3H10T1/2 (10T1/2) (Dainippon Sumitomo Pharma, Osaka, Japan), RAW264.7 macrophages (RAW) (RIKEN BioResource Center, Tsukuba, Japan) were cultured in DMEM with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL) at 37°C under a humidified 5% CO2 atmosphere, as previously described (37). The differentiation of 10T1/2 precursors, a mouse mesenchymal stem cell line, was induced by treatment with adipogenic agents (0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone, 10 μg/ml insulin, and 1 μM troglitazone) in DMEM containing 10% FBS for 2 days after the cells reached confluence (day 0). Then, the medium was replaced with DMEM containing 10% FBS and 5 μg/ml insulin every 2 days.
Then, the cells were serum-starved and maintained in the absence or presence of TNF-α at the indicated concentrations for different time periods as indicated. After that, the cells were cultured with or without ISO (10 μM). To inhibit ERK, cells were treated with the MEK1 inhibitor PD98059 at 20 μM.
Adipocytes and macrophages were cocultured in a contact system as previously described (15). In brief, RAW macrophages (5 × 105 cells/ml) were plated onto dishes with serum-starved 10T1/2 adipocytes. After 16 h, the coculture was incubated in serum-free DMEM with or without ISO at 10 μM for 8 h. As the control, adipocytes and macrophages, the numbers of which were equal to those in the contact system, were cultured separately and mixed after harvest.
RAW macrophages were cultured with or without DMEM containing 5 μg/ml LPS for 12 h and then, in serum-free DMEM for 24 h. Both media were collected as the control conditioned medium (Cont-CM) and LPS-conditioned medium (LPS-CM), respectively, and stored at −20°C until use, as previously described (15). Then, 10T1/2 adipocytes were pretreated with each conditioned medium for 16 h. After that, the cells were cultured with or without ISO (10 μM) for 8 h. To neutralize TNF-α in the LPS-CM, aliquots of LPS-CM were preincubated at 37°C for 60 min with 10 μg/ml neutralizing antibody against TNF-α (eBioscience, San Diego, CA; TN3–19, 10 μg/ml or the same concentration of isotype control). Then, 10T1/2 adipocytes were incubated in the resultant media for 16 h before ISO treatment (10 μM) for 8 h.
RNA preparation and quantification of gene expression.
Total RNA was prepared from cultured cells using Sepasol (R)-RNA I Super (Nacalai Tesuque) in accordance with the manufacturer's protocol. Total RNA was reverse-transcribed using M-MLV reverse transcriptase (Promega, WI) in accordance with the manufacturer's instructions using a thermal cycler (Takara PCR Thermal Cycler SP, Takara, Shiga, Japan). To quantify mRNA expression, real-time RT-PCR was performed with a LightCycler System (Roche Diagnostics, Mannheim, Germany) using SYBR Green fluorescence signals as described previously (37). The oligonucleotide primers were designed using a PCR primer selection program in the website of the Virtual Genomic Center from the GenBank database. The primers used for measuring mRNA expression levels of genes (upstream and downstream, respectively) were ACTGCCACACCTCCAGTCATT and CTTTGCCTCACTCAGGATTGG for mouse UCP1 mRNA; CCCTGCCATTGTTAAGACC and TGCTGCTGTTCCTGTTTTC for mouse PPARγ coactivator-1α (PGC1α) mRNA; AGCCCATGTAACCAGCACCGGA and CAGTCGCAC TGGCTCAGGAC for mouse type II iodothyronine deiodinase (Dio2) mRNA; TGTGTGTCTGCAGATCGGGTAC and CTTTGGCGGGATTAGTCGAAG for mouse 36B4 mRNA. To compare mRNA expression levels among the samples, the copy numbers of all transcripts were divided by that of mouse 36B4 showing a constant expression level in adipocytes. All mRNA expression levels are represented as a ratio relative to that of the control in each experiment. The mRNA expression level of 36B4 was stable under all conditions in 10T1/2 adipocytes.
mRNA stability assay.
The half-life of UCP1 mRNA was determined by treating 10T1/2 adipocytes with actinomycin D to block transcription. 10T1/2 adipocytes were cultured under ISO for 8 h, and then serum-starved DMEM, Cont-CM, and LPS-CM with actinomycin D (10 μg/ml) were added. Under these conditions, the cells were cultured for 3 and 6 h, total RNA was prepared, and real-time PCR was performed to quantify the amounts of UCP1 mRNA and 36B4 mRNA at each time point. The amount of UCP1 mRNA was corrected using the amount of 36B4 mRNA.
Plasmids and luciferase assay.
The 3.8-kb portion of the 5′-flanking region of the mouse UCP1 gene was obtained by PCR amplification using primers with a restriction enzyme site [forward primer with Xho I, 5′-CCGCTCGAGCGGTCCATTGGCCTCAAACCCTATGAG (the new Xho I site is underlined); reverse primer with BamHI 5′-CGGGATCCCGAGGCGTGAGTGCAAGAACAAAAGG (the new BamHI site is underlined)]. The luciferase reporter plasmid tk-LUC, which contains the Herpes simplex virus thymidine kinase (HSV-TK) promoter downstream of a multiple cloning site, was a gift from Dr. Yasutomi Kamei (Tokyo Medical and Dental University). The PCR product was digested with BamHI and Xho I and then ligated into the Sal I/BamHI sites of tk-LUC. We named the plasmids containing the mouse UCP1 promoter “pUCP1-pro-Luc”. The pCRE-TK-luciferase reporter plasmid (pCRE-Luc) was a kind gift from Dr. Yasutomi Kamei. The cloned RAW macrophages with the green fluorescence protein (GFP) reporter plasmids containing the mouse TNF-α promoter (TNF-α-pro-GFP/RAW) were previously described (32). We changed the FP reporter gene GFP to DD-tdTomato in the reporter plasmid and established the cloned RAW macrophages with the plasmid, which was named TNF-α-pro-DD-tdTomato/RAW. The detection and quantification of the FP signals were previously described (32). Undifferentiated 10T1/2 cells were transfected with plasmids using LipofectAMINE reagent (Invitrogen) in accordance with the manufacturer's instructions. In the luciferase assay, pUCP1-pro-Luc (5 μg/dish) or pCRE-Luc (5 μg/dish) and pRL-CMV (50 ng/dish) were transfected into undifferentiated 10T1/2 cells cultured on 10-cm culture dishes. Four hours after the transfection, the transfected cells were cultured on 96-well tissue plates. The cells were pretreated with TNF-α or Cont- or LPS-CM and then the cells were incubated with FSK at 20 μM or ISO at 10 μM for 8 h. The cells were lysed for luciferase assay performed using a Dual-Luciferase Reporter Gene Assay system (Promega) in accordance with the manufacturer's protocol.
