Abstract
Previously we demonstrated that viral-mediated increased expression of the anti-inflammatory cytokine interleukin-10 within the paraventricular nucleus of the hypothalamus significantly reduces blood pressure in normal rats made hypertensive by infusion of angiotensin II. However, the exact cellular locus of this interleukin-10 action within the paraventricular nucleus is unknown. In the present study we tested whether interleukin-10 exerts direct effects at its receptors located on hypothalamic neurons to offset the neuronal excitatory actions of angiotensin II via its type 1 receptors. The results indicated the presence of immunoreactive interleukin-10 receptors on neurons in normal rat paraventricular nucleus and that receptors for this cytokine were also expressed in neurons cultured from the hypothalamus. Patch-clamp electrophysiological recordings from these cultures revealed that extracellular application of interleukin-10 alone did not exert any alterations in neuronal membrane delayed rectifier or transient potassium currents. However, angiotensin II elicited a significant decrease in delayed rectifier potassium current, an effect that was abolished by interleukin-10 application. Since decreases in delayed rectifier potassium current contribute to increased neuronal excitability, this result is consistent with a direct inhibitory action of interleukin-10 on angiotensin-induced excitation of hypothalamic neurons. As such, these data are the first indication of a neuronal locus of action of interleukin-10 to temper the actions of angiotensin II in the hypothalamus.
Keywords: angiotensin II, neuron, interleukin-10, cytokine, potassium current
it is well known that the central nervous system (CNS) has an important role in the regulation of arterial blood pressure (14, 17). This regulation involves interplay between neural circuits that include connections among hypothalamic [paraventricular nucleus (PVN)], brain stem (rostral ventrolateral medulla), and spinal cord (intermediolateral column) sites to ultimately modulate sympathetic outflow and blood pressure (7, 17, 21). Critical to this regulation is the peptide angiotensin II (ANG II), which can elicit increases in sympathetic outflow and blood pressure in normotensive rats by acting via its type 1 receptors (AT1R) in the PVN (9, 11, 36). Further, in animal models of neurogenic hypertension such as the spontaneously hypertensive rat, it is clear that overactivity of ANG II/AT1R in the PVN makes a significant contribution to the raised blood pressure seen in these animals (2, 10, 16, 18). Until recently, investigations focused on the direct neuronal actions of ANG II via AT1R in the PVN and whether these effects are enhanced in hypertension (5, 6, 23). However, studies from our group and others indicate that there is another facet to the enhanced actions of ANG II in the PVN under conditions of chronically enhanced sympathoexcitation such as neurogenic hypertension and heart failure, namely a neuroimmune component (4, 15, 20, 29, 33). Specifically, our data indicate that during neurogenic hypertension there is ANG II-induced activation of microglia and production of inflammatory cytokines (PIC) in the PVN that contribute to the raised blood pressure, possibly via a paracrine stimulatory action on nearby sympathetic efferent neurons (29, 30).
The actions of PIC are normally counterbalanced in normal animals by anti-inflammatory cytokines (AIC) such as interleukin-10 (IL-10), which are secreted concomitantly from microglia and astroglia (1, 24, 25). Indeed, increased expression of IL-10 in the brain produced by intracerebroventricular gene transfer reduced hypothalamic inflammation during heart failure (33). In addition, studies from our group indicated that increased expression of IL-10 within the PVN reduces blood pressure in normal rats made hypertensive by infusion of ANG II but did not alter the blood pressure of normotensive control rats (29). However, the exact locus of this IL-10 action, whether it is at microglia or astroglia to prevent PIC synthesis (1, 24, 25) or directly at PVN sympathetic neurons to reduce their discharge, is unknown. The results presented in the current study provide the first evidence to support the latter scenario, demonstrating that while IL-10 does not alter basal K+ current in isolated hypothalamic neurons, it blocks the ANG II-induced decrease in delayed rectifier K+ current (IKv). Since decreases in IKv contribute to increased neuronal excitability, this result is consistent with a direct inhibitory action of IL-10 on ANG II-induced neuronal excitation.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (SD) rats or timed (E13–15) pregnant female SD rats were obtained from Charles River Laboratories (Wilmington, MA). Rats were kept on a 12:12-h light/dark cycle in a climate-controlled room. Rat chow (Harlan Teklad) and water were provided ad libitum. All animal use protocols were approved by the Institutional Animal Care and Use Committee of the University of Florida.
Chemicals
IL-10 was purchased from ProSpec-Tany TechnoGene (Ness Ziona, Israel) and ANG II, PD123319, poly-l-lysine hydrobromide, plasma-derived horse serum, and other basic chemicals were obtained from Sigma-Aldrich Chemical (St. Louis, MO). DMEM was purchased from Invitrogen (Carlsbad, CA). Anti-IL-10 receptor (R)α antibody (M-20) and the corresponding blocking peptide [IL-10Rα (M-20) P] were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse HuC/D neuronal protein monoclonal antibody (clone 16A11) was from Life Technologies (Carlsbad, CA). Mouse anti-NeuN monoclonal antibody was from Chemicon International (Temecula, CA). Alexa Fluor 594 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR). Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) was from Vector Labs (Burlingame, CA).
