Abstract
In the basolateral membrane of proximal-tubule cells, NBCe1-A (SLC4A4, variant A), operating with an apparent Na+:HCO3− stoichiometry of 1:3, contributes to the reclamation of HCO3− from the glomerular filtrate, thereby preventing whole body acidosis. Others have reported that NBCe1-like activity in human, rabbit, and rat renal preparations is substantially influenced by lithium, sulfite, oxalate, and harmaline. These data were taken as evidence for the presence of distinct Na+ and CO32− binding sites in NBCe1-A, favoring a model of 1 Na+:1 HCO3−:1 CO32−. Here, we reexamine these findings by expressing human or rabbit NBCe1-A clones in Xenopus oocytes. In oocytes, NBCe1-A exhibits a 1:2 stoichiometry and could operate in one of five thermodynamically equivalent transport modes: 1) cotransport of Na+ + 2 HCO3−, 2) cotransport of Na+ + CO32−, 3) transport of NaCO3−, 4) exchange of Na+ + HCO3− for H+, or 5) HCO3−-activated exchange of Na+ for 2 H+. In contrast to the behavior of NBCe1-like activity in renal preparations, we find that cloned NBCe1-A is only slightly stimulated by Li+, not at all influenced by sulfite or oxalate, and only weakly inhibited by harmaline. These negative data do not uniquely support any of the five models above. In addition, we find that NBCe1-A mediates a small amount of Na+-independent NO3− transport and that NBCe1-A is somewhat inhibited by extracellular benzamil. We suggest that the features of NBCe1-like activity in renal preparations are influenced by yet-to-be-identified renal factors. Thus the actual ionic substrates of NBCe1 remain to be identified.
Keywords: carbonate, NBC1, nitrate, benzamil, harmaline
electrogenic sodium/bicarbonate cotransport (NBCe) activity was first observed in salamander kidney proximal-tubule (PT) cells (7). The transporter responsible for the activity (NBCe1-A, encoded by the Slc4a4 gene) was cloned from cDNA prepared from salamander kidneys (42). Subsequently, mammalian orthologs of rat and human NBCe1-A were cloned from kidney cDNA libraries (10, 40). The Slc4a4 gene has the capability to encode at least five products, named NBCe1-A through -E (8, 30). NBCe1-A is mainly expressed in kidney (1), NBCe1-B is expressed in many tissues throughout the body but is particularly abundant in pancreas (1), and NBCe1-C is mainly expressed in brain (6). NBCe1-D and -E are comparatively minor variants originally cloned from cDNAs isolated from mouse reproductive tract tissues (30).
NBCe1-A activity is a critical component of the mechanism by which PT cells reclaim HCO3− from the PT lumen, preventing the loss of HCO3− into the urine that would otherwise result in metabolic acidosis. Briefly, carbonic anhydrase IV on the apical surface of PT cells combines luminal HCO3− with secreted H+, generating CO2, which enters PT cells. The intracellular CO2 is hydrated by carbonic anhydrase II, generating H+ and HCO3−. Whereas H+ is recycled into the PT lumen via Na/H exchanger 3, HCO3−-like species are transported across the basolateral membrane of PT cells via NBCe1-A and finally enter the blood (51). Thus, malfunction of NBCe1-A results in severe metabolic acidosis, a syndrome known as proximal renal tubular acidosis, pRTA (24). Features of pRTA in individuals with mutations in SLC4A4 include growth retardation, mental retardation, and ocular abnormalities (24). In most studies of PTs, or PT-like cell lines overexpressing NBCe1-A, NBCe1-A appears to transport 1 Na+ with 3 HCO3− (20, 41, 58). However, in most other cell types and heterologous expression systems, and even in one study of isolated rabbit PTs, the apparent stoichiometry of the transporter is 1 Na+: 2 HCO3− (20, 21, 33, 47, 49).
Although many aspects of the molecular physiology of NBCe1-A are well characterized, the substrates that NBCe1-A transports have not been determined. NBCe1-A, operating with a 1:2 stoichiometry or a 1:3 stoichiometry, could operate in one of five1 major, thermodynamically equivalent transport modes (e.g., see Refs. 9 and 35): 1) cotransport of 1 Na+ and 2 HCO3− (1:2) or 1 Na+ and 3 HCO3− (1:3); 2) cotransport of 1 Na+ and 1 CO32− (1:2), 1 Na+ plus 1 CO32− and 1 HCO3− (1:3), or exchange of 1 Na+ plus 1 CO32− for 1 H+ (1:3); 3) transport of the NaCO3− ion pair (1:2), or 1 NaCO3− and 1 HCO3− (1:3); 4) exchange of 1 Na+ plus 1 HCO3− for 1 H+ (1:2), 1 Na+ plus 2 HCO3− for 1 H+ (1:3), or 1 Na+ plus 1 HCO3− for 2 H+ (1:3); and 5) NBCe1-A could act as a HCO3−-stimulated Na-2 H exchanger (1:2) or a HCO3−-stimulated Na-3 H exchanger (1:3).
In rabbit renal cortical basolateral membrane vesicles (BLMVs) (2, 50) and in Xenopus oocytes injected with rabbit renal cortical poly(A)+ RNA (43), HCO3− application stimulates 22Na influx, an observation consistent with the action of NBCe1-A. The further addition of SO32− and, in one preliminary study, oxalate2− (2) to the BLMV preparation stimulates 22Na uptake (the proxy for NBCe1-A activity)2 to a greater extent than does HCO3− alone (2, 43, 52). This observation has been taken as evidence that NBCe1-A, operating with a presumed stoichiometry of 1 Na+: 3 HCO3− equivalents, is capable of Na/HCO3/SO3 cotransport and, therefore, Na/HCO3/CO3 cotransport. In other words, these data are consistent with the idea that the transporter has a distinct binding site for a divalent anion, which would rule out all transporter models except model 2.
Harmaline is a hallucinogenic alkaloid that inhibits sodium-dependent transport systems in intestinal and renal cells by, it is proposed, competing for Na+ binding sites (44, 50). Several studies report that harmaline blocks NBCe1-like activity in renal preparations and heterologous expression systems (4, 16, 52, 54), suggesting that NBCe1-A has a distinct binding site for a cation. This hypothesis is further supported by the near-total blockade of NBCe1-A (expressed in Xenopus oocytes) by the application of benzamil, another inhibitor proposed to act at Na+ binding sites, to the intracellular surface of excised membrane patches (14). These data appear to rule out model 3.
Taken together, the indirect evidence for discrete Na+ and CO32− binding sites in NBCe1-A, appear to rule out all transporter models except model 2. However, some of the properties associated with NBCe1-like activity in rabbit renal preparations have not been demonstrated to be intrinsic properties of NBCe1-A. Indeed cloned rat NBCe1-A expressed in Xenopus oocytes (19, 47) does not exhibit the substantial Li+- or SO32−-supported transport that is a feature of the NBCe1-like activity measured in rabbit renal preparations. Furthermore, a preliminary report suggests that cloned rat NBCe1-A expressed in oocytes mediates electrogenic NO3− transport (46a), even though NO3− does not stimulate 22Na uptake by the NBCe1-like activity detected in rabbit BLMVs (52). However, it could be argued that the rat and rabbit orthologs of NBCe1-A exhibit difference substrate specificities.
In the present study, we reexamine the earlier conclusions by expressing human, rabbit, or rat NBCe1-A in Xenopus oocyte in the absence of other renal factors. We find that, as expressed in Xenopus oocytes, 1) expression of rabbit NBCe1-A elicits the DIDS-sensitive, Na+- and HCO3−-dependent currents that are characteristic of expression of human NBCe1-A; 2) human and rabbit NBCe1-A exhibit similar intrinsic (i.e., per molecule) activities; 3) human and rabbit NBCe1-A exhibit a far stronger selectivity for Na+ over Li+ than suggested by earlier studies of renal preparations; 4) SO32− is neither a substrate nor an inhibitor of human or rabbit NBCe1-A; 5) oxalate2− is neither a substrate nor an inhibitor of human and rabbit NBCe1-A; 6) NO3− is a minor substrate of human, rabbit, and rat NBCe1-A in the absence of extracellular Na+; 7) 200 μM harmaline does not substantially inhibit human or rabbit NBCe1-A; and 8) 500 μM benzamil effects a 30% inhibition of human and rabbit NBCe1-A. Thus, evidence regarding the mode of HCO3−-equivalent transport by mammalian NBCe1-A is not adequately demonstrated by prior studies and it is premature to discount any of the five major transporter modes.
MATERIALS AND METHODS
Source of NBCe1-A Clones
We purchased a rabbit renal cDNA library (Zyagen, San Diego, CA) and amplified rabbit NBCe1-A cDNA by touchdown PCR. The forward primer was 5′-CGAAGCCCGGGCCACCATGTCCACTGAAAATGTGGAAG-3′ (in which the underlined sequence is an XmaI site, the italicized sequence is a Kozak sequence, and the boldfaced sequence is the initiator methionine) and the reverse primer was 5′-TGCTCTAGATCAGCATGATGTGTGGCG-3′ (in which the underlined sequence is an XbaI site and the boldfaced sequence is the termination codon). Primers were designed to match a previously deposited rabbit NBCe1-A sequence (GenBank accession no. AF119816). Pfu Ultra AD polymerase (Stratagene, La Jolla, CA) was used according to the manufacturer's recommendations in this 40-cycle PCR, with the exception that the annealing temperature was incrementally decreased from 75°C to 55°C for the first 20 cycles, and maintained at 55°C for the final 20 cycles. The resulting PCR product (>3 kb, which matches the estimated size of human NBCe1-A) was gel-purified using the QIAquick gel extraction kit (Qiagen, Valencia, CA). The PCR product was digested with XmaI and XbaI and subcloned into the pGH19 vector (56). The DNA sequence of two clones was confirmed by automated sequencing performed by the Keck Sequencing Center (Yale University, New Haven, CT). Sequence conflicts between the two clones were resolved by sequencing of two PCR product mixtures (representing two distinct fragments of NBCe1-A) that were amplified from the original cDNA library using Pfu Ultra AD polymerase. The two sets of primers were used to generate these fragments. The first set forward primer was 5′-CGAAGCCCGGGCCACCATGTCCACTGAAAATGTGGAAG-3′ and the reverse primer was 5′-GCATGTCAGTAGAAGAGACAGC-3′. The second set forward primer was 5′-CGCCAGACCCAGTTAATATC-3′ and the reverse primer was 5′-TGCTCTAGATCAGCATGATGTGTGGCG-3′. Sequencing of the PCR products was performed by Eurofins MWG Operon (Petaluma, CA). The construction of rat NBCe1-A.pTLN2 has been previously reported (40). The construction of human NBCe1-A-EGFP.pGH19 has been previously reported (55); the same method was used to generate rabbit NBCe1-A-EGFP.pGH19 from rabbit NBCe1-A.pGH19. The sequence of rabbit NBCe1-A.pGH19 was confirmed by automated sequencing performed by the Keck Sequencing Center and Eurofins MWG Operon.
cRNA Synthesis and Expression in Xenopus Oocytes
cDNA constructs in pGH19 were digested with NotI and then purified using the QIAquick PCR purification kit (Qiagen). Capped mRNAs was synthesized from the digested cDNAs using a T7 Message Machine kit (Ambion, Austin, TX) according to the manufacturer's instructions. The synthesized cRNAs were purified using an RNeasy MinElute RNA Cleanup kit (Qiagen). Oocytes were obtained by dissecting frogs in-house, and prepared as described previously (34, 36). The Institutional Animal Care and Use Committee at Case Western Reserve University approved the protocols for housing and handling of Xenopus laevis. One day after preparation, oocytes were injected with 25 nl of H2O or cRNA. The injected cRNA solution contained a final concentration of 1 ng/nl NBCe1-A cRNA. Thus each RNA-injected oocyte was injected with 25 ng of NBCe1-A cRNA. All experiments were performed at room temperature.
Solutions
The composition of solutions used in each protocol presented in the present study is presented in Tables 1–13. Briefly, our nominally CO2/HCO3−-free solution, ND96, contains (in mM) 93.5 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, adjusted to pH 7.50 using NaOH such that the final [Na+] is 96. Cation-substituted versions of our ND96 solution include 96 Li+ (ND96/Li+) or 96 NMDG+ (ND96/NMDG+) in place of 96 Na+. The anion-substituted version of our ND96 solution includes either 33 SO32− (ND96/SO32−) in place of 59.4 Cl−,3 33 oxalate2− (ND96/oxalate2−) in place of 66 Cl− or 33 NO3− (ND96/NO3−) in place of 33 Cl−. All solutions are adjusted to ∼200 mosM by the addition of mannitol. To avoid precipitation of insoluble calcium salts, we omitted CaCl2 from solutions that contained 33 SO32− or 33 oxalate2−, as well as from all other solutions in these protocols. Note that omission of magnesium from these solutions is not necessary because MgSO3 and MgOxalate are two orders of magnitude more soluble than their calcium-containing counterparts (38).
Table 1.
Solutions used in Fig. 1
| Component | ND96, mM | 10 mM HCO3− |
|---|---|---|
| Na+ | 96 | 96 |
| K+ | 2 | 2 |
| Mg2+ | 1 | 1 |
| Ca2+ | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 |
| Cl− | 101.1 | 91.1 |
| HCO3− | 0 | 10 |
| HEPES− | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 |
| pH | 7.5 | 7.5 |
Solutions are presented left to right in their order of application to the oocytes. Here and elsewhere, boldfaced text within a solution column indicates differences between that solution and the preceding solution. The 10-mM HCO3− solutions were equilibrated with 1.5% CO2/98.5% O2. Osmolarity of all solutions was ∼200 mosM.
Table 2.
| Component | ND96, mM | 33 mM HCO3− |
|---|---|---|
| Na+ | 96 | 96 |
| K+ | 2 | 2 |
| Mg2+ | 1 | 1 |
| Ca2+ | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 |
| HCO3− | 0 | 33 |
| HEPES− | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 |
| pH | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Note that the HCO3−-containing solutions used to gather the data in Fig. 16 included the 0.8% DMSO vehicle. Osmolarity of both solutions was ∼200 mosM.
Table 3.
Solutions used in Fig. 3
| Component | ND96/NMDG+, mM | 33 mM HCO3−/NMDG+ | 33 mM HCO3− |
|---|---|---|---|
| Na+ | 0 | 0 | 96 |
| NMDG+ | 96 | 96 | 0 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 | 68.1 |
| HCO3− | 0 | 33 | 33 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
Here and elsewhere, italicized and boldfaced text within the first solution column indicates differences between the starting solution and standard ND96 solution (left column in Table 1A). The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Osmolarity of all solutions was ∼200 mosM.
Table 4.
Solutions used in Fig. 4
| Component | ND96/Li+, mM | 33 mM HCO3−/Li+ | 33 mM HCO3− |
|---|---|---|---|
| Na+ | 0 | 0 | 96 |
| Li+ | 96 | 96 | 0 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 | 68.1 |
| HCO3− | 0 | 33 | 33 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Osmolarity of all solutions was ∼200 mosM.
Table 5.
| Component | ND96/10 mM Na+/86 mM NMDG+ | 33 mM HCO3−/10 mM Na+/86 mM NMDG+ | 33 mM HCO3−/10 mM Na+/86 mM Li+ |
|---|---|---|---|
| Na+ | 10 | 10 | 10 |
| NMDG+ | 86 | 86 | 0 |
| Li+ | 0 | 0 | 86 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 | 68.1 |
| HCO3− | 0 | 33 | 33 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Note that the HCO3−-containing solutions used to gather the data in Fig. 17 included 0.8% DMSO vehicle. Osmolarity of all solutions was ∼200 mosM.
