Abstract
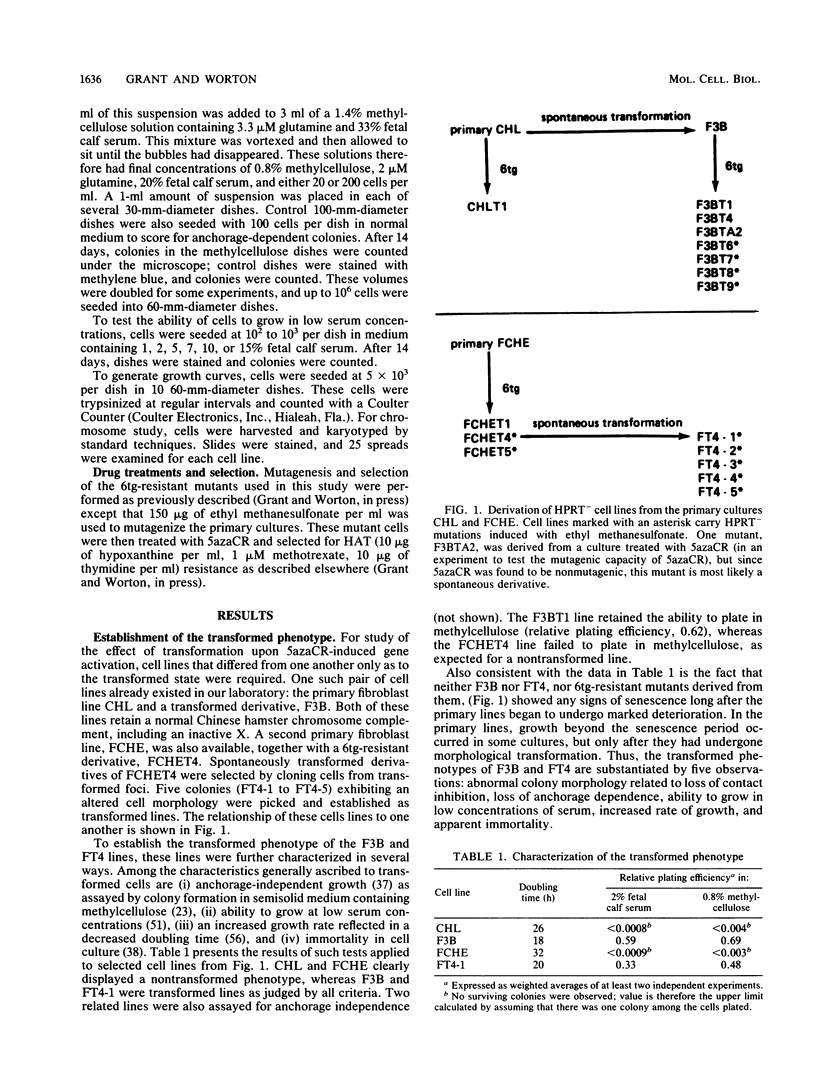
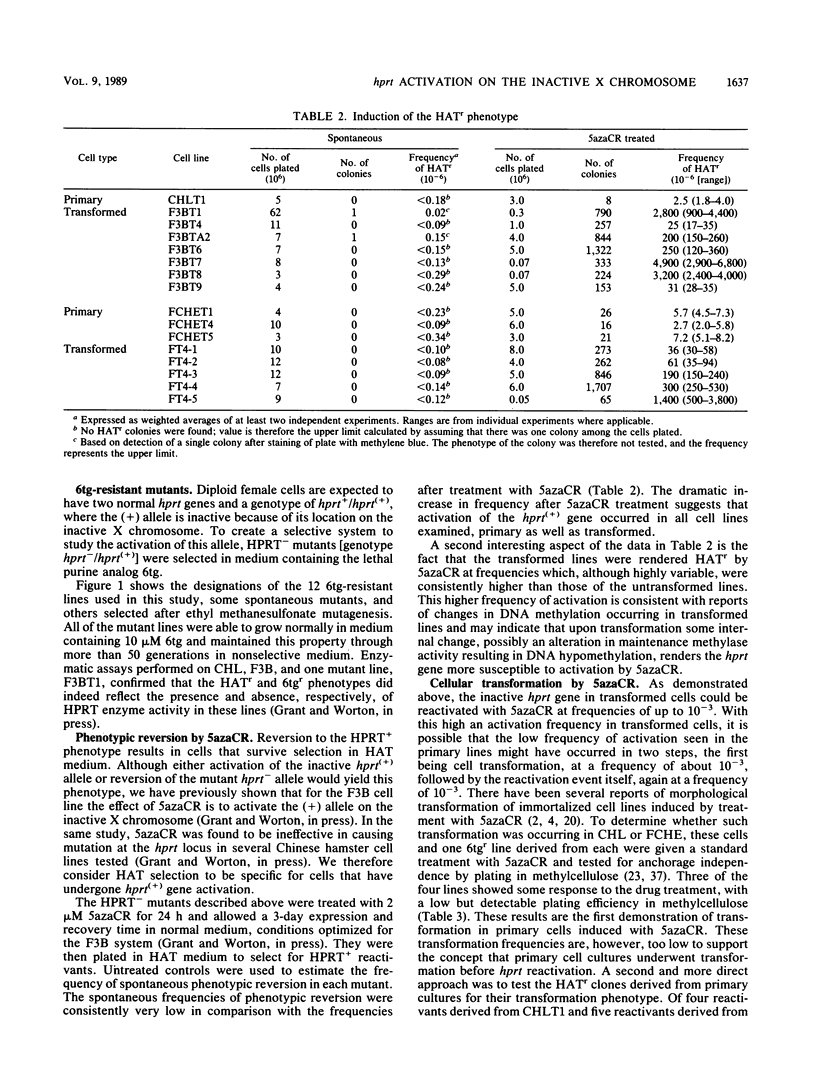
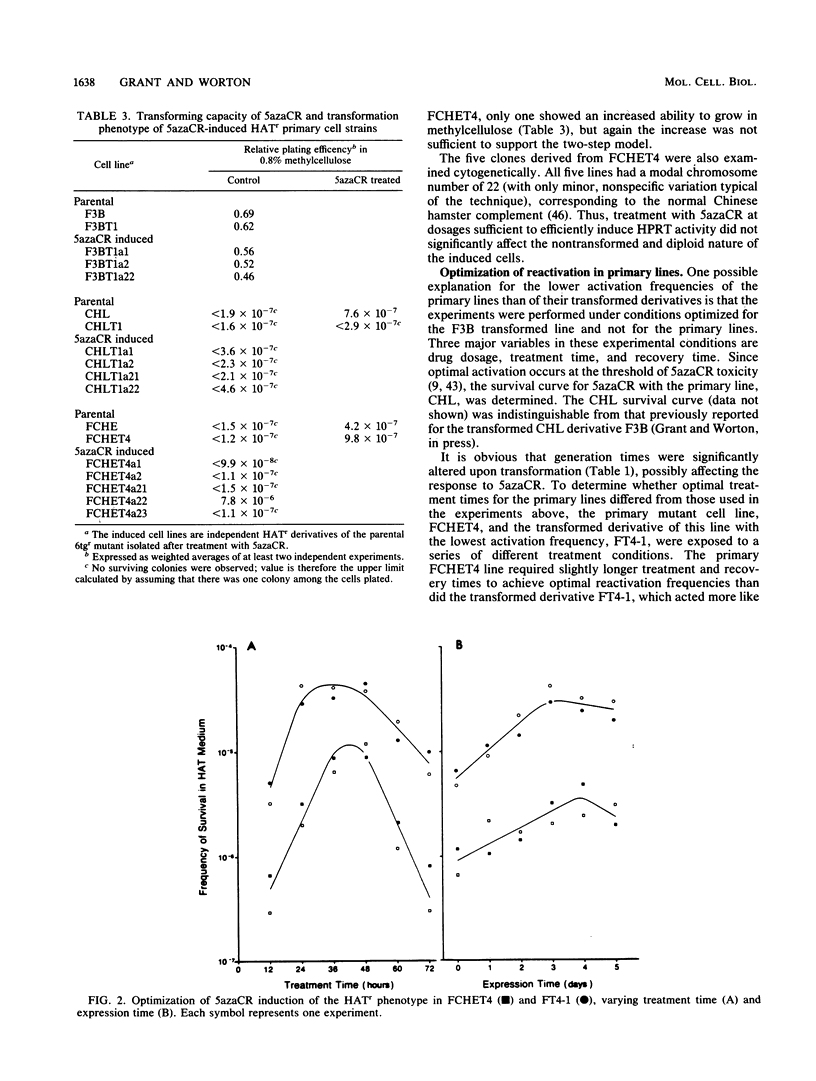
We have investigated the genetic activation of the hprt (hypoxanthine-guanine phosphoribosyltransferase) gene located on the inactive X chromosome in primary and transformed female diploid Chinese hamster cells after treatment with the DNA methylation inhibitor 5-azacytidine (5azaCR). Mutants deficient in HPRT were first selected by growth in 6-thioguanine from two primary fibroblast cell lines and from transformed lines derived from them. These HPRT- mutants were then treated with 5azaCR and plated in HAT (hypoxanthine-methotrexate-thymidine) medium to select for cells that had reexpressed the hprt gene on the inactive X chromosome. Contrary to previous results with primary human cells, 5azaCR was effective in activating the hprt gene in primary Chinese hamster fibroblasts at a low but reproducible frequency of 2 x 10(-6) to 7 x 10(-6). In comparison, the frequency in independently derived transformed lines varied from 1 x 10(-5) to 5 x 10(-3), consistently higher than in the nontransformed cells. This increase remained significant when the difference in growth rates between the primary and transformed lines was taken into account. Treatment with 5azaCR was also found to induce transformation in the primary cell lines but at a low frequency of 4 x 10(-7) to 8 x 10(-7), inconsistent with a two-step model of transformation followed by gene activation to explain the derepression of hprt in primary cells. Thus, these results indicate that upon transformation, the hprt gene on the inactive Chinese hamster X chromosome is rendered more susceptible to action by 5azaCR, consistent with a generalized DNA demethylation associated with the transformation event or with an increase in the instability of an underlying primary mechanism of X inactivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs A. H., Axelman J., Migeon B. R. Reactivation of X-linked genes in human fibroblasts transformed by origin-defective SV40. Somat Cell Mol Genet. 1986 Nov;12(6):585–594. doi: 10.1007/BF01671944. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Banerjee A., Gardner A., Jones P. A. Induction of morphological transformation in mouse C3H/10T1/2 clone 8 cells and chromosomal damage in hamster A(T1)C1-3 cells by cancer chemotherapeutic agents. Cancer Res. 1977 Jul;37(7 Pt 1):2202–2208. [PubMed] [Google Scholar]
