Abstract
The Notch pathway is an evolutionarily conserved signaling cascade that is critical in kidney development and has also been shown to play a pathogenetic role in a variety of kidney diseases. We have previously shown that the Notch signaling pathway is activated in human immunodeficiency virus-associated nephropathy (HIVAN) as well as in a rat model of the disease. In this study, we examined Notch signaling in the well established Tg26 mouse model of HIVAN. Notch signaling components were distinctly upregulated in the kidneys of these mice as well as in immortalized podocytes derived from these mice. Notch1 and Notch4 were upregulated in the Tg26 glomeruli, and Notch4 was also expressed in tubules. Notch ligands Jagged1, Jagged2, Delta-like1, and Delta-like 4 were all upregulated in the tubules of Tg26 mice, but glomeruli showed minimal expression of Notch ligands. To examine a potential pathogenetic role for Notch in HIVAN, Tg26 mice were treated with GSIXX, a gamma secretase inhibitor that blocks Notch signaling. Strikingly, GSIXX treatment resulted in significant improvement in both histological kidney injury scores and renal function. GSIXX-treated Tg26 mice also showed diminished podocyte proliferation and dedifferentiation, cellular hallmarks of the disease. Moreover, GSIXX blocked podocyte proliferation in vitro induced by HIV proteins Nef and Tat. These studies suggest that Notch signaling can promote HIVAN progression and that Notch inhibition may be a viable treatment strategy for HIVAN.
Keywords: human immunodeficiency virus-associated nephropathy, podocytes, glomerulosclerosis, notch
notch pathway activation plays a central role in kidney development as well as in the pathogenesis of many kidney diseases, especially those involving the glomerulus (32, 34). In mammals, there are four Notch receptors (Notch1–4) and five ligands (Delta-like1, 3, and 4, and Jagged1 and 2). Notch signaling is activated when, upon ligand binding, the receptors undergo a series of proteolytic steps initiated by presenilin-dependent gamma secretase-like protease. This results in release of the Notch intracellular (IC) domain and its translocation into the nucleus. In the nucleus, Notch IC domain associates with the RBP-jk transcription factor, activating the expression of Notch effector proteins such as Hes, Hey, or Cux1 (21, 40–42). These proteins recruit TLE co-repressors and repress the activity of several tissue-specific genes such as Mash1, MyoD, or p27. Although Notch signaling is absolutely essential for nephrogenesis, its suppression is necessary for terminal differentiation (5, 7, 10–12). Also, upregulated Notch signaling has been identified in kidney disease, including inflammation and fibrosis after renal injury, as well as in diseases involving glomerular injury like human immunodeficiency virus-associated nephropathy (HIVAN) (18, 32, 41).
HIVAN is characterized by collapsing glomerulopathy (CG), tubular cystic dilatation, and podocyte proliferation (3, 14, 16). In the CG of HIVAN, mature podocytes acquire a proliferative phenotype, expressing cell cycle reentry markers such as cyclin A and the proliferation marker Ki67, and downregulating expression of cyclin-dependent inhibitors p27 and p57 (4). There is associated foot process effacement, loss of actin cytoskeleton, loss of podocyte maturation markers, including WT1, synaptopodin, nephrin, Glepp1, and gain of podocyte dysregulation markers such as desmin. This phenotype in HIVAN has been shown to be a direct result of HIV-1 infection. In non HIV-1 CGs, it may be either due to viral infection, circulating factors, or a systemic reaction mediated by immune complex deposition (2, 8, 13, 43).
We have previously reported that Notch signaling is activated in HIVAN (41). We showed that Notch signaling members, especially Notch4, were activated in renal sections from HIVAN patients and in a rat model of HIVAN. Although a pathogenetic role for Notch in HIVAN was not established, such a role is suggested by studies in which transgenic mice ectopically expressing Notch1 IC in mature podocytes develop glomerulosclerosis, tubulointerstial fibrosis, and podocyte de-differentiation, features reminiscent of HIVAN (34, 46). In this study, we used an established mouse model of HIVAN, the Tg26 (TG) mice, to test the hypothesis that Notch signaling plays a role in the pathogenesis of HIVAN. Similar to our previous studies in humans and rats, we find that Notch signaling members are upregulated in these mice. We also find that pharmacological inhibition of Notch signaling can slow podocyte proliferation and ameliorate disease progression in this HIVAN model.
MATERIALS AND METHODS
Reagents and antibodies.
Gamma secretase inhibitors GSIXX (dibenzapine) and DAPT [N-(N-(3,5-difluorophenacetyl)-l-alanyl)-S-phenylglycine t-butyl ester] were purchased from EMD Millipore (Chicago, IL). Antibodies used to detect Jagged1, Jagged2, Notch2 IC, and Notch4 IC (catalog no. ab33163) were from Abcam (Cambridge, MA). Anti-Delta-like1, anti-Delta-like 4 were from LifeSpan Biosciences (Seattle, WA); anti-Notch1 IC (val1744), anti-Delta-like 3, anti-Notch3 IC, anti-phospho Stat3, anti-phospho S6 were from Cell Signaling Technology (Danvers, MA); and anti-PCNA and anti-β-actin were from Sigma Aldrich (St. Louis, MO). Rabbit anti-Nef and anti-Hes1 were purchased from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-Ki67 was purchased from Thermo-Scientific (Freemont, CA). Fluorescein labeled lotus tetragonolobus (LTA) and fluorescein labeled dolichos biflorus agglutinin (DBA) were from Vector Laboratories (Burlingame, CA).
Animals.
TG mice and wild-type (WT) FVB mice were housed under pathogen-free conditions in micro-isolator cages on a high-efficiency particulate air-filtered ventilated rack. The study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Kansas Medical Center (Kansas City, KS). Generation of Tg26 mouse model, detection of the transgene, and description of its kidney disorder have been reported earlier (30). Briefly, the Tg26 transgenic mice were generated using a 7.4-kb proviral DNA construct containing the 5′ and 3′ long terminal repeats and the env, tat, nef, rev, vif, vpr, and vpu genes. The proviral DNA construct carried a deletion encompassing most of the gag and pol genes to render it noninfectious. Streptozocin-induced diabetic mice were obtained as reported earlier (27). Briefly, the mice were used after 4 wk of a single intraperitoneal injection of streptozotocin (STZ) (180 mg/kg body wt; Sigma) dissolved in 10 mM sodium citrate buffer, pH 4.5. Animal weight and blood glucose levels (using glucose diagnostic reagents; Sigma) were measured 2 wk after STZ injection and every other week thereafter. Mice were included in the diabetic group if their whole blood glucose level, tested by tail vein sampling for intermediate measures and sampling from the decapitation pool for the terminal measure, was >16.0 mM at every measure. Mice were euthanized at 4 wk post-STZ. Kidneys were harvested for labeling.
Study design.
Tg26 (TG) mice from different colonies have variable severity of kidney phenotype (50). In our colony, ∼80% mice developed >300 mg/dl proteinuria at the age of 6 wk, based on urine dipstick (Siemens Healthcare Diagnostics, Tarrytown, NY) analysis in the urine samples. Although, as reported, the male and female mice exhibit similar disease severity, we restricted our studies to female mice only; all the mice had proteinuria of ∼300 mg/dl. Six-week-old female mice were divided into two groups: vehicle-treated group and GSIXX-treated group. Each of the vehicle and GSI-treated group consisted of at least three WT and three TG females. Before the study, all the TG mice displayed proteinuria of ∼300 mg/dl. GSIXX (500 μg/100 g body wt) or vehicle (0.5% Methocel E4M and 0.1% Tween 80) was administered intraperitoneally once a day for 9 consecutive days. On day 10, mice were euthanized by exposure to isoflurane followed by cervical dislocation. Urine, blood, and kidneys were collected for analysis.
