Abstract
This study investigated the molecular identity and impact of enhancing voltage-gated Na+ (NaV) channels in the control of vascular tone. In rat isolated mesenteric and femoral arteries mounted for isometric tension recording, the vascular actions of the NaV channel activator veratridine were examined. NaV channel expression was probed by molecular techniques and immunocytochemistry. In mesenteric arteries, veratridine induced potent contractions (pEC50 = 5.19 ± 0.20, Emax = 12.0 ± 2.7 mN), which were inhibited by 1 μM TTX (a blocker of all NaV channel isoforms, except NaV1.5, NaV1.8, and NaV1.9), but not by selective blockers of NaV1.7 (ProTx-II, 10 nM) or NaV1.8 (A-80347, 1 μM) channels. The responses were insensitive to endothelium removal but were partly (∼60%) reduced by chemical destruction of sympathetic nerves by 6-hydroxydopamine (2 mM) or antagonism at the α1-adrenoceptor by prazosin (1 μM). KB-R7943, a blocker of the reverse mode of the Na+/Ca2+ exchanger (3 μM), inhibited veratridine contractions in the absence or presence of prazosin. T16Ainh-A01, a Ca2+-activated Cl− channel blocker (10 μM), also inhibited the prazosin-resistant contraction to veratridine. NaV channel immunoreactivity was detected in freshly isolated mesenteric myocytes, with apparent colocalization with the Na+/Ca2+ exchanger. Veratridine induced similar contractile effects in the femoral artery, and mRNA transcripts for NaV1.2 and NaV1.3 channels were evident in both vessel types. We conclude that, in addition to sympathetic nerves, NaV channels are expressed in vascular myocytes, where they are functionally coupled to the reverse mode of Na+/Ca2+ exchanger and subsequent activation of Ca2+-activated Cl− channels, causing contraction. The TTX-sensitive NaV1.2 and NaV1.3 channels are likely involved in vascular control.
Keywords: veratridine, smooth muscle, reverse mode of Na+/Ca2+ exchanger, Ca2+-activated Cl− channels, tetrodotoxin
voltage-gated Na+ (NaV) channels are central to the initiation and propagation of action potentials in excitable cells, specifically neurons, as well as cardiac and skeletal myocytes (5). Nine NaV channel isoforms have been identified (NaV1.1–NaV1.9) on the basis of the underlying gene (SCNA1-9), rate of inactivation, and sensitivity to TTX; NaV1.5, NaV1.8, and NaV1.9 channels are much less sensitive to blockade by TTX (6, 14). Each NaV channel consists of a pore-forming ∼260-kDa α-subunit that is associated with one or more auxiliary ∼35-kDa β-subunit(s) (6).
In vascular smooth muscle cells, the presence and impact of NaV channels are less well defined. NaV channels have been characterized in freshly dispersed smooth muscle cells from rat or mouse mesenteric artery and portal vein (3, 27, 34) and are particularly prominent in cultured smooth muscle cells (26, 29, 31). There is also some molecular evidence supporting the expression of NaV channel isoforms in the smooth muscle of portal vein, which displays spontaneous and rhythmic contractions (34), as well as in rat aorta (12) and mouse cremaster arterioles (11). The physiological role of Nav channels in the vasculature is less well defined, because channel blockers such as TTX generally have little effect on vasoconstrictor responses (12, 34). However, a new blocker of persistent Nav currents, F 15845, prevents hypoxia-induced contractions of rat femoral artery (4), and the NaV channel activator veratridine enhances spontaneous contractions in mouse portal vein (34) or causes contraction of rat aorta (12). In the latter case, high concentrations of veratridine were required, and much of the effect was considered to be due to release of neurotransmitters from sympathetic nerve terminals (12).
In the present study we have determined the expression profile of NaV genes in the rat mesenteric resistance vessel, as well as the rat femoral artery, and have assessed the effect of the NaV channel activator veratridine on these vessels. We then utilized a panel of pharmacological agents selective for different NaV channel isoforms to determine the molecular identity of the channels contributing to changes in contractile state. We also used a Na+/Ca2+ exchange (NCX) inhibitor and a specific blocker of TMEM16A-encoded Ca2+-activated Cl− channels [2-(5-ethyl-4-hydroxy-6-methylpyrimidin-2-ylthio)-N-(4-(4-methoxyphenyl)thiazol-2-yl)acetamide (T16Ainh-A01)] (9) to define the vascular mechanisms underlying veratridine-induced contractions. These studies revealed that Nav channel activator produces robust contractions of resistance arteries, which were only partially driven by increased release of sympathetic neurotransmitters, and involved entry of Ca2+ by reverse-mode NCX and the subsequent activation of Ca2+-activated Cl− channels. These findings show that “silent” NaV channels represent a large contractile reserve with important consequences for vascular tone.
METHODS
Isometric tension recording.
Male Wistar rats (250–350 g; Charles River, Kent, UK) were stunned by a blow to the back of the neck and killed by cervical dislocation. All animal care and use were in accordance with the protocols approved under the UK Animal (Scientific Procedures) Act (1986). The third-order branches of the superior mesenteric artery were removed and cleaned of adherent tissue. Segments (2 mm long) were mounted in a Mulvany-Halpern-type wire myograph (model 610M, Danish Myo Technology, Aarhus, Denmark) and maintained at 37°C in gassed (95% O2-5% CO2) Krebs-Henseleit solution (mM: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 2 CaCl2, and 10 d-glucose), as previously described, unless otherwise stated (16). Arteries were equilibrated and set to a basal tension of 2–2.5 mN. The integrity of the endothelium was assessed by precontraction of the vessel with 10 μM methoxamine (an α1-adrenoceptor agonist) followed by relaxation with 10 μM carbachol (a muscarinic acetylcholine receptor agonist); vessels showing >90% relaxation were designated endothelium-intact. When endothelium was not required, it was removed by mechanical abrasion of the intima with a human hair; <10% carbachol-induced relaxation indicated successful removal. After the test for endothelial integrity, arteries were left for ≥30 min before they were exposed to veratridine.
Preliminary experiments indicated that veratridine induced contractions in some, but not all, quiescent mesenteric arteries, but consistent contractions were obtained when the vessels were prestimulated with a low concentration (1–3 μM) of methoxamine, which induced a small vascular tone. Thus all experiments, unless otherwise stated, were performed under a small methoxamine tone (average ∼2 mN, ∼15% of contraction attained in the endothelial integrity test at the start of each experiment). To obtain a concentration-contraction curve, veratridine (1–30 μM) was cumulatively added to the myograph bath, and the maximal tension achieved at each concentration was determined. To examine the involvement of NaV channel subtypes, vessels were first stimulated with methoxamine and then with a NaV channel inhibitor [TTX, ranolazine, ProTx-II, or 5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)-2-furancarboxamide (A803467)] or other putative modulators {2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea (KB-R7943 mesylate) or suramin} for 5–10 min followed by a single concentration (10 μM) of veratridine. The application of ranolazine and, in some cases, KB-R7943 was found to have relaxant effects. Thus the concentrations of methoxamine were adjusted, where necessary, to achieve a similar vascular tone (∼1–3 mN), in the absence and presence of the modulator, before the addition of veratridine. Some of these modulators were also tested against veratridine in mesenteric arteries precontracted with 60 mM KCl. All experiments were performed in matched vessels; effects of putative modulators or endothelium removal were compared with the control responses obtained in vessels of the same rat. The ethanol vehicle of veratridine [maximum 0.06% (vol/vol)] alone had no contractile effects.