Preparation of Neuronal Cultures
Neuronal cultures were prepared from the hypothalamus of newborn SD rats exactly as detailed previously (31). Cells were dissociated from this area, pooled, and resuspended in DMEM containing 10% plasma-derived horse serum and were cultured in poly-l-lysine-precoated plastic culture dishes for 10–17 days before use.
Immunostaining
PVN.
Immunoreactivity for IL-10R and the neuronal specific protein HuC/D was assessed within the PVN in brain sections as follows. Rats were deeply anesthetized with isoflurane, brains were perfused transcardially in situ with 0.9% saline containing 4% paraformaldehyde and then cryoprotected in 30% sucrose for 1 wk. Coronal sections (30 μm) were cut through the PVN and washed with PBS. The sections were blocked in 10% goat serum for 15 min, washed in PBS for 30 min, and then incubated overnight at 4°C in either mouse monoclonal anti-HuC/D (1:20) or rabbit polyclonal anti-IL-10Rα (1:100) antibody. Sections were then washed in PBS three times for 10 min and incubated for 1 h at room temperature with secondary antibodies, Alexa Fluor 594 goat anti-mouse IgG (1:500) or Alexa Flour 488 goat anti-rabbit IgG (1:500). Separate consecutive sections were treated as above with the following exceptions: 1) inclusion of the blocking peptide IL-10Rα (M-20) P (30-fold higher concentration than the anti-IL-10Rα antibody) to test the specificity of the primary antibody; and 2) omission of the rabbit polyclonal anti-IL-10R primary antibody to determine if this negated the observed IL-10R staining. Sections were mounted on glass slides with coverslips using Vectashield mounting medium with DAPI.
Cell cultures.
Immunoreactivity for IL-10R and the neuronal specific protein NeuN was assessed within hypothalamic neurons in culture as follows. Neuronal cultures were washed briefly with ice cold PBS and then fixed for 10 min with ice-cold 4% paraformaldehyde. The fixed cells were incubated with PBS containing 0.3% Triton X-100 (Sigma-Aldrich) for 20 min to improve antibody penetration. Goat serum (5%) in PBS was added to the dish for 30 min at 37°C to reduce nonspecific binding, followed by an additional wash with PBS. Immunostaining using mouse monoclonal anti-NeuN (1:200) or rabbit polyclonal anti-IL-10Rα (1:100) antibodies was carried out as described above for brain tissue. Following the immunostaining procedures, a drop of Vectashield mounting medium with DAPI was placed on each dish, followed by a coverslip.
Brain sections containing the PVN and hypothalamic cultures that underwent immunostaining procedures were examined at room temperature with an Olympus BX41 fluorescence microscope with ×10/0.3 UPlanFLN and ×40/0.75 UPlanFLN objectives. Digital images were captured using an Olympus DP71 microscope digital camera and processed with DP Manager software.
Real-Time RT-PCR
IL-10R and GAPDH mRNA levels in the PVN or cerebral cortex were analyzed via quantitative real-time RT-PCR. The PVN was dissected as detailed previously (19). Total RNA was isolated from the PVN or cerebral cortex using RNeasy kits (Qiagen, Valencia, CA). Isolated RNA underwent DNase I (Bio-Rad, Hercules, CA) treatment to remove genomic DNA, and 25 ng of the purified RNA were used to perform cDNA synthesis (Iscript cDNA synthesis kits; Bio-Rad) in a PCR machine (Applied Biosystems, Foster City, CA). Synthesized cDNA were used to perform real-time RT-PCR by using specific primers and probes using an ABI-PRISM 7000 sequence detection system (Applied Biosystems). Data for IL-10R were normalized to GAPDH mRNA.
Single Cell Real-Time RT-PCR
IL-10R gene expression in single hypothalamic neurons in culture was assessed by single cell real-time RT-PCR. Single cell contents from cultured hypothalamic neurons were collected as previously described (19). Briefly, presterilized glass electrodes were filled with lysis buffer from an Ambion Single Cell-to-CT kit (Life Technologies, Grand Island, NY) and were then used to obtain whole cell patches on hypothalamic neurons in culture. The intracellular contents (∼4–5 μl) were drawn into the tip of the patch pipette by applying negative pressure and were then transferred to RNase/DNase-free tubes. The volume in each tube was brought up to 10 μl by adding Single Cell DNase I/Single Cell Lysis solution, and then the contents were incubated at room temperature for 5 min. Following cDNA synthesis by performing reverse transcription in a thermal cycler (25°C for 10 min, 42°C for 60 min, and 85°C for 5 min), IL-10R gene expression primers were mixed with preamplification reaction mix based on the instructions from the kit (95°C for 10 min, 14 cycles of 95°C for 15 s, 60°C for 4 min, and 60°C for 4 min). The products from the preamplification stage were used for the real-time RT-PCT reaction (50°C for 2 min, 95°C 10 min, and 40 cycles of 95°C for 5 s and 60°C for 1 min). The products from the real-time RT-PCR were separated by electrophoresis on a 3% agarose gel containing 1 μl/ml ethidium bromide.