Renal function evaluation.
Mouse serum was collected for measurement of blood urea nitrogen (BUN) using the quantichrome urea assay kit (Bioassay Systems, Hayward, CA) according to manufacturer's protocol. Urinary albumin and creatinine were measured by immunoassay (DCA 2000 Systems; Bayer Diagnostics, Elkhart, IN), an analytical service provided by Vanderbilt O'Brien Kidney Disease Center. Results were expressed as albumin-to-creatinine ratio.
Histology.
Kidneys were fixed in 4% paraformaldehyde overnight followed by 70% ethanol. The tissues were embedded in paraffin. Five-micrometer-thick sections were processed by the core facilities at University of Kansas Medical Center. Sections were stained with routinely used stains, periodic acid-Schiff (PAS) and hematoxylin and eosin (H & E). Renal histology was blindly scored for tubulointerstitial disease (including tubular casts, tubular dilatation, and interstitial fibrosis), and the area affected was quantified by image analysis using ImageJ 64 (version 1.46, NIH.gov). Scores were assigned according to % area affected: 0, no disease; 1+, 0–1%; 2+, 1.1–2%; 3+, 2.1–3%; 3.1–4%; 4.1–5%; 6+, 5.1–6%. Glomerular injury (sclerosis, podocyte hyperplasia, and collapse) was also graded on a semi-quantitative scale. Scores were assigned according to the % glomeruli affected: 0, no disease; 1+, 0–10%; 2+, 11–20%; and 3+, 21–30%; 4+, 31–40%; 5+, 41–50%; 6+, 51–60%; 7+, 61–70%; and 8+, 71–80%. Total injury scores were calculated by the sum of these two scores.
Immunohistochemistry/immunoflourescence.
Immunohistochemistry/immunoflourescence (IHC/IF) was performed as described previously (41). Briefly, sections were deparaffinized with xylene and hydrated with graded ethanols. Sections were then boiled with antigen unmasking solution (Vector Laboratories, Burlingame, CA) and incubated for 30 min with 3% hydrogen peroxide for IHC and 0.5 M ammonium chloride for IF to block endogenous peroxidase/fluorescence activity. Subsequent washing in PBS and blocking with 10% normal serum (in PBS from the species the secondary antibody was raised in) for 1 h were followed by incubation for 1 h with primary antibodies in a humidified chamber. Slides were washed three times in PBS and incubated for 1 h in 1:400 diluted biotin-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA) for IHC and fluorescien/Texas red-conjugated antibodies for IF. Slides were washed four times in PBS for 5 min each. For IF, the slides were coverslipped using antifade (Molecular Probes, Life Technologies, Grand Island, NY). For IHC, the slides were further incubated with avidin-biotin-peroxidase complex (ABC Elite; Vector Laboratories, Burlingame, CA) and detected with diaminobenzidine (DAB; Sigma Aldrich, St. Louis, MO). Tissue sections were then dehydrated with graded ethanols, mounted with permount (Fisher Scientific, Pittsburg, PA), and covered with glass coverslips. Slides were viewed on a Leica DMR microscope equipped with an Optronics Magnafire digital camera.
Podocyte culture and notch inhibition.
WT and Tg26 immortal cell lines were maintained at 33°C in a growth medium containing RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1× Pen-Strep, 1 mM l-glutamine, 1× insulin-transferrin-selenium (ITS), and 10 U/ml murine interferon gamma (BD Biosciences, San Jose, CA) to promote expression of the SV-40 T antigen (TAg) in the presence of 5% CO2 (permissive conditions). Under these conditions, the Tg26 cells form hillocks and heaps and are not contact inhibited on confluency compared with the WT cells. At 37°C in absence of IFNγ, the temperature-sensitive TAg is inactivated (nonpermissive conditions) (25, 39). In 10–15 days, podocytes show foot processes with differentiated morphology. For the expression studies, both WT and Tg26 cells were harvested and lysed after 15 days of nonpermissive conditions. For inhibition studies, cells were grown in permissive conditions and then allowed to differentiate in nonpermissive conditions for 15 days in six-well plates. This was followed by treatment with recombinant Nef and Tat (100 ng/ml) with or without Notch inhibitors DAPT (1 μM) and GSIXX (100 nM) for 24 h. Cell were harvested and lysed.
Western blots.
Cells or tissues were lysed with RIPA buffer containing 0.01 M sodium phosphate, pH 7.2; 125 mM sodium chloride; 50 mM sodium fluoride; 0.1% SDS; 1 mM EDTA; 1% sodium deoxycholate; and 1% NP40 with protease inhibitor cocktail (Thermo Scientific, Freemont, CA). Protein was measured using BCA protein assay (Bio-Rad, Hercules, CA). Whole cell lysates (30 μg) were solubilized in SDS-PAGE sample buffer and electrophoresed on 4–15% gradient or 10% polyacrylamide gels. Proteins were transferred to PVDF membranes. The immunoblots were blocked in 5% nonfat dry milk in PBST (PBS containing 0.1% Tween 20) for 1 h at room temperature and then followed by PBS washes; the blots were incubated with appropriate dilutions of primary antibodies overnight. The blots were then washed three times at room temperature with PBST and incubated with secondary antibodies (1:10,000 dilution in blocking solution) for 1 h at room temperature. After three washes in PBST, bound antibody was detected by chemiluminescence (Super Signal West Pico Chemiluminescent Substrate; Pierce), according to manufacturer's protocol, and followed by exposure to autoradiography film.
Statistical analysis.
Unpaired t-test was used to compare mean values between two groups. One-way ANOVA was performed for multiple comparisons followed by Tukey's or Holm Sidak's test to calculate significance. Statistical significance was considered at P < 0.05. Results are presented as means ± SE.
RESULTS
Activation of notch pathway members in the kidneys of TG mice.