Measurement of inflammatory mediators.
The amount of nitric oxide (NO) released was measured using Griess reagent, as previously described (15). In brief, 100 μl of supernatant were mixed with an equal volume of Griess reagent [1:1 (vol/vol) composed of 0.1% N-1-naphthyl-ethylenediamine in distilled water and 1% sulfanilamide in 5% phosphoric acid] on a 96-well flat-bottom plate. The absorbance at 550 nm was measured after 10 min (using a microplate reader; Bio-Rad). The amount of NO was calculated from a standard curve plotted using known concentrations of NaNO2.
Immunoblotting.
Total cellular proteins were solubilized in lysis buffer [50 mM Tris·HCl, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS (pH 7.4), and a protease inhibitor cocktail]. The protein concentration of the samples was determined using a protein assay kit (Bio-Rad). Protein samples (45 μg) were subjected to SDS-PAGE on a 10% gel. Separated proteins were transferred electrophoretically to PVDF membranes (Millipore), which were blocked with 5% nonfat dried milk in phosphate-buffered saline. The membranes were incubated with antibodies to anti-p44/42 MAPK (ERK1/2) (Cell Signaling Technology), anti-phospho-p44/42 MAPK (ERK1/2) (Cell Signaling Technology) and then with peroxidase-conjugated anti-mouse and anti-rabbit IgG antibodies (Santa Cruz), respectively. Proteins were detected using an ECL Western blotting detection system (GE Healthcare).
Statistical analyses.
Means of two groups were compared by Student's t-test. To compare means of several groups, one-way ANOVA analysis of variance was used, followed by Tukey-Kramer's multiple-comparison test. The data are expressed as means ± SE. Differences were considered statistically significant at P < 0.05. The experiments described here were repeated several times with similar results in each case. The results shown are representative of the results from all experiments.
RESULTS
Coculture with and conditioned medium derived from RAW macrophages suppressed the increase in the level of UCP1 mRNA expression induced by ISO in 10T1/2 adipocytes.
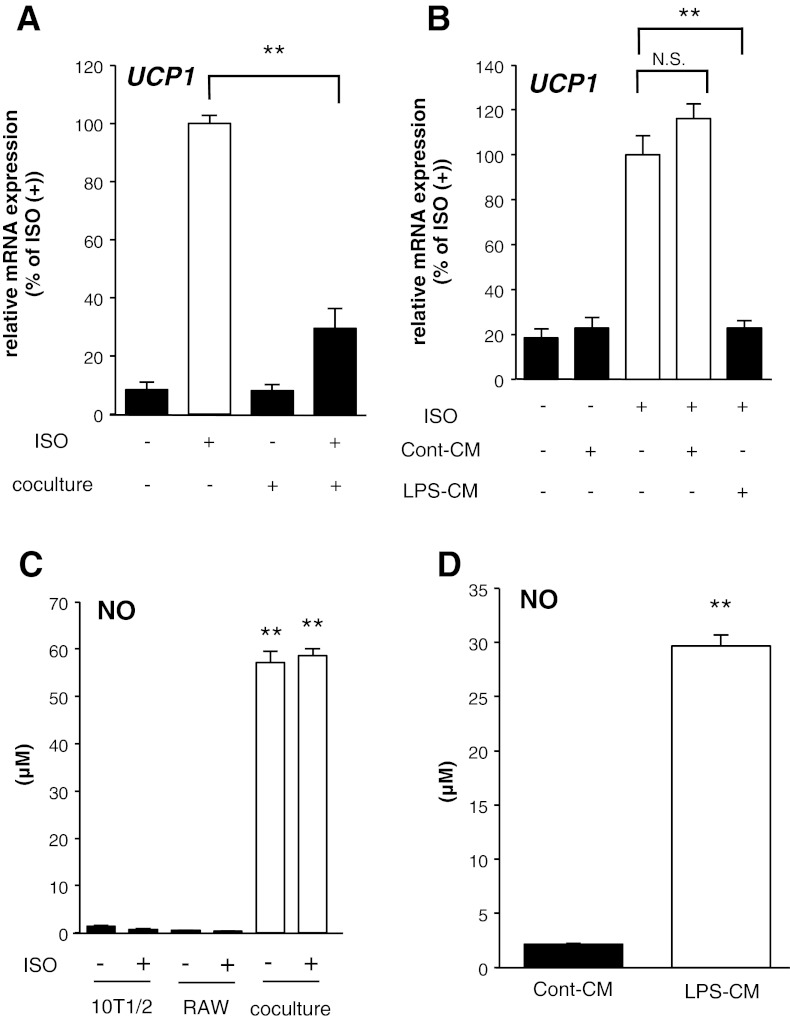
To determine the effects of inflammation in obese WAT on the mRNA expression level of UCP1 in white adipocytes, a coculture system of 10T1/2 adipocytes and RAW macrophages was used. As shown in Fig. 1A, 8 h after treatment with ISO, mRNA expression levels of UCP1 significantly increased in 10T1/2 adipocytes. However, coculture with RAW macrophages for 16 h suppressed the increase by 70.5%.
Fig. 1.
