Abstract
Objective
The aim of this study is to introduce a microvascular training model based on use of materials that can be easily obtained from the daily surroundings.
Methods
Simple microinstruments and a medical school laboratory microscope were used for anastomosis training. Chicken blood vessels were used as a material for this study. A long segment of blood vessel from the proximal brachial artery to the distal radial artery was used for training. End-to-side anastomosis was practiced first, and the training continued with end-to-end anastomosis of the appropriate segments.
Results
The instruments used for setting up this model were simple and easy to use; therefore, the time required for preparation of the materials and dissection of the chicken wings was only approximately five to ten minutes. The characteristics of 20 chicken wings were analyzed. The length of the brachial artery to the radial artery was 8 - 10 cm. The average diameter of the brachial artery was 1.3 mm ± 0.2 mm and that of the radial artery was 1.0 mm ± 0.2 mm. Taking advantage of these characteristics, the proximal brachial artery was grafted to the radial artery for practice of end-to-side anastomosis.
Conclusions
This study suggests an effective and feasible method for microvascular anastomosis training using chicken wing arteries and simple microinstruments. This model may simulate the conditions of a superficial temporal artery to middle cerebral artery anastomosis surgery.
Keywords: Chickens, Surgical anastomosis, Brachial artery, Training
INTRODUCTION
Cerebral revascularization has been useful in treatment of cerebral hemodynamic failure, in skull base surgery, and in complex aneurysm surgery. In addition, the beneficial effects of cerebral revascularization in treatment of acute stroke have been reported in recent years.3),8) Accordingly, there is an increasing demand for microvascular anastomosis training, which is considered a basic training technique of microsurgery in the neurosurgical department. A variety of materials and methods are used in microvascular anastomosis training, and some studies have reported on the advantages and disadvantages of each material.1),2),4) Skill in performing the microvascular suture technique can only be acquired through daily practice.14) Therefore, the training model must be feasible in daily life and as similar to real-life surgery conditions as possible. In reality, it is not easy for neurosurgeons to invest more than one hour per day in microvascular training. In addition, microvascular anastomosis training requires a great deal of preparation, including the preparation of instruments, microscopes, sutures, and blood vessels.
The aim of this study is to introduce a microvascular training model based on use of materials that are easily accessible from the daily surroundings.
MATERIALS AND METHODS
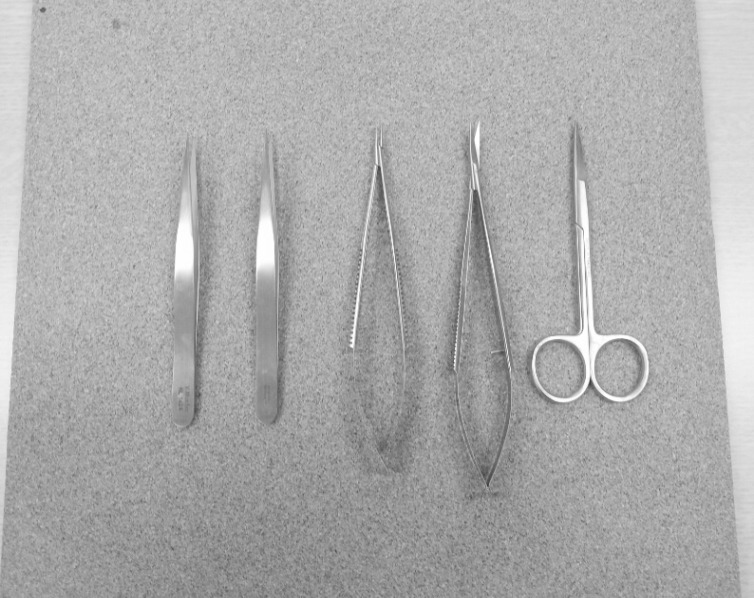
The microinstruments used in this study included two microforceps (jeweler's forceps No.3), one microneedle holder, one pair of microscissors, and one pair of Iris scissors (Fig. 1). All other necessary materials were obtained from the hospital. A desktop medical school laboratory microscope was used for the anastomosis training. The microscope had fixed magnification (10 × and 30 ×) and was easily available from the school or the hospital (Fig. 2). For this study, the 10 × magnification was mainly used.