To desensitize perivascular sensory nerves, some vessels were treated for 1 h with 10 μM capsaicin, which was then washed out. Capsaicin is an agonist of transient receptor potential vanilloid type 1 (TRPV1), and the prolonged treatment in this protocol was demonstrated to successfully abolish activation of TRPV1-expressing sensory nerves and the subsequent vasorelaxation (18). To test the involvement of sympathetic nerves, 10 μM veratridine was tested in the presence of the α1-adrenoceptor antagonist prazosin (1 μM). In these experiments, a complete blockade of contractions to 10 μM methoxamine verified an effective treatment, and a small vascular tone was induced by 5-HT (0.3–1 μM) before addition of veratridine in control and prazosin-treated vessels. Responses to veratridine were also tested after treatment with chemical sympatholytics, guanethidine (5 μM for 30 min) (33) or 6-hydroxydopamine (2 mM for 20 min, with 0.3% ascorbic acid) (36), followed by a small 5-HT (0.3–1 μM)-induced tone. The control vessels for 6-hydroxydopamine treatment were incubated with 0.3% ascorbic acid.
In separate sets of experiments, rat femoral artery and aorta were isolated and mounted in a myograph (Danish Myo Technology), as described above; basal tensions of 5 mN (4) and 10 mN (7) were used for femoral artery and aorta, respectively.
RNA extraction, cDNA synthesis, and PCR.
To explore the expression of NaV isoforms (NaV1.1–1.9), we used conventional RT-PCR of total RNA extracted from the isolated vessels. Three separate experiments (using vessels from 3 rats) were performed. Similar to previous studies (8, 22, 28), total RNA was extracted from rat mesenteric and femoral arteries using the RNeasy Micro kit (Qiagen) according to the manufacturer's instruction, which includes an on-column DNase treatment step. RNA quality was measured using a spectrophotometer (Nanodrop, Agilent Technologies), and oligo(dT)12–18 primers and Maloney's murine leukemia virus were used for RT. Negative controls (RT−) were carried out in the absence of RT and used to check for genomic contamination. Primers for all genes were taken from Fort et al. (12) and synthesized by Invitrogen.
Semiquantitative PCR was performed using Platinum Taq DNA polymerase (Invitrogen) with an initial denaturation step at 94°C followed by 35 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 1 min; the reaction was completed with a 10-min extension step. β-Actin served as an internal control; no-template controls were performed with every PCR. PCR product amplification was confirmed with 1% agarose gel electrophoresis and subsequent band quantification with National Institutes of Health ImageJ (http://rsb.info.nih.gov/ij/, 1997–2005).
Isolation of mesenteric myocytes.
Mesenteric artery smooth muscle cells were isolated as previously described (7). Briefly, mesenteric arteries were transferred to Ca2+-free physiological saline solution (mM: 6.0 KCl, 120 NaCl, 1.2 MgCl2, 10.0 d-glucose, and 10.0 HEPES, pH 7.3) supplemented with protease type X (0.5 mg/ml) and collagenase type IA (1.5 mg/ml) and incubated at 37°C for 15 min. Dispersed cells were maintained in 0.75 mM Ca2+ physiological saline solution and adhered to polylysine slides for 30 min at room temperature.
Immunocytochemistry.
Isolated myocytes were fixed and stained for confocal microscopy as previously described (7). Protein expression and localization were determined by immunofluorescence using antibodies against Nav (1:200 dilution; catalog no. AB5210, Millipore), NCX-1 (1:500 dilution; catalog no. R3F1, Swant), Na+-K+-ATPase (1:100 dilution; Ab 7671, Abcam), and smooth muscle α-actin (1:500 dilution; catalog no. A2547, Sigma). For control experiments, primary antibodies were omitted. The labeling was visualized with tetramethylrhodamine isothiocyanate donkey anti-rabbit IgG (1:100 dilution; catalog no. 711-025-152, Jackson ImmunoResearch) and FITC sheep anti-mouse IgG (1:100 dilution; catalog no. 515-095-062, Jackson ImmunoResearch). Images were gain-matched to ensure accuracy between samples.
Data and statistical analysis.
Vascular responses are given as absolute changes in tension (mN) or as percent contraction of the tone induced by methoxamine or KCl. Values are means ± SE; n represents the number of animals. Where concentration-response curves were obtained, the data were fitted using a sigmoidal logistic equation (Prism 4, GraphPad Software, San Diego, CA): y = bottom + (top − bottom)/{1 + 10[(logEC50−x)∗Hill slope]}, where x is a logarithm of drug concentration and y is the response that starts from the bottom and goes to the top in a sigmoid shape. The sigmoidal curves were also used to calculate pEC50 (the negative logarithm of the concentration of agent giving 50% of maximum response) and Emax (the maximal response). Statistical comparisons of vascular responses were made by Student's t-tests or one-way analysis of variance followed by Bonferroni's post hoc tests, where appropriate (Prism 4, GraphPad Software). P < 0.05 was taken as statistically significant.
Drugs.
Methoxamine, carbachol, TTX, suramin, guanethidine, 6-hydroxydopamine, and ascorbic acid (Sigma-Aldrich, Dorset, UK) and 5-HT and ranolazine (Tocris Biosciences, Bristol, UK) were dissolved in deionized water. Veratridine and capsaicin (Sigma) and A-803467 (Ascent Scientific, Bristol, UK) were dissolved in 100% ethanol. ProTx-II (kindly provided by Prof. John Wood, University College London), 9,11-dideoxy-9a,11a-methanoepoxyprostaglandin F2α (U46619) and KB-R7943 mesylate (Tocris), T16Ainh-A01 (Millipore), and prazosin (Sigma) were dissolved in dimethyl sulfoxide.
RESULTS
Complex effects of veratridine in the rat small mesenteric artery.
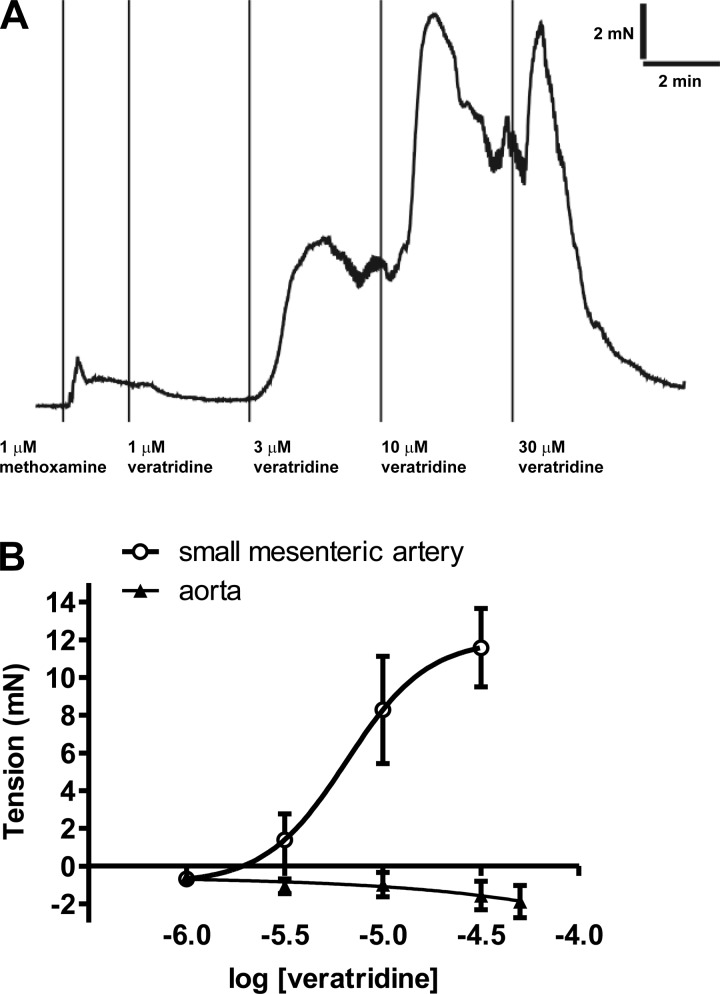
Veratridine induced concentration-dependent contractions (pEC50 = 5.19 ± 0.20, Emax = 12.0 ± 2.7 mN, n = 4; Fig. 1) in small mesenteric arteries; these contractions were rapid in onset and rarely sustained (Fig. 1A). Endothelium removal had no effect on contractions to a submaximal concentration (10 μM) of veratridine [9.2 ± 1.0 and 8.7 ± 2.4 mN with (n = 6) and without (n = 4) endothelium, respectively]. These responses were comparable to contractions induced by 10 μM methoxamine in the same vessels [8.1 ± 1.6 and 9.4 ± 3.0 mN with (n = 6) and without (n = 4) endothelium, respectively]. In contrast, ≤50 μM veratridine was a relatively ineffective spasmogen of rat aortic segments (Fig. 1B).