Coculture with and conditioned medium (CM) derived from activated RAW macrophages suppressed the increase in UCP1 mRNA expression in C3H10T1/2 (10T1/2) adipocytes. A: mRNA expression levels of UCP1 in 10T1/2 adipocytes cocultured with RAW macrophages for 16 h before isoproterenol (ISO) treatment (10 μM) for 8 h. Values are means ± SE for 4 wells. **P < 0.01, compared with ISO-stimulated 10T1/2 adipocytes. B: mRNA expression levels of UCP1 in 10T1/2 adipocytes incubated with CM from nonactivated macrophages (Cont-CM) or CM from LPS-activated macrophages (LPS-CM) for 16 h before ISO treatment (10 μM) for 8 h. Values are means ± SE for 3–4 wells. **P < 0.01, compared with ISO-stimulated 10T1/2 adipocytes. NS, statistically not significant. C: nitric oxide (NO) concentrations in the coculture system of 10T1/2 adipocytes and RAW macrophages. Values are means ± SE for 3–4 wells. **P < 0.01, compared with nonstimulated 10T1/2 adipocytes. D: NO concentrations in Cont-CM or LPS-CM when these media were harvested from RAW macrophages, respectively. Values are means ± SE for 4 wells. **P < 0.01, compared with Cont-CM.
To exclude the possible involvement of cell-to-cell contact in the suppression of UCP1 mRNA induction, we used a conditioned medium (CM) derived from LPS-activated RAW macrophages. Eight hours after the ISO treatment together with the control CM derived from nonactivated RAW macrophages (Cont-CM), there was no effect on the induction of UCP1 mRNA. However, CM derived from LPS-activated RAW macrophages (LPS-CM) significantly suppressed the induction by 77% (Fig. 1B). NO production, a general inflammation indicator, was increased by the coculture and in the LPS-CM, indicating that the experimental conditions were sufficient to induce inflammation (Figs. 1, C and D). These findings suggest that proinflammatory cytokines derived from activated macrophages suppressed the ISO-dependent induction of UCP1 mRNA in 10T1/2 adipocytes.
Inflammation suppressed the activation of the UCP1 promoter in 10T1/2 adipocytes.
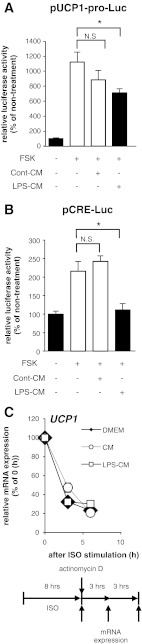
To examine the activity of the UCP1 promoter under inflammatory condition, we measured the activity of a 3.8-kb mouse UCP1 promoter using luciferase assay. Forskolin (FSK), which directly increases intracellular cAMP amounts, increased the luciferase activity of a reporter plasmid (pUCP1-pro-Luc). The increase was suppressed by treatment with LPS-CM but not with Cont-CM (Fig. 2A). Under the same conditions, the activity of transcriptional factors binding to the cAMP response element (CRE) such as the CRE binding protein (CREB) and activating transcription factor-2 (ATF2) was also suppressed by the LPS-CM treatment (Fig. 2B); these are downstream transcriptional factors following the increase in intracellular cAMP amounts, leading to the activation of the UCP1 promoter (5). To determine whether the change in UCP1 mRNA expression levels is due to transcriptional regulation, we examined the degradation of UCP1 mRNA under inflammatory conditions. 10T1/2 adipocytes were treated with actinomycin D, a blocker of mRNA synthesis, in serum-free DMEM, Cont-CM, or LPS-CM after ISO stimulation. The amount of UCP1 mRNA thereafter was measured by a real-time PCR technique. We detected no significant difference between the half-lives of UCP1 mRNA in 10T1/2 adipocytes incubated in any medium for 3 and 6 h after ISO stimulation (Fig. 2C). These data strongly suggest that the activity of the UCP1 promoter is suppressed by inflammatory conditions in 10T1/2 adipocytes.
Fig. 2.
Inflammation suppressed the ISO-dependent activation of the UCP1 promoter in 10T1/2 cells. Luciferase activity levels derived from the construct with UCP1 promoter (A) and cAMP response element (CRE; B) in undifferentiated 10T1/2 cells incubated with Cont-CM or LPS-CM for 16 h before forskolin (FSK) treatment (20 μM) for 8 h. Values are means ± SE for 3–5 wells. *P < 0.05, compared with FSK-stimulated undifferentiated 10T1/2 cells. C: mRNA expression level of UCP1 in 10T1/2 adipocytes cultured under serum-free DMEM or Cont-CM or LPS-CM with actinomycin D (10 μg/ml). Total RNA samples were collected every 3 h. Quantitative analysis of UCP1 mRNA stability by real-time PCR. Comparison of UCP1 mRNA levels in 10T1/2 adipocytes at each time point was achieved by normalization to 36B4 mRNA.
TNF-α was involved in the suppression of UCP1 promoter activity.
