Abstract
This study tested the hypothesis that Rho kinase contributes to the enhanced pressor response to acute angiotensin II in intact male growth-restricted and gonadectomized female growth-restricted rats. Mean arterial pressure (MAP) and renal function were determined in conscious animals pretreated with enalapril (250 mg/l in drinking water) for 1 wk to block the endogenous renin-angiotensin system and normalize blood pressure (baseline). Blood pressure and renal hemodynamics did not differ at baseline. Acute Ang II (100 ng·kg−1·min−1) induced a greater increase in MAP and renal vascular resistance and enhanced reduction in glomerular filtration rate in intact male growth-restricted rats compared with intact male controls (P < 0.05). Cotreatment with the Rho kinase inhibitor fasudil (33 μg·kg−1·min−1) significantly attenuated these hemodynamic changes (P < 0.05), but it did not abolish the differential increase in blood pressure above baseline, suggesting that the impact of intrauterine growth restriction on blood pressure in intact male growth-restricted rats is independent of Rho kinase. Gonadectomy in conjunction with fasudil returned blood pressure back to baseline in male growth-restricted rats, and yet glomerular filtration rate remained significantly reduced (P < 0.05). Thus, these data suggest a role for enhanced renal sensitivity to acute Ang II in the developmental programming of hypertension in male growth-restricted rats. However, inhibition of Rho kinase had no effect on the basal or enhanced increase in blood pressure induced by acute Ang II in the gonadectomized female growth-restricted rat. Therefore, these studies suggest that Rho kinase inhibition exerts a sex-specific effect on blood pressure sensitivity to acute Ang II in growth-restricted rats.
Keywords: sex differences, intrauterine growth restriction, developmental programmed hypertension, angiotensin II, Rho kinase
placental insufficiency is one of the causes of intrauterine growth restriction (IUGR), with effects on adult vascular function (15, 18, 19, 39). A reduction in the supply of nutrients and oxygen to the fetus mediated via placental insufficiency can induce IUGR and program hypertension and alter responsiveness to vasoconstrictors in male growth-restricted rats (2, 28, 39, 40). Several regulatory systems have been linked to the increased vasoconstrictor response observed in experimental models of hypertension associated with IUGR (6, 15, 29, 41); however, the exact mechanistic pathways are still poorly understood.
We utilize a rat model of placental insufficiency induced by reduction in uterine perfusion during late gestation to investigate the mechanisms underlying the developmental origins of hypertension (2). This model of placental insufficiency results in growth-restricted rats that develop hypertension with sex-specific differences (2, 23, 24). Previously, we reported that adult male growth-restricted rats are hypertensive, whereas female growth-restricted rats are normotensive (2). Gonadectomy completely abolishes hypertension in male growth-restricted rats (24). In female growth-restricted rats, hypertension is induced by gonadectomy and reversed by hormone replacement with estradiol (23), indicating a key role for sex hormone involvement. Male growth-restricted rats exhibit an enhanced sensitivity to acute angiotensin II (Ang II) that is androgen dependent (27); gonadectomy induces enhanced Ang II sensitivity in female growth-restricted rats (26). Thus, these data indicate that testosterone is permissive in the programming of hypertension and enhanced sensitivity to acute Ang II in male growth-restricted rats, whereas estradiol is protective against the programming of cardiovascular risk in female growth-restricted rats. Sex differences in blood pressure and impaired vascular function are observed in several different models of compromised fetal growth related to later increased cardiovascular risk (19, 42, 43, 45). Yet the exact mechanism(s) responsible for mediating the sex difference in adult blood pressure and modulation of sensitivity to acute Ang II in growth-restricted rats remains unknown.
Despite an enhanced sensitivity to acute Ang II, no difference in renal Ang II type 1 receptor (AT1R) density is observed in male growth-restricted rats relative to male controls (9). The male New Zealand genetically hypertensive (NZGH) rat also exhibits a potentiated sensitivity to Ang II that is not associated with a change in renal AT1R expression (33). Similar to male growth-restricted rats, sensitivity to Ang II in the male NZGH rat is also androgen dependent (33). Inhibition of Rho kinase suppresses the differential effects of acute Ang II on blood pressure and renal vascular resistance in the male NZGH rat, indicating that activation of the Rho kinase pathway contributes to androgen-mediated hypersensitivity to acute Ang II in this genetic model of hypertension (33). Therefore, one aim of this study was to test the hypothesis that post-AT1R signaling mediated via Rho kinase contributes to the enhanced pressor response to acute Ang II in male growth-restricted rats. Whether the vasoprotective effects of estradiol are mediated via inhibition of the RhoA/Rho kinase pathway is not clear (5, 7, 14). Thus, the second aim was to determine the importance of the Rho kinase pathway in mediating enhanced sensitivity to acute Ang II in female gonadectomized growth-restricted rats.