Table 6.
Solutions used in Fig. 6
| Component | Ca2+-free ND96, mM | ND96/SO32−, mM | 33 mM HCO3− |
|---|---|---|---|
| Na+ | 96 | 96 | 96 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 0 | 0 | 0 |
| Total cations, meq | 100 | 100 | 100 |
| Cl− | 97.5 | 38.1 | 64.5 |
| HCO3− | 0 | 0 | 33 |
| SO32− | 0 | 26.4 | 0 |
| HSO3− | 0 | 6.6 | 0 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 100 | 100 | 100 |
| Mannitol | 0 | 33 | 0 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Osmolarity of all solutions was ∼200 mosM.
Table 7.
Solutions used in Fig. 7
| Component | ND96, mM | 10 mM HCO3− | 10 mM HCO3−/SO32− |
|---|---|---|---|
| Na+ | 96 | 96 | 96 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 91.1 | 73.1 |
| HCO3− | 0 | 10 | 10 |
| SO32− | 0 | 0 | 8 |
| HSO3− | 0 | 0 | 2 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| Mannitol | 0 | 0 | 10 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 10 mM HCO3− solutions were equilibrated with 1.5% CO2/98.5% O2. Osmolarity of all solutions was ∼200 mosM.
Table 8.
Solutions used in Fig. 8
| Component | Ca2+-free ND96, mM | ND96/Oxalate2−, mM | 33 mM HCO3− |
|---|---|---|---|
| Na+ | 96 | 96 | 96 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 0 | 0 | 0 |
| Total cations, meq | 100 | 100 | 100 |
| Cl− | 97.5 | 31.5 | 64.5 |
| HCO3− | 0 | 0 | 33 |
| Oxalate2− | 0 | 33 | 0 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 100 | 100 | 100 |
| Mannitol | 0 | 33 | 0 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Osmolarity of all solutions was ∼200 mosM.
Table 9.
Solutions used in Fig. 9
| Component | ND96, mM | ND96/NO3−, mM | 33 mM HCO3− |
|---|---|---|---|
| Na+ | 96 | 96 | 96 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 | 68.1 |
| HCO3− | 0 | 0 | 33 |
| NO3− | 0 | 33 | 0 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Osmolarity of all solutions was ∼200 mosM.
Table 10.
Solutions used in Fig. 10
| Component | ND96/NMDG+, mM | ND96/NMDG+/33 mM NO3− |
|---|---|---|
| Na+ | 0 | 0 |
| NMDG+ | 96 | 96 |
| K+ | 2 | 2 |
| Mg2+ | 1 | 1 |
| Ca2+ | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 |
| NO3− | 0 | 33 |
| HEPES− | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 |
| pH | 7.5 | 7.5 |
Osmolarity of both solutions was ∼200 mosM.
Table 11.
Solutions used in Fig. 11
| Component | ND96, mM | ND96/93.5 NO3−, mM | ND96/NMDG+/93.5 mM NO3− |
|---|---|---|---|
| Na+ | 96 | 96 | 0 |
| NMDG+ | 0 | 0 | 96 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 7.6 | 7.6 |
| NO3− | 0 | 93.5 | 93.5 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
Osmolarity of all solutions was ∼200 mosM.
Table 12.
Solutions used in Fig. 12
| Component | ND96, mM | 10 mM HCO3− | 10 mM HCO3−/NO3− |
|---|---|---|---|
| Na+ | 96 | 96 | 96 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 91.1 | 81.1 |
| HCO3− | 0 | 10 | 10 |
| NO3− | 0 | 0 | 10 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 10 mM HCO3− solutions were equilibrated with 1.5% CO2/98.5% O2. Osmolarity of all solutions was ∼200 mosM.
Table 13.
Solutions used in Fig. 15
| Component | ND96, mM | 33 mM HCO3−/NMDG+ | 33 mM HCO3− |
|---|---|---|---|
| Na+ | 96 | 0 | 96 |
| NMDG+ | 0 | 96 | 0 |
| K+ | 2 | 2 | 2 |
| Mg2+ | 1 | 1 | 1 |
| Ca2+ | 1.8 | 1.8 | 1.8 |
| Total cations, meq | 103.6 | 103.6 | 103.6 |
| Cl− | 101.1 | 68.1 | 68.1 |
| HCO3− | 0 | 33 | 33 |
| HEPES− | 2.5 | 2.5 | 2.5 |
| Total anions, meq | 103.6 | 103.6 | 103.6 |
| HEPES (neutral) | 2.5 | 2.5 | 2.5 |
| pH | 7.5 | 7.5 | 7.5 |
The 33-mM HCO3− solutions were equilibrated with 5% CO2/95% O2. Osmolarity of all solutions was ∼200 mosM.
Our CO2/HCO3−-containing solutions are similar to ND96 except that 10 or 33 NaHCO3 replaces 10 or 33 NaCl and these solutions are equilibrated with either 1.5% CO2/balanced O2 or 5% CO2/balanced O2. We refer to these solutions as 10 mM HCO3− and 33 mM HCO3−. Anion and cation substituted versions of these solutions were created by the same substitution maneuvers used to create our modified ND96 solutions.
The ion transport inhibitors used in this study were 1) DIDS (Invitrogen, Carlsbad, CA), which was used at final concentration of 200 μM and prepared by dissolving powdered DIDS directly into our 33-mM HCO3− solution; 2) harmaline (Sigma-Aldrich, St. Louis, MO), which was used at a final concentration of 200 μM and prepared by dissolving powdered harmaline directly into our 33-mM HCO3− solution; and 3) benzamil (Enzo Life Sciences, Farmingdale, NY), which was used at a final concentration of 500 μM and prepared as a 25 mg/ml stock in DMSO. The final working solution included 0.8% (vol/vol) DMSO. A 33-mM HCO3− solution, containing 0.8% DMSO but no benzamil, was used in place of our standard 33-mM HCO3− solution in the experiments shown in Fig. 16.
Fig. 16.
Influence of benzamil upon I-V relationships for human and rabbit NBCe1-A in the presence of 96 mM Na+. Experiments similar to those shown in Fig. 14, A–D, except that the oocytes were exposed sequentially to 1) ND96, 2) 33 mM HCO3−, 3) a 33-mM HCO3− solution that contained 500 μM benzamil, and finally 4) to the benzamil-free 33 mM HCO3−. The composition of solutions used in this protocol is shown in Table 2. Horizontal bars, *P < 0.05.
Electrophysiological Measurements
We measured ionic currents in intact oocytes using a two-electrode voltage clamp. We recorded current-voltage (I-V) relationships using a model OC-725C oocyte clamp (Warner Instruments, Hamden, CT). We pulled electrodes from thin-walled borosilicate glass (Harvard Apparatus, Holliston, MA), each of which had a resistance of 0.5–2.0 MΩ when filled with 3 M KCl (Fisher, Pittsburgh, PA). In each experiment, an oocyte was placed in the recording chamber in one of our CO2/HCO3−-free solutions (e.g., ND96 or ND96/NMDG+) and sequentially impaled with two KCl-filled microelectrodes, one to measure membrane potential (Vm) and one to pass current. The cell was superfused with the CO2/HCO3−-free solution until Vm had reached a stable value, indicating that the cell membrane had resealed around the electrode impalement sites. The voltage clamp was applied to hold Vm at its spontaneous value and then the voltage-clamp protocol was initiated. The voltage-clamp protocol used to generate I-V relationships stepped Vm from its spontaneous value to a holding potential (Vh) of −160 mV for 100 ms and then back to the spontaneous Vm for an additional 100 ms prior to the next step, which was 20 mV more positive than the last. This cycle was repeated until the final Vh step was +20 mV. After the first set of voltage-clamp recordings in the CO2/HCO3−-free solution, the superfusion solution was changed and another set of voltage-clamp recordings was gathered. Most protocols included additional solution changes and the gathering of additional voltage-clamp recordings. Note that when the superfusion solution was switched from a CO2/HCO3−-free solution to a CO2/HCO3−-containing solution, the oocytes were superfused with the CO2/HCO3− solution for at least 5 min prior to obtaining voltage-clamp data to make sure that CO2 was equilibrated across the oocyte membrane (e.g., see Refs. 12 and 18). In other cases, voltage-clamp recordings were performed ∼1 min after the solution change.
Biotinylation
Proteins expressed in the oocyte plasma membrane were biotinylated and isolated using the protocol described in Ref. 28. Groups of 10 oocytes were biotinylated and processed using the Cell Surface Protein Isolation Kit (Pierce, Rockford, IL), according to the manufacturer's instructions. Briefly, the oocytes were incubated with biotinylating agent for 1 h and then lysed. An aliquot of total oocyte protein was set aside for Western blot analysis. The remaining homogenate was passed through a neutravidin-agarose-packed column to isolate the biotinylated oocyte protein. Total and biotinylated oocyte protein fractions were resolved by SDS-PAGE on Novex 3–8% Tris-acetate gels (Invitrogen) and transferred onto polyvinylidene difluoride membranes using the iBlot dry blotting system (Invitrogen). NBCe1-A was detected using the NBC-3 anti-NBCe1-A rabbit-polyclonal primary antibody (46), followed by a horseradish peroxidase-conjugated goat anti-rabbit polyclonal antibody (MP Biomedicals, Solon, OH). Western blots were developed using ECL Plus reagents (GE Healthcare Biosciences, Piscataway, NJ), and signals were visualized on a ChemFluor E (Protein Simple, Santa Clara, CA). The signals were quantified with Image J software (NIH). Cells were processed in triplicate batches of 10, and each of the biotinylated protein samples was resolved and analyzed in triplicate.
Data Analysis
pClamp and Clampfit software (version 10; Axon Instruments, Foster City, CA) were used to collect and analyze voltage-clamp data. Data were further analyzed with Microsoft Excel 2010. Values are given as means ± SE together with the number of replicate experiments (n). Membrane conductance was calculated between −20 mV and +20 mV, where extracellular HCO3−-dependent currents associated with NBCe1 expression dominate HCO3−-independent currents (31). Statistical analyses (ANOVA with Tukey's post hoc analysis, and t-tests) were performed on data using Minitab 16 (Minitab, State College, PA) or Microsoft Excel 2010.
RESULTS
Cloning Rabbit NBCe1-A
Using a primer pair specific for rabbit NBCe1-A, we amplified and isolated a 3.2-kb PCR product from rabbit kidney cDNA. Ligation of this product into pGH19 resulted in the isolation of two clones that we determined by DNA sequencing to represent full-length rabbit NBCe1-A in pGH19. We noted two nucleotide differences between these clones within the NBCe1-A open reading frame. It was not obvious which of these differences were PCR errors because 1) the rabbit genome sequence is not well characterized, 2) we do not know if the partly sequenced rabbit genome is isogenic with the rabbit strain from which our cDNA originated, and 3) we noted eight nucleotide differences between each of our two clones and a previously deposited rabbit NBCe1-A sequence. Thus, to determine which of the mismatches between our two clones arose from PCR errors, we compared the sequence of our clones to a consensus NBCe1-A sequence generated by sequencing a population of partial rabbit NBCe1-A PCR products. In this way, we determined that each of our two clones includes a single, synonymous nucleotide difference compared with the consensus sequence. Thus, both clones faithfully encode the NBCe1-A protein from our source rabbit. We present an alignment of the protein sequence of our rabbit NBCe1-A clone vs. human, mouse, and rat NBCe1-A in Supplemental Fig. 1. We have deposited the consensus nucleotide sequence of our rabbit NBCe1-A in GenBank (nucleotide accession no. JX426114 and protein accession no. AFS49951).
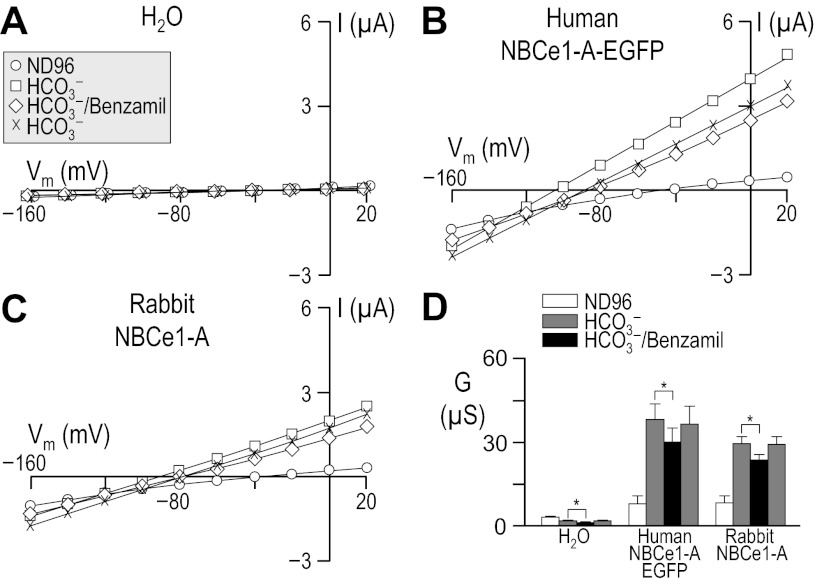
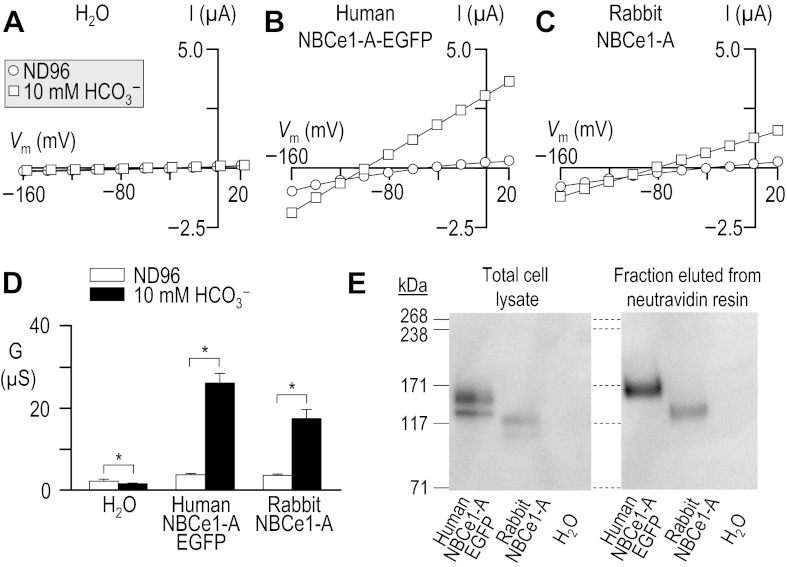
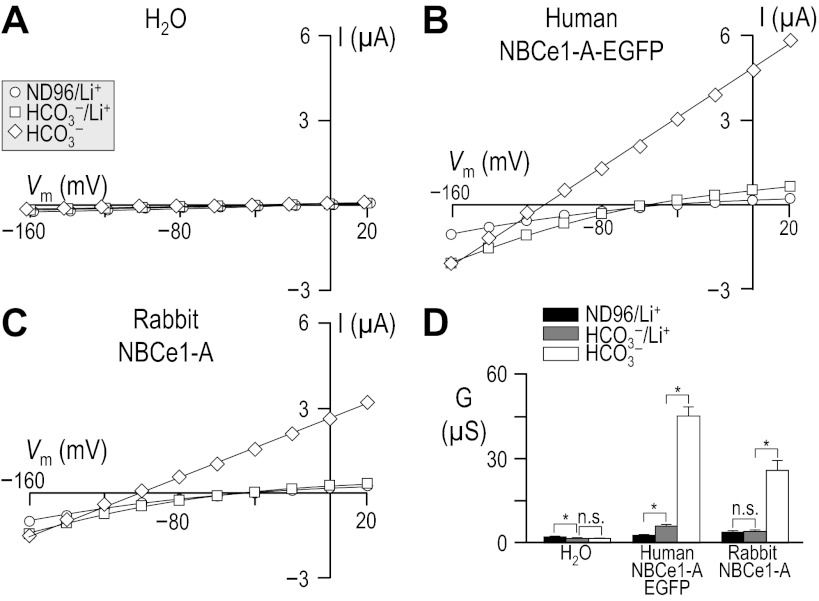
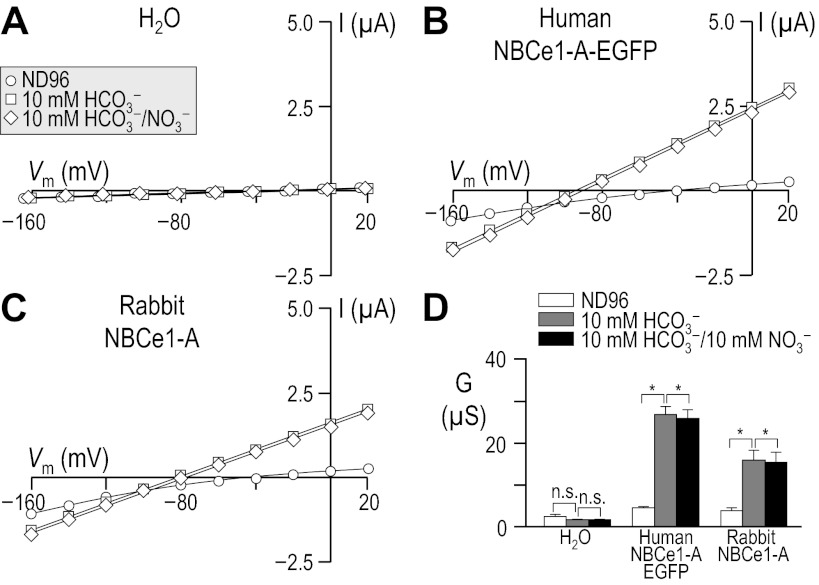
Fig. 1.