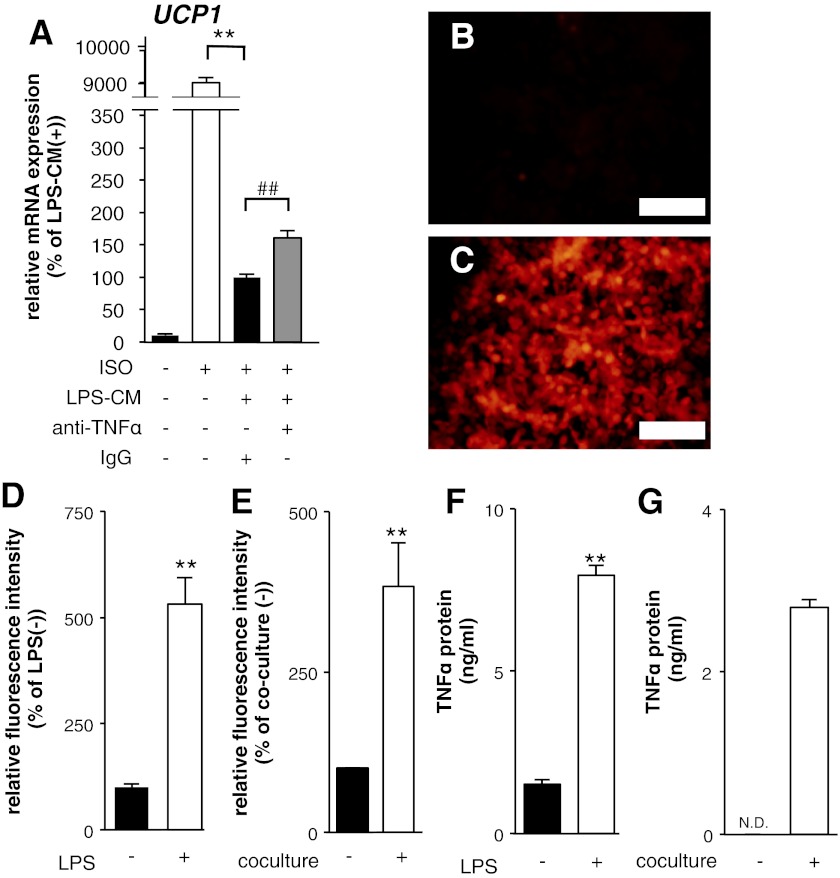
In the inflammation suppressing the induction of UCP1 mRNA expression, we focused on TNF-α, which is involved in metabolic disorders including insulin resistance and atherosclerosis due to inflammation in obese WAT (14). As shown in Fig. 3A, neutralizing antibodies against TNF-α partially suppressed the inhibitory effects of LPS-CM on UCP1 mRNA induction. In addition, the reporter fluorescence protein expression driven by the mouse TNF-α promoter was induced by LPS treatment in RAW macrophages (Fig. 3, B–D). Similar to the LPS treatment, the coculture induced the reporter expression, suggesting that these stimulations increase TNF-α promoter activity in RAW macrophages (Fig. 3E). The protein levels of TNF-α in the medium were increased by both the LPS stimulation and the coculture (Fig. 3, F and G). These results indicate that LPS stimulation and coculture increase the expression levels of TNF-α in RAW macrophages, suggesting a possibility that TNF-α is involved in the inhibitory effects of LPS-CM on UCP1 mRNA induction, although the involvement is partial.
Fig. 3.
TNF-α was involved in the suppression of the UCP1 promoter activity in 10T1/2 adipocytes. A: mRNA expression levels of UCP1 in 10T1/2 adipocytes cultured with LPS-CM with or without neutralizing antibody against TNF-α for 16 h before the ISO treatment (10 μM) for 8 h. Values are means ± SE for 4 wells. **P < 0.01, compared with ISO-stimulated 10T1/2 adipocytes. ##P < 0.01, compared with ISO and LPS-CM-stimulated 10T1/2 adipocytes. B and C: photographs of TNF-α promoter-DD-tdTomato reporter-transfected RAW macrophages. B: nonstimulated cells as controls. C: cells stimulated with LPS (5 μg/ml) for 16 h. Scale bars, 200 μm. D and E: detection of fluorescence intensities derived from reporter-transfected RAW macrophages stimulated with LPS (5 μg/ml) for 16 h (D) and cocultured with 10T1/2 adipocytes for 16 h (E). Values are means ± SE for 3–4 wells. **P < 0.01, compared with LPS (−) or coculture (−). F and G: concentrations of TNF-α protein in the medium derived from LPS-activated RAW macrophages (F) and the coculture (G). Values are means ± SE for 4 wells. **P < 0.01, compared with LPS (−) or coculture (−). ND, statistically not detected.
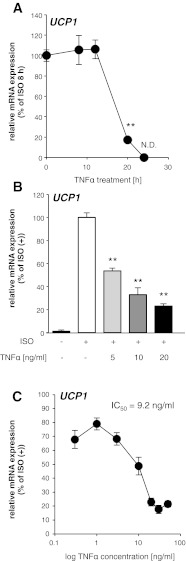
To examine the TNF-α effects on mRNA expression levels of UCP1 induced by ISO, we treated 10T1/2 adipocytes with TNF-α at various preincubation periods in combination with a constant-period ISO and TNF-α treatment (8 h). The mRNA expression of UCP1 induced by ISO was suppressed in a preincubation time-dependent manner (Fig. 4A). The simultaneous treatment with ISO and TNF-α as well as the TNF-α addition 4 h before the ISO treatment (12-h TNF-α treatment) did not suppress mRNA induction of UCP1. However, TNF-α addition 12 h before (20-h TNF-α treatment) significantly suppressed mRNA expression by 82.8%.
Fig. 4.
TNF-α treatment suppressed UCP1 mRNA induction in 10T1/2 adipocytes in a time- and dose-dependent manner. A and B: time-dependent (A) and dose-dependent (B) effects of the TNF-α treatment on UCP1 mRNA induction in 10T1/2 adipocytes. Values are means ± SE for 3–4 wells. **P < 0.01, compared with ISO-stimulated 10T1/2 adipocytes. C: concentration-response curve for the suppression of UCP1 mRNA induction in 10T1/2 white adipocytes after 20 h of exposure to 0.3, 1, 3, 10, 20, 30, and 50 ng/ml of TNF-α. Each data point represents the mean of data from 3 wells with error bars representing the SE.
Then, to examine details of the TNF-α effects on UCP1 mRNA suppression, we treated 10T1/2 adipocytes with TNF-α at various concentrations for 20 h (pretreatment with TNF-α, 12 h; treatment with ISO and TNF-α 8 h). The TNF-α treatment suppressed mRNA induction of UCP1 in a dose-dependent manner (Fig. 4B). The induction of UCP1 was significantly suppressed at 5 ng/ml TNF-α. According to the TNF-α concentration-response curve for the suppression of the UCP1 mRNA induction (Fig. 4C), the IC50 of TNF-α was 9.2 ng/ml. These data indicate that, at least, TNF-α is involved in the suppression of the UCP1 mRNA induction in 10T1/2 adipocytes. The mRNA expression levels of cAMP-induced genes such as PGC1α and Dio2 were not suppressed by TNF-α treatment (data not shown). Therefore, it is likely that the suppressive effects of TNF-α are specific in the UCP1 mRNA induction.