Fig. 1.
A: original trace showing veratridine responses in small mesenteric artery. B: concentration-dependent responses to veratridine in rat small mesenteric artery (n = 4) and aorta (n = 4).
Veratridine also induced relaxations of rat mesenteric artery that were evident at concentrations as low as 1 μM (Fig. 1A) and often preceded the contractile response to 10 μM veratridine (Fig. 2A). However, these relaxations were very variable, particularly at higher concentrations of veratridine. In vessels pretreated with 10 μM capsaicin followed by washout, which has previously been shown to desensitize perivascular sensory nerves (18), relaxation to 1 μM veratridine was abolished [−1.8 ± 0.6 mN (control) and 0.9 ± 0.6 mN (with capsaicin), P < 0.05, n = 4–7], while no significant effects on contractions to veratridine were observed [at 10 μM: 9.8 ± 2.2 mN (control) and 9.4 ± 2.4 mN (with capsaicin), n = 4–7]. Relaxant responses to veratridine were also seen in TTX-treated or endothelium-denuded, precontracted mesenteric arteries [%relaxation of methoxamine tone at 10 μM: 44.9 ± 13.3% (control), 34.2 ± 7.6% (without endothelium), and 54.6 ± 27.5% (with TTX), n = 5–7].
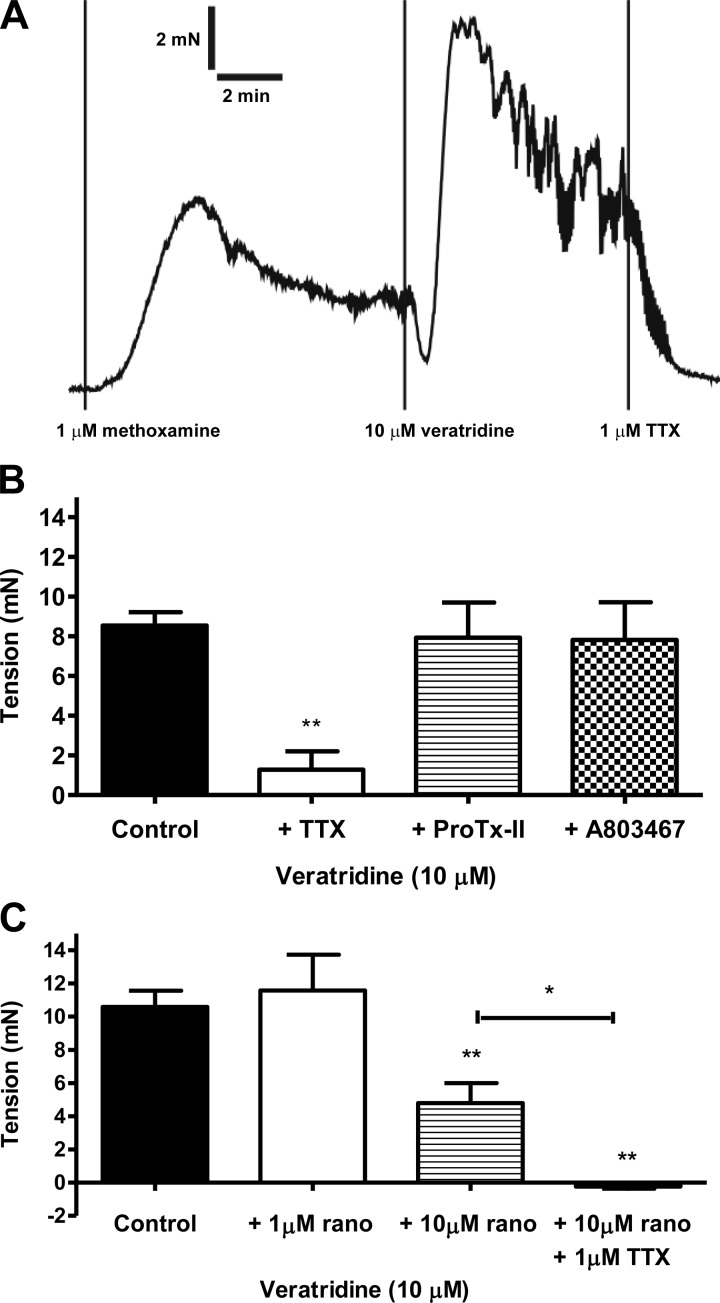
Fig. 2.
A: original trace showing veratridine contraction and its rapid reversal by application of TTX in small mesenteric artery. B: mesenteric contraction to veratridine in the absence (n = 12) and presence of TTX (1 μM, n = 4), ProTx-II (10 nM, n = 4), and A803467 (1 μM, n = 4). C: mesenteric contraction to veratridine in the absence (n = 6) and presence of ranolazine [rano; 1 μM (n = 4) and 10 μM (n = 7)] and ranolazine (10 μM) + TTX (1 μM, n = 4). **P < 0.01 vs. control; *P < 0.05 between treated groups (by 1-way ANOVA, followed by Bonferroni's post hoc tests).
Effects of TTX and other NaV channel blockers on veratridine-induced mesenteric contractions.
Removal of Na+ (replaced by choline) from the Krebs-Henseleit solution abolished contractions to veratridine (n = 3), suggesting that veratridine effects were mediated by Na+ influx. We then used an array of pharmacological agents to ascertain the nature of the ion channel proteins involved. TTX at 1 μM is a pore blocker of all NaV isoforms, except NaV1.5, NaV1.8, and NaV1.9 (25). Pretreatment with 1 μM TTX greatly inhibited (P < 0.01) contractions induced by veratridine (Fig. 2B). Application of TTX after veratridine also caused a rapid reversal of veratridine-induced contraction and sometimes reduced the tone to below the pre-veratridine level (Fig. 2A).
On the other hand, the selective NaV1.7 channel blocker ProTx-II (10 nM) (35) and the selective NaV1.8 channel blocker A-803467 (1 μM) (21) had no significant effect on veratridine (10 μM) responses (Fig. 2B). The NaV channel blockers TTX, ProTx-II, and A-803467 had no effect on the small methoxamine tone prior to veratridine additions [2.5 ± 0.8 mN (control) and 2.6 ± 0.5 mN (with toxin), n = 12].
Effects of ranolazine on veratridine-induced mesenteric contractions.