METHODS
Animals.
All experimental procedures were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and with approval by the Animal Care and Use Committee at the University of Mississippi Medical Center. Timed pregnant Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle with food and water available ad libitum. On day 14 of gestation, rats destined for reduced uteroplacental perfusion underwent reduced uterine perfusion surgical procedure. Control pregnant rats underwent a sham surgical procedure, as described below. All dams delivered at term (21–22 days of gestation), with birth weight recorded within 12 h of delivery. Forty-eight hours after birth, offspring in each litter were culled to eight pups per dam to ensure equal nutrient access for all offspring. Animal weights were recorded twice per week; pups were weaned at 3 wk of age. Male and female offspring from eight control pregnant and 10 reduced-uterine perfusion pregnant litters were assigned randomly into eight groups: male control intact, n = 6; male IUGR intact, n = 6; male control gonadectomized, n = 6; male IUGR gonadectomized, n = 6; female control intact, n = 6; female IUGR intact, n = 6; female control gonadectomized, n = 6; and female IUGR gonadectomized, n = 6. To block the endogenous production of Ang II and normalize blood pressure in all groups, animals were treated with enalapril 1 wk before measurement of systemic and renal hemodynamics. Systemic and renal hemodynamic parameters were measured in all rats during baseline, an acute infusion of Ang II, and an infusion of Ang II plus fasudil. All animals undergoing surgical procedures were anesthetized using 2–5% isoflurane by inhalation. All experimental end points were measured at 16 wk of age to ensure complete passage through puberty and into adulthood.
Reduced uterine perfusion in the pregnant rat.
Reduced uteroplacental perfusion, as described previously (2), was utilized for induction of IUGR. Briefly, on day 14 of gestation, a silver clip (0.203 mm ID) was placed around the lower abdominal aorta above the iliac bifurcation. Since compensation of blood flow occurs through an adaptive increase in ovarian blood flow, a silver clip was slipped around each branch of the ovarian arteries (0.100 mm ID), followed by closure of the incision in two layers, muscle and skin. Control dams were exposed to sham procedure, which involved opening of the abdominal cavity with visualization of the uterine horn and closure of the incision, as described previously (2).
Gonadectomy in male and female rats.
Male gonadectomy was performed as described previously (24). Briefly, at 10 wk of age, a small median incision was made at the distal tip of the scrotum. Subcutaneous connective tissue was cleared, and the testes were visualized. The muscular sac of the testes was excised and exposed by gently pulling on the cauda epididymis. The blood vessels were tied and the vas deferens with the testes removed (gonadectomized males), followed by closure of the muscle and the skin. The sham operation involved exposure of the testes without isolation and removal. Female gonadectomy was performed as described previously (23). Briefly, the procedure was performed at 10 wk of age with the skin prepared for aseptic surgery, followed by a dorsal incision. The dorsal musculature was incised, and the ovaries were visualized. In the gonadectomized group, the ovarian vessels were tied off and the ovaries removed (gonadectomized females). The sham operation involved a dorsal midline incision followed by visualization of the ovaries without removal. The incision was closed in two layers, muscle and skin.
Drug administration.
The angiotensin converter enzyme (ACE) inhibitor enalapril (40 mg·kg−1·day−1; Sigma-Aldrich, St. Louis, MO) was administered in the drinking water from 15 to 16 wk of age at a dose previously shown to block the endogenous production of Ang II in the rat (27). Water consumption was monitored daily for the duration of the treatment period. At 16 wk of age, the rats received a 30-min infusion of each of the following: 1) 0.9% saline solution, 2) Ang II (Sigma-Aldrich) at a dose of 100 ng·kg−1·min−1 in 0.9% saline solution, and 3) Ang II at a dose of 100 ng·kg−1·min−1 plus fasudil (Sigma-Aldrich) at a dose of 33 mg·kg−1·min−1 in 0.9% saline solution. The order of infusion of Ang II and Ang II plus fasudil was interchanged in a subset of rats to ensure that preadministration of Ang II did not alter the pressor response to coadministration of Ang II plus fasudil. Systemic and renal hemodynamic parameters were measured during each 30-min infusion period. Blood pressure values were allowed to return to baseline between each infusion during 0.9% saline solution infusion.
Measurement of systemic and renal hemodynamics.