ERK activation induced by TNF-α mediated the suppression of UCP1 mRNA induction.
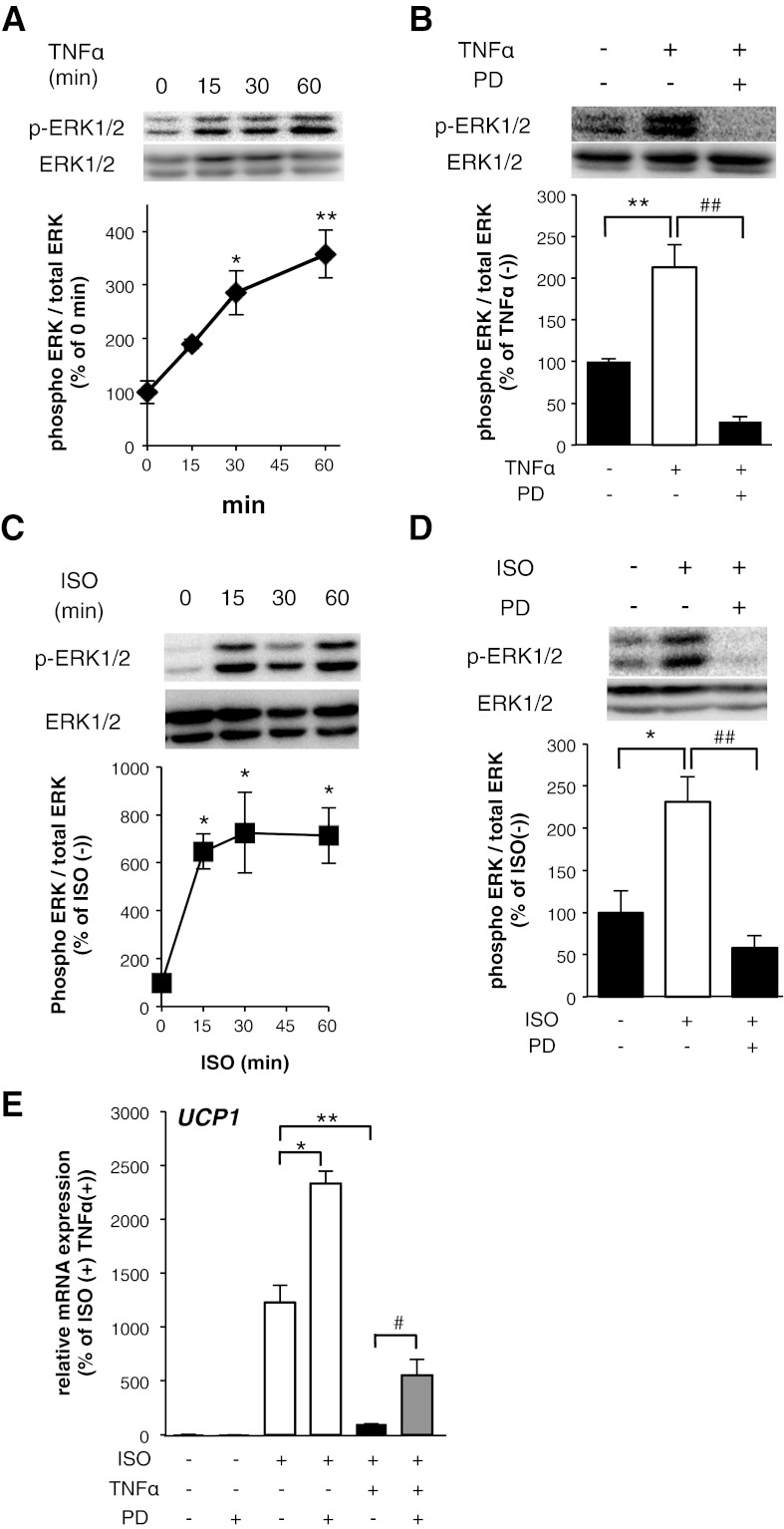
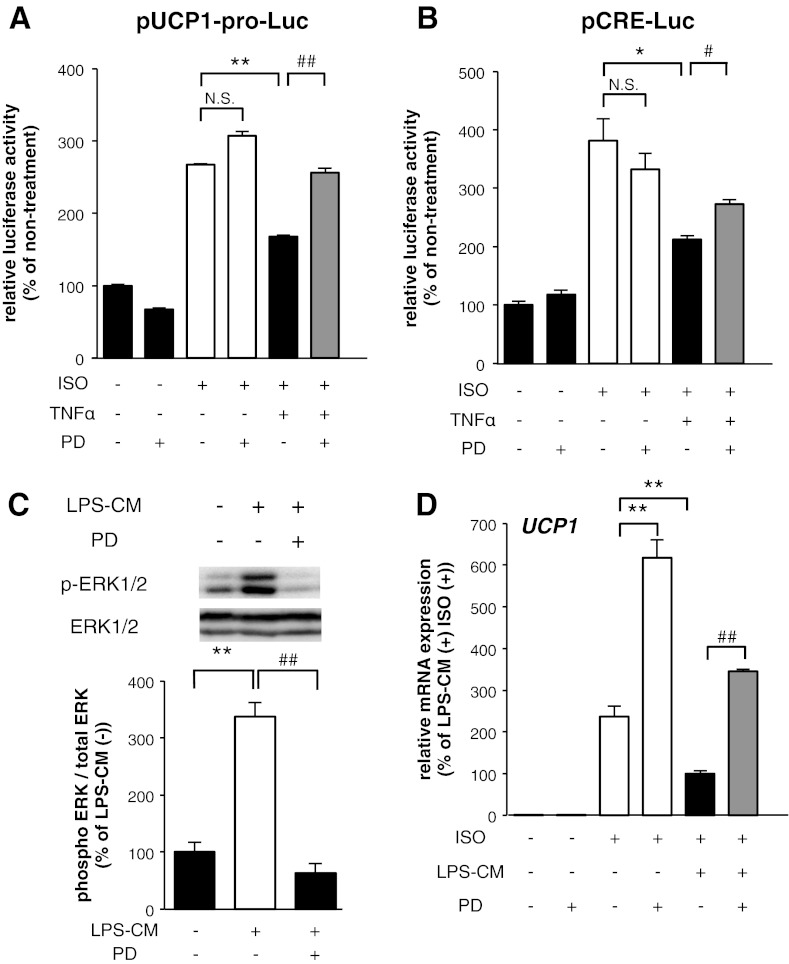
We elucidated signaling events by which TNF-α suppresses the induction of UCP1 mRNA expression. Proinflammatory cytokines activate MAPKs such as ERKs, c-Jun NH2-terminal kinase (JNK), and p38MAPK, leading to multiple signal transductions in adipocytes (4, 14). Moreover, ERK activation downregulates UCP1 expression in brown adipocytes (38). Western blot analysis using phosphospecific antibodies revealed that ERK phosphorylation was enhanced within 15 min of the treatment of 10T1/2 adipocytes with 10 ng/ml TNF-α, as shown in Fig. 5A. This TNF-α-induced activation was prevented by the treatment with an ERK inhibitor, PD98059 (Fig. 5B). The ISO treatment also enhanced ERK phosphorylation, and the activation was prevented by the PD98059 treatment in 10T1/2 adipocytes (Fig. 5, C and D). The PD98059 treatment with TNF-α increased the ISO-induced UCP1 mRNA expression, compared with the induction in TNF-α-treated 10T1/2 adipocytes, although single PD treatment also enhanced the ISO-induced UCP1 mRNA expression (Fig. 5E). To determine whether ERK is involved in the regulation of the UCP1 promoter by TNF-α, we performed transient transfection experiments in undifferentiated 10T1/2 cells with pUCP1-pro-Luc. The TNF-α treatment suppressed the ISO-dependent activation of the UCP1 promoter and the suppression was abrogated by PD98059 treatment, although PD98059 treatment did not significantly increase the ISO-induced UCP1 promoter activation (Fig. 6A). A similar effect of PD98059 was observed in the FSK-induced activation of the UCP1 promoter (data not shown). Under the same conditions, the suppression of activities in CRE binding transcriptional factors by TNF-α was partially also abrogated by PD98059 treatment (Fig. 6B). Finally, to investigate the involvement of ERK in the suppression of UCP1 mRNA induction, we incubated 10T1/2 adipocytes in LPS-CM with PD98059 and then measured the mRNA expression level of UCP1 induced by ISO. As shown in Fig. 6C, LPS-CM treatment enhanced ERK phosphorylation and the activation was prevented by PD98059 treatment in 10T1/2 adipocytes. The PD98059 treatment with LPS-CM increased the ISO-induced UCP1 mRNA expression, compared with the induction in LPS-CM-treated 10T1/2 adipocytes, although single PD treatment also increased ISO-induced UCP1 mRNA expression (Fig. 6D). Together, these data strongly suggest that ERK activation suppresses the induction of UCP1 mRNA expression and that TNF-α-induced ERK activation is partially involved in the suppression of UCP1 mRNA induction.