We found that ranolazine, a NaV1.4, NaV1.5, NaV1.7, and NaV1.8 channel blocker (13, 32, 37), alone relaxed precontracted tone induced by methoxamine (pEC50 = 5.93 ± 0.10, Emax = 99.4 ± 8.4%, n = 3), but not 60 mM KCl (relaxation at 10 μM = 3.8 ± 8.0%, n = 3). Because of the relaxant effects of ranolazine, a slightly modified protocol was used: vessels were contracted with a higher concentration of methoxamine (3–10 μM), followed by addition of ranolazine (1 or 10 μM) and then veratridine. Using this protocol, ranolazine, at 10 μM (P < 0.01) but not 1 μM, significantly reduced contractions to 10 μM veratridine (Fig. 2C). Treatment with 10 μM ranolazine + 1 μM TTX abolished (P < 0.01) the veratridine-induced contractions (P < 0.01; Fig. 2C).
Contractions to veratridine in mesenteric arteries precontracted with 60 mM KCl.
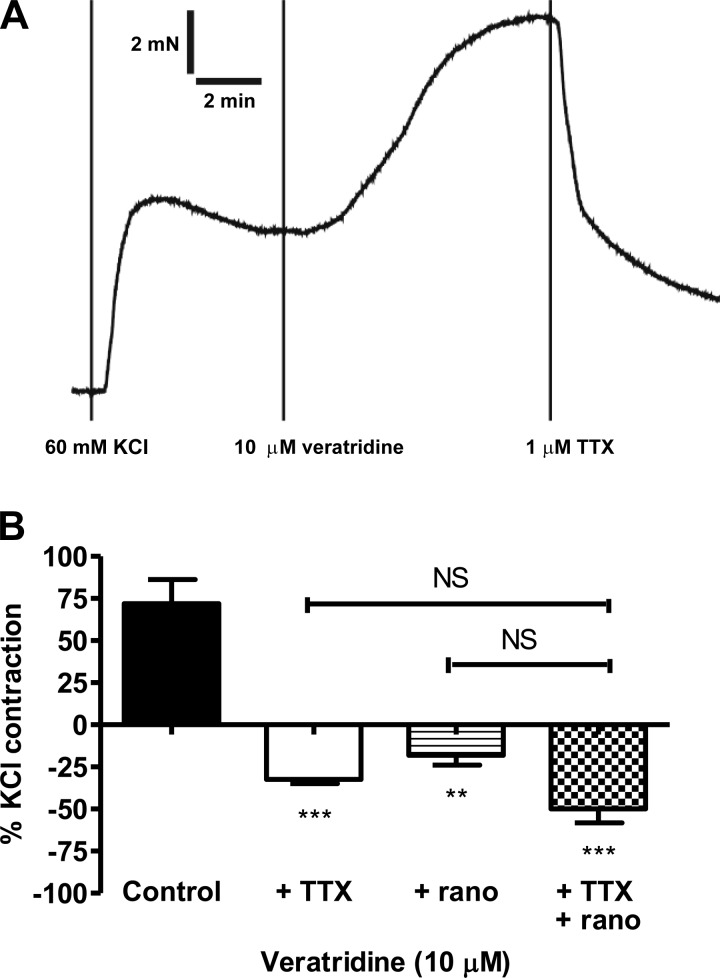
In vessels precontracted with 60 mM KCl, veratridine (10 μM) induced consistent, monophasic contractions (Fig. 3A). Pretreatment with 1 μM TTX (P < 0.05) or 10 μM ranolazine (P < 0.05) reverted the contractile responses to relaxation (Fig. 3B). Application of TTX after veratridine also reversed the veratridine-induced contraction (Fig. 3A). Treatment with TTX + ranolazine tended to produce an additive effect, but it did not reach statistical significance (Fig. 3B). TTX or ranolazine alone had no effect on KCl-induced tone [3.8 ± 0.8 mN (control) and 3.5 ± 0.6 mN (with toxin), n = 10].
Fig. 3.
A: original trace showing veratridine contraction (in the presence of 60 mM KCl) and its reversal by application of TTX in small mesenteric artery. B: mesenteric contraction to veratridine in the presence of 60 mM KCl. Responses were obtained in the absence (n = 8) and presence of TTX (1 μM, n = 4) or ranolazine (10 μM, n = 5) and TTX + ranolazine (n = 4). **P < 0.01, ***P < 0.001 vs. control (by 1-way ANOVA, followed by Bonferroni's post hoc tests). NS, not significant.
Effects of sympathetic inhibition on veratridine-induced mesenteric contractions.
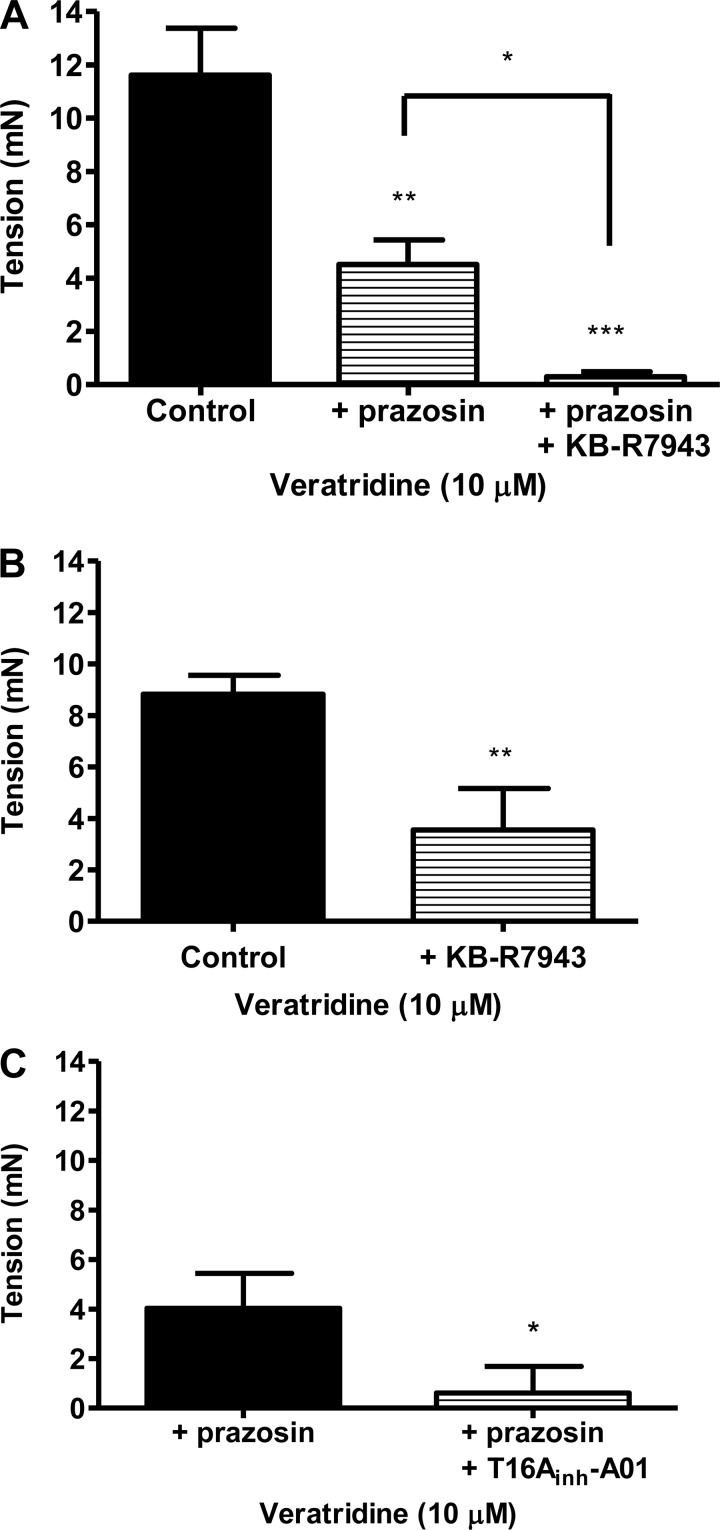
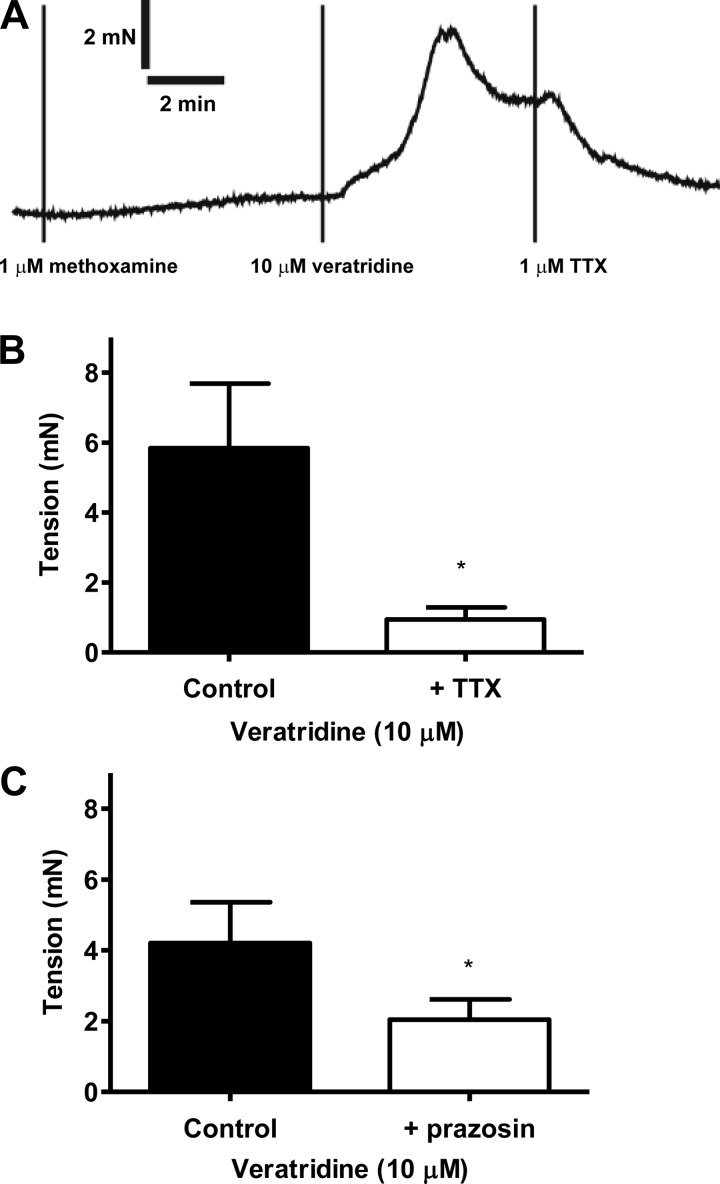
Activation of NaV channels on perivascular sympathetic nerves could lead to release of norepinephrine and ATP, which cause mesenteric contractions via activation of α1-adrenoceptors and P2 purinergic receptors, respectively (17, 23). The presence of suramin (100 μM), a nonselective P2Y receptor, had no significant effect on contraction to 10 μM veratridine [9.5 ± 2.2 mN (control) and 9.6 ± 0.3 mN (with suramin), n = 4 for both]. In contrast, prazosin (1 μM), an α1-adrenoceptor antagonist, which completely blocks methoxamine contractions (see methods), partially inhibited (by 61%) veratridine responses (Fig. 4A). Interestingly, the prazosin-resistant contraction to veratridine was abolished by KB-R7943 (Fig. 4A).
Fig. 4.