Electrophysiological Recordings
Electrophysiological recordings of delayed rectifier K+ current (IKv), A-type K+ current (IA), and total outward K+ current (IKv + IA) were made using whole cell patch-clamp procedures in the voltage-clamp mode, as detailed previously (19, 32, 35). Experiments were performed at room temperature (23–24°C) with an Axopatch 200B amplifier and a Digidata 1200B interface (Axon Instruments, Burlingame, CA). Patch electrodes were pulled (Flaming/Brown P-97, Sutter Instrument, Novato, CA) from borosilicate glass (Friedrich & Dimmock). Data acquisition and analyses were performed using pClamp 8.0. Cells were bathed in modified Tyrode's solution containing (in mM) 137 NaCl, 5.4 KCl, 1.35 CaCl2·2H2O, 2.0 MgSO4·7H2O, 0.3 NaH2PO4, 10 HEPES, 10 dextrose, 0.3 CdCl2, and 0.001 tetrodotoxin, pH adjusted to 7.4 with NaOH. Neurons in the culture dish (volume 2 ml) were superfused at a rate of 1–2 ml/min. The patch electrodes had resistances of 2–4 MΩ when filled with an internal pipette solution containing (in mM) 130 KCl, 2 MgCl2, 0.25 CaCl2 3 ATP, 0.1 GTP, 8 dextrose, 5 EGTA, and 10 HEPES, pH adjusted to 7.2 with KOH. The whole cell configuration was formed by applying negative pressure to the patch electrode. Whole cell capacitance was compensated 70–80%; series resistance (Rs) was compensated 40–60% with Axopatch 200B compensation circuitry. Leak current was subtracted online using the P/4 method. Cell capacitance was determined in voltage-clamp mode using the “Membrane Test” function in the ClampEx software (v 8.0) software utility. The average cell capacitance for neurons used in this study was 26.43 ± 1.74 pF (range from 15 to 40 pF). Standard recording conditions for IKv were elicited by stepping to a conditioning voltage of −40 mV from the holding potential of −60 mV for 300 ms; the membrane was then depolarized from −40 to +60 mV in increments of 10 mV for 200 ms. A voltage of +60 mV produced the largest peak current in each recording. Standard recording conditions for total outward K+ current were elicited by stepping from a holding potential of −120 to +60 mV in increments of 10 mV for 200 ms. IA was derived by subtraction (total outward K+ current −IKv). Data are expressed as current density (current amplitude divided by its capacitance; pA/pF). ANG II and IL-10 were applied via a gravity fed pipette that was placed ∼100 μm from the cell. For the ANG II experiments, the angiotensin type 2 receptor (AT2R) blocker PD123319 (0.001 mM) was included in the extracellular perfusate.
Statistical Analyses
Results are expressed as means ± SE. Analyses were performed with Student's t-test when comparing only two groups or with ANOVA followed by Bonferroni analysis for multiple comparisons. Differences were considered significant at P < 0.05.