Fig. 5.
ERK activation induced by TNF-α mediated the suppression of UCP1 mRNA induction in 10T1/2 adipocytes. Total cell lysates (45 μg protein/lane) were examined by Western blot assay using the indicated antibodies. 10T1/2 adipocytes were exposed to TNF-α for the indicated times (A) and with PD98059 (PD) simultaneously for 15 min (B). 10T1/2 adipocytes were exposed to ISO for the indicated times (C) and with PD98059 simultaneously for 15 min (D). The band intensities were quantified using ImageJ software. The results were calculated using data of independent experiments repeated multiple times. *P < 0.05, **P < 0.01, compared with nonstimulated 10T1/2 adipocytes. ##P < 0.01, compared with TNF-α- or ISO-stimulated 10T1/2 adipocytes. E: mRNA expression levels of UCP1 in 10T1/2 adipocytes cultured with TNF-α with or without PD98059 for 12 h before ISO treatment (10 μM) for 8 h. Values are means ± SE for 3–4 wells. *P < 0.05, **P < 0.01, compared with ISO-stimulated 10T1/2 adipocytes. #P < 0.05, compared with ISO and TNF-α-stimulated 10T1/2 adipocytes.
Fig. 6.
ERK activation induced by TNF-α was involved in the suppression of UCP1 promoter activation in 10T1/2 adipocytes. A and B: luciferase activity levels derived from the construct with UCP1 promoter (A) and CRE (B) in undifferentiated 10T1/2 cells incubated with TNF-α with or without PD98059 for 16 h before ISO treatment (10 μM) for 8 h. Values are means ± SE for 3–5 wells. *P < 0.05, **P < 0.01, compared with ISO-stimulated undifferentiated 10T1/2 cells. #P <0.05, ##P < 0.01, compared with ISO and TNF-α-stimulated undifferentiated 10T1/2 cells. C: 10T1/2 adipocytes were exposed to LPS-CM with PD98059 simultaneously for 15 min. The band intensities were quantified using ImageJ software. The results were calculated using data of independent experiments repeated multiple times. **P < 0.01, compared with nonstimulated 10T1/2 adipocytes. ##P < 0.01, compared with LPS-CM-stimulated 10T1/2 adipocytes. D: mRNA expression level of UCP1 in 10T1/2 adipocytes incubated with LPS-CM with or without PD98059 for 16 h before ISO treatment (10 μM) for 8 h. Values are means ± SE for 3–4 wells. **P < 0.01, compared with ISO-stimulated 10T1/2 adipocytes. ##P < 0.01, compared with LPS-CM and ISO-stimulated 10T1/2 adipocytes.