A: mesenteric contraction to veratridine in the absence (n = 5) and presence of prazosin (1 μM, n = 5) and prazosin (1 μM) + KB-R7943 (3 μM, n = 5). **P < 0.01, ***P < 0.001 vs. control; *P < 0.05 between treated groups (by 1-way ANOVA, followed by Bonferroni's post hoc tests). B: mesenteric contraction to veratridine in the absence (n = 8) and presence of KB-R7943 (3 μM, n = 8). **P < 0.01 vs. control (by Student's t-test). C: mesenteric contraction to veratridine in the presence of prazosin (1 μM, n = 4) and prazosin + T16Ainh-A01 (10 μM, n = 4). *P < 0.05 vs. control (by Student's t-test).
Additional experiments found that destruction of sympathetic nerves with 6-hydroxydopamine (2 mM) caused a 65% reduction in veratridine contractions [8.0 ± 1.3 mN (control) and 2.8 ± 1.3 mN (after 6-hydroxydopamine), n = 6 for both, P < 0.05]. However, it was somewhat surprising that inhibition of vesicular release of norepinephrine/ATP by guanethidine (5 μM) had no effect [10.7 ± 2.3 mN (control) and 10.1 ± 1.0 mN (with guanethidine), n = 4 for both]. We verified that the same guanethidine treatment abolished contractions elicited by electrical stimulation of sympathetic nerves in small mesenteric artery [at 12 Hz, 10 V for 0.5 ms: 5.4 ± 1.4 mN (control) and 0.3 ± 0.1 mN (with guanethidine), n = 6] and vas deferens (data not shown) of the rat. The sympathetic-mediated contractions in mesenteric artery were also blocked by prazosin (by 76 ± 2%, n = 4).
Effects of inhibitors of NCX and Ca2+-activated Cl− channels on veratridine-induced mesenteric contractions.
It has been postulated (3, 12, 34) that Na+ accumulation following NaV channel activity results in a rise in Ca2+ concentration because the NCX is driven into reverse mode (20, 30). Consequently, we studied whether KB-R7943, a selective inhibitor of the reverse mode of NCX (20), modified veratridine-induced contractions. It is clear from Fig. 4B that, at concentrations selective for NCX, KB-R7943 significantly reduced the veratridine-induced contraction by ∼60% (P < 0.01). We postulated that the rise in intracellular Ca2+ concentration ([Ca2+]i) due to reverse-mode NCX would be sufficient to activate Ca2+-activated Cl− channels, leading to membrane depolarization and enhanced opening of voltage-dependent Ca2+ channels. In rat mesenteric arteries incubated in 1 μM prazosin, preapplication of the TMEM16A-encoded Ca2+-activated Cl− channel blocker T16Ainh-A01 (9) resulted in a markedly impaired contractile response to 10 μM veratridine compared with arteries bathed in the vehicle control (Fig. 4C).
Effects of NaV channel blockers on U46619-induced mesenteric contractions.
Pretreatment with TTX (1 μM) or ranolazine (10 μM) had no significant effect on mesenteric contractions to the thromboxane analog U46619 [at 3 μM: 7.4 ± 1.3 mN (control), 5.9 ± 1.4 mN (with TTX), and 8.9 ± 1.7 mN (with ranolazine), n = 4–5].
Contractions to veratridine in rat femoral artery.