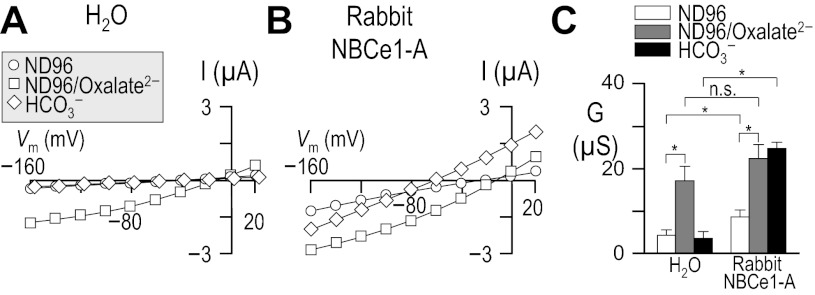
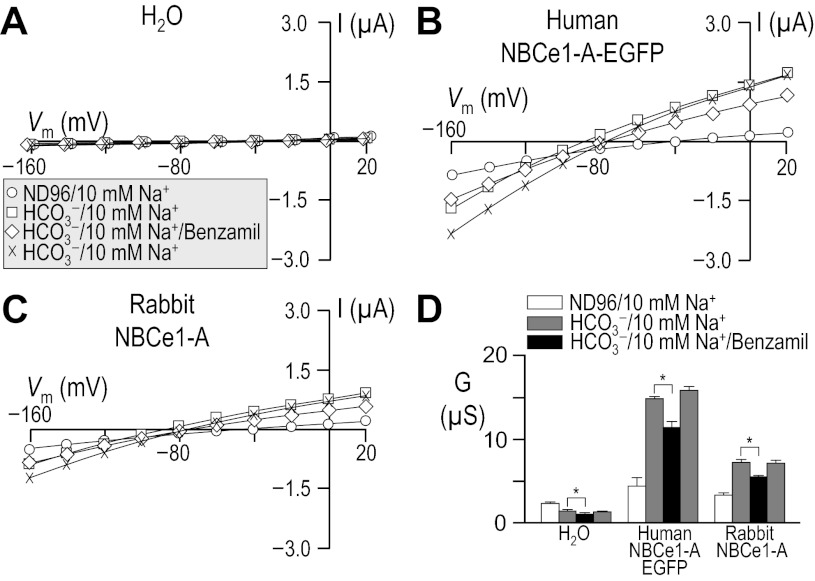
Influence of HCO3− upon current-voltage (I-V) relationships for human vs. rabbit electrogenic sodium/bicarbonate cotransporter 1-A (NBCe1-A). Oocytes were injected with either H2O (A), cRNA encoding human NBCe1-A-EGFP (B), or cRNA encoding rabbit NBCe1-A (C). I-V relationships were obtained by voltage clamp while the cells were sequentially exposed to the nominally CO2/HCO3−-free solution, ND96 and to our 10-mM HCO3− solution. Note that the squares in A are artificially offset by +3 mV from the circles to make both data sets visible. Averaged slope conductances (G) measured between −20 and +20 mV for 6 cells are shown in D, in which the results of paired one-tailed t-tests are shown as horizontal bars (*P < 0.05). A representative Western blot analysis of total (left) and biotinylated (right) NBCe1 protein from oocytes similar to those assayed in A–D is shown in E, detected using an anti-NBCe1 antibody. The composition of solutions used in this protocol is shown in Table 1.
Demonstrating the Functionality of Rabbit NBCe1-A
Because cloned rabbit NBCe1 had never been characterized functionally, we compared our rabbit data with results from the better-characterized human NBCe1-A. We injected Xenopus oocytes with H2O, human NBCe1-A-EGFP cRNA, or cRNA that encodes non-EGFP-tagged rabbit NBCe1-A.4 Three to four days after injection, we performed a voltage-clamp protocol that would allow us to determine the membrane slope conductance, between −20 and +20 mV in ND96 and in 10-mM HCO3− solutions (see Table 1). The difference between these values is a measure of HCO3−-dependent NBCe1-A activity. The membrane potential of H2O-injected cells did not substantially change in response to the application of CO2/HCO3− (not shown). As expected, cells expressing an electrogenic NBC hyperpolarized immediately upon exposure to CO2/HCO3−, cells expressing human NBCe1-A-EGFP hyperpolarized by 76 ± 1 mV (n = 6, not shown), and those expressing rabbit NBCe1-A hyperpolarized by 60 ± 1 mV (n = 6, not shown).
Figure 1 shows representative I-V relationship data gathered in the absence (circles) and presence (squares) of 1.5% CO2/10 mM HCO3− for oocytes injected with H2O (Fig. 1A) or with cRNA encoding either human NBCe1-A-EGFP (Fig. 1B) or rabbit NBCe1-A (Fig. 1C). Figure 1D summarizes the mean slope conductances that we obtained from a larger number of cells such as those in Fig. 1, A–C. The mean HCO3−-dependent conductance in these experiments, determined oocyte by oocyte, was −0.7 ± 0.3 μS (n = 6) for H2O-injected cells, 22 ± 2.2 μS (n = 6) for oocytes expressing human NBCe1-A-EGFP, and 14 ± 1.8 μS (n = 6) for cells expressing rabbit NBCe1-A. The HCO3−-dependent conductance of cells expressing human and rabbit NBCe1-A was significantly greater than exhibited by H2O-injected cells (P < 0.05, n = 6, ANOVA). Moreover, the HCO3−-dependent conductance of cells expressing human NBCe1-A-EGFP was significantly greater than that exhibited by cells expressing rabbit NBCe1-A (P = 0.007, n = 6, unpaired one-tailed t-test).
The lesser functional expression5 of rabbit NBCe1-A vs. human NBCe1-A-EGFP could be explained by the reduced abundance in the plasma membrane of rabbit NBCe1-A vs. human NBCe1-A-EGFP, as evidenced by the representative blots of total and biotinylated NBCe1-A (Fig. 1E). In five independent biotinylation experiments, we found rabbit NBCe1-A protein to be consistently less abundant than human NBCe1-A-EGFP protein (P < 0.001, one-tailed, paired t-test, n = 6). On average, rabbit NBCe1-A exhibited 54 ± 12% of the total abundance and 68 ± 15% of the plasma membrane abundance of human NBCe1-A-EGFP. We estimate that no more than ∼20% of the total human NBCe1-A-EGFP and no more than ∼15% of the total rabbit NBCe1-A is resident in the oocyte plasma membrane, indicating a small but statistically significant difference in protein trafficking (P = 0.011, paired one-tailed t-test, n = 5).
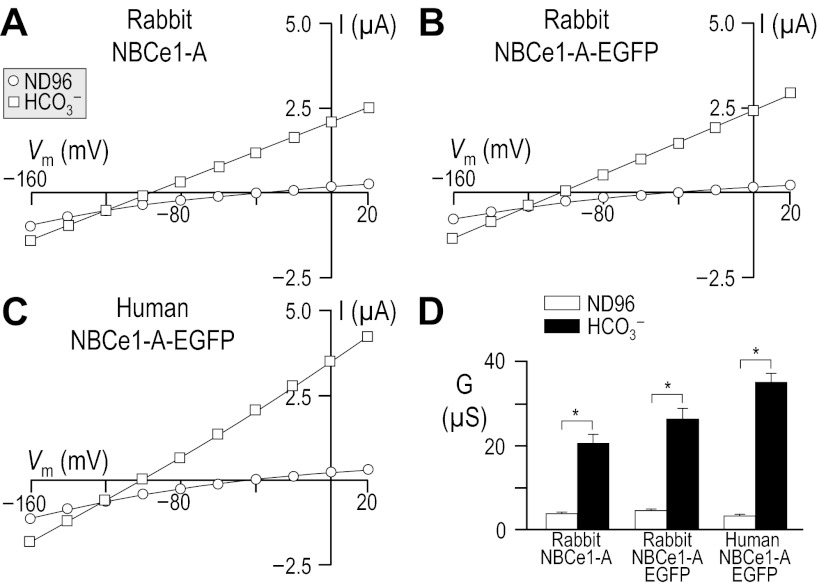
A comparison of the functional expression of human NBCe1-A-EGFP, rabbit NBCe1-A-EGFP, and non-EGFP-tagged rabbit NBCe1-A assayed in 33 mM HCO3− (Fig. 2; see Table 2) shows that the presence of the EGFP tag confers a small but statistically insignificant increase in HCO3−-dependent slope conductance to rabbit NBCe1-A (P > 0.05, n = 6, unpaired one-tailed t-test). However, cells expressing human NBCe1-A-EGFP exhibit a significantly greater HCO3−-dependent conductance than cells expressing either rabbit NBCe1-A or rabbit NBCe1-A-EGFP (P < 0.05, n = 6, ANOVA with post hoc analysis), indicating that human NBCe1-A exhibits a greater functional expression than its rabbit ortholog.
Fig. 2.
Influence of the EGFP-tag upon I-V relationships for human vs. rabbit NBCe1-A. Oocytes were injected with cRNA encoding either rabbit NBCe1-A (A) rabbit NBCe1-A-EGFP (B), or human NBCe1-A-EGFP (C). I-V relationships were obtained by voltage clamp while the cells were sequentially exposed to ND96 solution and to our 33-mM HCO3− solution. Averaged slope conductances measured between −20 and +20 mV for at least 6 cells are shown in D, in which the results of certain one-tailed paired t-tests are shown as horizontal bars (*P < 0.05). The composition of solutions used in this protocol is shown in Table 2.
Cation Specificity of Human and Rabbit NBCe1-A
Li+ supports the NBCe1-like activity of human NBCe1-A heterologously expressed in HEK cells and the native NBCe1-like activity of rabbit renal preparations better than Li+ supports rat NBCe1-A expressed in Xenopus oocytes (see Refs. 4, 50, and 52 vs. Ref. 46). To compare the cation selectivities of human and rabbit NBCe1-A in the same cell type, we expressed these transporters in Xenopus oocytes and assayed the ability of NMDG+ or Li+ to support electrogenic HCO3− transport.
NMDG+.
We superfused oocytes with our ND96/NMDG+, 33-mM HCO3−/NMDG+, and 33-mM HCO3− solutions (Table 3) in sequence and performed our voltage-clamp protocol during each superfusion period. Neither solution change caused the Vm of H2O-injected oocytes to exhibit a substantial, instantaneous response (not shown). However, application of the 33-mM HCO3−/NMDG+ solution to oocytes expressing human NBCe1-A-EGFP caused the cells to depolarize by 45 ± 2 mV (n = 6, not shown) and oocytes expressing rabbit NBCe1-A to depolarize by 21 ± 2 mV (n = 6, not shown). However, a rapid hyperpolarization accompanied the subsequent application of 33 mM HCO3− to cells expressing human NBCe1-A-EGFP (ΔVm = −115 ± 3 mV, n = 6, not shown) or cells expressing rabbit NBCe1-A (ΔVm = −91 ± 3 mV, n = 6, not shown).
Figure 3, A–C shows representative I-V relationships for oocytes injected with H2O or with the cRNA encoding either human or rabbit NBCe1-A and then subjected to above-mentioned protocol. The average slope conductances for a larger number of cells subjected to this protocol are shown in Fig. 3D. We note that, at positive Vm, all three cell populations exhibit outwardly rectifying currents in ND96/NMDG+ (e.g., circle at +20 mV in Fig. 3A) that are larger than the currents of similar cells measured in ND96 (e.g., circle at +20 mV in Fig. 1A). For example, H2O-injected cells exhibited an average membrane conductance of 10 ± 5 μS (n = 6) in ND96/NMDG+ (Fig. 3D) compared with an average membrane conductance of 2.3 ± 0.4 μS (n = 6) in ND96 (Fig. 1D), although the difference does not achieve statistical significance in our data set (P = 0.072, n = 6, one-tailed unpaired t-test).
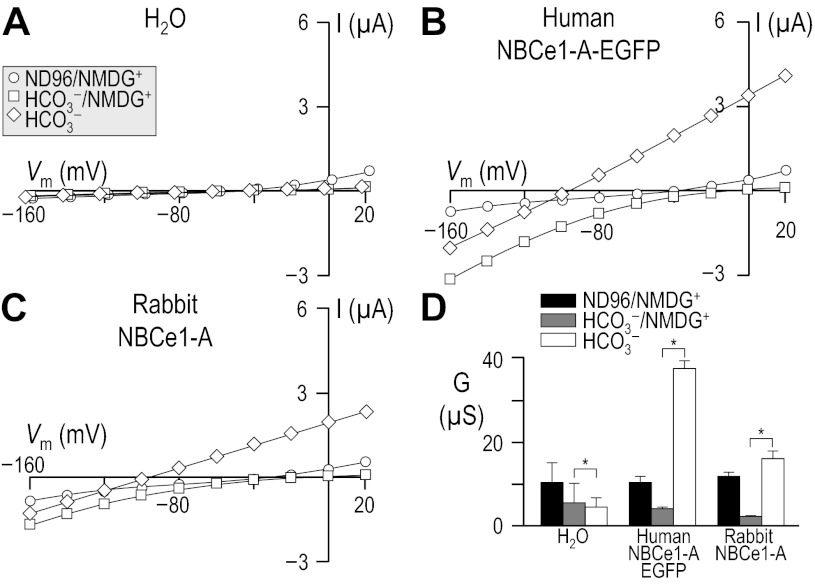
Fig. 3.