Fig. 1.
The microinstruments used in this study include two microforceps (jeweler's forceps No.3), one microneedle holder, one pair of microscissors and one pair of Iris scissors.
Fig. 2.
A desktop medical school laboratory microscope with fixed magnification is used for the anastomosis training because it is easily available from the school or the hospital. The magnifications of the microscope are 10× and 30×. The 10× magnification is primarily used.
In this study, the materials used for performance of anastomosis were the blood vessels of chicken wings. Chicken wings were easily purchased from an online market; whole chicken wings were needed for this study, however, they were difficult to obtain from offline markets in Korea. The wings were from eight- to ten-week-old chickens weighing between 1.3 and 1.4 kg. Twelve to 13 chicken wings were purchased at the same time and kept in a freezer. Three of the frozen chicken wings were thawed together to be used at the same time. Once extracted, the blood vessels were kept in a refrigerator. The authors were able to practice three times with these blood vessels.
First, we practiced daily with gauze using 10-0 nylon suture. After approximately three months of training with gauze, training with the blood vessels extracted from chicken wings was started. A long segment of blood vessel from the proximal brachial artery to the distal radial artery was used for the training. The blood vessels were extracted from the proximal shoulder joint to the distal wrist joint. The extraction was initiated from the inside of the elbow joint where the radial artery is clearly visible and continued toward the proximal and distal parts (Fig. 3). The proximal end of the brachial artery was considered as the superficial temporal artery (STA) and the radial artery was considered as the cortical branch of the middle cerebral artery (MCA) (Fig. 4).
Fig. 3.
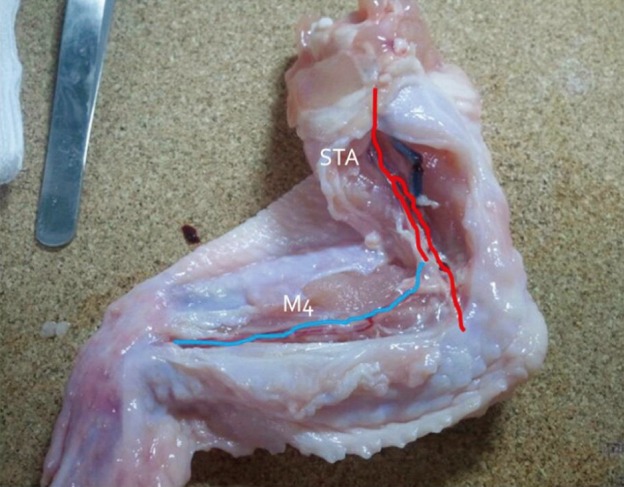
Blood vessels were extracted from the proximal brachial artery to the distal radial artery. The dissection was initiated from the inside of the elbow joint where the radial artery is clearly visible and continued toward the proximal and distal parts.
Red: brachial artery of a chicken wing, blue: radial artery of a chicken wing, STA: superficial temporal artery, M4: middle cerebral artery cortical branch.
Fig. 4.
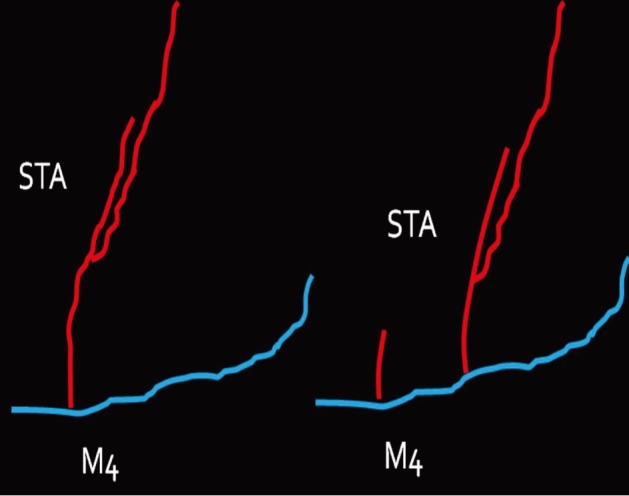
The proximal end of the brachial artery was considered to be the superficial temporal artery and the radial artery the cortical branch of the middle cerebral artery.