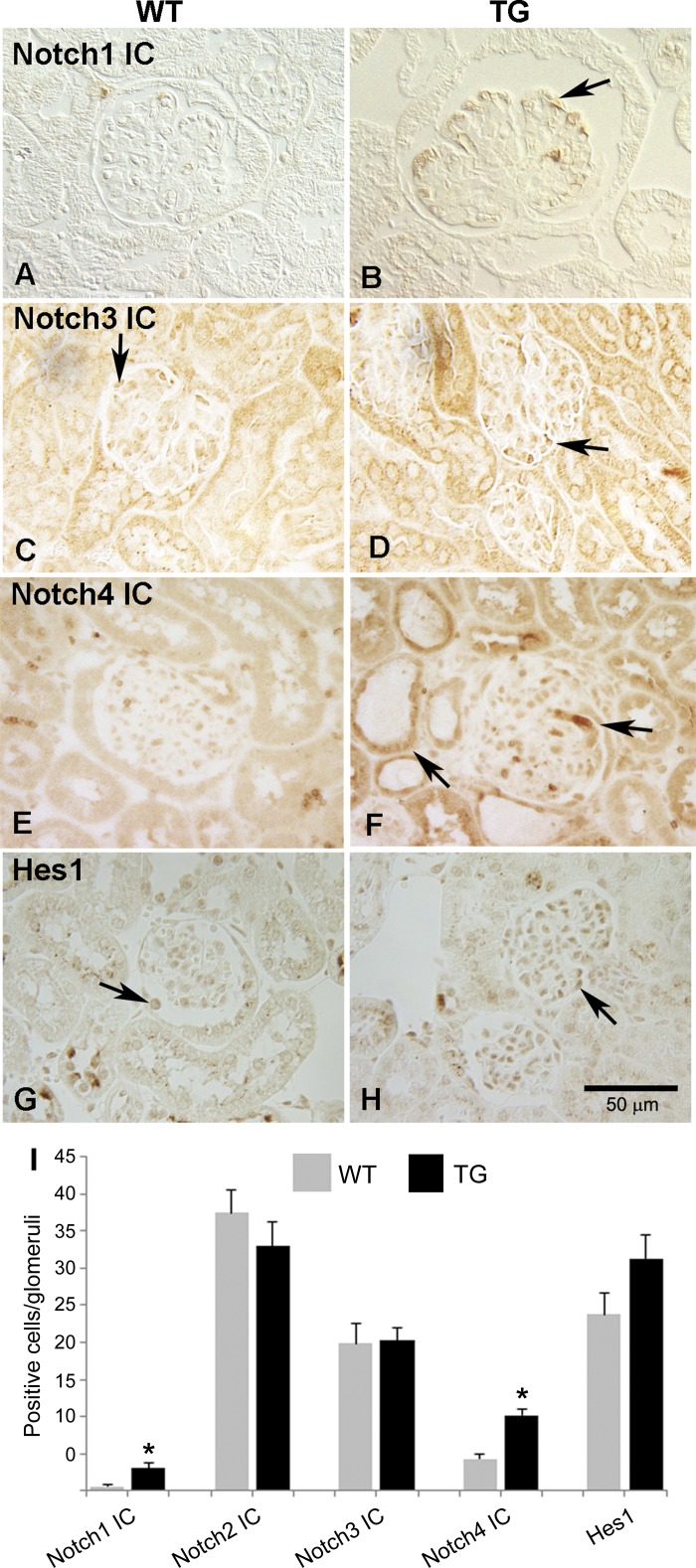
The Notch pathway has been shown to be upregulated in a wide variety of glomerular diseases, and increased expression of the Notch proteins in these diseases is correlated with proteinuria (32). In HIVAN, which is characterized by proteinuria, podocyte proliferation, and glomerular collapse, we provided the first evidence of Notch activation (41), but it is not clear what role Notch signaling plays in HIVAN pathophysiology. To address this question, we turned to a robust animal model of HIVAN, the TG mice. These transgenic mice express HIV proteins and exhibit disease manifestations that mimic HIVAN (3, 30). Waters et al. (46) showed that induced Notch1 IC expression in mice starting at the capillary-staged podocytes results in podocyte proliferation but no collapse, so there is evidence to suggest that Notch signaling can promote at least some of the elements of HIVAN in mice. To examine Notch signaling in the mice, we first determined whether TG mice exhibit increased expression of Notch intracellular (IC) domain. These findings of glomerular expression are summarized in Fig. 1I. Although Notch1 IC expression was significantly upregulated, the labeling was weak and confined to the glomeruli (most likely podocytes, based on the location), as shown in Fig. 1B. Notch2 IC and Notch 3 IC were present in both WT and TG sections, and their expression was not altered (Fig. 1, C and D) (only Notch3 IC is shown). Similar to what has been observed in human HIVAN and the rat HIVAN model, Notch4 IC exhibited a stronger and broader labeling with positive cells in glomeruli and tubules (Fig. 1, E and F). Hes1 expression appeared to be increased; however, the difference is not statistically significant (Fig. 1, G–I). These data, together with our previous findings, indicate that Notch is activated in rodent models and suggest that Notch4 IC may play a unique role in HIVAN pathogenesis.
Fig. 1.
Immunostaining of activated Notch receptors in kidneys of TG mice. A–H: immunostaining was performed in paraffin sections of kidneys from Tg26 (TG) mice and compared with their littermates (WT) using anti Notch1 IC (A and B), Notch3 IC (C and D), Notch4 IC (E and F), and Hes1 (G and H) antibodies. Arrows indicate cells expressing respective marker. I: semi-quantitative analysis of the labeling was performed. First 30 random glomeruli from each section (n = 3) were used to count cells positive for labeling. *Significant difference between the WT and Tg26 groups (P < 0.01).
Upregulation of Notch ligands in tubules, but weak expression in glomeruli of TG mice.
To identify Notch ligands expressed in TG mice, we performed immunohistochemical expression analysis for all five Notch ligands: Jagged1 (J1), Jagged2 (J2), Delta-like1 (Dll1), Delta-like3 (Dll3), and Delta-like4 (Dll4). All the ligands were upregulated in the tubules of TG mice compared with the WT mice (Fig. 2, A–J). Control sections with no primary antibody did not label any cells (Fig. 2K). In addition to the tubular cells, J2 and Dll4 ligands showed some expression in cells in close proximity to Bowman's capsule (Fig. 2, D and J, right arrows). Of the Notch ligands, Dll4 expression was most robust. Since Dll4 has been reported to be a specific ligand for Notch4 IC (47), it is possible that the increased Notch4 IC expression observed in the tubular and the parietal epithelial cells of TG mice (Fig. 1F) is the result of Notch4 receptor binding to the Dll4 that is expressed in this region. There was also focal staining of glomerular cells for J1, J2, Dll1, and Dll3; however, this labeling was not much different from that of the respective control WT sections and not localized to the membranes where ligands would typically express, so there does not appear to be significant expression of these ligands. The expression of both Notch4 and J1 at mRNA level has previously been found to be increased in streptozocin (STZ)-induced diabetic nephropathy (DN) in mice (34). In humans, the expression of J1, N1, and N2 was upregulated in a variety of diseases including DN (32). We labeled the sections obtained from DN mice and determined the expression of J1 and Notch4 at protein level. We were able to demonstrate robust membrane staining for J1 in glomeruli and tubules of STZ-treated, diabetic mice consistent with previous reports (Fig. 2L, arrows) (27). Interestingly, Notch4 IC expression was very weak in the diabetic mice (Fig. 2M) compared with the HIVAN mice (Fig. 1F). Thus the Notch signaling components upregulated in HIVAN are distinct.
Fig. 2.