RESULTS
Localization of IL-10R on Neurons in the PVN
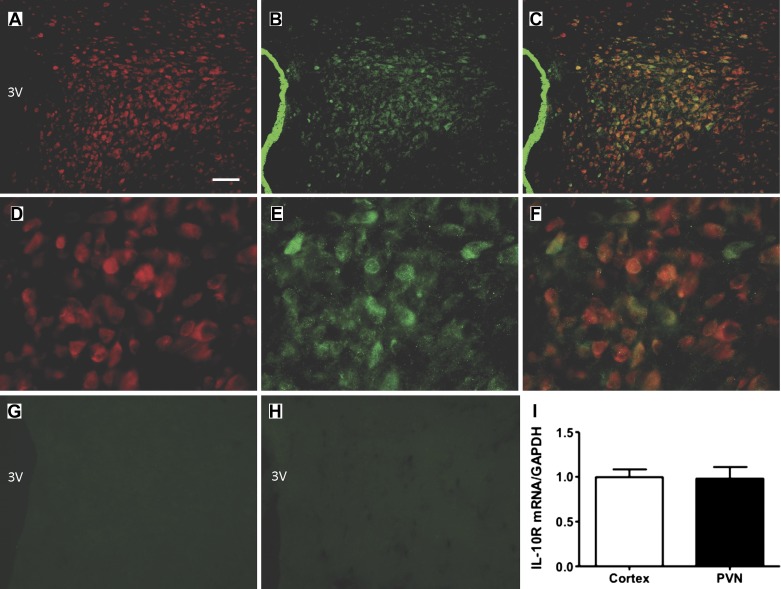
Since our overall goal was to determine if IL-10 modulates the inhibitory effects of ANG II on neuronal IKv, it was important to demonstrate the presence of IL-10R on neurons in the PVN, and on hypothalamic neurons in culture. The representative low (×10) and high (×40) power fluorescence micrographs in Fig. 1, A–C and D–F, are taken from the PVN at approximately −1.8 mm from bregma. They show IL-10R immunoreactivity in the PVN (green) colocalized with immunoreactivity for the neuron-specific marker HuC/D (red). IL-10R immunoreactivity was observed to be colocalized with neurons throughout the PVN (−1.6 to −2.2 mm relative to the bregma; not shown). In addition, either omission of the IL-10R antibody or preabsorption using the blocking peptide IL-10Rα (M-20) P resulted in no specific IL-10R immunostaining (Fig. 1, G and H). Based on this qualitative view, it is apparent that neurons within the PVN express immunoreactive IL-10R. The presence of IL-10R in the PVN was confirmed by real-time RT-PCR. The results presented in Fig. 1I show the presence of IL-10R mRNA in the PVN compared with that present in the cerebral cortex.
Fig. 1.
Localization of IL-10 receptor (R) on neurons in the paraventricular nucleus. A–C: low-power fluorescence micrographs (×10 magnification) showing IL-10R (green) and HuC/D (red; neuron-specific marker) immunoreactivities in the paraventricular nucleus (PVN) of a Sprague-Dawley (SD) rat. Also shown are overlays between IL-10R and NeuN. 3V, 3rd cerebroventricle; bar = 100 µm. D–F: higher power view (×40 magnification) of IL-10R and HuC/D immunoreactivities in the PVN. G and H: representative micrographs showing a lack of specific IL-10R immunoreactivity in the PVN when the primary antibody was omitted (G) or preabsorbed with an IL-10R blocking peptide (H). I: bar graph of real-time RT-PCR data showing IL-10R mRNA levels in cerebral cortex and in hypothalamic tissue containing the PVN. Immunostaining was performed on 5 SD rats. Data are means ± SE (n = 3 rats per area).
Localization of IL-10R on Hypothalamic Neurons in Culture
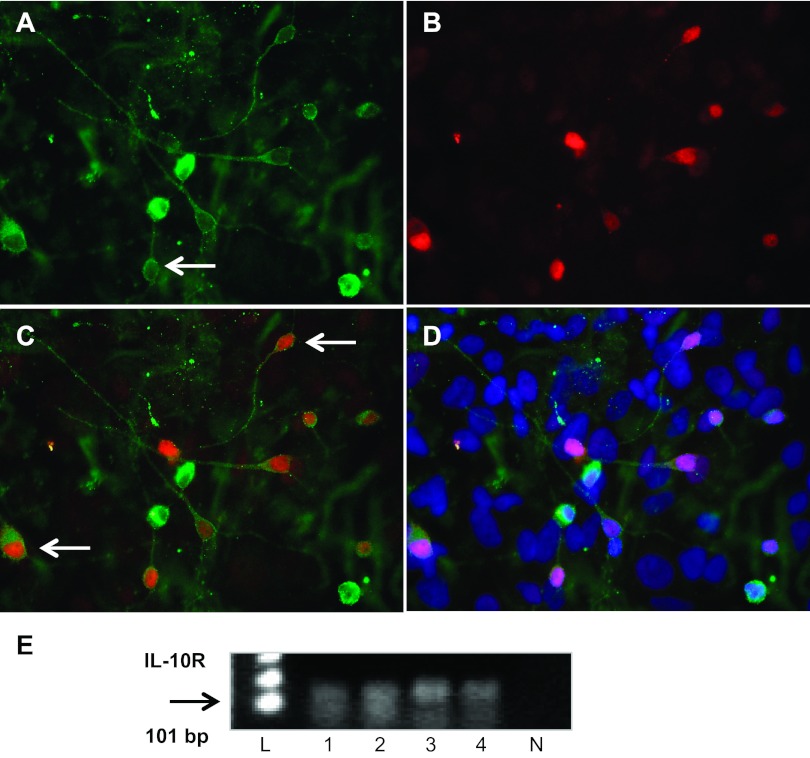
Having demonstrated that IL-10R are present in PVN neurons it was important to demonstrate that this expression was maintained in hypothalamic neurons, in culture that were to be used for electrophysiological recordings. These cultured neurons have been used by us previously in multiple experiments, and contain functional AT1R (33). The representative high-power (×40 magnification) fluorescence micrographs in Fig. 2 are taken from hypothalamic neuronal cultures and show IL-10R immunoreactivity (green) colocalized with the neuron-specific marker NeuN (red) and DAPI nuclear stain (blue). These micrographs indicate the presence of IL-10R on neurons within hypothalamic cultures, and this was confirmed by the single cell RT-PCR analyses that revealed that IL-10R transcripts are present in single neuron samples (Fig. 2E).