Red: brachial artery of a chicken wing, blue: radial artery of a chicken wing, STA: superficial temporal artery, M4: middle cerebral artery cortical branch.
End-to-side anastomosis was practiced first, followed by end-to-end anastomosis using appropriate segments. Saline was injected to check the patency of the anastomosis sites, and the vessels were incised to check for the presence of any intima damage (Fig. 5).
Fig. 5.
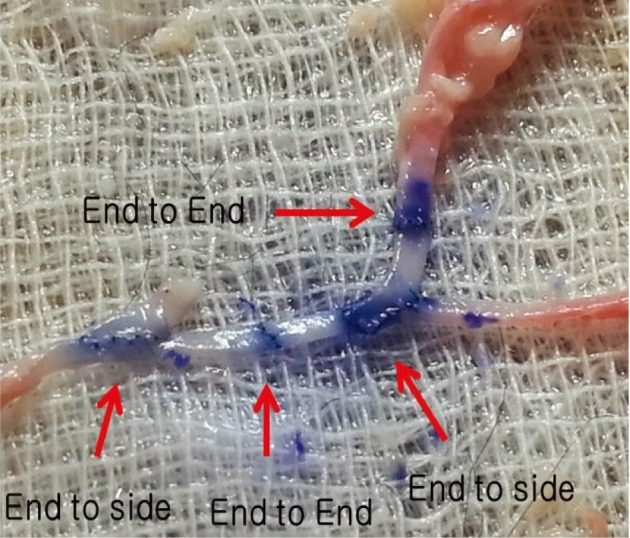
End-to-side anastomosis is practiced first, followed by end-to-end anastomosis training using appropriate segments.
RESULTS
The instruments used in this study were simple and easy to use; therefore, the time required for preparation of the materials and dissection of the chicken wings was only approximately five to ten minutes. The practice was stopped in order to attend to an emergency situation, if any, and the practice was resumed afterward. However, it was found that the interruption did not have a significant effect on the training, and practicing more than three times per week was feasible.
The characteristics of 20 chicken wings, which were purchased in three batches, were analyzed. The average diameter of the brachial artery was larger than that of the radial artery. The length from the brachial artery to the radial artery was 8 - 10 cm. The average diameter of the brachial artery was 1.3 mm ± 0.2 mm and that of the radial artery was 1.0 mm ± 0.2 mm (Fig. 6). With the long segment of blood vessel, considering that the vessel becomes finer as it progresses from the proximal part to the distal part, the end of the proximal brachial artery was anastomosed to the side of the radial artery for practice of end-to-side anastomosis. Careful dissection of the periadventitial tissue was required before anastomosis. One blood vessel was used several times.
Fig. 6.
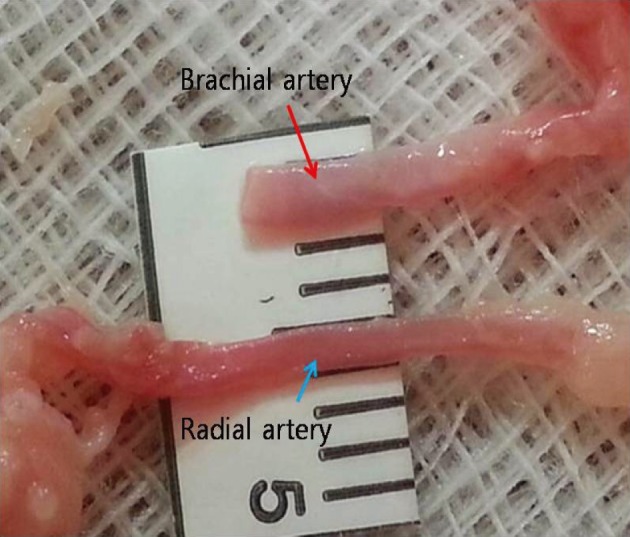
The average diameter of the brachial artery is 1.3 mm ± 0.2 mm and that of the radial artery is 1.0 mm ± 0.2 mm.