Recently, it has been shown that hypoxia induced contractions of rat femoral artery that were prevented by a NaV channel blocker (4). We found that, under normoxic conditions, veratridine was an effective constrictor of rat femoral arteries and its response was ablated by application of TTX before or during veratridine challenge (Fig. 5, A and B). In addition, prazosin (1 μM) attenuated veratridine responses by ∼51% (P < 0.05; Fig. 5C). The results with 6-hydroxydopamine (2 mM) treatment were more variable; it reduced contraction to veratridine only in three of six vessels [5.0 ± 2.3 mN (control) and 2.3 ± 0.7 mN (after 6-hydroxydopamine), not significantly different, n = 6 for both]. Veratridine contractions were insensitive to 5 μM guanethidine [3.2 ± 1.0 mN (control) and 4.2 ± 0.9 mN (with guanethidine), n = 3 for both].
Fig. 5.
A: original trace showing veratridine contraction and rapid reversal by application of TTX in femoral artery. B: femoral contraction to veratridine in the absence (n = 5) and presence of TTX (1 μM, n = 4). *P < 0.05 vs. control (by Student's t-test). C: femoral contraction to veratridine in the absence (n = 7) and presence of prazosin (1 μM, n = 7). *P < 0.05 vs. control (Student's t-test).
Expression of NaV isoforms in rat small mesenteric and femoral arteries.
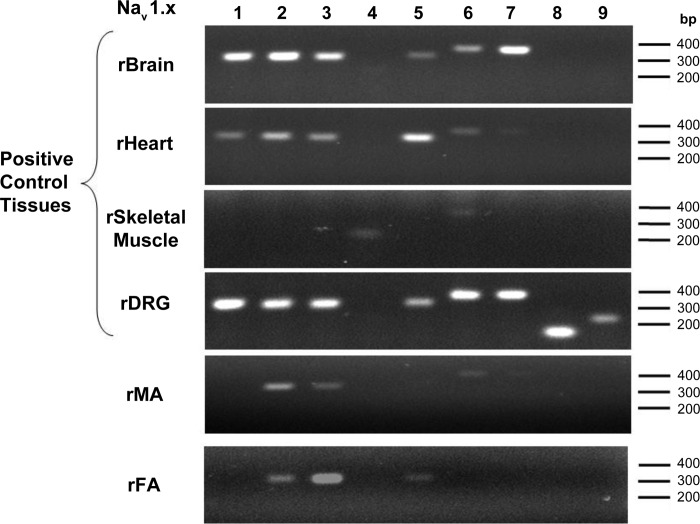
Among the NaV isoforms, mRNA transcripts for the α-subunits of NaV1.2 and NaV1.3 channels (both are TTX-sensitive) were consistently detected in all samples of mesenteric and femoral arteries (Fig. 6). On the other hand, expression of NaV1.5 (TTX-resistant) and NaV1.6 and NaV1.7 (TTX-sensitive) α-subunits was variable; for example, NaV1.5 was detected in two of three femoral arteries and one of three mesenteric arteries. The housekeeping gene β-actin was used as internal positive control, as well as no template controls, for all PCRs (see methods; data not shown). The expression pattern of all NaV isoforms in control tissues, brain, heart (mainly NaV1.5), skeletal muscle (mainly NaV1.4), and dorsal root ganglia (NaV1.8 and NaV1.9), is also shown in Fig. 6.
Fig. 6.
Analysis of voltage-activated Na+ (NaV) channel transcripts in rat small mesenteric (rMA) and femoral (rFA) arteries. Brain, heart, skeletal muscle, and dorsal root ganglia (DRG) from the rat were positive controls for various NaV isoforms of α-subunits.
Expression of NaV and NCX proteins in mesenteric smooth muscle cells.
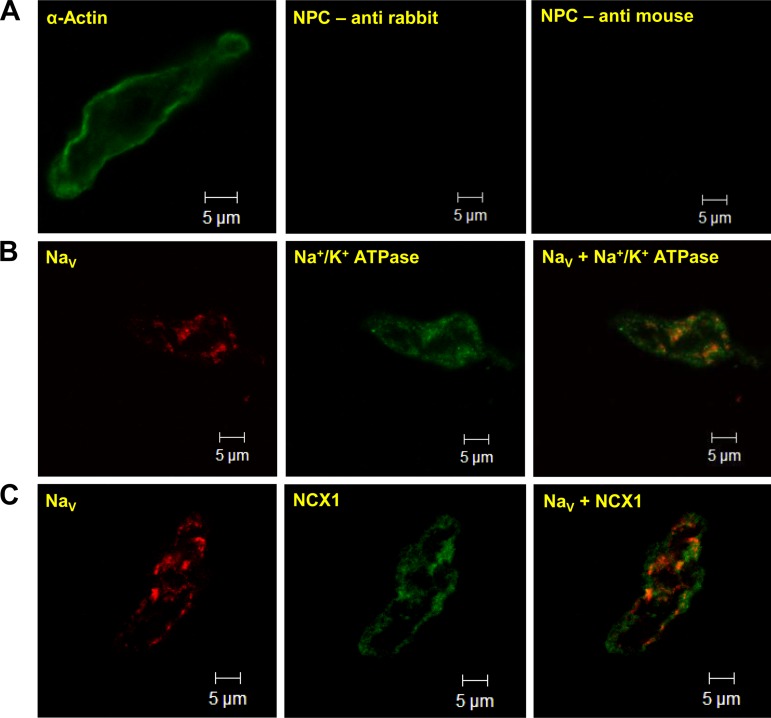
The working hypothesis is that veratridine-induced contractions are due to accumulation of Na+ and NCX being forced into reverse mode. This would necessitate the close association of NaV and NCX proteins to allow the creation of functional microdomains. We therefore ascertained whether NaV and NCX colocalized in rat mesenteric artery. Single-cell immunocytochemistry on mesenteric myocytes (Fig. 7), confirmed by positive staining for α-actin, showed significant expression of NaV protein. This represents the overall level of NaV expression, since a pan-NaV antibody was used to visualize all NaV isoforms in the myocytes. Immunoreactivity for NaV also costained with the membrane marker Na+-K+-ATPase, indicating that significant NaV expression was localized to the plasma membrane (Fig. 7B). Importantly, NaV displayed considerable colocalization with NCX1 protein (Fig. 7C), the predominant NCX isoform in vascular smooth muscle (40).
Fig. 7.
Identification of NaV protein in freshly isolated mesenteric smooth muscle cells. Cell type was confirmed by positive staining of smooth muscle α-actin (A; catalog no. A2547, Sigma; 1:500 dilution). NPC, control cells with no primary antibodies. B and C: representative fluorescent images of isolated smooth muscle cells coincubated with anti-NaV (pan-NaV; Ab 5210, Millipore; 1:200 dilution) and the membrane marker anti-Na+-K+-ATPase (Ab 5210, Abcam; 1:200 dilution) or anti-NaV and anti-Na+/Ca2+ exchanger type 1 (NCX1; R3F1, Swant; 1:500 dilution). Overlay images indicate coexpression of NaV channel with Na+-K+-ATPase and with NCX1. Control cells with no primary antibodies (NPC) showed minimal fluorescence (A), confirming specificity of signals in B and C.