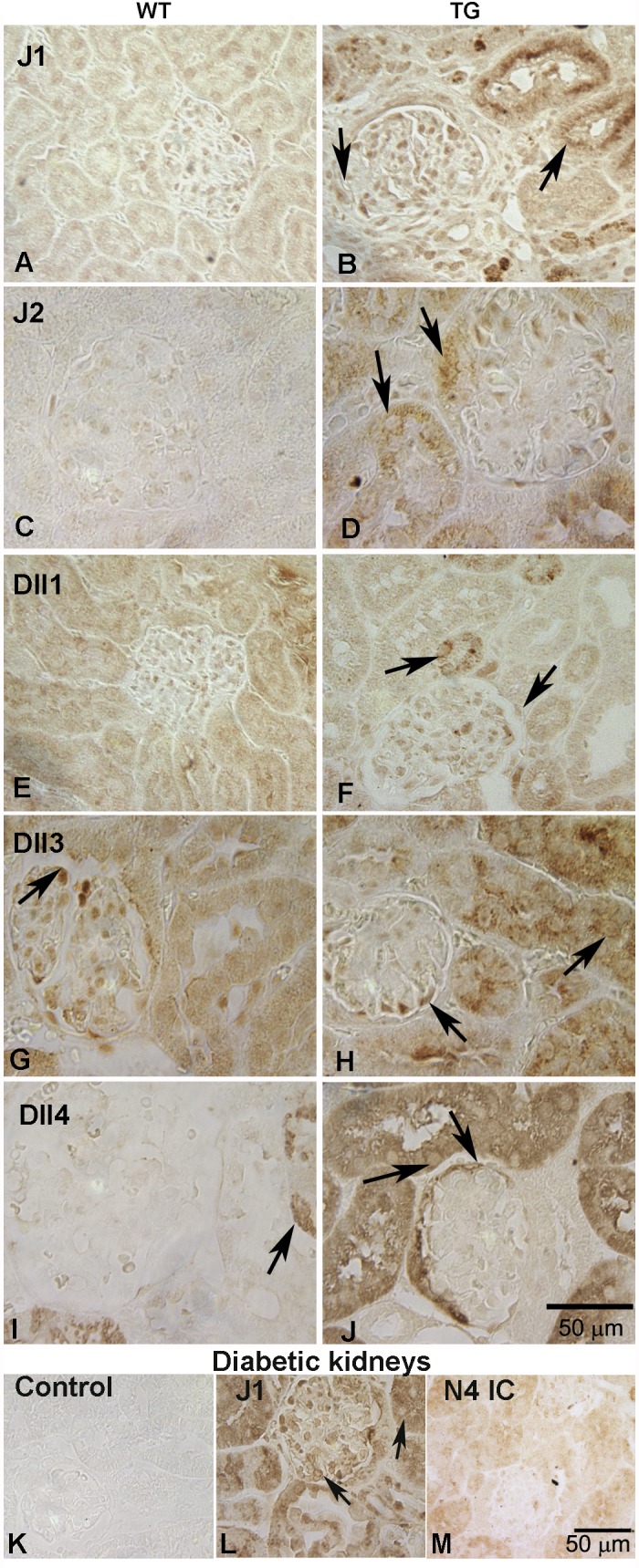
Immunostaining of Notch ligands in kidneys of Tg26 mice. Immunotaining was performed for the presence of Notch ligands in the TG kidneys and compared with the WT controls. Antibodies for anti-Jagged1 (J1; A and B), Jagged 2 (J2; C and D), Delta-like 1 (Dll1; E and F), Delta-like 3(Dll3; G and H), and Delta-like 4 (Dll4; I and J) were used. Arrows pointing to the tubules reflect membrane staining in cells; however, some intracellular staining was also observed. Dll4 and J2 appeared to be increased in the cells close to the urinary pole (top arrows in D and J). Diabetic kidney sections were assesed for the expression of Jagged1 and Notch4 IC (L and M). K: no labeling was observed in the rabbit IgG control. Notch4 IC was weakly expressed (M), and Jagged1 was upregulated in both glomeruli and tubules (L; arrows).
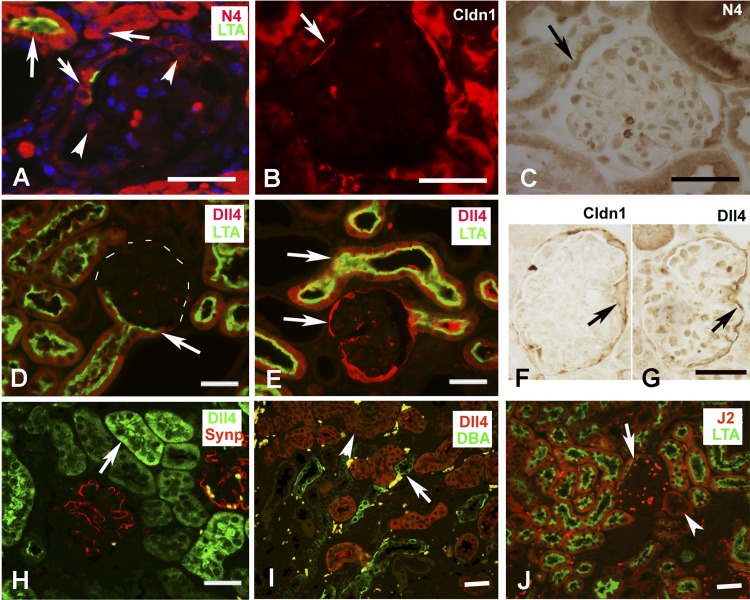
Notch4 IC and Dll4 expression appeared to be most robust among the Notch receptors and ligands in the Tg26 renal sections. We next sought to identify the cells positive for Notch4 IC and Dll4 using double-labeling immunoflourescence or serial section labeling in the renal sections. Notch4 IC (red) was expressed well in the LTA (Lotus tetraagglutinin, green)-positive cells, indicating its presence in proximal tubules. Interestingly, Notch4 IC expression was also observed in the tubular cells, in close proximity to the urinary pole, which is a site of origin of parietal cells. All LTA-positive cells expressed high levels of Notch4 IC. Notch4 IC was also expressed in the cells of Bowman's capsule that were not positive for LTA in addition to other glomerular cells (Fig. 3A, arrowheads). To determine whether Notch4 IC positive cells are parietal epithelial cells, serial sections from TG and WT mice were labeled for Claudin-1 (Fig. 3B) and Notch4 IC (Fig. 3C). Claudin 1 (Cldn1) is a marker of parietal epithelial cells and was expressed in both WT and TG mice (only TG sections are shown). Arrows in Fig. 3, B and C, indicate the same cell expressing both Cldn1 and Notch4 IC. Colocalization of LTA and Dll4 was observed in both WT and TG mice. Dll4 expression was not just restricted to proximal tubules, since some LTA-negative tubules also expressed Dll4. Dll4 was not expressed in collecting ducts or podocytes since its expression did not overlap with DBA and synaptopodin, respectively (Fig. 3, H and I). Compared with WT mice (Fig. 3D), TG mice (Fig. 3E) had much stronger expression of Dll4 (revealed by bright red labeling in the tubules). Interestingly, Dll4 was robustly expressed in the cells lining Bowman's capsule in TG sections. We confirmed these cells to be parietal epithelial cells by labeling serial sections from TG mice for Cldn1 and Dll4, and the same cells expressed both Dll4 and Cldn 1 (Fig. 3, F and G, arrows). J2 was also expressed in higher amounts in TG sections and was found to colocalize with LTA, although some non-LTA-positive tubules were also positive for J2 (Fig. 3J, arrowheads). J2 was expressed in the tubular cells close to the urinary pole, but unlike Dll4 its expression in parietal epithelial cells was rarely noted.
Fig. 3.
Localization of Notch4 IC (N4 IC), Delta-like 4 (Dll4), and Jagged 2 (J2). Double immunolabeling serial section labeling was performed to identify cells positive for N4 IC, Dll4, and J2. A: N4 IC was expressed broadly in tubular cells (arrows), which included proximal tubules (LTA positive cells; green); arrowheads represent glomerular cells positive for N4 IC. B and C: in Bowman's capsule, N4 IC was expressed in the cells labeled positive for Claudin1 (cldn1) (marker for parietal epithelial cells). The arrows reflect same cell expressing both Cldn1 (red) and N4 (brown). D and E: note strong expression of Dll4 in the proximal tubules (LTA positive) from Tg26 mice (E) compared with the WT littermates (D). There was also an increased expression of Dll4 in the cells lining Bowman's capsule (between urinary pole and vascular pole) in the TG sections (bottom arrow; E). F and G: serial sections from TG mice kidneys were labeled for Cldn1 (to identify parietal epithelial cells; F) and Dll4 (G). Cells expressing high levels of Dll4 were identified as parietal epithelial cells. Arrows represent same cell expressing both Dll4 and Cldn1. H and I: Dll4 was not expressed in podocytes or collecting ducts [reflected by synaptopodin staining in red and by DBA (dolichus biflorous agglutinin) staining in green]. J: J2 (red) was found expressed and elevated in proximal tubules (green) from TG mice; however, parietal epithelial cells of Bowman's capsule did not reveal much difference in the labeling between WT and TG sections (only TG section shown).