DISCUSSION
Preparation of animal models and simulation models for microvascular training is difficult for young neurosurgeons. Inoue et al.7) recommended microvascular training using gauze and a desk-type microscope, which minimized the preparation time to almost "zero" and allowed surgeons to spend their limited time on their actual practice. Young neurosurgeons are thought to need to practice of approximately 10,000 stitches before actually performing an STA-to-MCA anastomosis.7) However, Lascar et al.12) reported that there was no significant statistical difference in patency rates between the expert group and the beginner group after practicing 50 times. Therefore, it was estimated that beginners would need to practice approximately 2000 stitches using gauze for three months before starting training with blood vessels of chicken wings. At our institution, at least three months of gauze training was required and then artificial vessels were used. Chicken wing arteries have very similar consistency to that of the STA and MCA; therefore they are very practical and useful for simulation of real operation conditions. Nevertheless, more and more practice leads to good results.
Another issue was the surgical microscope. Surgical microscopes are available in operation rooms in most hospitals. However, because chicken wings or other live animals are considered to be pathogen bearing, they are not permitted in operation rooms. Training with a surgical microscope would have been ideal; however, it was not feasible to obtain one because of the high cost. Hence, a medical school laboratory microscope (with fixed magnification) was used in this study, and no problems were reported with regard to its use in the training with either gauze or chicken wing arteries.
Many materials, such as synthetic materials, chicken neck, chicken wing, pig leg, placenta and cadaver vessels, can be used for microvascular training.11) Chicken wing arteries have been proven as suitable materials for microvascular anastomosis training because they are cheap, easy to purchase, convenient to manage, do not require a specific facility nor anesthesia use (like maintaining living animals do), and are similar to human vessels.2),4),5),6),10),14) In addition, use of this model can reduce the numbers of live animals used in experiments by 80 - 100%.15) The average size of the cortical branch of the MCA is known to be 1.0 mm (range, 0.6 - 1.4 mm), which is similar to the size of the radial artery of chicken wings.9) The structure of a chicken wing resembles that of a human arm. According to Levinsohn et al.,13) the blood vessels in a chicken wing stretch down to the brachial artery lying parallel to the humerus and are divided into the radial artery and the ulnar artery at the middle of the humerus and stretch further down to the end of the wings. Among these, the brachial artery, radial artery and ulnar artery are considered to be the most appropriate for training, although dissection of the ulnar artery takes a long time. In our study, a long segment of blood vessel from the proximal brachial artery to the distal radial artery was used for training. As long as the anatomy of a chicken wing is well understood, vascular dissection in a chicken wing can be completed within approximately five minutes.
The diameters of the brachial and radial arteries of domestically distributed chicken wings were found to be relatively similar to the average diameters of the human brachial artery (1.2 mm) and radial artery (1.0 mm); therefore, they were judged to be suitable for use in training. In addition, the diameter of the cortical branch of the MCA in patients with moyamoya disease is often < 1 mm. With the use of a long segment of blood vessel, considering that the vessel becomes finer as it progresses from the proximal part to the distal part, the above anastomosis methods were devised and performed. The anastomosis methods practiced in this study were similar to those of STA-to-MCA anastomosis. In addition, one blood vessel could be used several times.
Between chicken wing blood vessels and human blood vessels, it was found that more meticulous suturing was required for the blood vessels of chicken wings because there was more suture leakage.14) Chicken wings require more extensive dissection of the periadventitial tissue layers than intracranial vessels. However, this also provides essential training in dissection and prepares the STA for anastomosis.1) On completion of anastomosis, infusion of saline was administered in order to check patency and the vessels were incised to check for the presence of any intima damage.10),14)
This report has some differences from previous reports. First, a long segment of blood vessel from the proximal brachial artery to the distal radial artery was used for training. Second, the authors took advantage of the difference in blood vessel diameters. Third, the equipment used is introduced in detail. Therefore, on the basis of this article, even without other information, readers could start microvascular anastomosis training.
CONCLUSION
A desktop microscope, simple and inexpensive microinstruments, and a long segment of chicken blood vessel are sufficient for repeated microvascular training. The practice of anastomosing the brachial artery to the radial artery is useful and provides very similar conditions to those of STA-to-MCA anastomosis surgery.