DISCUSSION
In the present study, we established an experimental system for evaluating mouse UCP1 promoter activity and its mRNA expression in differentiated 10T1/2 adipocytes. The brown-like adipocytes emerge in most WAT upon prolonged cold exposure in vivo (2, 9, 12, 24). In the in vivo experimental systems, it is difficult to identify which factors are important for the induction of UCP1 expression in white adipocytes, because cold exposure increases many factors such as the growth of blood vessels (19) and sympathetic nerve fibers in WAT (22). On the other hand, in vitro systems have revealed the molecular mechanisms of UCP1 expression in white adipocytes. The experimental system we used in this study may be useful for elucidating the mechanisms of transcriptional regulation of UCP1 induction. However, we could not detect the protein expression of UCP1 in 10T1/2 adipocytes. That might be because the expression levels of PRD1-BF1-RIZ1 homologous-domain-containing protein 16 (PRDM16), which is an important factor for UCP1 expression, are lower in 10T1/2 adipocytes than immortalized BAT cell lines (35). Further improvements are required to detect UCP1 protein expression in 10T1/2 adipocytes or other cell lines.
In the present study, we showed that humoral factors derived from LPS-activated RAW macrophages suppress the induction of UCP1 mRNA in 10T1/2 adipocytes. Moreover, we also demonstrated that LPS stimulation and coculture with 10T1/2 adipocytes increased TNF-α production from RAW macrophages and that the suppression of UCP1 mRNA induction by LPS-CM was partially mediated by TNF-α. Under severe obese conditions, macrophages infiltrate WAT and are activated by chemokines and FFA derived from hypertrophied adipocytes. The activated macrophages then secrete various proinflammatory cytokines such as TNF-α (36, 43). TNF-α has been indicated to be a potent inhibitor of insulin-induced adipocyte-specific gene expression in white adipocytes (14). Some of these TNF-α effects have been due to the impairment of insulin signals, leading to insulin resistance in adipocytes (14). Our results suggest that proinflammatory cytokines including TNF-α derived from activated macrophages suppress the induction of UCP1 mRNA expression, leading to energy expenditure in white adipocytes. A major cell type that produces proinflammatory cytokines in obese adipose tissue is macrophages, but hypertrophied adipocytes also produce proinflammatory cytokines (36). Therefore, the proinflammatory cytokines derived from these adipocytes could suppress the inductions of UCP1 expression in vivo. Under healthy obese conditions induced by a short-term, 2-days of high fat diet (HFD) feeding, the UCP1 expression has been shown to increase in WAT (17). Inflammation is not induced in such obese conditions, because macrophages significantly infiltrate WAT due to a long-term, several weeks of HFD-feeding (43). Therefore, the UCP1 expression might be dependent on the duration of HFD feeding, although further investigations are needed.
In the present study, PD98059, an ERK inhibitor, partially abrogated the suppression of UCP1 promoter activation and the luciferase activity derived from a CRE-luciferase reporter plasmid in the TNF-α-treated 10T1/2 adipocytes. These results suggest that TNF-α suppresses the activity of transcriptional factors binding to CREs such as CREB and ATF2 in the UCP1 promoter via ERK activation. Our results in white adipocytes coincide with previous results of brown adipocytes. There is a report that an injection of TNF-α suppresses UCP1 expression in BAT (21). In addition, gene disruption of TNF-α receptors reduced the rate of brown adipocyte apoptosis and increased the expression levels of the β3-adrenergic receptor and UCP1 in obese mice (25, 28). In primary culture brown adipocytes, TNF-α suppresses UCP1 expression via ERK activation, although p38 is a positive regulator of UCP1 expression (38) and the relationship between UCP1 transcriptional regulation and JNK activation remains controversial (27, 45). Moreover, a constitutively active form of Ras, an upstream factor of ERK, suppresses mRNA expression levels of UCP1, but dominant interfering Ras causes increased UCP1 expression in brown adipocytes (23). In our study, PD98059 treatment with TNF-α or LPS-CM reverted the suppression of UCP1 mRNA induction, although single PD98059 treatment enhanced ISO-induced UCP1 mRNA expression (Figs. 5E and 6D). This is because ISO enhanced ERK phosphorylation as well as TNF-α and LPS-CM (Fig. 5C), and PD98059 treatment also inhibited the ISO-induced ERK activation (Fig. 5D). Together, these data suggest that ERK activation suppresses UCP1 mRNA expression and that ERK activation induced by TNF-α or LPS-CM treatment is partially involved in the suppression of UCP1 mRNA induction in 10T1/2 adipocytes. The present study is the first report showing that ERK is involved in the suppression of UCP1 induction in white adipocytes.
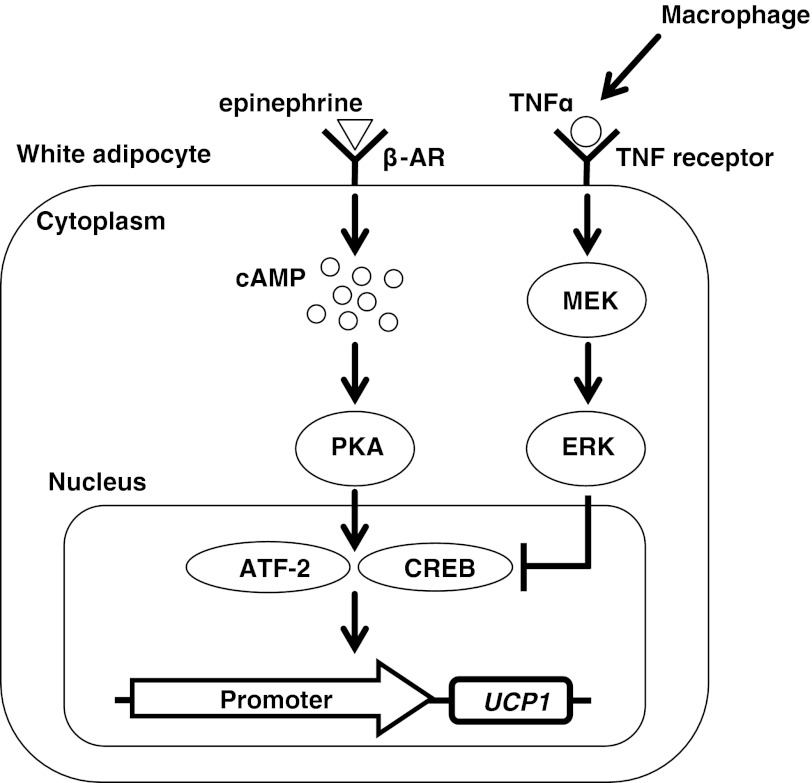
In conclusion, inflammation induced by activated RAW macrophages could suppress the induction of UCP1 expression in white adipocytes via ERK activation in 10T1/2 adipocytes. We identified TNF-α as a possible proinflammatory cytokine suppressing the UCP1 expression (Fig. 7). This report suggests a possible mechanism of UCP1 transcriptional suppression in white adipocytes associated with obese and diabetic conditions. Elucidating such a mechanism will provide clues for not only understanding the physiological and pathological regulation of the UCP1 expression in WAT but also developing a new treatment for the decrease in energy expenditure under obese conditions.