These studies suggest that canonical Notch signaling takes place in tubular and parietal epithelial cells in the kidneys of HIVAN mice. However, absence or weak expression of most Notch ligands from the glomeruli despite presence of Notch1C, Notch 2 IC, Notch3 IC, and Notch4 IC implies that noncanonical Notch signaling is present within the glomeruli and may be involved in the glomerular phenotype in HIVAN. This may also indicate that Notch signaling plays differential roles in tubular vs. glomerular injury in HIVAN.
Notch inhibition can ameliorate HIVAN progression in TG mice.
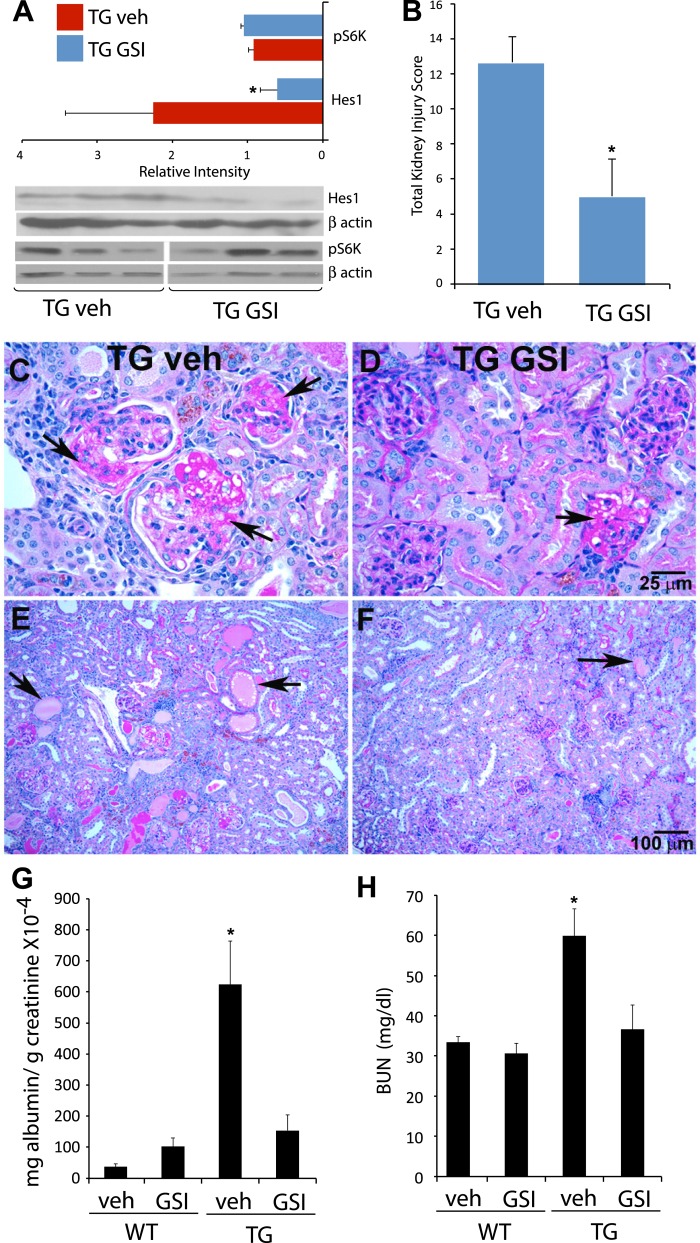
Our data indicate that Notch signaling components are upregulated and that the pathway is active in the HIVAN mice (Figs. 1–3), as it is in the human disease and the rat model (41). To determine whether Notch signaling plays a role in HIVAN pathology, we treated mice with a gamma secretase inhibitor (GSIXX) that inhibits Notch signaling by preventing cleavage of receptor and has previously been shown to be effective in treating other glomerular diseases that involve Notch. TG mice from different colonies have variable severity of kidney phenotype (50). In our colony, ∼80% mice developed >300 mg/dl proteinuria at the age of 6 wk, based on urine dipstick analysis. Although, the male and female mice exhibit similar disease severity, we restricted our studies to female mice only; all the mice had proteinuria of ∼300 mg/dl. Both TG and age-matched WT littermates were intraperitoneally injected with GSIXX (500 μg/100 mg body wt) or vehicle control (0.5% Methocel, 0.1% Tween 80) for 9 consecutive days. This dose was selected based on previous study (34), where rats treated with GSIXX and puromycin aminonucleotide (PAN), (used to induce glomerular injury) had significantly less albuminuria compared with rats treated with PAN only. We collected urine and blood on day 10, and kidneys were harvested for histological evaluation. Although GSIs have shown to effectively inhibit Notch signaling, they are not entirely specific for Notch pathway. Other pathways, such as mTOR, have been shown to be inhibited by GSIs (19). In our studies, GSIXX treatment effectively inhibited Notch pathway, as evidenced by a significant reduction in expression of the Notch target gene Hes1, whereas phosphorylation of ribosomal protein S6 (S6K), a downstream target of mTOR, was not much altered at this concentration (Fig. 4A). Kidney sections, stained by hemotoxylin and eosin (H & E) and periodic acid-Schiff (PAS) methods, revealed marked improvement in glomerular pathology (only PAS shown in Fig. 4, C–F), with reduced scarring, sclerosis, and foam-cell accumulation (compare Fig. 4C with Fig. 4D, arrows). GSIXX treatment also significantly improved tubulointerstitial disease and cystic dilatations as shown in Fig. 4F compared with the vehicle-treated controls (Fig. 4E). The renal lesions were scored and quantified, as illustrated in Fig. 4B; GSIXX treatment resulted in a significant improvement, almost threefold reduction in overall kidney injury (glomeruli + tubulointerstitial disease).
Fig. 4.
Effects of GSIXX treatment on disease progression in TG mice. WT and TG mice were intraperitoneally injected with gamma secretase inhibitor (GSIXX) every day, consecutively for 9 days. Kidneys, urine, and blood were collected for disease evaluation. A: Western blots were carried out from kidney lysates obtained from Tg26 vehicle (TG veh) and GSIXX-treated (TG GSI) mice for Notch target Hes1 and mTOR pathway activation marker S6 kinase (S6K). Hes1 expression was significantly downregulated, whereas S6K was not affected. B: total kidney injury score was quantitatively evaluated in sections stained for hemotoxylin and eosin (H and E) and periodic acid Schiff (PAS). C–F: PAS staining in TG veh and TG GSI sections revealed an improvement in glomerular lesions following GSIXX treatment in TG mice; arrows reflect scarred glomerul, the number of which was significantly less in GSI-treated group (C and D). GSIXX treatment also resulted in drastic reduction in cystic dilatations (E and F). Each evaluation is done in three mice and averaged to obtain scores in B. G and H: protein-to-creatinine ratio values and BUN levels were evaluated. In each case, protein-to-creatinine or BUN levels were significantly elevated in TG veh group compared with either WT veh or TG GSI groups (n = 4). Data is represented as means ± SE. *Significant difference (P < 0.01).
To evaluate effects on renal function, we measured blood urea/nitrogen (BUN) and urine albumin-to-creatinine ratio (Fig. 4, G and H). The albumin-to-creatinine ratio was significantly elevated ∼10-fold in the TG vehicle-treated group compared with the WT vehicle group. Remarkably, despite only a 9-day treatment, GSIXX was able to significantly reduce the ratio close to the level observed in WT mice treated with the drug. Similarly, serum BUN was increased significantly in TG vehicle-treated mice compared with the WT vehicle controls, and GSIXX was able to significantly reduce this increase in TG mice.