DISCUSSION AND CONCLUSIONS
In rat isolated small mesenteric artery, we demonstrated that veratridine induced endothelium-independent contractions at concentrations (pEC50 ∼5.2) considerably less than those required to contract rat aorta (present study; 12). We did not observe contractile responses to ≤50 μM veratridine in rat aorta. The mesenteric contractions were predominantly inhibited by TTX, suggesting the involvement of TTX-sensitive NaV channels (NaV1.1, NaV1.2, NaV1.3, NaV1.4, NaV1.6, and NaV1.7). In an attempt to further explore the functional NaV isoforms in mesenteric arteries, the effects of a range of NaV channel inhibitors against veratridine contractions were also tested. The selective blockers of NaV1.7 (ProTx-II) (35) and NaV1.8 (A-803467) channels (21) had no significant effect. In contrast, the novel antianginal agent ranolazine, which mainly blocks NaV1.5, but also NaV1.4, NaV1.7, and NaV1.8 (13, 32, 37), channels, partly inhibited veratridine-induced contraction. Ranolazine also abolished the TTX-resistant contraction to veratridine, suggesting the presence of TTX-sensitive NaV channels (NaV1.1, NaV1.2, NaV1.3, 1.4, and NaV1.6) and the TTX-resistant NaV1.5 channel. However, ranolazine, at concentrations commonly used to block NaV, has been found to antagonize vasocontractions to methoxamine, but not KCl. This could be due to an antagonist action of ranolazine at the α1-adrenoceptor (1). Thus the ranolazine data must be interpreted cautiously. Additional experiments revealed that, in arteries depolarized with 60 mM KCl [depolarization of ∼20–30 mV (10)], veratridine contractions were also sensitive to TTX and ranolazine, but the two blockers did not exert a significant additive effect. Thus it remains unclear if the NaV1.5 channel plays a role in the contractile effects of veratridine. Also, although mRNA transcripts for NaV1.2 and NaV1.3 α-subunits were consistently found in isolated mesenteric arteries, the NaV1.5 α-subunit was detected in only some of the arteries. NaV1.2 and NaV1.3 channels are sensitive to TTX, and their expression would be consistent with the above-mentioned pharmacological data. We therefore conclude that mesenteric contraction to veratridine is predominantly mediated by NaV1.2 and NaV1.3 channels. This represents the first study to characterize the effects of NaV channel modulators on contractility of the rat small mesenteric artery, which is frequently used to model systemic resistance arteries crucial for blood pressure regulation. Our pharmacological and molecular data also corroborate the detection of NaV currents, with high sensitivity to TTX, in freshly isolated myocytes from the same vessel (3). Selective blockers of the TTX-sensitive, NaV1.2 and NaV1.3 channels are unavailable, but future immunohistochemical studies using isoform-selective antibodies would help pinpoint the isoforms(s) involved.
NCX plays a critical role in regulating [Ca2+]i in vascular myocytes. It operates in a forward mode (for Ca2+ extrusion) or a reverse mode (for Ca2+ entry), depending on electrochemical gradients of Na+ and Ca2+ across the plasma membrane. Increasing evidence suggests that elevation of intracellular Na+ concentration, which drives NCX into the reverse mode, contributes to agonist-mediated Ca2+ entry and contraction (19, 30). Genetic deletion of NCX1 in vascular smooth muscle and pharmacological inhibitors of the reverse mode of NCX lower blood pressure (19, 40). NCX1 also underlies salt-sensitive hypertension (19). Thus there has been much interest in the therapeutic potential of NCX inhibitors. Here, we found that inhibition by KB-R7943 (3 μM) of the reverse mode of NCX (20) compromised mesenteric contraction to veratridine, suggesting a functional coupling between NaV and NCX. Using single-cell immunocytochemistry in mesenteric myocytes, we have now demonstrated the close proximity of NaV and NCX1 proteins on the plasma membrane. Although NCX reversal has increasingly been implicated in Ca2+ influx and vascular contractility, the ion channel or transporter responsible for the localized Na+ accumulation remains unclear. Our data suggest that this could be mediated by activation of NaV channels, which represents a novel route of Na+ entry in myocytes. Our data also suggest that recruitment of Ca2+-activated Cl− channels is involved in this process. Reverse-mode NCX driven by raised intracellular Na+ concentration activates Ca2+-activated Cl− currents in rabbit portal vein myocytes (24), and the electrophysiological interplay of Nav, NCX, and Ca2+-activated Cl− channels in rat mesenteric artery will be the focus of future experiments.
In this study, we also found that veratridine induced TTX-sensitive contraction in the rat femoral artery. This is consistent with a role for the NaV channel in resistance (e.g., small mesenteric) and conduit (e.g., femoral and aorta) arteries, but our data also suggest a larger impact of the NaV channel on the diameter of smaller arteries; veratridine efficacy ranked as follows: small mesenteric > femoral >>> aorta. As in the mesenteric artery, messages for the α-subunits of NaV1.2 and NaV1.3 are evident in the femoral artery. NaV1.5, NaV1.6, and NaV1.7 channels can also be detected in some, but not all, femoral arteries. This corroborates a recent report of the NaV1.5 channel in rat femoral artery (4). Interestingly, only the Nav1.2 channel was detected in the smooth muscle layer of rat aorta (12). Together, these data provide strong evidence for a role of NaV channels in vascular myocytes throughout the vasculature. It is worth stressing that veratridine contractions in mesenteric and femoral arteries were significantly reduced by sympathetic inhibition, implicating the involvement of NaV channels in sympathetic nerves surrounding the vessels and the subsequent release of norepinephrine. However, a considerable contraction to veratridine remained in mesenteric and femoral arteries, despite complete blockade of α1-adrenoceptor-mediated contraction by prazosin or chemical destruction of sympathetic nerves by 6-hydroxydopamine. In contrast, Fort et al. (12) reported that, in rat aorta, TTX-sensitive contractions to veratridine are completely inhibited by prazosin, although they used much higher concentrations of veratridine (100 μM) than those used in the current study. These data point to a differential role of NaV in vascular myocytes vs. sympathetic nerves depending on the vascular regions, which could have important pathophysiological implications. It is also worth noting that veratridine also evoked relaxation in mesenteric arteries under certain conditions. This could be due to NaV channels expressed in the endothelium, resulting in relaxation, counteracting the contraction mediated by NaV channels in vascular smooth muscle (11). This is unlikely, since the relaxation was also observed in mesenteric arteries without endothelium or in the presence of TTX. It was, however, inhibited by capsaicin treatment, indicating the involvement of TRPV1-expressing sensory nerves and the release of vasodilatory neuropeptides (18, 39). Indeed, relaxation to veratridine was absent in femoral arteries, which display little capsaicin-sensitive relaxation (15). Further investigation is warranted to clarify the role of NaV channels, especially the TTX-resistant isoforms, in vasorelaxation to veratridine.
Our working model is that activation of NaV channels in vascular myocytes increases intracellular Na+, which then induces Ca2+ influx through the reverse mode of NCX. The subsequent rise in [Ca2+]i is sufficient to activate Ca2+-activated Cl− channels, leading to membrane depolarization, and the subsequent influx of Ca2+ through voltage-dependent Ca2+ channels results in arterial contractions. This is supported by our pharmacological, molecular, and immunocytochemical evidence. However, contractions induced by methoxamine or KCl were not significantly attenuated by TTX alone. Similarly, TTX and ranolazine do not significantly modify contractions to a thromboxane analog. This suggests that NaV channels are not recruited by these vasoconstrictor mechanisms and would appear to have little physiological impact. However, veratridine acts by binding to open NaV channels to increase activation at more negative membrane potential and greatly reduces inactivation of the channels, resulting in sustained Na+ influx (2, 38). The generation of large contractions by this agent implies that inactivated NaV channels are present in these arteries and represent a considerable contractile “reserve.” Consequently, any pathophysiological mechanism that enhances NaV channel activity will have a large impact on arterial contraction and blood pressure. Our data would also indicate that the NaV channel contractile reserve in vascular smooth muscle is much larger in resistance arteries, which play a major role in the regulation of regional blood flow and blood pressure, than in conduit arteries, especially the aorta. It has been shown previously that hypoxia can produce robust contractions, which are sensitive to NaV channel blockers (4), and the focus of future studies will be to ascertain the mechanisms that allow NaV channel activity to be manifest in blood vessels. Interestingly, application of TTX after veratridine often reduced the tone to below the pre-veratridine level (cf. Fig. 2B), a phenomenon observed on spontaneous activity in mouse portal vein (34). These consistent findings suggest that the role of NaV channels and NCX in vascular smooth muscle is considerably more complex than envisaged and that once NaV channels are activated, inhibition of the Na+ influx leads to an overdrive of Ca2+ extrusion via NCX.