- Bouck N., Kokkinakis D., Ostrowsky J. Induction of a step in carcinogenesis that is normally associated with mutagenesis by nonmutagenic concentrations of 5-azacytidine. Mol Cell Biol. 1984 Jul;4(7):1231–1237. doi: 10.1128/mcb.4.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M. J., Burdon R. H. The sequence specificity of vertebrate DNA methylation. Nucleic Acids Res. 1977 Apr;4(4):1025–1037. doi: 10.1093/nar/4.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Evidence obtained by induced mutation frequency analysis for functional hemizygosity at the emt locus in CHO cells. Somatic Cell Genet. 1979 Jan;5(1):51–65. doi: 10.1007/BF01538786. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Diala E. S., Cheah M. S., Rowitch D., Hoffman R. M. Extent of DNA methylation in human tumor cells. J Natl Cancer Inst. 1983 Oct;71(4):755–764. [PubMed] [Google Scholar]
- Diala E. S., Plent M. M., Coalson D. W., Hoffman R. M. DNA methylation in normal and SV40-transformed human fibroblasts. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1379–1384. doi: 10.1016/s0006-291x(81)80164-7. [DOI] [PubMed] [Google Scholar]
- Ellis N., Keitges E., Gartler S. M., Rocchi M. High-frequency reactivation of X-linked genes in Chinese hamster X human hybrid cells. Somat Cell Mol Genet. 1987 May;13(3):191–204. doi: 10.1007/BF01535202. [DOI] [PubMed] [Google Scholar]
- Ferraro M., Lavia P. Activation of human ribosomal genes by 5-azacytidine. Exp Cell Res. 1983 May;145(2):452–457. doi: 10.1016/0014-4827(83)90024-1. [DOI] [PubMed] [Google Scholar]
- GROUPE V., MANAKER R. A. Discrete foci of altered chicken embryo cells associated with Rous sarcoma virus in tissue culture. Virology. 1956 Dec;2(6):838–840. doi: 10.1016/0042-6822(56)90064-2. [DOI] [PubMed] [Google Scholar]
- Gama-Sosa M. A., Slagel V. A., Trewyn R. W., Oxenhandler R., Kuo K. C., Gehrke C. W., Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983 Oct 11;11(19):6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Dyer K. A., Graves J. A., Rocchi M. A two step model for mammalian X-chromosome inactivation. Prog Clin Biol Res. 1985;198:223–235. [PubMed] [Google Scholar]
- Grant S. G., Chapman V. M. Mechanisms of X-chromosome regulation. Annu Rev Genet. 1988;22:199–233. doi: 10.1146/annurev.ge.22.120188.001215. [DOI] [PubMed] [Google Scholar]