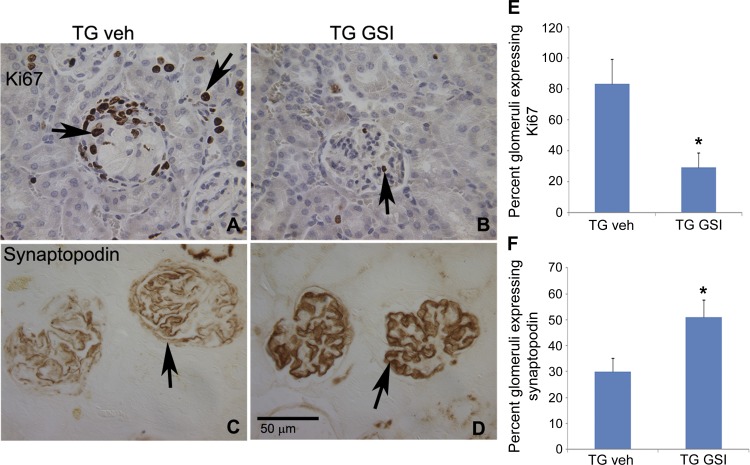
GSI XX treatment reverses podocyte dedifferentiation and reduces Ki67 expression in the TG mice glomeruli. Podocyte proliferation is a hallmark of the CG associated with HIVAN (3), and this proliferation is associated with extensive loss of podocyte maturation markers, such as synaptopodin. To identify the cells potentially targeted by GSIXX, we evaluated proliferation, as indicated by expression of the proliferation marker Ki67 and synaptopodin expression after GSIXX treatment of TG mice. Representative pictures are shown in Fig. 5, A–D. As expected, the WT mice treated or untreated did not show any glomerular expression of Ki67 (not shown). In vehicle-treated TG mice, 80% of the glomeruli had some expression of Ki67 (Fig. 5, A and E). GSIXX treatment significantly reduced this to ∼12% (Fig. 5, B and E). Ki67 was also expressed in tubules, and GSIXX treatment appeared to reduce this effect (Fig. 5, A and B, arrows). Synaptopodin, on the other hand, was expressed in 100% glomeruli in WT treated or untreated mice (not shown). In TG vehicle-treated mice, 30% of glomeruli showed full synaptopodin expression (Fig. 5, C and F). GSIXX treatment resulted in a significant recovery of synaptopodin expression, with 51% of glomeruli exhibiting synaptopodin expression (Fig. 5, D and F). These studies suggest that GSIXX plays a protective role specifically in the podocyte in HIVAN. Moreover, this implies that Notch signaling promotes the pathological podocyte phenotype observed in HIVAN.
Fig. 5.
Effects of GSIXX treatment on Ki67 and synaptopodin expression. Kidneys obtained from GSIXX-treated or vehicle control TG mice were evaluated for the expression of Ki67 and synaptopodin by immunostaining on the paraffin sections. A and B: Ki67 is shown both in glomeruli and tubules. Note a collapsed glomeruli with many Ki67 positive cells in A. GSIXX treatment appeared to reduce Ki67-positive cells in both glomeruli and tubules. C and D: a regain of synaptopodin expression is shown in sections from GSI-treated TG mice (TG GSI) compared with TG veh control. E and F: glomeruli positive for any Ki67 labeling were counted as positive stained, and glomeruli with full expression of synaptopodin were counted positive; others were counted negative for staining. Semiquatitative analysis of the Ki67 and synaptopodin expression in glomeruli is shown. P < 0.05 represents statistical difference compared with WT vehicle and TG vehicle groups (n = 3). *Significant difference vs. the WT vehicle-treated group (P < 0.05).
Notch inhibition can reduce HIV protein-mediated proliferation.
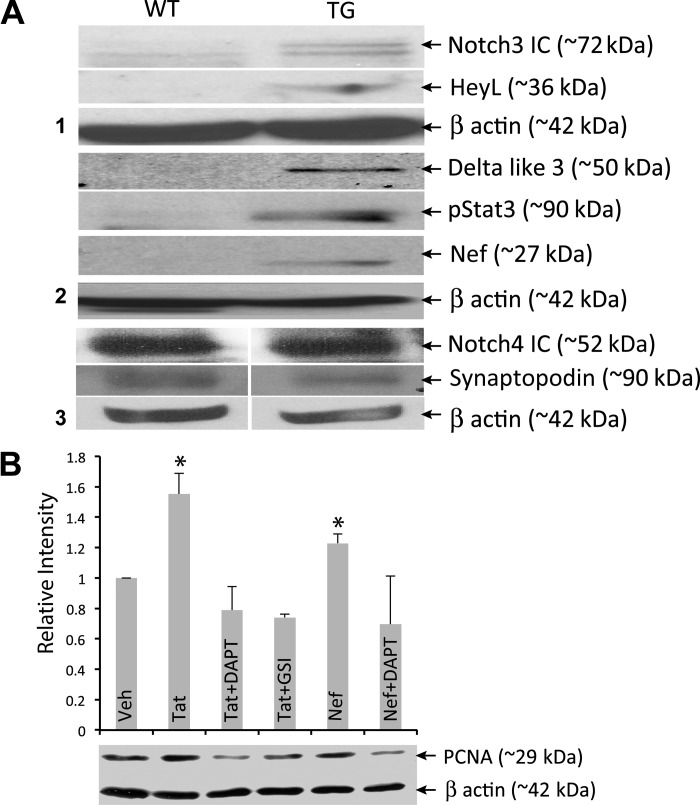
To evaluate the relationship between Notch signaling and the podocyte phenotype associated with HIVAN directly, we used cultured podocytes. First, Notch pathway expression was evaluated in a clone of immortalized podocyte line derived from TG mice (23) and compared with that derived from WT mice. These cells (Tg26) proliferate and expand under permissive conditions (33°C in the presence of IFNγ). When these cells are transferred to nonpermissive conditions (37°C, no IFNγ), they differentiate in 10–15 days and exhibit multiple foot processes. After 15 days of nonpermissive conditions, the Tg26 cell line showed expression of Nef and phospho-Stat3, as expected and shown previously (20). In addition, Notch3 IC, Hey L, and Dll3 were increased in the immortalized Tg26 cells compared with the WT podocytes (Fig. 6A). Unexpectedly, we did not find any increase in the Notch4 IC expression (Fig. 6A); in contrast, an increase in Notch3 IC expression was observed (Fig. 1F). Thus the immortalized cells behaved differently and did not exactly mimic the in vivo situation.
Fig. 6.
In vitro assessment of the effects of HIV proteins and Notch inhibition in immortal podocytes. A: immortal podocytes obtained from WT and TG mice were allowed to differentiate for 15 days followed by assessment of various Notch pathway members, Notch3 IC, HeyL, Delta like 3, Notch4 IC, and for Phospho-Stat3, Nef, and synaptopodin by Western blots. B: WT differentiated immortal podocytes were incubated with HIV proteins Nef and Tat alone or with Notch inhibitors DAPT or GSIXX (GSI). The effects on PCNA levels were evaluated. One-way ANOVA was used to make comparisons between groups. *Significant difference between vehicle-treated and Tat- or Nef-treated cells (P < 0.05). Significant difference was also observed between Tat- or Nef-treated cells and cells treated with either inhibitor, GSI, or DAPT. Data are representative of three experiments performed in triplicate.