Influence of Na+ and HCO3− upon I-V relationships for human vs. rabbit NBCe1-A. Oocytes were injected with either H2O (A), cRNA encoding human NBCe1-A-EGFP (B), or cRNA encoding rabbit NBCe1-A (C). I-V relationships were obtained by 2-electrode voltage clamp while the cells were sequentially exposed to a Na+-free ND96 solution that contained 96 mM NMDG+ in place of 96 mM Na+, a 33-mM HCO3− solution in the continued absence of Na+, and finally a 33-mM HCO3− solution containing 96 mM Na+. Note that the circles in A are artificially offset by +2 mV, and the diamonds by −2 mV, from the squares to make all data sets visible. Averaged slope conductances measured between −20 and +20 mV for 6 cells are shown in D, in which the results of certain paired one-tailed t-tests are shown as horizontal bars (*P < 0.05). The composition of solutions used in this protocol is shown in Table 3.
Application of 33 mM HCO3− in the continued absence of Na+/presence of NMDG+ did not elicit an increase in outwardly directed currents, which would have indicated the net, inward action of an electrogenic cation, 2 HCO3− cotransporter. In fact, for all three groups of injected oocytes, the addition of 33 mM HCO3− in the continued absence of Na+ (squares) reduced the conductance between −20 mV and +20 mV (Fig. 3A–D). On the other hand, for oocytes expressing human NBCe1-A-EGFP (Fig. 3B) or rabbit NBCe1-A (Fig. 3C), the application of CO2/HCO3− increased the magnitude of inwardly directed currents (squares), which likely represent electrogenic Na/2 HCO3 efflux, supported by intracellular Na+ and HCO3−.
The presence of NBCe1-A activity in oocytes injected with human NBCe1-A-EGFP or rabbit NBCe1-A cRNA was confirmed by replacing NMDG+ with Na+ in the continued presence of HCO3− (diamonds). This maneuver elicited substantial Na+- and HCO3−-dependent currents in these cells (Fig. 3, B–D), but not in H2O-injected oocytes (Fig. 3, A and D). Thus, neither human NBCe1-A-EGFP nor rabbit NBCe1-A exhibit detectable electrogenic NMDG/HCO3 cotransport activity in oocytes.
Lithium.
We superfused oocytes with our ND96/Li+, 33-mM HCO3−/Li+, and 33-mM HCO3− solutions (Table 4) in sequence, and then performed the voltage-clamp protocol. In H2O-injected oocytes, Vm did not change instantaneously in response to either solution change. However, application of CO2/HCO3− in the presence of Li+ induced a rapid hyperpolarization in oocytes expressing human NBCe1-A-EGFP (ΔVm = −33 ± 2 mV, n = 6, not shown) and in oocytes expressing rabbit NBCe1-A (ΔVm = −24 ± 2 mV, n = 6, not shown). Subsequently, replacing Li+ with Na+ in the superfusion solution elicited hyperpolarizations of even greater magnitude: ΔVm = −79 ± 1 mV for human NBCe1-A-EGFP (n = 6, not shown) and ΔVm = −74 ± 1 mV for rabbit NBCe1-A (n = 6, not shown).
Figure 4, A–C shows representative I-V relationships for oocytes injected with H2O or with cRNA encoding human NBCe1-A-EGFP or rabbit NBCe1-A. Figure 4D shows the slope conductances extracted from data such as these for a larger number of cells. The switch from ND96 to 33 mM HCO3− in the presence of Li+ (i.e., absence of Na+) did not elicit an increase in membrane conductance (measured between −20 mV and +20 mV) in H2O-injected cells (Fig. 4A). In fact, we measured a small but significant decrease (P = 0.002, paired one-tailed t-test). The same was true of cells expressing rabbit NBCe1-A (Fig. 4C; P = 0.306, paired one-tailed t-test). However, in the six cells expressing human NBCe1-A-EGFP (Fig. 4B), the same maneuver elicited a small but significant increase in slope conductance (P < 0.001, paired one-tailed t-test). By comparing the HCO3−-dependent slope conductances measured in the presence of Na+ vs. the presence of Li+ for these same six cells, we estimate that Li+ supports about 8% of the electrogenic cation/HCO3 cotransport activity supported by Na+ when the two cation species are present at a level of ∼96 mM.6 Thus, human NBCe1-A-EGFP exhibits detectable electrogenic Li/HCO3 cotransport activity in oocytes. Li/HCO3 cotransport by rabbit NBCe1-A is evidenced by a Li+-and HCO3−-dependent hyperpolarization (see above), but the cotransport activity was not sufficiently robust to produce a measureable increase in membrane conductance.
Fig. 4.
Influence of Li+ upon I-V relationships for human vs. rabbit NBCe1-A. Similar experiments to those shown in Fig. 3, A–D, except that the Na+ replacement in this case is Li+ rather than NMDG+ (*P < 0.05, n.s., not significant).The composition of solutions used in this protocol is shown in Table 4.
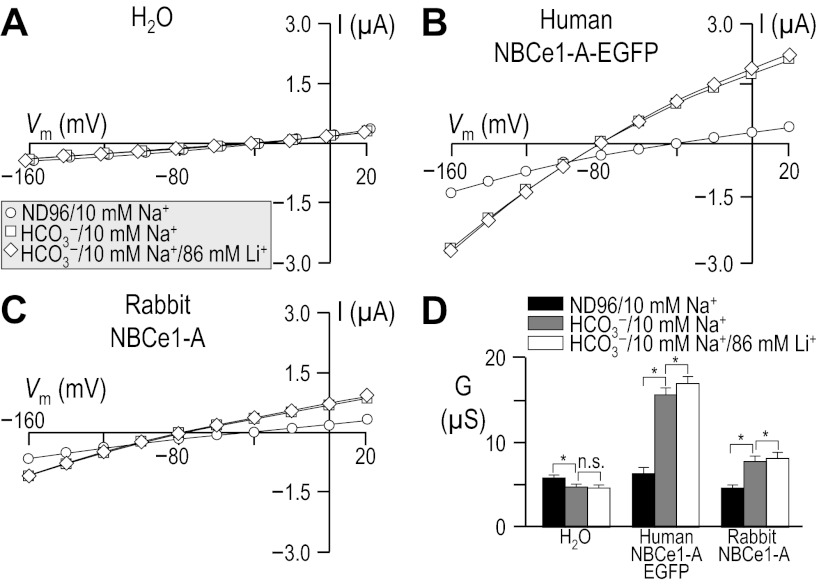
To determine whether Li+ can inhibit NBCe1-A activity in oocytes, a feature of NBCe1-like activity in renal preparations, we assayed the influence of Li+ upon NBCe1-A activity in the continued presence of 10 mM Na+ (i.e., close to the Km of NBCe1-A for Na+; see Refs. 33 and 46). The composition of the solutions used in this protocol is provided in Table 5. Figure 5, A–C shows representative I-V relationships for oocytes injected with H2O or with cRNA encoding human NBCe1-A-EGFP or rabbit NBCe1-A. From a starting point of a HCO3−-free solution containing 10 mM Na+/86 mM NMDG+, the addition of 33 mM HCO3− causes substantial increases in slope conductance that are, at most, slightly affected by replacing 86 mM NMDG+ with 86 mM Li+. The slope conductances (between −20 and +20 mV) extracted from data such as these are shown for a larger number of cells in Fig. 5D. We note that such conductances measured in oocytes expressing human or rabbit NBCe1-A in the presence of 10 mM Na+/33 mM HCO3− were less than half the value measured in the presence of 96 mM Na+/33 mM HCO3− (e.g., see Fig. 3). Thus, the Km for Na+ is somewhat >10 mM for both human and rabbit NBCe1-A. The addition of 86 mM Li+ to the 10 mM Na+/33 mM HCO3− containing bathing solution did not reduce the HCO3−-dependent slope conductance for either human or rabbit NBCe1-A (Fig. 5D). Instead we detected a small but significant increase in slope conductance (P < 0.001, n = 6 for oocytes expressing human NBCe1-A-EGFP; P = 0.045, n = 6, for oocytes expressing rabbit NBCe1-A, paired one-tailed t-test).
Fig. 5.
Influence of Li+, in the presence of 10 mM Na+, upon I-V relationships for human vs. rabbit NBCe1-A. Experiments similar to those shown in Fig. 3 except that the cells were sequentially exposed to 1) a modified ND96 solution that contained 10 mM Na+ + 86 mM NMDG+ in place of 96 mM Na+, 2) a 33-mM HCO3− solution that also contained 10 mM Na+ + 86 mM NMDG+ in place of 96 mM Na+, and finally 3) a 33-mM HCO3− solution containing 10 mM Na+ + 86 mM Li+. The composition of solutions used in this protocol is shown in Table 5. Horizontal bars, *P < 0.05.
Anion Specificity of Human and Rabbit NBCe1-A
Sulfite.
The NBCe1-like activity expressed in rabbit renal preparations (2, 52) and in Xenopus oocytes injected with rabbit kidney poly(A)+ RNA (43) is stimulated by sulfite.3 However, the NBCe1-like activity of Xenopus oocytes injected with cRNA encoding rat NBCe1-A is neither stimulated nor blocked by SO32− in the extracellular solution (19). Because all of the data supporting the involvement of SO32− were obtained on rabbit material, and none of the experiments involved cloned NBCe1, we assessed the ability of heterologously expressed rabbit NBCe1-A to interact with SO32−.
In the first set of experiments (Fig. 6), we performed our voltage-clamp protocol on H2O-injected oocytes, or oocytes expressing either human NBCe1-A-EGFP or rabbit NBCe1-A, as they were superfused with (in order) our ND96, ND96/SO32−, and 33-mM HCO3− solutions. Note that, in this sequence, we first replaced 59.2 mM Cl− with 33 mM SO32−, and subsequently replaced 33 mM SO32− with 33 mM Cl− plus 33 mM HCO3− (see Table 6). Moreover, to prevent precipitation of CaSO3, all solutions in this protocol were nominally Ca2+ free. The omission of Ca2+ from the ND96 solution resulted in a noticeable increase in inward current in all experimental cells. For example, in the case of H2O-injected cells, the inward current at −160 mV in Fig. 6A is substantially greater than in Fig. 1A, which was obtained in the presence of Ca2+ (P < 0.001, n = 6, one-tailed unpaired t-test). Furthermore, these 0-Ca2+ oocytes were more depolarized at rest than similar cells bathed in Ca2+-containing ND96 (P < 0.001, n = 6, not shown, one-tailed unpaired t-test).
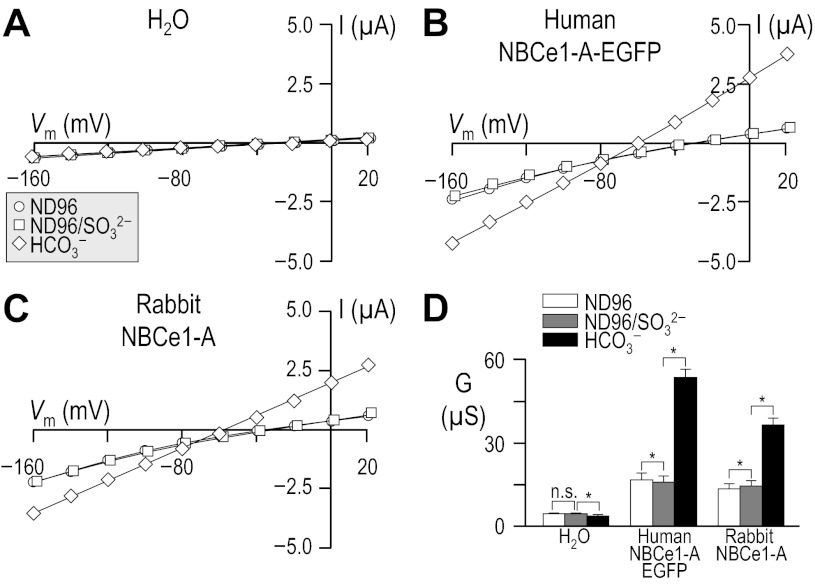
Fig. 6.
Influence of sulfite, in the absence of HCO3−, upon I-V relationships for human vs. rabbit NBCe1-A. Experiments similar to those shown in Fig. 3 except that the cells were sequentially exposed to 1) a Ca2+-free ND96 solution; 2) a modified a Ca2+-free ND96 solution that contained 33 mM sulfite in place of 59.4 mM Cl−; and finally 3) a Ca2+-free 33-mM HCO3− solution that, compared with the preceding solution, contained 33 mM HCO3− + 33 mM Cl− in place of 33 mM sulfite. Note that, in addition to A, the squares in B and C are artificially offset by +2 mV from the circles to make all data sets visible. The composition of solutions used in this protocol is shown in Table 6. Horizontal bars, *P < 0.05.
The switch from ND96 to ND96/SO32− did not elicit a detectable hyperpolarization in any of our three experimental cell populations (not shown), indicating that SO32− could not replace a HCO3−-like species in supporting transport by NBCe1-A. Figure 6, A–C shows representative I-V relationships for oocytes injected with H2O or with cRNA encoding either human or rabbit NBCe1-A. Average slope conductances extracted from data such as these are summarized for a large number of cells in Fig. 6D. The application of ND96/SO32− did not cause a significant increase in membrane conductance with H2O-injected oocytes (P = 0.323, n = 6, paired one-tailed t-test), but caused a small but significant decrease with oocytes expressing human NBCe1-A-EGFP (P = 0.002, n = 6, paired one-tailed t-test) and caused a small but significant increase with oocytes expressing rabbit NBCe1-A (P = 0.032, n = 6, paired one-tailed t-test). In neither group of NBC oocytes did the addition of SO32− change the steady-state Vm.
When we replaced SO32− with HCO3− in the bathing solution, H2O-injected cells exhibited a small but significant decrease in membrane conductance (P = 0.026, n = 6, paired one-tailed t-test). As expected, both of the oocyte populations expressing NBCe1-A exhibited substantial increases in membrane conductance upon replacement of SO32− with HCO3− (P < 0.001 for human and P = 0.001 for rabbit; for both, n = 6, paired one-tailed t-test).
To assay the ability of SO32− to block human and rabbit NBCe1-A, we voltage clamped oocytes as they were exposed to our ND96, 10 mM HCO3−, and 10 mM HCO3−/SO32− solutions. Note that, in this sequence, our first solution change replaced 10 mM Cl− with 10 mM HCO3− and our second solution replaced a further 18 mM Cl− with 10 mM SO32− in the continued presence of 10 mM HCO3− (see Table 7). Figure 7, A–C shows representative I-V relationships for H2O-injected oocytes as well as oocytes expressing human NBCe1-A-EGFP or rabbit NBCe1-A. Figure 7D summarizes these data for a larger number of cells and leads to two conclusions. First, the application of 10 mM HCO3− significantly increased the membrane conductance of oocytes expressing NBCe1-A (P < 0.001 for human and P < 0.001 for rabbit; for both, n = 6, paired one-tailed t-test), but not of oocytes injected with H2O, which exhibited a small decrease in membrane conductance (P = 0.029, n = 6, paired one-tailed t-test). Second, the subsequent application of SO32− did not significantly influence the membrane conductance of oocytes expressing NBCe1-A and bathed in 10 mM HCO3− (P = 0.494 for human and P = 0.280 for rabbit; for both n = 6, paired two-tailed t-test).