- Graves J. A. 5-azacytidine-induced re-expression of alleles on the inactive X chromosome in a hybrid mouse cell line. Exp Cell Res. 1982 Sep;141(1):99–105. doi: 10.1016/0014-4827(82)90072-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Gunthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. J., Anisowicz A., Gadi I. K., Raffeld M., Sager R. Azacytidine-induced tumorigenesis of CHEF/18 cells: correlated DNA methylation and chromosome changes. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6606–6610. doi: 10.1073/pnas.80.21.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellkuhl B., Grzeschik K. H. Partial reactivation of a human inactive X chromosome in human-mouse somatic cell hybrids. Cytogenet Cell Genet. 1978;22(1-6):527–530. doi: 10.1159/000131016. [DOI] [PubMed] [Google Scholar]
- Hors-Cayla M. C., Heuertz S., Frezal J. Coreactivation of four inactive X genes in a hamster x human hybrid and persistence of late replication of reactivated X chromosome. Somatic Cell Genet. 1983 Nov;9(6):645–657. doi: 10.1007/BF01539470. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Pluznik D. H., Sachs L. In vitro control of the development of macrophage and granulocyte colonies. Proc Natl Acad Sci U S A. 1966 Aug;56(2):488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Hemimethylated duplex DNAs prepared from 5-azacytidine-treated cells. Nucleic Acids Res. 1981 Jun 25;9(12):2933–2947. doi: 10.1093/nar/9.12.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B., DeMars R. Autonomous gene expression on the human inactive X chromosome. Somatic Cell Genet. 1980 May;6(3):309–323. doi: 10.1007/BF01542785. [DOI] [PubMed] [Google Scholar]
- Kahan B., DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. doi: 10.1073/pnas.72.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow D. C., Migeon B. R. DNA methylation stabilizes X chromosome inactivation in eutherians but not in marsupials: evidence for multistep maintenance of mammalian X dosage compensation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6210–6214. doi: 10.1073/pnas.84.17.