To address whether Notch signaling influences the HIV-mediated podocyte phenotype, we used the Notch signaling inhibitors in conjunction with treatment of cells with the HIV proteins Nef and Tat. Nef and Tat, as well as Vpr, have all been shown to induce podocyte proliferation and dedifferentiation in vitro (15, 29, 36, 49, 51). As expected, treatment of podocytes with both Tat and Nef significantly stimulated proliferation compared with vehicle-treated cells, as measured by expression of the proliferation marker PCNA (Fig. 5B, columns 2 and 5). Notably, the presence of Notch inhibitors (DAPT or GSIXX; Fig. 6B, columns 3, 4, and 6) significantly blocked these proliferative effects. These results provide evidence that Notch is involved in inducing proliferation in podocytes and that HIV-1 proteins may induce Notch pathway activation. Thus these studies indicate that Notch signaling upregulation is not merely a consequence of the disease progression but plays a role in HIVAN pathophysiology.
DISCUSSION
HIVAN is the most prevalent and common cause of end-stage renal disease in HIV-1 seropositive patients and affects individuals with a marked racial bias for African descent. Currently, HIVAN prevalence is between 3.5 and 12%, affecting an estimated 1–3 million people worldwide (1, 33, 35). With the advent of highly active antiretroviral therapy (HAART), the incidence of HIVAN has greatly declined. These drugs, however, are associated with side effects including nephropathies (17). Therefore, despite the improvements, kidney disease in HIV remains a significant cause of morbidity and mortality.
In our studies, both Notch1 IC and Notch4 IC were upregulated in renal sections from TG mice. The expression of Notch1 IC was weak and confined to podocytes, whereas Notch4 IC expression was widespread and strong (Figs. 1 and 3) and localized to proximal tubules, cells lining Bowman's capsule, and other glomerular cells. In contrast, diabetic kidneys expressed Jagged1 in both tubules and glomeruli as previously described (34), whereas Notch4 IC expression was very weak. These observations suggest that Notch signaling is differentially upregulated in HIVAN. Conditional expression of the Notch1 IC in the mature podocytes has shown to result in glomerulosclerosis, proteinuria, and podocyte apoptosis in mice (34). In contrast, transgenic expression of Notch1 IC starting at the capillary loop stage of podocyte development results in glomerulosclerosis, proteinuria, and podocyte proliferation (46). In the latter study, although podocyte proliferation was observed, there was no glomerular collapse. Moreover, in the above two studies, only Notch1 IC was overexpressed in podocytes. We believe that disease pathogenesis is not dependent on single Notch isotype, and thus Notch1 IC expression may not be solely responsible for the glomerular phenotype, and perhaps other Notch members/receptors may be involved. Thus, depending on its spatial and temporal expression, Notch may initiate proliferative or apoptotic responses. In HIVAN, differential upregulation of Notch pathway may indicate its distinct role. All the ligands appeared to be increased in the proximal tubules especially; the expression of Dll4 reported to be a specific ligand for Notch4 was most striking and was also found to be increased in the cells lining Bowman's capsule (Figs. 2 and 3) in TG mice. It is therefore possible that Notch4 engages with the DLL4 ligand to activate canonical Notch4 signaling in cells lining the tubules and Bowman's capsule. Increased expression of Dll4 and Notch4 IC in the cells lining Bowman's capsule localized between the urinary and vascular pole in TG mice was interesting since Notch1, 2, and 3 activation in these cells has been shown to induce regeneration of podocytes from renal progenitors in the event of injury (31, 37). Although all Notch receptors were expressed in glomerular cells, Notch ligands did not show much labeling in glomeruli except in the cells lining Bowman's capsule. These studies suggest that Notch signaling in the glomerular cells may be induced via some unknown factors (perhaps a virus) and is most likely non-canonical in HIVAN. In this regard, it is interesting that Tat has been shown to directly interact with EGF repeats of Notch receptors (44). These EGF repeats are important in the binding of ligands to the Notch receptors (38, 44). One possibility is that Notch receptors are directly activated by viral protein via interaction with the EGF repeats in HIVAN. Although this is a tempting possibility, other HIV-1 proteins, Nef and Vpr proteins, have been shown to be sufficient to induce HIVAN phenotype in mice (51). The in vivo and in vitro significance of HIV proteins and Notch interaction in HIVAN remains uncertain and needs further investigation.
Treatment with GSIXX ameliorated the disease progression in TG mice. GSI treatment significantly reduced the total kidney injury that included cystic dilatations, interstitial fibrosis, glomerulosclerosis, and collapse. This was associated with significant downregulation of cell proliferation and increase in the podocyte redifferentiation. Moreover, renal function was significantly recovered in the GSI-treated TG mice compared with the vehicle-treated mice. These studies show that Notch plays an important role in HIVAN pathogenesis and that GSI XX can ameliorate the disease progression. GSIs were also able to inhibit Tat- and Nef-mediated cell proliferation, which provides evidence that Notch pathway is induced by HIV proteins and is not a consequence of disease progression.
Many signaling pathways including Wnt, TGF-β, Stat3, and mTOR pathway have been shown to be involved in HIVAN progression (20, 24, 43, 45, 48). Interestingly, many of these pathways have also been shown to interact with the Notch pathway (9, 28, 34). GSIs have also been shown to inhibit mTOR pathway (19). We did not see any altered activity of mTOR pathway protein S6K on treatment with GSIXX in our studies. In clinical situations, however, GSIs have limitations because of the non-specificity and are associated with gastrointestinal toxicity (22, 26). Thus identifying and targeting specific members of Notch pathway and/or specific cells in the body may be a viable approach.
We are carrying out further studies to determine the importance of context-dependent Notch inhibition to identify and narrow down the receptors that may be important in the pathogenesis of HIVAN. Our studies indicate that Notch4 inhibition may be an effective approach for treatment of HIVAN. Antibodies for Notch1, Notch2, and Notch3 have been developed and are being evaluated in clinical trials for treatment of several cancers (22). Although more Notch inhibitors are being developed and tested for their efficacy, our study taken together suggests that Notch is distinctly regulated in HIVAN, and Notch inhibitors provide protection against the disease progression in HIVAN. To our knowledge, this is the first evidence of Notch inhibition playing a protective role in HIV-associated diseases.
GRANTS
This work was supported by intramural grants from Lied, Department of Internal Medicine, and departmental endowment funds provided to M. Sharma.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S. conception and design of research; M.S., L.K.M., T.H., and K.N.T. performed experiments; M.S., P.C.S., G.B.V.H., and T.A.F. analyzed data; M.S., D.P.H., P.E.K., G.B.V.H., and T.A.F. interpreted results of experiments; M.S. and T.A.F. prepared figures; M.S. drafted manuscript; M.S., P.C.S., and T.A.F. edited and revised manuscript; M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Alan Yu for the intellectual input and for critically reading the manuscript, Dr. Dale Abrahamson for useful suggestions, Dr. Dipika Sharma for help with tissue collection, Dr. Gaurav Chaturvedi for critically reading the manuscript, and Rossetta Barkley and Carol Carlton for technical help. Methocel E4M was a kind gift from Dow Chemical, (Midland, MI). HIV-1 Nef protein was obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID): Nef Protein (catalog no. 11478). Tat recombinant protein was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 Tat protein was from Dr. John Brady and DAIDS, NIAID (6).