References
- 1.Abla AA, Uschold T, Preul MC, Zabramski JM. Comparative use of turkey and chicken wing brachial artery models for microvascular anastomosis training. J Neurosurg. 2011;115(6):1231–1235. doi: 10.3171/2011.7.JNS102013. [DOI] [PubMed] [Google Scholar]
- 2.Douglas HE, Mackay IR. Microvascular surgical training models. J Plast Reconstr Aesthet Surg. 2011 Aug;64(8):e210–e212. doi: 10.1016/j.bjps.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011 Mar;68(3):723–729. doi: 10.1227/NEU.0b013e318207a9de. discussion 729-30. [DOI] [PubMed] [Google Scholar]
- 4.Hwang G, Oh CW, Park SQ, Sheen SH, Bang JS, Kang HS. Comparison of different microanastomosis training models: model accuracy and practicality. J Korean Neurosurg Soc. 2010 Apr;47(4):287–290. doi: 10.3340/jkns.2010.47.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hino A. Training in microvascular surgery using a chicken wing artery. Neurosurgery. 2003 Jun;52(6):1495–1497. doi: 10.1227/01.neu.0000065174.83840.62. discussion 1497-8. [DOI] [PubMed] [Google Scholar]
- 6.Hong JW, Kim YS, Lee WJ, Hong HJ, Roh TS, Song SY. Evaluation of the efficacy of microsurgical practice through time factor added protocol: microsurgical training using nonvital material. J Craniofac Surg. 2010 May;21(3):876–881. doi: 10.1097/SCS.0b013e3181d7f2c7. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Tsutsumi K, Adachi S, Tanaka S, Saito K, Kunii N. Effectiveness of suturing training with 10-0 nylon under fixed and maximum magnification (× 20) using desk type microscope. Surg Neurol. 2006 Aug;66(2):183–187. doi: 10.1016/j.surneu.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 8.JET Study group. [Japanese EC-IC Trial (JET Study). Study design and interim analysis] Surg Cereb Stroke. 2002;30(2):97–100. Japanese. [Google Scholar]
- 9.Kadri PA, Krisht AF, Gandhi GK. An anatomic mathematical measurement to find an adequate recipient M4 branch for superficial temporal artery to middle cerebral artery bypass surgery. Neurosurgery. 2007 Sep;61(3 Suppl):74–78. doi: 10.1227/01.neu.0000289716.25921.9a. discussion 78. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan KG, Dramm P, Schackert G. Simple and viable in vitro perfusion model for training microvascular anastomoses. Microsurgery. 2004;24(4):335–338. doi: 10.1002/micr.20031. [DOI] [PubMed] [Google Scholar]
- 11.Lannon DA, Atkins JA, Butler PE. Non-vital, prosthetic, and virtual reality models of microsurgical training. Microsurgery. 2001;21(8):389–393. doi: 10.1002/micr.21709. [DOI] [PubMed] [Google Scholar]
- 12.Lascar I, Totir D, Cinca A, Cortan S, Stefanescu A, Bratianu R, et al. Training program and learning curve in experimental microsurgery during the residency in plastic surgery. Microsurgery. 2007;27(4):263–267. doi: 10.1002/micr.20352. [DOI] [PubMed] [Google Scholar]
- 13.Levinsohn EM, Packard DS, Jr, West EM, Hootnick DR. Arterial anatomy of chicken embryo and hatchling. Am J Anat. 1984 Apr;169(4):377–405. doi: 10.1002/aja.1001690403. [DOI] [PubMed] [Google Scholar]
- 14.Olabe J, Olabe J. Microsurgical training on an in vitro chicken wing infusion model. Surg Neurol. 2009 Dec;72(6):695–699. doi: 10.1016/j.surneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Schöffl H, Froschauer SM, Dunst KM, Hager D, Kwasny O, Huemer GM. Strategies for the reduction of live animal use in microsurgical training and education. Altern Lab Anim. 2008 May;36(2):153–160. doi: 10.1177/026119290803600206. [DOI] [PubMed] [Google Scholar]