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Gowans B. J., Lieberman M. W. Methylation of deoxycytidine incorporated by excision-repair synthesis of DNA. Cell. 1982 Sep;30(2):509–516. doi: 10.1016/0092-8674(82)90248-3. [DOI] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M., Lambert H., Evans R. E., Liskay R. M. Differences in the DNA of the inactive X chromosomes of fetal and extraembryonic tissues of mice. Cell. 1983 May;33(1):37–42. doi: 10.1016/0092-8674(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Lapeyre J. N., Becker F. F. 5-Methylcytosine content of nuclear DNA during chemical hepatocarcinogenesis and in carcinomas which result. Biochem Biophys Res Commun. 1979 Apr 13;87(3):698–705. doi: 10.1016/0006-291x(79)92015-1. [DOI] [PubMed] [Google Scholar]
- Leshin M. 5-Azacytidine and sodium butyrate induce expression of aromatase in fibroblasts from chickens carrying the henny feathering trait but not from wild-type chickens. Proc Natl Acad Sci U S A. 1985 May;82(9):3005–3009. doi: 10.1073/pnas.82.9.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Lock L. F., Melton D. W., Caskey C. T., Martin G. R. Methylation of the mouse hprt gene differs on the active and inactive X chromosomes. Mol Cell Biol. 1986 Mar;6(3):914–924. doi: 10.1128/mcb.6.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Melton D. W., Caskey C. T., Martin G. R. Methylation of the mouse hprt gene differs on the active and inactive X chromosomes. Mol Cell Biol. 1986 Mar;6(3):914–924. doi: 10.1128/mcb.6.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Takagi N., Martin G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987 Jan 16;48(1):39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Sprenkle J. A., Do T. T. Studies of human-mouse cell hybrids with respect to X-chromosome inactivation. Basic Life Sci. 1978;12:329–337. doi: 10.1007/978-1-4684-3390-6_23. [DOI] [PubMed] [Google Scholar]
- Migeon B. R. Studies of skin fibroblasts from 10 families with HGPRT deficiency, with reference in X-chromosomal inactivation. Am J Hum Genet. 1971 Mar;23(2):199–210. [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Axelman J., Kaslow D. C., Schmidt M. Incomplete X chromosome dosage compensation in chorionic villi of human placenta. Proc Natl Acad Sci U S A. 1985 May;82(10):3390–3394. doi: 10.1073/pnas.82.10.