Fig. 2.
Localization of IL-10R in hypothalamic neurons in culture. Fluorescence micrographs (×40 magnification) showing IL-10R (A) and NeuN (B) immunoreactivities in neuronal cultures prepared from the hypothalamus of SD rats. Also shown are overlays between IL-10R and NeuN (C) and among IL-10R, NeuN, and the nuclear stain DAPI (D). Arrows indicate examples of PVN neurons containing IL-10R immunoreactivity. E: presence of IL-10R in single hypothalamic neurons. Ethidium bromide-stained agarose gel shows RT-PCR products of the expected size for IL-10R (101 bp) from 4 hypothalamic neurons in culture (lanes 1–4). Substitution of RNAse free water for the cell sample mRNA produced no such RT-PCR products (lane N). L, DNA ladder.
IL-10 Does Not Alter Basal Potassium Currents
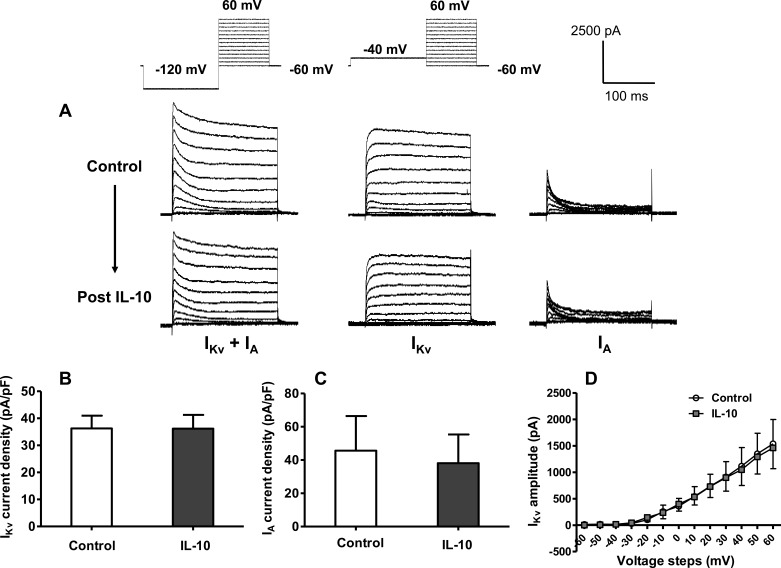
Considering that hypothalamic neurons in culture contain IL-10R, we first tested whether IL-10 elicits any changes in baseline neuronal K+ currents. This concentration of IL-10 (10 ng/ml) was chosen based on doses that were effective in studies from other neuronal systems (3, 34). The representative current tracings presented in Fig. 3A demonstrate that extracellular application of this AIC has little effect on baseline IKv, IA, and total outward K+ current (IKv + IA). This was confirmed by analyses that demonstrated no significant effect of IL-10 on IKv and IA current densities (pA/pF; Fig. 3, B and C). Furthermore, calculation of the I-V relationship of neuronal IKv before and after application of IL-10 revealed that this cytokine did not alter the threshold, density, or amplitude of this current (Fig. 3D). Similar preliminary I-V analyses for IA from two neurons indicated that IL-10 did not alter the voltage properties of this current (not shown). In addition to these results using 10 ng/ml IL-10, we also determined in preliminary studies that a lower concentration of IL-10 (1 ng/ml) was also ineffective in altering IKv + IA.
Fig. 3.
IL-10 does not alter baseline potassium currents in hypothalamic neurons in culture. A: representative tracings of current-voltage (I-V) step currents showing total outward K+ current (IKv + IA), delayed rectifier K+ current (IKv), and A-type K+ current (IA) before and after extracellular application of IL-10 (10 ng/ml). B and C: bar graphs show IKv current densities (±SE) from 13 neurons (B) and IA current densities from 4 neurons (C) before and after extracellular application of IL-10 (10 ng/ml). Current amplitude values used to calculate the pA/pF values shown in B and C were recorded at +10 mV. D: I-V relationship of IKv current before and after extracellular application of IL-10 (10 ng/ml). Data are means (±SE) from 3 neurons.