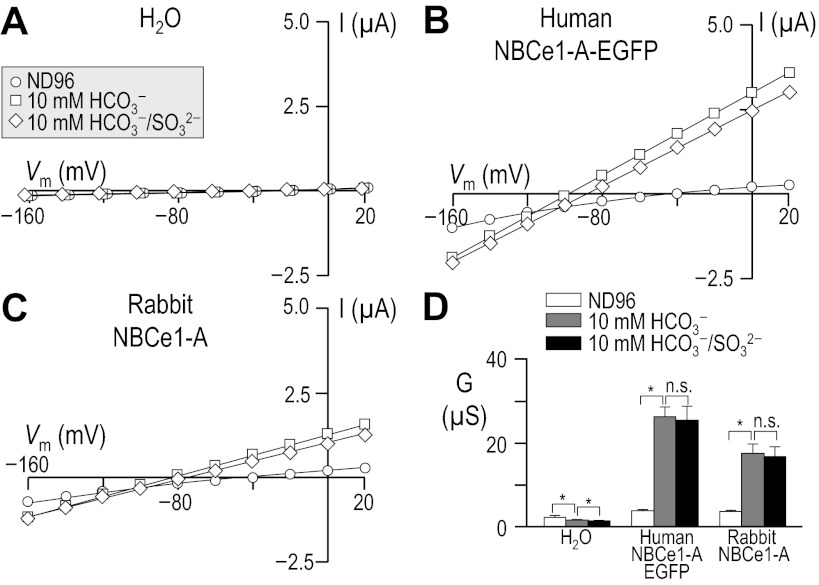
Fig. 7.
Influence of sulfite, in the presence of HCO3−, upon I-V relationships for human vs. rabbit NBCe1-A. Experiments similar to those shown in Fig. 3 except that the cells were sequentially exposed to 1) ND96 solution; 2) a 10-mM HCO3− solution; and 3) finally a 10-mM HCO3− solution that, compared with the preceding solution, contained 10 mM sulfite in place of 18 mM Cl−. The composition of solutions used in this protocol is shown in Table 7. Horizontal bars, *P < 0.05.
Because SO32− in solution readily undergoes photocatalyzed oxidation into SO42− and S2O62− (11), we repeated the HCO3−/SO32− assay on rabbit NBCe1-A using freshly prepared solutions, equilibrated with 1.5% CO2/98.5% N2, and shielded the solution-bearing syringes from ambient light using aluminum foil. Even under these conditions, SO32− application did not increase the slope conductance in the presence of HCO3− (P = 0.35, n = 6, results of a paired, one-tailed t-test, data not shown). Thus, we find no evidence that human or rabbit NBCe1-A perform substantial electrogenic Na/SO3 cotransport or electrogenic Na/HSO3 cotransport. Nor do we find evidence that SO32− stimulates or inhibits the electrogenic Na/HCO3 cotransport activity of human or rabbit NBCe1-A expressed in Xenopus oocytes.
Oxalate.
One preliminary report suggests that 20 mM oxalate2− can enhance the NBCe1-like activity of rabbit BLMVs (2). To test the hypothesis that oxalate is a substrate of rabbit NBCe1-A, we sequentially exposed H2O-injected oocytes and oocytes expressing rabbit NBCe1-A to our ND96, ND96/oxalate2−, and 33-mM HCO3− solutions (Table 8). Note that the solutions used in this protocol were nominally Ca2+-free to prevent precipitation of calcium oxalate. Cells exposed to a Ca2+-free ND96 solution were more depolarized at rest (not shown) and exhibited greater membrane conductances than similar cells bathed in Ca2+-containing ND96-an observation that we made above.
Figure 8, A and B shows representative I-V relationships for H2O-injected oocytes and oocytes expressing rabbit NBCe1-A. Figure 8C summarizes these data for a larger number of cells. Following the application of oxalate, H2O-injected oocytes depolarized by ∼18 mV to −16 ± 1 mV (n = 6, not shown) and rabbit NBCe1-A-expressing cells depolarized by ∼9 mV to −18 ± 1 mV (n = 6, not shown). Furthermore, the application of oxalate resulted in a substantial increase in the membrane conductance, both for oocytes injected with H2O and those expressing rabbit NBCe1-A (Fig. 8; P < 0.002 for both, n = 6, one-tailed paired t-test). The membrane conductance of oocytes expressing rabbit NBCe1-A was slightly greater than that of H2O-injected oocytes in our ND96 solution (P = 0.030, n = 6, one-tailed t-test), but was not different from that of H2O-injected cells when measured in our ND96/oxalate2− solution (P = 0.142, n = 6, one-tailed t-test). As expected, the membrane conductance of oocytes expressing rabbit NBCe1-A was much greater than that of H2O-injected oocytes in our HCO3−-containing solution (P < 0.001, n = 6, one-tailed t-test). In summary, oocytes injected with H2O or expressing rabbit NBCe1-A exhibit similar oxalate-dependent currents (P = 0.536, n = 6, two-tailed unpaired t-test). However, only oocytes expressing rabbit NBCe1-A exhibit HCO3−-stimulated currents.
Fig. 8.
Influence of oxalate, in the absence of HCO3−, upon I-V relationships for rabbit NBCe1-A. Oocytes were injected with either H2O (A) or cRNA encoding rabbit NBCe1-A (B). I-V relationships were obtained by voltage clamp while the cells were sequentially exposed to a 1) Ca2+-free ND96 solution; 2) a modified Ca2+-free ND96 solution that contained 33 mM oxalate in place of 66 mM Cl−; and finally 3) a Ca2+-free 33-mM HCO3− solution that, compared with the preceding solution, contained 33 mM HCO3− + 33 mM Cl− in place of 33 mM oxalate. Note that the diamonds in A are artificially offset by +3 mV from the circles to make all data sets visible. Averaged slope conductances measured between −20 and +20 mV for 6 cells are shown in C, in which the results of certain paired t-tests are shown as horizontal bars (*P < 0.05). The composition of solutions used in this protocol is shown in Table 8.
The endogenous oxalate-dependent current exhibited by H2O-injected cells was substantially stimulated by the application of 200 μM DIDS as evidenced by a 75 ± 5% increase in slope conductance between −20 and +20 mV (P < 0.001, n = 6, paired one-tailed t-test, data not shown).
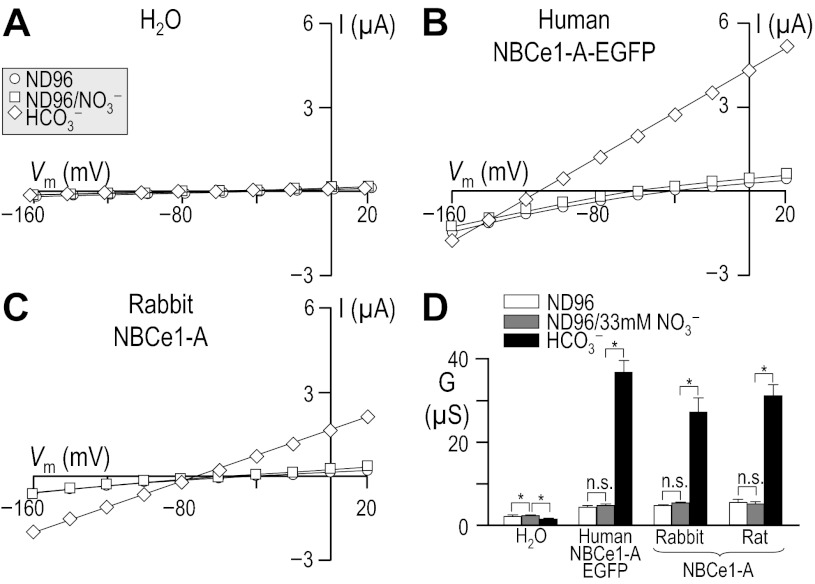
Nitrate.
Because some members of the SLC4 family of solute carriers are capable of substantial NO3− transport (e.g., AE2, see Ref. 23) and because rat NBCe1-A has been reported to transport NO3− (46a), we assayed the ability of human, rabbit, and rat NBCe1 to mediate electrogenic Na/NO3 cotransport. In this set of experiments (Fig. 9), we performed our voltage-clamp protocol on H2O-injected oocytes (Fig. 9A) or oocytes that were expressing either human NBCe1-A-EGFP (Fig. 9B), rabbit NBCe1-A (Fig. 9C), or rat NBCe1-A (not shown), as they were superfused with (in order) our ND96, ND96/NO3−, and 33-mM HCO3− solutions. In this sequence, we first replaced 33 mM Cl− with 33 mM NO3− and subsequently replaced 33 mM NO3− with 33 mM HCO3− (see Table 9). The application of ND96/NO3− did not elicit a detectable hyperpolarization in H2O-injected oocytes or oocytes expressing rat NBCe1-A but elicited small, though significant, hyperpolarizations in both groups of oocytes expressing human or rabbit NBCe1-A (ΔVm = −13 ± 1 mV for human and ΔVm = −10 ± 1 mV for rabbit; for both, n = 6). Even though NBCe1-A appears to mediate a conductive flux of NO3− in the case of the human and rabbit clones, this flux was not sufficiently robust to cause a measurable increase in membrane conductance in any of the cells, as shown in the averaged data in Fig. 9D.
Fig. 9.
Influence of 33 mM nitrate, in the absence of HCO3− upon I-V relationships for human vs. rabbit NBCe1-A. Experiments similar to those shown in Fig. 6, A–D, except that the partial Cl− replacement in this case was 33 mM NO3− rather than sulfite and the solutions were not Ca2+-free. The composition of solutions used in this protocol is shown in Table 9. Horizontal bars, *P < 0.05.
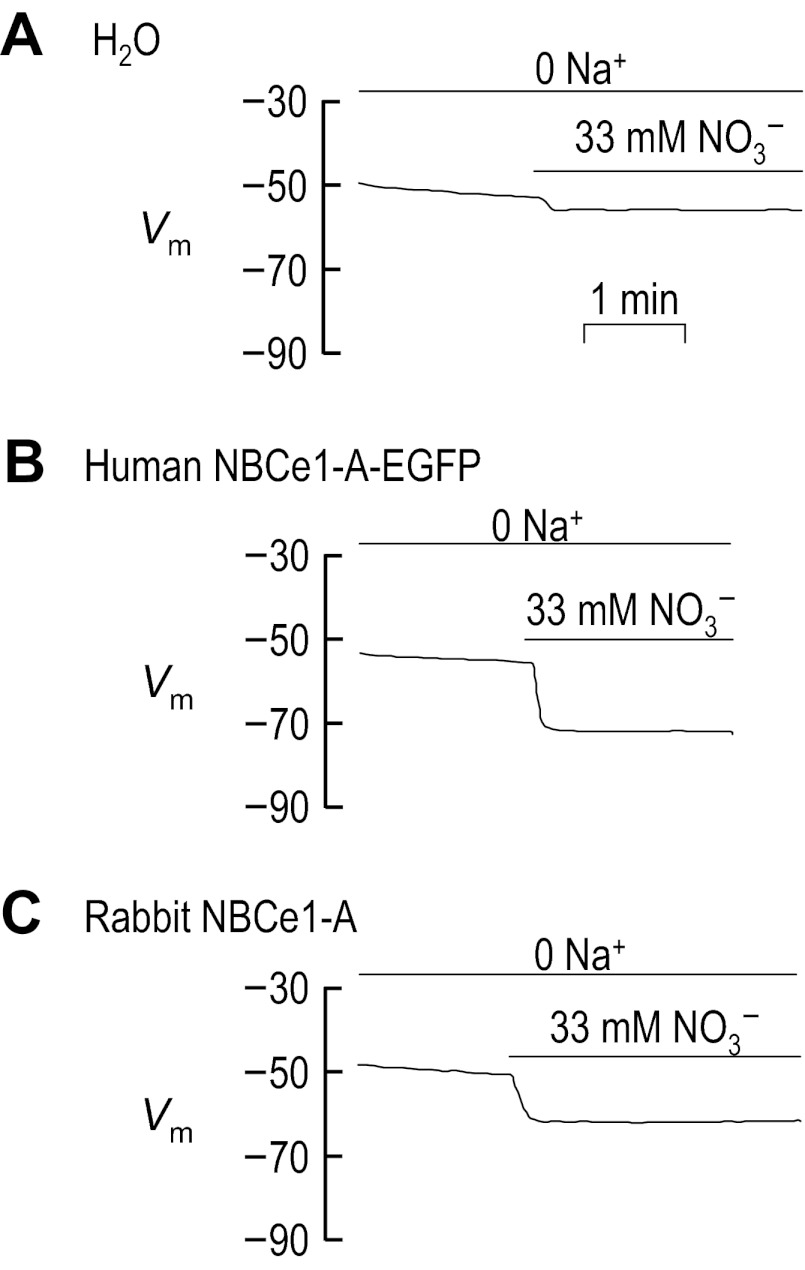
Moreover, the apparent NO3− flux does not require extracellular Na+. The application of NO3− in the absence of Na+ (see solution Table 10) causes H2O-injected cell to hyperpolarize by 4 ± 1 mV (n = 6, Fig. 10A), cells expressing human NBCe1-A-EGFP to hyperpolarize by 16 ± 1 mV (n = 6, P < 0.001 compared with H2O-injected cells, one-tailed t-test; see Fig. 10B) and cells expressing rabbit NBCe1-A to hyperpolarize by 11 ± 1 mV (n = 6, P < 0.001 compared with H2O-injected cells, one-tailed t-test; see Fig. 10C). Thus the hyperpolarizations elicited by the application of NO3− to cells expressing NBCe1-A are unlikely to represent electrogenic Na/NO3 cotransport.
Fig. 10.
Influence of nitrate, in the absence of Na+, upon the membrane potential (Vm) of oocytes expressing human and rabbit NBCe1-A. Representative Vm traces gathered from oocytes that were injected with either H2O (A), cRNA encoding human NBCe1-A-EGFP (B), or cRNA encoding rabbit NBCe1-A (C). Cells were initially bathed in a Na+-free ND96 solution and the Vm of each cell was continuously monitored as each cell was exposed to 33 mM NO3− in the continued absence of Na+. The composition of solutions used in this protocol is shown in Table 10.