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Mareni C., Axelman J. Derepression with decreased expression of the G6PD locus on the inactive X chromosome in normal human cells. Cell. 1982 Jun;29(2):595–600. doi: 10.1016/0092-8674(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Paterno G. D., Adra C. N., McBurney M. W. X chromosome reactivation in mouse embryonal carcinoma cells. Mol Cell Biol. 1985 Oct;5(10):2705–2712. doi: 10.1128/mcb.5.10.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind W. H., Gartler S. M. X chromosome inactivaton and SV40 transformation of mammalian cells. Somatic Cell Genet. 1979 Nov;5(6):945–955. doi: 10.1007/BF01542653. [DOI] [PubMed] [Google Scholar]
- Ray M., Mohandas T. Proposed banding nomenclature for the Chinese hamster chromosomes (Cricetulus griseus). Cytogenet Cell Genet. 1976;16(1-5):83–91. doi: 10.1159/000130559. [DOI] [PubMed] [Google Scholar]
- Romeo G., Migeon B. R. Stability of X chromosomal inactivation in human somatic cells transformed by SV-40. Humangenetik. 1975 Sep 10;29(2):165–170. doi: 10.1007/BF00430356. [DOI] [PubMed] [Google Scholar]
- STANNERS C. P., TILL J. E., SIMINOVITCH L. STUDIES ON THE TRANSFORMATION OF HAMSTER EMBRYO CELLS IN CULTURE BY POLYOMA VIRUS. I. PROPERTIES OF TRANSFORMED AND NORMAL CELLS. Virology. 1963 Nov;21:448–463. doi: 10.1016/0042-6822(63)90206-x. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Interclonal variation in methylation patterns for expressed and non-expressed genes. Nucleic Acids Res. 1982 Jul 24;10(14):4293–4304. doi: 10.1093/nar/10.14.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Martini G., Migeon B. R., Dono R. Expression of the G6PD locus on the human X chromosome is associated with demethylation of three CpG islands within 100 kb of DNA. EMBO J. 1988 Feb;7(2):401–406. doi: 10.1002/j.1460-2075.1988.tb02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. L., Jones P. A. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983 Jan;32(1):239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Dintzis S., Toniolo D., Persico G., Lunnen K. D., Axelman J., Migeon B. R. Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3' CpG clusters: implications for X chromosome dosage compensation. Nucleic Acids Res. 1984 Dec 21;12(24):9333–9348. doi: 10.1093/nar/12.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Studies of X chromosome DNA methylation in normal human cells. Nature. 1982 Feb 25;295(5851):667–671. doi: 10.1038/295667a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]