IL-10 Inhibits ANG II-Induced Decreases in Neuronal IKv
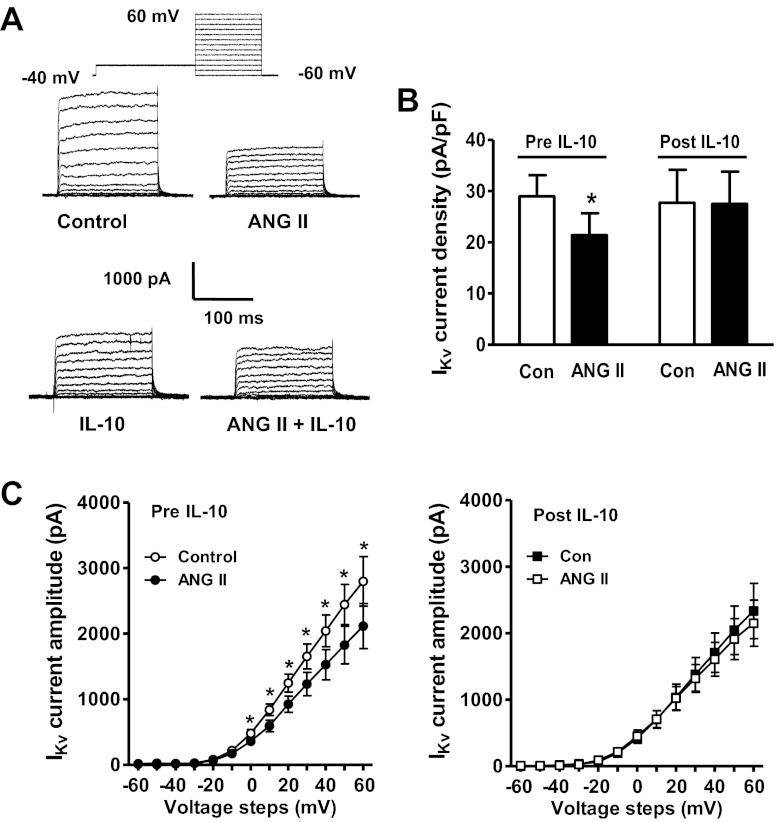
In numerous previous studies we demonstrated that application of ANG II, acting via AT1R, produced a decrease of neuronal IKv (19, 35). Even though IL-10 did not alter basal IKv in hypothalamic neurons in culture, we tested whether it would modulate the inhibitory actions of ANG II on this current. In agreement with previous studies, extracellular perfusion of ANG II (100 nM) made in the presence of the AT2R blocker PD123319 (0.001 mM) produced a significant decrease in neuronal IKv, with almost 50% of the neurons tested responding to ANG II (Fig. 4, A and B). After the inhibitory action of ANG II on IKv was recorded neurons underwent washout with normal extracellular perfusate for 5–7 min. This was followed by extracellular application of IL-10 (10 ng/ml) onto the same neuron for 5 min, followed by a second application of ANG II in the presence of IL-10. In this way we were able to test any effect of IL-10 in ANG II-responsive neurons. The results shown in Fig. 4 demonstrate that ANG II alone produced a significant (∼30%) decrease in IKv current density. The decrease in neuronal IKv produced by ANG II was abolished by application of IL-10 (Fig. 4, B and C). Recordings of the effects of ANG II on IKv in the absence or presence of IL-10 were made during different voltage steps and enabled us to assess the current-voltage relationship in each treatment condition. As shown in the I-V curves in Fig. 4C, left, ANG II produced a significant decrease in the amplitude of IKv at every voltage step from 0 to +60 mV. No such decreases in IKv amplitude were evident when ANG II was applied in the presence of IL-10 (Fig. 4C, right). Importantly, repeated application of ANG II (100 nM) using the above protocol in the absence of IL-10 perfusion produced no significant lessening of the ANG II-induced decrease in IKv. Without IL-10 perfusion, the reduction in IKv current density produced by the first and second applications of ANG II (100 nM) was 9.7 ± 1.25 and 8.8 ± 1.5 pA/pF, respectively (n = 5 neurons).
Fig. 4.
IL-10 decreases the inhibitory effect of ANG II on neuronal IKv. A: representative tracings of the changes in IKv elicited by extracellular application of ANG II (100 nM), IL-10 (10 ng/ml), and ANG II/IL-10 in combination. B: bar graph shows the ANG II-induced decrease in IKv current density before and after application of IL-10 (10 ng/ml). All recordings were made in the presence of the AT2R blocker PD123319 (0.001 mM). Data are means ± SE from 6 neurons. *P < 0.02 vs. control (pre-IL-10); there was no significant difference between control and ANG II groups post-IL-10. C, left: I-V relationship of IKv current before and after extracellular application of ANG II in the absence of IL-10 treatment. Data are means ± SE from 7 neurons. *P < 0.05. C, right: I-V relationship of IKv current before and after extracellular application of ANG II, in the presence of IL-10 treatment. Data are means ± SE from 7 neurons.