REFERENCES
- 1. Ahuja TS, Borucki M, Funtanilla M, Shahinian V, Hollander M, Rajaraman S. Is the prevalence of HIV-associated nephropathy decreasing? Am J Nephrol 19: 655–659, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Avila-Casado C, Fortoul TI, Chugh SS. HIV-associated nephropathy: experimental models. Contrib Nephrol 169: 270–285, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Barisoni L, Bruggeman LA, Mundel P, D'Agati VD, Klotman PE. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int 58: 173–181, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int 58: 137–143, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Barisoni L. Notch signaling: a common pathway of injury in podocytopathies? J Am Soc Nephrol 19: 1045–1046, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bohan CA, Kashanchi F, Ensoli B, Buonaguro L, Boris-Lawrie KA, Brady JN. Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr 2: 391–407, 1992 [PMC free article] [PubMed] [Google Scholar]
- 7. Bonegio RG, Beck LH, Kahlon RK, Lu W, Salant DJ. The fate of Notch-deficient nephrogenic progenitor cells during metanephric kidney development. Kidney Int 79: 1099–1112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D'Agati VD, Winston JA, Klotman ME, Klotman PE. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 11: 2138–2140, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Casalbore P, Budoni M, Ricci-Vitiani L, Cenciarelli C, Petrucci G, Milazzo L, Montano N, Tabolacci E, Maira G, Larocca LM, Pallini R. Tumorigenic potential of olfactory bulb-derived human adult neural stem cells associates with activation of TERT and NOTCH1. PLos One 4: e4434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134: 801–811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng HT, Kopan R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int 68: 1951–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130: 5031–5042, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Chugh SS, Clement LC, Macé C. New insights into human minimal change disease: lessons from animal models. Am J Kidney Dis 59: 284–292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen AH, Nast CC. HIV-associated nephropathy. A unique combined glomerular, tubular, and interstitial lesion. Mod Pathol 1: 87–97, 1998 [PubMed] [Google Scholar]
- 15. Conaldi PG, Bottelli A, Baj A, Serra C, Fiore L, Federico G, Bussolati B, Camussi G. Human immunodeficiency virus-1 tat induces hyperproliferation and dysregulation of renal glomerular epithelial cells. Am J Pathol 161: 53–61, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Agati V, Suh JI, Carbone L, Cheng JT, Appel G. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int 35: 1358–1370, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Daugas E, Rougier JP, Hill G. HAART-related nephropathies in HIV-infected patients. Kidney Int 67: 393–403, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Djudjaj S, Chatziantoniou C, Raffetseder U, Guerrot D, Dussaule JC, Boor P, Kerroch M, Hanssen L, Brandt S, Dittrich A, Ostendorf T, Floege J, Zhu C, Lindenmeyer M, Cohen CD, Mertens PR. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol 228: 286–299, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, Buser C, Yeatman T, Coppola D, Winter C, Clark EA, Draetta GF, Strack PR, Majumder PK. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res 15: 2476–2484, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Feng X, Lu TC, Chuang PY, Fang W, Ratnam K, Xiong H, Ouyang X, Shen Y, Levy DE, Hyink D, Klotman M, D'Agati V, Iyengar R, Klotman PE, He JC. Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20: 2138–2146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Fernandez F, Tan J, Shytle RD. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain 4: 23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groth C, Fortini ME. Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin Cell Dev Biol 23: 465–472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He JC, Husain M, Sunamoto M, D'Agati VD, Klotman ME, Iyengar R, Klotman PE. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest 114: 643–651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hiramatsu N, Hiromura K, Shigehara T, Kuroiwa T, Ideura H, Sakurai N, Takeuchi S, Tomioka M, Ikeuchi H, Kaneko Y, Ueki K, Kopp JB, Nojima Y. Angiotensin II type 1 receptor blockade inhibits the development and progression of HIV-associated nephropathy in a mouse model. J Am Soc Nephrol 18: 515–527, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, Schwartz EJ, Cara A, Klotman PE. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol 13: 1806–1815, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Imbimbo BP, Giardina GA. γ-Secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes. Curr Top Med Chem 11: 1555–1570, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain 140: 35–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signaling. Nat Cell Biol 6: 547–554, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kaminska M, Francin M, Shalak V, Mirande M. Role of HIV-1 Vpr-induced apoptosis on the release of mitochondrial lysyl-tRNA synthetase. FEBS Lett 581: 3105–3110, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA 89: 1577–1581, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D, Gacci M, Carini M, Lazzeri E, Romagnani P. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28: 1674–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int 78: 514–522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naicker S, Han TM, Fabian J. HIV/AIDS: dominant player in chronic kidney disease. Ethn Dis 16: S2–56–60, 2006 [PubMed] [Google Scholar]
- 34. Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 14: 290–298, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Papeta N, Sterken R, Kiryluk K, Kalyesubula R, Gharavi AG. The molecular pathogenesis of HIV-1 associated nephropathy: recent advances. J Mol Med 89: 429–436, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Quaranta MGVO, Felli C, Spadaro F, Silano M, Moricoli D, Giordani L, Viora M. Exogenous HIV-1 Nef upsets the IFN-γ-induced impairment of human intestinal epithelial integrity. PLos One 6: e23442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakamoto K, Chao WS, Katsube K, Yamaguchi A. Distinct roles of EGF repeats for the Notch signaling system. Exp Cell Res 302: 281–291, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Schwartz EJ, Cara A, Snoeck H, Ross MD, Sunamoto M, Reiser J, Mundel P, Klotman PE. Human immunodeficiency virus-1 induces loss of contact inhibition in podocytes. J Am Soc Nephrol 12: 1677–1684, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Sharma M, Brantley JG, Vassmer D, Chaturvedi G, Baas J, Vanden Heuvel GB. The homeodomain protein Cux1 interacts with Grg4 to repress p27(kip1) expression during kidney development. Gene 15: 87–94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma M, Callen S, Zhang D, Singhal PC, Vanden Heuvel GB, Buch S. Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS 24C: 2161–2170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma M, Fopma A, Brantley JG, Vanden Heuvel GB. Coexpression of Cux-1 and notch signaling pathway components during kidney development. Dev Dyn 231: 828–838, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Shkreli M, Sarin KY, Pech MF, Papeta N, Chang W, Brockman SA, Cheung P, Lee E, Kuhnert F, Olson JL, Kuo CJ, Gharavi AG, D'Agati VD, Artandi SE. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med 18: 111–119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shoham N, Cohen L, Yaniv A, Gazit A. The Tat protein of the human immunodeficiency virus type 1 (HIV-1) interacts with the EGF-like repeats of the Notch proteins and the EGF precursor. Virus Res 98: 57–61, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Shukla RR, Kumar A, Kimmel PL. Transforming growth factor beta increases the expression of HIV-1 gene in transfected human mesangial cells. Kidney Int 44: 1022–1029, 1993 [DOI] [PubMed] [Google Scholar]
- 46. Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol 19: 1139–1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 107: 931–939, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto T, Noble NA, Miller DE, Gold LI, Hishida A, Nagase M, Cohen AH, Border WA. Increased levels of transforming growth factor-beta in HIV-associated nephropathy. Kidney Int 55: 579–592, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Zhong J, Zuo Y, Ma J, Fogo AB, Jolicoeur P, Ichikawa I, Matsusaka T. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int 68: 1048–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Zhong Y, Wu Y, Liu R, Deng Y, Mallipattu SK, Klotman PE, Chuang PY, He JC. Roflumilast enhances the renal protective effects of retinoids in an HIV-1 transgenic mouse model of rapidly progressive renal failure. Kidney Int 81: 856–864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zuo Y, Matsusaka T, Zhong J, Ma J, Ma LJ, Hanna Z, Jolicoeur P, Fogo AB, Ichikawa I. HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J Am Soc Nephrol 17: 2832–2843, 2006 [DOI] [PubMed] [Google Scholar]