In conclusion, the present study confirms that NaV channels in vascular myocytes are functionally coupled to contraction. Arterial contractions to agonists of α1-adrenoceptors and thromboxane receptors or a depolarizing concentration of KCl are not dependent on NaV channel activity. However, these channels can be “activated” by veratridine, leading to contractions via the reverse mode of NCX. Our results reveal that NaV channels represent a means to increase arterial tone considerably and highlight the need to elucidate the function of vascular NaV channels in physiological and pathological conditions.
GRANTS
P. S. Chadha and A. J. Davis were funded by British Heart Foundation Grants PG/09/104 and PG/07/127/24235 to I. A. Greenwood.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.-S.V.H. and I.A.G. are responsible for conception and design of the research; W.-S.V.H., A.J.D., and P.S.C. performed the experiments; W.-S.V.H., A.J.D., and P.S.C. analyzed the data; W.-S.V.H., A.J.D., P.S.C., and I.A.G. interpreted the results of the experiments; W.-S.V.H., A.J.D., and P.S.C. prepared the figures; W.-S.V.H. and I.A.G. drafted the manuscript; W.-S.V.H., A.J.D., P.S.C., and I.A.G. edited and revised the manuscript; W.-S.V.H., A.J.D., P.S.C., and I.A.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the staff at the Image Resource Facility (St. George's University of London) for technical assistance with fluorescence microscopy. We are grateful to Dr. R. Docherty (King's College London) for the single-copy genes used in end-point PCR and Harry Pritchard and Dr. James Moffatt (St. George's University of London) for technical support.
REFERENCES
- 1. Allely MC, Brown CM, Kenny BA, Kilpatrick AT, Martin A, Spedding M. Modulation of α1-adrenoceptors in rat left ventricle by ischaemia and acyl carnitines: protection by ranolazine. J Cardiovasc Pharmacol 21: 869–873, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Barnes S, Hille B. Veratridine modifies open sodium channels. J Gen Physiol 91: 421–443, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berra-Romani R, Blaustein MP, Matteson DR. TTX-sensitive voltage-gated Na+ channels are expressed in mesenteric artery smooth muscle cells. Am J Physiol Heart Circ Physiol 289: H137–H145, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bocquet A, Sablayrolles S, Vacher B, Le Grand B. F 15845, a new blocker of the persistent sodium current, prevents consequences of hypoxia in rat femoral artery. Br J Pharmacol 161: 405–415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13–25, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XXXIX Compendium of voltage-gated ion channels: sodium channels. Pharmacol Rev 55: 575–578, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chadha PS, Zunke F, Davis AJ, Jepps TA, Linders JT, Schwake M, Towart R, Greenwood IA. Pharmacological dissection of Kv7.1 channels in systemic and pulmonary arteries. Br J Pharmacol 166: 1377–1387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol 299: C948–C959, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis AJ, Shi J, Pritchard HA, Chadha PS, Leblanc N, Vasilikostas G, Yao Z, Verkman AS, Albert AP, Greenwood IA. Potent vasorelaxant activity of the TMEM16A inhibitor T16Ainh-A01. Br J Pharmacol 168: 773–784, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Favaloro JL, McPherson GA. Vasorelaxation and hyperpolarisation of rat small mesenteric artery by the quaternary anion tetraphenylboron. Naunyn Schmiedebergs Arch Pharmacol 369: 367–373, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol 293: H1371–H1383, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Fort A, Cordaillat M, Thollon C, Salazar G, Mechaly I, Villeneuve N, Vilaine JP, Richard S, Virsolvy A. New insights in the contribution of voltage-gated Nav channels to rat aorta contraction. PLos One 4: e7360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol 148: 16–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol 63: 871–894, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Gyimah P, Ho V. Actions of calcium-sensing receptor ligands in mesenteric, femoral and pulmonary arteries from the rat (Abstract). Proc Br Pharmacol Soc 9: 23, 2011 [Google Scholar]
- 16. Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol 150: 641–651, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hogestatt ED, Andersson KE. On the postjunctional α-adrenoreceptors in rat cerebral and mesenteric arteries. J Auton Pharmacol 4: 161–173, 1984 [DOI] [PubMed] [Google Scholar]
- 18. Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991 [PubMed] [Google Scholar]
- 19. Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem 271: 22391–22397, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104: 8520–8525, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 124: 602–611, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Lagaud GJ, Stoclet JC, Andriantsitohaina R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J Physiol 492: 689–703, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leblanc N, Leung PM. Indirect stimulation of Ca2+-activated Cl− current by Na+/Ca2+ exchange in rabbit portal vein smooth muscle. Am J Physiol Heart Circ Physiol 268: H1906–H1917, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Lee CH, Ruben PC. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels (Austin) 2: 407–412, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Meguro K, Iida H, Takano H, Morita T, Sata M, Nagai R, Nakajima T. Function and role of voltage-gated sodium channel NaV1.7 expressed in aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 296: H211–H219, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Mironneau J, Martin C, Arnaudeau S, Jmari K, Rakotoarisoa L, Sayet I, Mironneau C. High-affinity binding sites for [3H]saxitoxin are associated with voltage-dependent sodium channels in portal vein smooth muscle. Eur J Pharmacol 184: 315–319, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162: 42–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Platoshyn O, Remillard CV, Fantozzi I, Sison T, Yuan JX. Identification of functional voltage-gated Na+ channels in cultured human pulmonary artery smooth muscle cells. Pflügers Arch 451: 380–387, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van Breemen C. Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res 101: 1030–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Quignard JF, Ryckwaert F, Albat B, Nargeot J, Richard S. A novel tetrodotoxin-sensitive Na+ current in cultured human coronary myocytes. Circ Res 80: 377–382, 1997 [PubMed] [Google Scholar]
- 32. Rajamani S, Shryock JC, Belardinelli L. Block of tetrodotoxin-sensitive, NaV1.7, and tetrodotoxin-resistant, NaV18, Na+ channels by ranolazine. Channels (Austin) 2: 449–460, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Ralevic V, Burnstock G. Mesenteric arterial function in the rat in pregnancy: role of sympathetic and sensory-motor perivascular nerves, endothelium, smooth muscle, nitric oxide and prostaglandins. Br J Pharmacol 117: 1463–1470, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saleh S, Yeung SY, Prestwich S, Pucovsky V, Greenwood I. Electrophysiological and molecular identification of voltage-gated sodium channels in murine vascular myocytes. J Physiol 568: 155–169, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, Kaczorowski GJ, Garcia ML, Koltzenburg M, Priest BT. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol 74: 1476–1484, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Shiraki H, Kawasaki H, Tezuka S, Nakatsuma A, Kurosaki Y. Endogenous calcitonin gene-related peptide (CGRP) mediates adrenergic-dependent vasodilation induced by nicotine in mesenteric resistance arteries of the rat. Br J Pharmacol 130: 1083–1091, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol 73: 940–948, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal 15: 151–159, 2003 [DOI] [PubMed] [Google Scholar]
- 39. White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol 134: 921–929, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP, Iwamoto T, Philipson KD, Kotlikoff MI, Santana LF, Wier WG, Matteson DR, Blaustein MP. Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol 298: H1472–H1483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]