DISCUSSION
While it is known that IL-10R are present on astroglia, microglia, and neurons in the CNS, the immunostaining shown in the current study is the first demonstration that receptors for this AIC are located on neurons in the PVN. Furthermore, our immunostaining and single cell RT-PCR data demonstrate that IL-10R are maintained on isolated hypothalamic neurons in culture and the electrophysiological studies presented here demonstrate that IL-10 can act at these receptors to inhibit the decreases in neuronal IKv elicited by ANG II. Since a decrease in IKv contributes to increased neuronal excitability, our studies provide the first demonstration that IL-10 can exert direct actions to inhibit ANG II-induced increases in neuronal excitation. Further, our results suggest that this is one mechanism by which increased IL-10 levels in the PVN act to decrease hypertension induced by elevated ANG II. These findings raise a number of questions that have yet to be answered.
First of all, the electrophysiological recordings performed here were performed on hypothalamic neurons in culture and as such are a first step. While the data clearly show that IL-10 can inhibit ANG II actions on neurons, the next step will be to determine if this AIC can inhibit ANG II/AT1R actions on defined preautonomic neurons in the PVN. Such information would allow us to further link antihypertensive action of increased IL-10 expression in the PVN to inhibition of the neuronal actions of ANG II.
A further question concerns the mechanism by which IL-10 exerts its inhibitory action over ANG II. The mechanism is unlikely to involve a direct action of IL-10 on the K+ channels that underlie IKv or on certain of the signaling mechanisms that directly influence these channels, since IL-10 alone does not influence basal IKv (Fig. 3). In addition, even though IL-10R are well known to signal via activation of Janus kinase and phosphorylation of signal transducer and activator of transcription-3 (STAT3; Refs. 28, 38), the latter is a transcription factor and its time course of induction is not consistent with the rapid inhibitory action of IL-10 on ANG II (Fig. 4). However, it is also known that IL-10 can inhibit NADPH oxidase and decrease the levels of reactive oxygen species (ROS) in the CNS and peripheral cells (22, 26, 27). Since activation of NADPH oxidase and induction of ROS are critical to the ANG II/AT1R -induced decrease in neuronal IKv and increase in neuronal excitation (32) and the CNS actions of ANG II to raise blood pressure (37), we considered the possibility that the inhibition of ANG II's actions observed in the current work are due to a decrease in ROS levels. Our preliminary findings would suggest that this is not the case, since ANG II (100 nM; 1–30 min)-induced increases in ROS levels (whole culture dish) were not affected by IL-10 (10 ng/ml) treatment (data not shown). This will be further investigated by testing the effects of IL-10 on ROS in individual ANG II-responsive neurons.
While the present results suggest a neuronal locus for the inhibitory action of IL-10 over ANG II, they do not mean that this is the only site of IL-10 action. For example, it is well known that microglia contain IL-10R (24). Since our previous studies implicate a role for microglial activation in the PVN in neurogenic hypertension, a further locus of action of IL-10 in decreasing the hypertensive effect of ANG II might be inhibition of production of PIC such as IL-1β, which is known to activate PVN parvocellular neurons (12).
In conclusion, our results demonstrate that IL-10 has the ability to inhibit the actions of ANG II on one of the membrane currents (IKv) that underlies the neuronal excitatory actions of this peptide (32, 35). Whether IL-10 can alter the actions of ANG II on other membrane currents/channels such as calcium current remains to be investigated. Nonetheless, the results imply that IL-10, produced from immune cells in the CNS (microglia and astroglia), can temper the neuronal actions of ANG II to increase sympathetic outflow and blood pressure. By extrapolation, while this IL-10-mediated mechanism may be of importance to temper ANG II-induced increases in sympathetic outflow in normal rats, it is clearly inoperative in neurogenic hypertension, where our previous results indicate that ANG II inhibits IL-10 production by microglia (20), tipping the balance in favor of PIC in hypertension. Another interesting point concerns the stimulus for IL-10 secretion. One study has demonstrated that activation of AT2R on CD8 T-regulatory cells increases IL-10 production (8). Considering this and the evidence for AT2R-mediated inhibition of sympathetic activity via brain cardiovascular control centers (13), opposite to the stimulatory effects of AT1R, it is possible to speculate that a stimulus for IL-10 production in the PVN is AT2R. However, while we have preliminary evidence that the PVN contains AT2R mRNA (Shi P and Sumners C, unpublished observations), whether or not IL-10 producing glia at this site contain AT2R is not known.