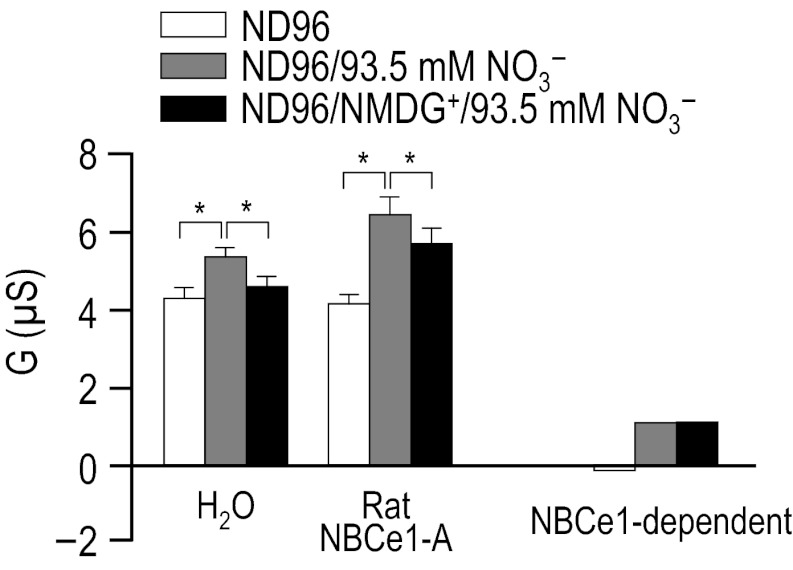
Because the original preliminary data presented by Sciortino (46a) in support of the ability of rat NBCe1-A to mediate electrogenic NO3− transport was gathered in the presence of 100 mM NO3−, we performed a series of experiments in which we expressed rat NBCe1-A in oocytes and gathered I-V plots as we sequentially exposed the cells to ND96, a modified ND96 solution that contained 93.5 mM NO3− in place of 93.5 mM Cl−, and finally a modified ND96 solution that contained 100 mM NMDG+ and 93.5 mM NO3− in place of 100 mM Na+ and 93.5 mM Cl− (see Table 11). The application of 93.5 mM NO3− elicited a small hyperpolarization of H2O-injected oocytes (6 ± 2 mV, n = 6, not shown) and caused a significantly larger hyperpolarization of oocytes expressing rat NBCe1-A (16 ± 2 mV, n = 6, not shown, P = 0.001, unpaired t-test). Moreover, the application of 93.5 mM NO3− caused a small but significant increase in the membrane conductance of H2O-injected oocytes and of oocytes expressing rat NBCe1-A (Fig. 11). The replacement of extracellular Na+ with NMDG+ in the continued presence of 93.5 mM NO3− caused the membrane conductance of H2O-injected oocytes and of cells expressing rat NBCe1-A to decrease by a small amount (Fig. 11). When we subtract the membrane conductance of H2O-injected cells from the conductance of cells expressing rat NBCe1-A, we obtain a measure of NBCe1-dependent conductance (Fig. 11). These data confirm the observation by Sciortino that rat NBCe1-A is capable of electrogenic NO3− transport and extend that study to demonstrate that these currents do not represent electrogenic Na/NO3 cotransport, but the same Na+-independent conductive transport of NO3− that we have observed in the present study to be a capability of cloned human and rabbit NBCe1-A.
Fig. 11.
Influence of 93.5 mM nitrate, in the presence and absence of Na+, upon the I-V relationships for rat NBCe1-A. Experiments similar to those shown in Fig. 9, A–D, except that 93.5 mM Cl− was replaced with 93.5 mM NO3− and finally, in the continued presence of 93.5 mM NO3−, 96 mM Na+ was replaced with 96 mM NMDG+. The composition of solutions used in this protocol is shown in Table 11. Horizontal bars, *P < 0.05.
To assay the ability of NO3− to block human and rabbit NBCe1-A, we voltage clamped oocytes as they were exposed, in turn, to our ND96, 10 mM HCO3−, and 10 mM HCO3−/NO3− solutions. In this sequence, we first replaced 10 mM Cl− with 10 mM HCO3− and subsequently replaced a further 10 mM Cl− with 10 mM NO3− in the continued presence of 10 mM HCO3− (see Table 12). Figure 12, A–C shows representative I-V relationships for H2O-injected oocytes, as well as of oocytes expressing human NBCe1-A-EGFP or rabbit NBCe1-A. Figure 12D summarizes these data for a larger number of cells and leads to two conclusions. First, the application of 10 mM HCO3− significantly increases the membrane conductance of oocytes expressing NBCe1-A (P < 0.001 for human and P = 0.001 for rabbit; for both, n = 6, paired one-tailed t-test) but has no significant effect on oocytes injected with H2O (P = 0.057, n = 6, paired one-tailed t-test). Second, the application of NO3− does not significantly influence the membrane conductance of H2O-injected oocytes bathed in 10 mM HCO3− (P = 0.901, n = 6, paired two-tailed t-test) but does result in a small reduction in membrane conductance of oocytes expressing NBCe1-A (P < 0.001 for human and P = 0.001 for rabbit; for both, n = 6, paired one-tailed t-test).
Fig. 12.
Influence of nitrate, in the presence of HCO3−, upon I-V relationships for human vs. rabbit NBCe1-A. Experiments similar to those shown in Fig. 7, A–D, except that the partial Cl− replacement in this case is 10 mM NO3− rather than sulfite. The composition of solutions used in this protocol is shown in Table 12. Horizontal bars, *P < 0.05.
In conclusion, although human, rabbit, and rat NBCe1-A mediate a limited conductive flux of NO3− (as evidenced by the NO3−-induced hyperpolarization of NBCe1-A expressing cells), the flux is not robust enough to produce a measurable increase in the membrane conductance of NBCe1-A-expressing oocytes and is unlikely to represent electrogenic Na/NO3 cotransport. Nevertheless, NO3− exerts a small inhibitory effect on Na-HCO3 cotransport mediated by human or rabbit NBCe1-A.
Inhibitor Sensitivity of Human and Rabbit NBCe1-A
DIDS.
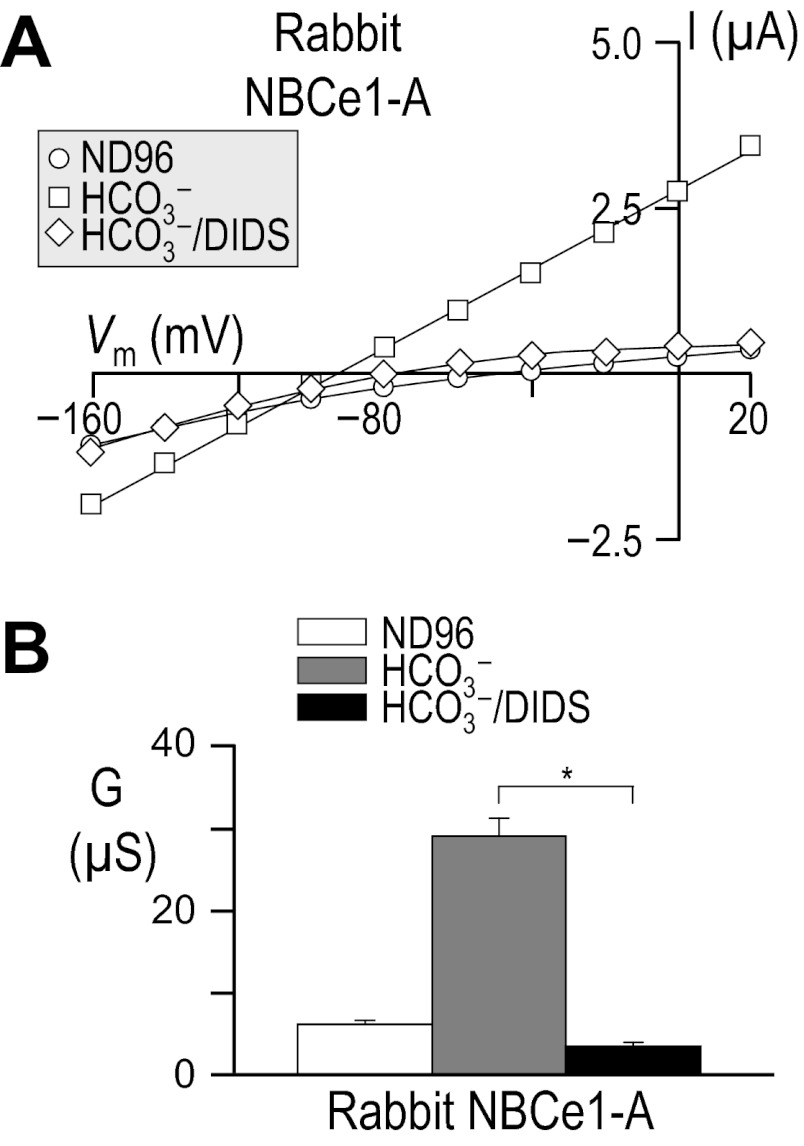
Previous work has shown that DIDS produces a substantial inhibition not only of human and rat NBCe1-A as expressed in oocytes (31, 47), but also of NBCe1-like activity in rat and rabbit BLMVs (15, 16). However, DIDS-blockade of rabbit NBCe1-A has not been formally demonstrated for the transporter as heterologously expressed in oocytes. As shown in the representative I-V plots in Fig. 13A, the application of 200 μM DIDS causes a substantial reduction in the magnitudes of HCO3−-dependent currents in oocytes expressing rabbit NBCe1-A. Figure 13B summarizes the averaged data for a larger number of cells. Comparing the currents at +20 mV, where outward currents representing Na/HCO3 influx are maximal, we estimate that DIDS blocks ∼97 ± 1% of the HCO3−-dependent current mediated by rabbit NBCe1-A (P < 0.01, n = 6, paired one-tailed t-test).7
Fig. 13.
Influence of DIDS upon I-V relationships for rabbit NBCe1-A. Oocytes were injected with cRNA encoding rabbit NBCe1-A, and I-V relationships were obtained by voltage clamp while the cells were sequentially exposed to 1) our ND96 solution, 2) a 33-mM HCO3− solution, and finally 3) 33-mM HCO3− solution that contained 200 μM DIDS (A). Averaged slope conductances measured between −20 and +20 mV for 6 cells are shown in B in which the results of certain paired t-tests are shown as horizontal bars (*P < 0.05). The composition of solutions used in this protocol is shown in Table 2.
Harmaline.
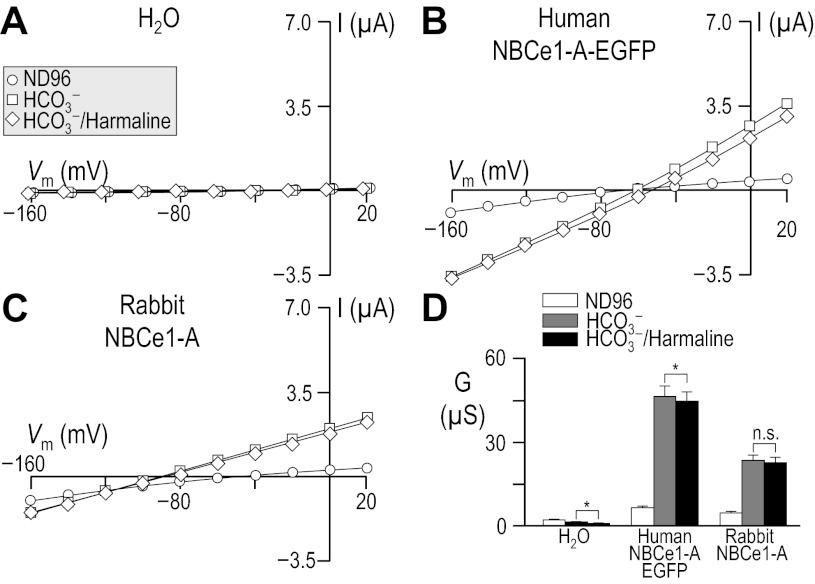
Others report that 200 μM harmaline substantially (4, 16, 52, 54) inhibits the NBCe1-like activity in human and rabbit renal preparations. However, we know of no reports on the effect of the drug on any ortholog of NBCe1 as expressed in oocytes. To test the hypothesis that harmaline is a blocker of human and rabbit NBCe1-A, we exposed our experimental oocyte groups first to ND96, then to 33 mM HCO3−, and finally to 33 mM HCO3− plus 200 μM harmaline (see Table 2), gathering voltage-clamp data for each cell in each solution. Figure 14, A–C shows representative data from H2O-injected cells and cells expressing either human or rabbit NBCe1-A. Figure 14D summarizes these data for a larger number of cells and confirms that the application of 33 mM HCO3− does not increase the membrane conductance of H2O-injected oocytes, but increases the membrane conductance of oocytes expressing NBCe1-A (P < 0.001, n = 6, for human NBCe1-A-EGFP; P < 0.001, n = 6, for rabbit NBCe1-A, paired one-tailed t-test).
Fig. 14.
Influence of harmaline upon I-V relationships for human and rabbit NBCe1-A. Experiments similar to those shown in Fig. 3, A–D, except that the oocytes were sequentially exposed to 1) ND96, 2) 33 mM HCO3−, and finally 3) 33-mM HCO3− solution that contained 200 μM harmaline. Composition of solutions used in this protocol is shown in Table 2. Horizontal bars, *P < 0.05.
Applying harmaline to H2O-injected oocytes in the continued presence of our 33-mM HCO3− solution results in a statistically significant but small reduction in membrane conductance (P < 0.001, n = 6, paired one-tailed t-test). In the case of oocytes expressing human NBCe1-A-EGFP, harmaline again causes a significant but small (i.e., 4 ± 1%) reduction in the HCO3−-dependent membrane conductance (P = 0.012, n = 6, paired one-tailed t-test). This small degree of inhibition is fully reversible upon washout of harmaline (not shown). Finally, for oocytes expressing rabbit NBCe1-A, harmaline caused a slight decrease in mean slope conductance in each case (mean decrease, 4 ± 2%, n = 6), although the effect did not reach statistical significance (P = 0.070, n = 6, paired one-tailed t-test). The absolute magnitude of the reduction in slope conductance was ∼0.4 μS for H2O oocytes, ∼1.7 μS for human NBCe1-A, and ∼0.6 μS for rabbit NBCe1-A.