Fig. 7.
Inflammation induced by activated macrophages has inhibitory effects on UCP1 transcriptional activation via ERK activation in white adipocytes. Activated macrophages produce proinflammatory cytokines including TNF-α. TNF-α can suppress UCP1 transcriptional activation induced by β-adrenergic receptor (β-AR)-PKA signaling. ERK activation induced by TNF-α is partially involved in the suppression of the UCP1 mRNA induction.
GRANTS
This work was largely supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (nos. 22228001, 22380075, and 24229).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.S. and N.T. conception and design of the research; T.S. and Y.S. performed the experiments; T.S., N.T., S.N., and T.G. analyzed the data; T.S., N.T., S.N., R.Y., T.G., and T.K. interpreted the results of the experiments; T.S. prepared the figures; T.S. drafted the manuscript; N.T., S.N., R.Y., T.G., and T.K. edited and revised the manuscript; T.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Sayoko Shinoto for secretarial assistance and Yukiko Tada and Takahiro Nitta for technical assistance.
Footnotes
This article is the topic of an Editorial Focus by Narasimham L. Parinandi and Ulysses J. Magalang (25a).
REFERENCES
- 1. Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci USA 104: 2366– 2371, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244– E1253, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200– 205, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 87: 51– 56, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24: 3057– 3067, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106: 563– 573, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49: 784– 791, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 25: 9383– 9391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103: 931– 942, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509– 1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203– 209, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord 21: 465– 475, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 102: 412– 420, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367– 377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirai S, Kim YI, Goto T, Kang MS, Yoshimura M, Obata A, Yu R, Kawada T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci 81: 1272– 1279, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860– 867, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab 14: 658– 670, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 96: 2914– 2923, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim S, Honek J, Xue Y, Seki T, Cao Z, Andersson P, Yang X, Hosaka K, Cao Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat Protoc 7: 606– 615, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Lowell BB, SSusulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740– 742, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Masaki T, Yoshimatsu H, Chiba S, Hidaka S, Tajima D, Kakuma T, Kurokawa M, Sakata T. Tumor necrosis factor-alpha regulates in vivo expression of the rat UCP family differentially. Biochim Biophys Acta 1436: 585– 592, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat 214: 171– 178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murholm M, Dixen K, Hansen JB. Ras signalling regulates differentiation and UCP1 expression in models of brown adipogenesis. Biochim Biophys Acta 1800: 619– 627, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, Kawada T, Saito M. Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic beta 3-adrenergic agonist. J Clin Invest 97: 2898– 2904, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nisoli E, Briscini L, Giordano A, Tonello C, Wiesbrock SM, Uysal KT, Cinti S, Carruba MO, Hotamisligil GS. Tumor necrosis factor alpha mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc Natl Acad Sci USA 97: 8033– 8038, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a. Parinandi NL, Magalang UJ. Avatars of adipose tissue: the saga of transformation of white fat, the villain into brown fat, the protector. Focus on “Inflammation induced by RAW macrophages suppresses the UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes.” Am J Physiol Cell Physiol (January 30, 2013) doi:10.1152/ajpcell.00022.2013 [DOI] [PubMed] [Google Scholar]
- 26. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153– 7164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Porras A, Valladares A, Alvarez AM, Roncero C, Benito M. Differential role of PPAR gamma in the regulation of UCP-1 and adipogenesis by TNF-alpha in brown adipocytes. FEBS Lett 520: 58– 62, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, Hirabara S, Boschero AC, Curi R, Velloso LA. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 284: 36213– 37222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31– 35, 1979 [DOI] [PubMed] [Google Scholar]
- 30. Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes 53: S130– S135, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526– 1531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakamoto T, Yamaguchi Y, Goto T, Takahashi N, Kawada T. An in vitro analysis system using a fluorescence protein reporter for evaluating anti-inflammatory effects in macrophages. Biosci Biotechnol Biochem 75: 1582– 1587, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961– 967, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96– 105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab 61: 38– 54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 25: 2062– 2068, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Takahashi N, Kawada T, Goto T, Yamamoto T, Taimatsu A, Matsui N, Kimura K, Saito M, Hosokawa M, Miyashita K, Fushiki T. Dual action of isoprenols from herbal medicines on both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett 514: 315– 322, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Valladares A, Roncero C, Benito M, Porras A. TNF-alpha inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett 493: 6– 11, 2001 [DOI] [PubMed] [Google Scholar]
- 39. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500– 1508, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518– 1525, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281– 1289, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wlodek D, Gonzales M. Decreased energy levels can cause and sustain obesity. J Theor Biol 225: 33– 44, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821– 1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 19: 1755– 1760, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Yubero P, Barberá MJ, Alvarez R, Viñas O, Mampel T, Iglesias R, Villarroya F, Giralt M. Dominant negative regulation by c-Jun of transcription of the uncoupling protein-1 gene through a proximal cAMP-regulatory element: a mechanism for repressing basal and norepinephrine-induced expression of the gene before brown adipocyte differentiation. Mol Endocrinol 12: 1023– 1037, 1998 [DOI] [PubMed] [Google Scholar]