From a global standpoint, the current work aids in our understanding of the regulatory mechanisms that are involved in controlling ANG II's neuronal actions in the brain. Since these regulatory mechanisms may ultimately fail or not be enough to combat the prohypertensive stimuli, the current findings may open up a new avenue for development of future antihypertensive therapeutics.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-076803 (to C. Sumners) and American Heart Association Scientist Development Grant 11SDG770006 (to P. Shi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.J. and C.S. conception and design of research; N.J., P.S., F.D., and M.C.K.-P. performed experiments; N.J., P.S., F.D., and C.S. analyzed data; N.J., P.S., M.C.K.-P., and C.S. interpreted results of experiments; N.J., M.C.K.-P., and C.S. prepared figures; N.J. and C.S. drafted manuscript; N.J., P.S., F.D., M.C.K.-P., and C.S. edited and revised manuscript; N.J., P.S., F.D., M.C.K.-P., and C.S. approved final version of manuscript.
REFERENCES
- 1. Balasingam V, Yong VW. Attenuation of astroglial reactivity by interleukin-10. J Neurosci 16: 2945–2955, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braga VA, Medeiros IA, Ribeiro TP, França-Silva MS, Botelho-Ono MS, Guimarães DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension. Braz J Med Biol Res 44: 871–876, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Burkovetskaya ME, Levin SG, Godukhin OV. Neuroprotective effects of interleukin-10 and tumor necrosis factor-alpha against hypoxia-induced hyperexcitability in hippocampal slice neurons. Neurosci Lett 416: 236–240, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59: 113–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen QH, Andrade MA, Calderon AS, Toney GM. Hypertension induced by angiotensin II and a high salt diet involves reduced SK current and increased excitability of RVLM projecting PVN neurons. J Neurophysiol 104: 2329–2337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifró W, Miteva K, Kaschina E, Thiel A, Imboden H, Wang J, Steckelings U, Steinhoff G, Unger T, Li J. Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. J Immunol 185: 6286–6293, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Dupont AG, Brouwers S. Brain angiotensin peptides regulate sympathetic tone and blood pressure. J Hypertens 28: 1599–1610, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Fan ZD, Zhang L, Shi Z, Gan XB, Gao XY, Zhu GQ. Artificial microRNA interference targeting AT(1a) receptors in paraventricular nucleus attenuates hypertension in rats. Gene Ther 19: 810–817, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 226: 85–96, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Ferri CC, Ferguson AV. Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol 15: 126–133, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gao L, Zucker IH. AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol 11: 124–130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens 23: 1052–1060, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail 10: 625–634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J Hypertens 6: 79–84, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gyurko R, Wielbo D, Phillips MI. Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept 49: 167–174, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Jiang N, Shi P, Li H, Lu S, Braseth L, Cuadra AE, Raizada MK, Sumners C. Phosphate-activated glutaminase-containing neurons in the rat paraventricular nucleus express angiotensin type 1 receptors. Hypertension 54: 845–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295: H227–H236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand 177: 7–15, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kuga S, Otsuka T, Niiro H, Nunoi H, Nemoto Y, Nakano T, Ogo T, Umei T, Niho Y. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp Hematol 24: 151–157, 1996 [PubMed] [Google Scholar]
- 23. Lebrun CJ, Blume A, Herdegen T, Möllenhoff E, Unger T. Complex activation of inducible transcription factors in the brain of normotensive and spontaneously hypertensive rats following central angiotensin II administration. Regul Pept 66: 19–23, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Ledeboer A, Brevé JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, Tilders FJ, Van Dam AM. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci 16: 1175–1185, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lodge PA, Sriram S. Regulation of microglial activation by TGF-beta, IL-10, and CSF-1. J Leukoc Biol 60: 502–508, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Park KW, Lee HG, Jin BK, Lee YB. Interleukin-10 endogenously expressed in microglia prevents lipopolysaccharide-induced neurodegeneration in the rat cerebral cortex in vivo. Exp Mol Med 39: 812–819, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Qian L, Hong JS, Flood PM. Role of microglia in inflammation-mediated degeneration of dopaminergic neurons: neuroprotective effect of interleukin 10. J Neural Transm Suppl 70: 367–371, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 64: 621–651, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension 56: 297–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol 37: e52–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci 24: 9944–9952, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res 96: 659–666, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res 101: 304–312, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. J Neurochem 110: 1617–1627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu M, Gelband CH, Posner P, Sumners C. Angiotensin II decreases neuronal delayed rectifier potassium current: role of calcium/calmodulin-dependent protein kinase II. J Neurophysiol 82: 1560–1568, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol 282: H2039–H2045, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Zocchia C, Spiga G, Rabin SJ, Grekova M, Richert J, Chernyshev O, Colton C, Mocchetti I. Biological activity of interleukin-10 in the central nervous system. Neurochem Int 30: 433–439, 1997 [DOI] [PubMed] [Google Scholar]