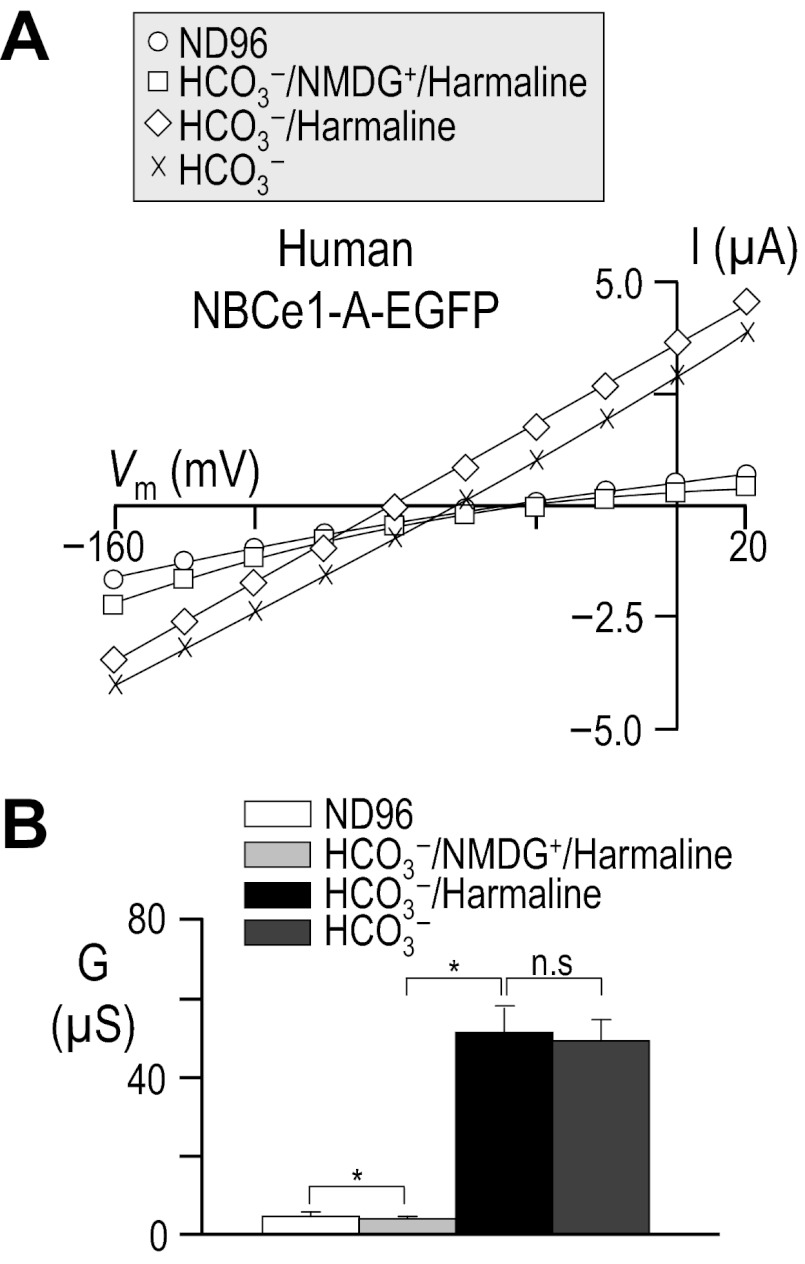
In a previous study that addressed the inhibition of cloned human NBCe1-A by harmaline, the authors monitored intracellular pH while exposing HEK cells to HCO3− in the absence of Na+, and then adding either 1) Na+ + amiloride or 2) Na+ + amiloride + harmaline followed by Na+ + amiloride. To mimic this protocol more closely, we expressed human NBCe1-A-EGFP in oocytes and exposed the cells first to our Na+-free HCO3−/NMDG+ solution containing 200 μM harmaline and then elicited NBCe1-A activity by adding Na+ in the continued presence of harmaline (see Fig. 15A and solution Table 13). Finally we washed harmaline away to disclose the full extent of NBCe1-A action. Using this alternative protocol and consistent with Fig. 14, we found no evidence that 200 μM harmaline interacts substantially with NBCe1-A in oocytes.
Fig. 15.
Influence of harmaline upon I-V relationships for human NBCe1-A, following a period in Na+-free solution. Representative I-V relationships from a cell expressing human NBCe1-A-EGFP (A) as the cell is exposed to ND96 solution, Na+-free 33-mM HCO3− solution that includes 200 μM harmaline, Na+-containing 33-mM HCO3 solution that includes 200 μM harmaline, and finally a Na+-containing 33-mM HCO3− solution without harmaline. Averaged slope conductances measured between −20 and +20 mV for 6 cells are shown in B, in which the results of paired one-tailed t-tests shown as horizontal bars (*P < 0.05). The composition of solutions used in this protocol is shown in Table 13.
Benzamil.
In giant inside-out membrane patches from oocytes expressing rat NBCe1-A, applying 500 μM benzamil to the cytosolic face blocks NBCe1 activity (14). However, we know of no studies assessing the inhibitory effect of benzamil applied to the outside of an intact oocyte. We compared the membrane conductance of H2O-injected oocytes and of oocytes expressing human or rabbit NBCe1-A in the presence of our 33-mM HCO3− solution and in the subsequent presence of 33 mM HCO3− plus 500 μM benzamil. Finally, we superfused the cells with a 33-mM HCO3− solution that contained no benzamil. Figure 16, A–C shows representative I-V plots from these experiments, and Fig. 16D summarizes membrane conductances for a larger number of cells. We find that 500 μM benzamil effects a ∼30% blockade of NBCe1-A activity (30 ± 7%, P = 0.006, n = 6 for human NBCe1-A-EGFP; 28 ± 5%, P < 0.001, n = 6 for rabbit NBCe1-A, paired one-tailed t-test). A comparison of the membrane conductances measured before and after the application of benzamil (P = 0.180, n = 5 for human NBCe1-A-EGFP; P = 0.080, n = 5 for rabbit NBCe1-A, paired one-tailed t-test) indicates that the inhibition of NBCe1-A by benzamil is fully reversible.
To test the hypothesis that benzamil competes with Na+ for a binding site on NBCe1-A, we assayed the potency of 500 μM benzamil in the presence of 10 mM Na+ (i.e., close to the estimated Km of NBCe1-A for Na+, see above). Under these conditions, a competitive inhibitor ought to be more potent than in 96 mM Na+ (see discussion). Representative I-V plots from these experiments are shown in Fig. 17, A–C and membrane conductances calculated from data such as these are summarized for a larger number of cells in Fig. 17D. Note that the membrane conductances measured in oocytes expressing NBCe1-A in Fig. 17 are less than one half the magnitude of the conductances measured from similar cells assayed in the presence of 96 mM Na+ (e.g., in Fig. 16), indicating that 10 mM is close to the Km of NBCe1-A for Na+. We calculate that the inhibitory effect of benzamil upon human NBCe1-A-EGFP in the presence of 10 mM Na+ (31 ± 5% inhibition) is indistinguishable from the extent of block effected by benzamil in the presence of 96 mM Na+ (P = 0.961, n = 6, unpaired two-tailed t-test assuming equal variances). However, the inhibitory effect of benzamil upon rabbit NBCe1-A in the presence of 10 mM Na+ (45 ± 3% inhibition) is significantly greater than the block effected by benzamil in the presence of 96 mM Na+ (P = 0.023, n = 6, unpaired two-tailed t-test assuming equal variances). Thus, the inhibition of rabbit, but not human, NBCe1-A appears to be enhanced by lowering external [Na+] but, as we will see in the discussion, this phenomenon is not quantitatively consistent with a model of competitive inhibition.
Fig. 17.
Influence of benzamil upon I-V relationships for human and rabbit NBCe1-A in the presence of 10 mM Na+. Experiments similar to those shown in Fig. 14, A–D, except that the protocol was performed in solutions that contained 10 mM Na+ and 86 mM NMDG+ in place of 96 mM Na+. The composition of solutions used in this protocol is shown in Table 5. Horizontal bars, *P < 0.05.
DISCUSSION
The Deduced Amino-Acid Sequence and the Functional Expression of Human and Rabbit NBCe1-A
Human and rabbit NBCe1-A share 97% sequence identity at the amino acid level, with 15 of the 36 aa differences between these orthologs occurring in the lengthy third extracellular loop (EL3; Supplemental Fig. 1 can be accessed on the Journal website). EL3 of NBCe1-A includes three putative glycosylation sites (13) as well as four cysteine residues that can form intra- and intermolecular disulfide bridges (25, 32, 45). We are not aware of any reports that ascribe a contribution of EL3 to the functional expression of NBCe1-A. Furthermore, none of the differences between the human and rabbit sequences is predicted to disrupt the EL3 glycosylation sites or disulfide bridges.
In our experiments, human and rabbit NBCe1-A are distinguished by the robustness of their expression in oocytes. For reasons that are unclear, total rabbit NBCe1-A protein fails to accumulate in oocytes to the same extent as human NBCe1-A-EGFP (Fig. 1E and Fig. 2). However, the ratio of surface protein to total protein is about the same for human and rabbit NBCe1-A, indicating that the fundamental difference is in RNA stability or protein synthesis/degradation, not trafficking. Moreover, we estimate that, normalized for protein at the plasma membrane, human and rabbit NBCe1-A have the same activity. Our analysis indicates that the reduced accumulation of total rabbit vs. human NBCe1-A protein is predominantly due to sequence differences, rather than the presence of the EGFP tag on human NBCe1-A. We cannot predict which of the sequence differences (either at the level of cRNA or protein) between human and rabbit NBCe1-A are responsible for this phenomenon. Even a single, relatively conservative amino acid change in NBCe1-A has the potential to produce a substantial effect on the functional expression of NBCe1-A. An example is the substantial loss of functional expression (both surface expression and per-molecule activity) of NBCe1-A caused by an Ala to Val substitution (A799V) that is associated with pRTA (22, 37).
Cation Specificity of Human and Rabbit NBCe1-A in Xenopus Oocytes
Four lines of evidence, taken at face value, suggest that human and rabbit orthologs of NBCe1-A interact substantially with Li+: 1) in rabbit BLMVs, HCO3−-stimulated 22Na uptake is substantially inhibited by external Li+ (52); 2) rabbit BLMVs loaded with Li+ acidify in the presence of HCO3−, as if BLMVs have a Li/HCO3−-efflux mechanism (54); 3) in rabbit BLMVs, HCO3−-stimulated 22Na uptake is enhanced by outwardly directed gradients of Na+ and of Li+, an activity proposed to represent HCO3−-dependent cation-cation exchange by NBCe1 (54); and 4) in the case of human NBCe1-A overexpressed in HEK cells, Li+ is ∼25% as effective as Na+ in supporting DIDS-sensitive, HCO3−-dependent acid-extrusion (4).
Speaking against a substantial interaction of Li+ with NBCe1 are voltage-clamp experiments performed by Sciortino and Romero (47) on oocytes expressing rat NBCe1-A. In this case, substitution of Na+ with Li+ in the bathing solution results in a ∼97% reduction in HCO3−-stimulated currents across the voltage range tested. If these data are comparable with the BLMV and HEK data, they would suggest that the human and rabbit orthologs of NBCe1-A are better able to interact with Li+ than is rat NBCe1-A.
In the present experiments on human and rabbit NBCe1-A expressed in oocytes, we find that both clones mediate electrogenic, Na+-coupled transport of HCO3− equivalents (e.g., Fig. 1 and Fig. 3). Moreover, both orthologs mediate a small amount of electrogenic Li/HCO3 cotransport (Fig. 4) that we estimate to be no greater than 8% as robust as the electrogenic cation-HCO3 cotransport activity supported by Na+ under equivalent conditions.
Taken together these data suggest that, although NBCe1-A is capable of mediating some electrogenic Li/HCO3 cotransport in oocytes, Li+ is a poor substitute for Na+ in inwardly directed transport cycles. We have not studied the ability of Li+ vs. Na+ to support HCO3− efflux mediated by NBCe1-A, per points 2 and 3 above. It is likely that the data gathered in HEK cells (point 4 above), which was not obtained under voltage clamped conditions, cannot be used to reliably estimate the relative affinities of NBCe1-A for Na+ vs. Li+, because the driving forces acting upon NBCe1 in the presence of extracellular Na+ vs. Li+ are unlikely to be equal. That is to say, the driving force for Na+ and HCO3− entry rapidly dissipates due to robust Na/HCO3 cotransport, as evidenced by how rapidly Vm approaches the reversal potential (Erev) of NBCe1-A. On the other hand, the driving force for Li+ and HCO3− entry would dissipate more slowly due to feeble Li/HCO3 cotransport. Thus, the extent of Li/HCO3 vs. Na/HCO3 cotransport would be overestimated under nonvoltage-clamped conditions, an effect that would increase in severity with reduced time resolution. On the other hand, the Na+-driven Cl-HCO3 exchanger from squid axons appears to be more selective for Na+ over Li+ in situ than when heterologously expressed in oocytes (57), providing a precedent for the apparent cation selectivity of SLC4 proteins being cell-specific.
Anion Specificity of Human and Rabbit NBCe1-A in Xenopus Oocytes
Four lines of evidence, taken at face value, suggest that rabbit NBCe1-A can interact substantially with anions other than HCO3− or HCO3− equivalents: 1) in rabbit BLMVs, SO32− substantially stimulates HCO3−-dependent, DIDS-sensitive 22Na uptake (2, 52). However, SO32− does not support 22Na uptake in the absence of HCO3− (52); 2) in Xenopus oocytes injected with poly (A)+ RNA isolated from rabbit renal cortex, HCO3−-dependent 22Na influx is substantially stimulated by SO32− (43). However, as in point 1, SO32− does not support 22Na uptake in the absence of HCO3− (43); 3) one preliminary report suggests that oxalate2− slightly enhances the HCO3−-dependent 22Na uptake exhibited by rabbit BLMVs (2), although another group reports that oxalate2− does not influence HCO3−-dependent 22Na influx in the same preparation (52); and 4) one preliminary report suggests that NO3− increases the membrane conductance of oocytes expressing rat NBCe1-A (46a).
In voltage-clamp experiments performed by Grichtchenko et al. (19) on rat NBCe1-A expressed in oocytes, neither the presence of 33 mM SO42− nor 33 mM SO32− (that in solution is actually 26.4 mM SO32− in equilibrium with 6.6 mM HSO3−) stimulates or inhibits HCO3−-induced currents. If these data are comparable with points 1–3 above, and the SO32−-dependent stimulation of NBCe1-like activity represents Na/SO3 cotransport, they would suggest that rabbit NBCe1-A is better able to carry SO32− than is rat NBCe1-A.
In our experiments on human and rabbit NBCe1-A expressed in oocytes, we find no evidence that NBCe1-A supports electrogenic Na/SO3 cotransport (Fig. 6). It is true that we observed a small but statistically significant increase in the membrane conductance (between −20 and +20 mV) of oocytes expressing rabbit NBCe1-A when we applied SO32− in the absence of HCO3−. However, the introduction of SO32− did not elicit a hyperpolarization (starting from a resting Vm in Ca2+-free ND96 of approximately −25 mV). Thus, we have no evidence for electrogenic Na/SO3 cotransport activity in the absence of HCO3−. Moreover, in contrast to the findings of points 1 and 2 above, and consistent with the findings of Grichtchenko et al. (19), we find no evidence in oocytes that SO32− stimulates human or rabbit NBCe1-A in the presence of HCO3− (Fig. 7).
Our study presented in Fig. 8 indicates that NBCe1-A is unable to perform electrogenic Na/oxalate cotransport in oocytes, although these experiments were complicated by endogenous currents elicited by the application of 33 mM oxalate. To our knowledge, the present experiments are the first to reveal such oxalate-stimulated endogenous currents.
The studies presented in support of SO32− or oxalate2− transport by NBCe1 do not consider the potential impact of other basolateral, DIDS-sensitive, HCO3− transporters. For example, in the membranes of PT cells (26), sat-1 (encoded by the Slc26a1 gene) is capable of HCO3−-oxalate as well as HCO3-SO4 exchange (27). Furthermore, SO42− uptake mediated by sat-1 is inhibited by SO32−, indicating that sat-1 may also be capable of HCO3-SO3 exchange (27). If the BLMVs and oocytes injected with rabbit poly (A)+ RNA express a transporter such sat-1 (in addition to NBCe1), the application of SO32− (or oxalate2−) would stimulate HCO3− extrusion, in turn promoting Na/HCO3 influx by NBCe1. However, if NBCe1 was supported by sat-1 action, we might also expect SO42− to indirectly promote NBCe1-like activity in renal preparations, which it does not (43, 52).
The original hypothesis was that NBCe1 can perform the cotransport of 1 Na+ + 1 SO32− + 1 HCO3−. Thus, the apparent 1:3 stoichiometry of NBCe1-A in situ might be better explained by the cotransport of 1 Na+ + 1 CO32− + 1 HCO3− rather than by the cotransport of 1 Na+ + 3 HCO3−. However, our data show that the ability of SO32− to stimulate NBCe1-like activity in renal preparations is not a feature of NBCe1-A expressed in oocytes.
Finally, in this set of anion-related experiments, we assayed the ability of NO3− to support electrogenic Na-anion cotransport by NBCe1-A. The data presented in Figs. 9–12 are consistent with the ability of NBCe1 to mediate a small amount of conductive NO3− transport. However, the NO3−-induced hyperpolarizations (Fig. 10) and conductances (Fig. 11) do not require extracellular Na+, consistent with the idea that NBCe1 can mediate a small amount of uncoupled NO3− conduction. Thus it is not surprising that others do not detect NO3−-supported NBCe1-like activity in 22Na influx assays performed on renal preparations (43, 52).
Inhibitor Sensitivity of Human and Rabbit NBCe1-A in Xenopus Oocytes
Because harmaline is proposed to act at cation binding sites (3, 5, 44, 50), others have cited the harmaline sensitivity of the NBCe1-like activity in renal preparations (4, 16, 52, 54)8 as evidence that NBCe1 includes a discrete cation binding site. If correct, this result would lead to the conclusion that NBCe1 transports Na+ plus a HCO3−-like species as opposed to transporting the NaCO3− ion pair. However, we find that harmaline does not substantially inhibit either human or rabbit NBCe1-A, as expressed heterologously in oocytes (Fig. 14 and Fig. 15).
Another compound, benzamil, is also thought to act at cation binding sites, and previous workers have shown that this drug blocks heterologously expressed rat NBCe1-A when applied to the intracellular face of oocyte patches (14). In the present study, we assayed the ability of benzamil to block human and rabbit NBCe1-A when applied to the extracellular face of the transporter expressed in intact oocytes. We detected a ∼30% inhibition of human NBCe1-A by 500 μM benzamil, both in the presence of 96 mM Na+ (Fig. 16) and in the presence of 10 mM Na+ (Fig. 17). In the case of rabbit NBCe1-A, 0.5 mM benzamil appeared to be more effective by in the presence of 10 mM Na+ (∼45% inhibition) than in the presence of 96 mM Na+ (∼30%). If benzamil were a competitive inhibitor (where benzamil and Na+ compete for the same binding site), benzamil ought to be predictably more potent at lower [Na+]o (see Ref. 38)
where, V is the HCO3−-dependent slope conductance, Vmax is the maximal HCO3−-dependent slope conductance, [S] is [Na+]o, Km is the apparent Michaelis constant for extracellular Na+, [I] is [benzamil]o, and Ki is the apparent inhibitory constant for benzamil binding.
Using an experimentally determined Km for NBCe1-A in oocytes (20 mM Na+, see Ref. 33), and calculating Vmax from data gathered in the presence of 10 mM or 96 mM Na+, we estimate that the Ki for benzamil is 0.23 mM. Based on these values, a model of competitive inhibition predicts that 0.5 mM benzamil ought to inhibit NBCe1-A activity by 30% in the presence of 96 mM Na+ but by 60% in the presence of 10 mM Na+. Neither our rabbit data nor especially our human data are consistent with this prediction.
We can perform a similar calculation for a model of noncompetitive inhibition (where the benzamil binds equally well to the free and substrate-bound transporter, reducing Vmax; see Ref. 38)
Using this equation, we calculate that benzamil has a Ki of 1.33 mM and that 0.5 mM benzamil should produce a 45% block in the presence of 10 mM Na+. Thus, this model is consistent with our data on rabbit NBCe1-A, but of course is inconsistent with our human data. A firm conclusion regarding the mode of action of benzamil would require a rigorous kinetic analysis, involving multiple [Na+]o and [benzamil]o values. Nevertheless, it appears that benzamil does not simply compete with extracellular Na+ for a common binding site on NBCe1-A.
Substrate Roster of NBCe1-A
Both the human and rabbit orthologs of NBCe1-A exhibit very different features when expressed in oocytes as opposed to renal preparations. Notably, the cloned NBCe1-A expressed in oocytes is more selective for Na+ over Li+, is more selective for HCO3−/CO32− over HSO3−/SO32−, and is not substantially blocked by harmaline. Thus, the previous data presented in support of the hypothesis that NBCe1 include distinct binding sites for Na+ (as evidenced by harmaline block) and CO32− (as evidenced by SO32− stimulation of NBCe1-like activity) are not applicable to either cloned human or cloned rabbit NBCe1-A, as expressed in a system in isolation from other renal factors. It is unlikely that the aforementioned observations are due to oocyte-specific factors or to differences in membrane curvature or membrane lipid composition among systems because SO32− stimulation and harmaline inhibition of HCO3−-dependent 22Na uptake were also observed in oocytes injected with rabbit renal poly (A)+ RNA (43).
In oocytes, NBCe1-A is calculated to operate with a 1:2 stoichiometry (21, 47), which is different from the apparent 1:3 stoichiometry estimated for NBCe1-A operating in most renal preparations examined (20, 53, 58). It has been suggested that NBCe1-A in renal preparations carries 1 Na+ + 1 CO32− (or 1 SO32−) + 1 HCO3−. In Xenopus oocytes, however, NBCe1-A operates 1:2 stoichiometry, and thus presumably carries either 1 Na+ + 2 HCO3− or 1 Na+ + 1 CO32− (or 1 SO32−). One might suggest that the switch from a 1:3 to a 1:2 stoichiometry occurs as NBCe1-A switches the substrate at its first anion site from CO32− (or SO32−) to HCO3−. However, preliminary studies of cloned human NBCe1-A in oocytes, work that uses surface-pH changes at the plasma membrane to distinguish HCO3− vs. CO32− transport, suggest that NBCe1-A prefers CO32− over HCO3− (17, 29). If this conclusion proves correct, a stoichiometry shift from 1:3 to 1:2 could not explain the altered anion selectivity for SO32−.
Others report that HSO3−/SO3− stimulates NBCe1-like activity in renal preparations (presumably operating with a 1:3 stoichiometry) only in the presence of HCO3− (43, 52). This interpretation would imply that HSO3− could not substitute for HCO3−. We suggest that SO32− may indirectly stimulate NBCe1-A, perhaps by promoting HCO3− extrusion via a functionally coupled transporter such as Sat-1 (see anion specificity of human and rabbit nbce1-a in xenopus oocytes), also present in the system. Note that our data do not address the likelihood that NBCe1-A includes a distinct cation binding site, only that previous data presented in support of such a feature should be interpreted with caution.
In conclusion, cloned human and rabbit NBCe1-A exhibit some important differences compared with the previously reported NBCe1-like activities in BLMVs, HEK cells, and oocytes injected with renal poly (A)+ RNA. The simplest explanation is that the harmaline block and the SO32−-stimulation of NBCe1-like activity in the renal preparations reflects the influence of other renal proteins upon NBCe1-A action and are not features of NBCe1-A per se.
GRANTS
This work was funded by National Institutes of Health Grants DK-30344 and NS-18400 (to W. F. Boron), and EY-021646 (to M. D. Parker and M. L. Jennings).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.-K.L., W.F.B., and M.D.P. conception and design of research; S.-K.L. and M.D.P. performed experiments; S.-K.L. and M.D.P. analyzed data; S.-K.L., W.F.B., and M.D.P. interpreted results of experiments; S.-K.L. and M.D.P. prepared figures; S.-K.L. and M.D.P. drafted manuscript; S.-K.L., W.F.B., and M.D.P. edited and revised manuscript; S.-K.L., W.F.B., and M.D.P. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENT
We thank Dale Huffman at Case Western Reserve University for computer support.
Footnotes
We ignore a large number of models involving the exchange of carbon-containing species.
Hereafter we refer to transport activities that cannot be specifically attributed to NBCe1-A alone as NBCe1-like activities.
At pH 7.5, 33 mM SO32− equilibrates in solution to 26.4 mM SO32−/6.6 mM HSO3−.
The data presented in Figs. 1–12 were gathered from oocytes expressing either EGFP-tagged human NBCe1-A or non-EGFP-tagged rabbit NBCe1-A. As discussed and shown in Figure 2, the presence or absence of an EGFP-tag at the carboxy-terminus of rabbit NBCe1-A does not significantly alter the HCO3−-dependent slope conductance of oocytes expressing the clone.
Combination of per-molecule (i.e., intrinsic) activity and cotransporter abundance in the plasma membrane.
We calculate the Li+- vs. Na+-supported component of the HCO3−-dependent conductance as (GHCO3,Li − GND96,Li)*100/(GHCO3,Na − GND96,Na), assuming that GND96,Na, a quantity that we did not measure in this set of experiments, has the same magnitude as GND96,Li.
We calculate DIDS-sensitive component of outward currents at +20 mV as (IHCO3 − IHCO3,DIDS)*100/(IHCO3−IND96).
REFERENCES
- 1. Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273: 17689–17695, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Akiba T, Warnock D. Evidence for Na+/carbonate cotransport in rabbit renal cortical basolateral membrane-vesicles (BLMV). Clin Res 35: A633–A633, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvarado F, Brot-Laroche E, L'Herminier M, Murer H, Stange G. The effect of harmaline on intestinal sodium transport and on sodium-dependent d-glucose transport in brush-border membrane vesicles from rabbit jejunum. Pflügers Arch 382: 35–41, 1979 [DOI] [PubMed] [Google Scholar]
- 4. Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3− cotransporter. J Biol Chem 273: 16810–16815, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Aronson PS, Bounds SE. Harmaline inhibition of Na-dependent transport in renal microvillus membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 238: F210–F217, 1980 [DOI] [PubMed] [Google Scholar]
- 6. Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na/HCO3 cotransporter (NBC) with a novel C terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–C1211, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3− transport. J Gen Physiol 81: 53–94, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol 212: 1697–1706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boron WF. Intracellular-pH-regulating mechanism of the squid axon: relation between the external Na+ and HCO3− dependences. J Gen Physiol 85: 325–345, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem 272: 19111–19114, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Capewell S, Hefter G, Sipos P, May P. Protonation and sodium ion-pairing of the sulfite ion in concentrated aqueous electrolyte solutions. J Solution Chem 26: 957–972, 1997 [Google Scholar]
- 12. Choi I, Aalkjær C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1). Am J Physiol Renal Physiol 284: F1199–F1206, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Ducoudret O, Diakov A, Muller-Berger S, Romero MF, Frömter E. The renal Na-HCO3− cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflügers Arch 442: 709–717, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Grassl SM, Aronson PS. Na+/HCO3− co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem 261: 8778–8783, 1986 [PubMed] [Google Scholar]
- 16. Grassl SM, Holohan PD, Ross CR. HCO3− transport in basolateral membrane vesicles isolated from rat renal cortex. J Biol Chem 262: 2682–2687, 1987 [PubMed] [Google Scholar]
- 17. Grichtchenko II, Boron WF. Surface-pH measurements in voltage-clamped Xenopus oocytes co-expressing NBCe1 and CAIV: evidence for CO3= transport (Abstract). FASEB J 16: A795, 2002 [Google Scholar]
- 18. Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem 276: 8358–8363, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Grichtchenko II, Romero MF, Boron WF. Extracellular HCO3− dependence of electrogenic Na/HCO3 cotransporters cloned from salamander and rat kidney. J Gen Physiol 115: 533–545, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol 531: 597–603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heyer M, Muller-Berger S, Romero MF, Boron WF, Frömter E. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflügers Arch 438: 322–329, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol 16: 2270–2278, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol Cell Physiol 267: C1295–C1307, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem 283: 26782–26794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karniski LP, Lotscher M, Fucentese M, Hilfiker H, Biber H, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol 275: F79–F87, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Krick W, Schnedler N, Burckhardt G, Burckhardt BC. Ability of sat-1 to transport sulfate, bicarbonate, or oxalate under physiological conditions. Am J Physiol Renal Physiol 297: F145–F154, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na/HCO3 cotransporter NBCe1-B: role of IRBIT versus amino-terminal truncation. Am J Physiol Cell Physiol 302: C518–C526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SK, Grichtchenko II, Boron WF. Distinguishing HCO3− from CO3= transport by NBCe1-A (Abstract). FASEB J 25: 656.–9., 2011 [Google Scholar]
- 30. Liu Y, Xu JY, Wang DK, Wang L, Chen LM. Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: a comparative study of NCBT genes. Genomics 98: 112–119, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjær C. Immunoelectron microscopic localization of the electrogenic Na/HCO3 cotransporter in rat and Ambystoma kidney. J Am Soc Nephrol 11: 2179–2189, 2000 [DOI] [PubMed] [Google Scholar]
- 33. McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol 127: 639–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Musa-Aziz R, Boron WF, Parker MD. Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51: 134–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem 283: 12777–12788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parker MD, Qin X, Williamson RC, Toye AM, Boron WF. HCO3−-independent conductance with a mutant Na+/HCO3− cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalemic paralysis. J Physiol 590: 2009–2034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perry DL, Phillips SL. Handbook of Inorganic Compounds. Boca Raton, FL: CRC, 1995 [Google Scholar]
- 39. Rawn JD. Proteins, energy, metabolism. Burlington, NC: N. Patterson, 1989 [Google Scholar]
- 40. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch 447: 495–509, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387: 409–413, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Ruiz OS, Qiu YY, Arruda JA. The renal cortical Na-HCO3 cotransporter: V. Expression in Xenopus oocytes. Proc Soc Exp Biol Med 211: 199–204, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Samarzija I, Kinne-Saffran E, Baumann K, Frömter E. The mechanism of action of harmaline on renal solute transport. Pflügers Arch 368: 83–88, 1977 [DOI] [PubMed] [Google Scholar]
- 45. Sassani P, Pushkin A, Abuladze N, Azimov R, Kao L, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Role of S-S bond formation in the oligomerization of kNBC1 (NBCe1-A) (Abstract). FASEB J 21: 916.–3., 2007 [Google Scholar]
- 46. Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+/HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276: F27–F36, 1999 [DOI] [PubMed] [Google Scholar]
- 46a. Sciortino CM. Characterization and Localization of the Sodium Mediated Bicarbonate Transporters NBC and NDAE1 (PhD thesis) Cleveland, OH: Case Western Reserve University, 2001 [Google Scholar]
- 47. Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Seki G, Coppola S, Frömter E. The Na+-HCO3− cotransporter operates with a coupling ratio of 2 HCO3− to 1 Na+ in isolated rabbit renal proximal tubule. Pflügers Arch 425: 409–416, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Sepulveda FV, Robinson JW. Harmaline, a potent inhibitor of sodium-dependent transport. Biochim Biophys Acta 373: 527–531, 1974 [DOI] [PubMed] [Google Scholar]
- 51. Skelton LA, Boron WF, Zhou Y. Acid-base transport by the renal proximal tubule. J Nephrol 23: 4–18, 2010 [PMC free article] [PubMed] [Google Scholar]
- 52. Soleimani M, Aronson PS. Ionic mechanism of sodium bicarbonate cotransport in rabbit renal basolateral membrane vesicles. J Biol Chem 264: 31: 18302–18308, 1989 [PubMed] [Google Scholar]
- 53. Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3−- cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest 79: 1276–1280, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soleimani M, Lesoine GA, Bergman JA, Aronson PS. Cation specificity and modes of the Na+:CO32−:HCO3− cotransporter in renal basolateral membrane vesicles. J Biol Chem 266: 8706–8710, 1991 [PubMed] [Google Scholar]
- 55. Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier on the voltage-gated potassium channel family. Science 269: 92–95, 1995 [DOI] [PubMed] [Google Scholar]
- 57. Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285: C771–C780, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Yoshitomi K, Burckhardt BC, Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflügers Arch 405: 360–366, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.