As described previously (22) during isoflurane anesthesia, rats were surgically instrumented with flexible catheters (PE 50 tubing) in the right jugular vein for infusion and in the right carotid artery for measurement of arterial pressure and collection of blood; the bladder was instrumented with a flexible catheter (PE 90 tubing) for collection of urine. All catheters were tunneled to the nape of the neck and exteriorized. Renal function and arterial pressure measurements were performed in the conscious state after a 24-h recovery phase. Mean arterial pressure (MAP) was monitored in conscious, chronically instrumented rats via connection of the arterial catheter to a pressure transducer and a data acquisition kit (DATAQ Instruments, Akron, OH) with a computer for continuous recording. Glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) were calculated from radioactivity of [125I]iothalamate and concentration of para-aminohippuric acid, respectively, in plasma and urine. Renal vascular resistance (RVR) and filtration fraction (FF) were calculated as RVR = (MAP/ERPF) × (1 − hematocrit) and FF = (GFR/ERPF), respectively. Data were collected for 30 min each during the baseline clearance, during the acute infusion of Ang II, and during Ang II plus fasudil for comparison between groups.
Statistics.
GraphPad Prism version 5 and IBMR SPSS Statistics version 19 were utilized for all statistical analysis. Comparisons made between groups utilized ANOVA, with adjustment for multiple comparisons. Bonferroni's post hoc test was utilized for multiple comparisons. The general linear model (GLM-SPSS) univariate with three-way interactions was used to calculate interactions related to birth weight, gonadectomy, and treatment with fasudil in males and females separately. Statistical significance of interaction was set with P < 0.05 or lower. The sample size for all experiments was calculated to attain a statistical power of ≥0.85.
RESULTS
Birth weight, body weight, kidney weight, and water consumption.
Birth weight was significantly reduced in growth-restricted male (5.30 ± 0.2 vs. 6.25 ± 0.1 g, P < 0.05) and female rats (5.00 ± 0.1 vs. 5.96 ± 01 g, P < 0.05) compared with their control counterparts. At 16 wk of age, body weight did not differ upon comparison between same-sex groups. Male rats were significantly heavier than females in the control and growth-restricted groups (Table 1). Kidney weight and the kidney/body weight ratio at 16 wk of age did not differ upon comparison between same-sex groups. However, male rats had larger kidneys compared with female rats in both the control and growth-restricted groups. (Table 1). Water consumption was monitored daily during the administration of the ACE inhibitor enalapril, and the average daily volume did not differ between same-sex growth-restricted vs. control groups. (Table 1). Gonadectomy had no significant effect on body weight, kidney weight, or water intake among groups (Table 1).
Table 1.
Birth weight, body weight, kidney weight, and water consumption in intact and gonadectomized male and female control and IUGR offspring
| Experimental Groups | Body Weight at Birth, g | Body Weight at 16 wk of Age, g | Kidney Weight at 16 wk of Age, g | Kidney/Body Weight Ratio at 16 wk of Age | Water Intake at 16 wk of Age, ml/day |
|---|---|---|---|---|---|
| Males | |||||
| Control intact | 6.7 ± 0.2 | 406.3 ± 8.4 | 3.2 ± 0.2 | 0.78 ± 0.05 | 52.6 ± 1.2 |
| IUGR intact | 5.5 ± 0.1* | 408.3 ± 9.1 | 2.9 ± 0.1 | 0.71 ± 0.01 | 51.5 ± 0.9 |
| Control gonadectomized | 6.8 ± 0.1 | 424.5 ± 12.9 | 2.9 ± 0.1 | 0.67 ± 0.02 | 51.7 ± 1.2 |
| IUGR gonadectomized | 5.1 ± 0.3* | 415.7 ± 13.3 | 2.8 ± 0.1 | 0.67 ± 0.01 | 52.2 ± 1.1 |
| Females | |||||
| Control intact | 5.7 ± 0.2 | 260.0 ± 4.6 | 1.7 ± 0.1‡ | 0.65 ± 0.02 | 41.5 ± 1.4 |
| IUGR intact | 4.9 ± 0.1* | 259.0 ± 5.8 | 1.9 ± 0.1‡ | 0.72 ± 0.02 | 39.0 ± 1.5 |
| Control gonadectomized | 6.2 ± 0.1 | 306.8 ± 11.9† | 2.0 ± 0.1‡ | 0.67 ± 0.05 | 40.5 ± 1.7 |
| IUGR gonadectomized | 5.1 ± 0.1* | 287.2 ± 16.6*† | 1.9 ± 0.2‡ | 0.67 ± 0.03 | 40.3 ± 1.3 |
Values represent means ± SE. IUGR, intrauterine growth restricted.
P < 0.05 vs. control counterpart;
P < 0.05 vs. intact counterpart;
P < 0.05 vs. male counterpart.
Systemic hemodynamic: MAP.
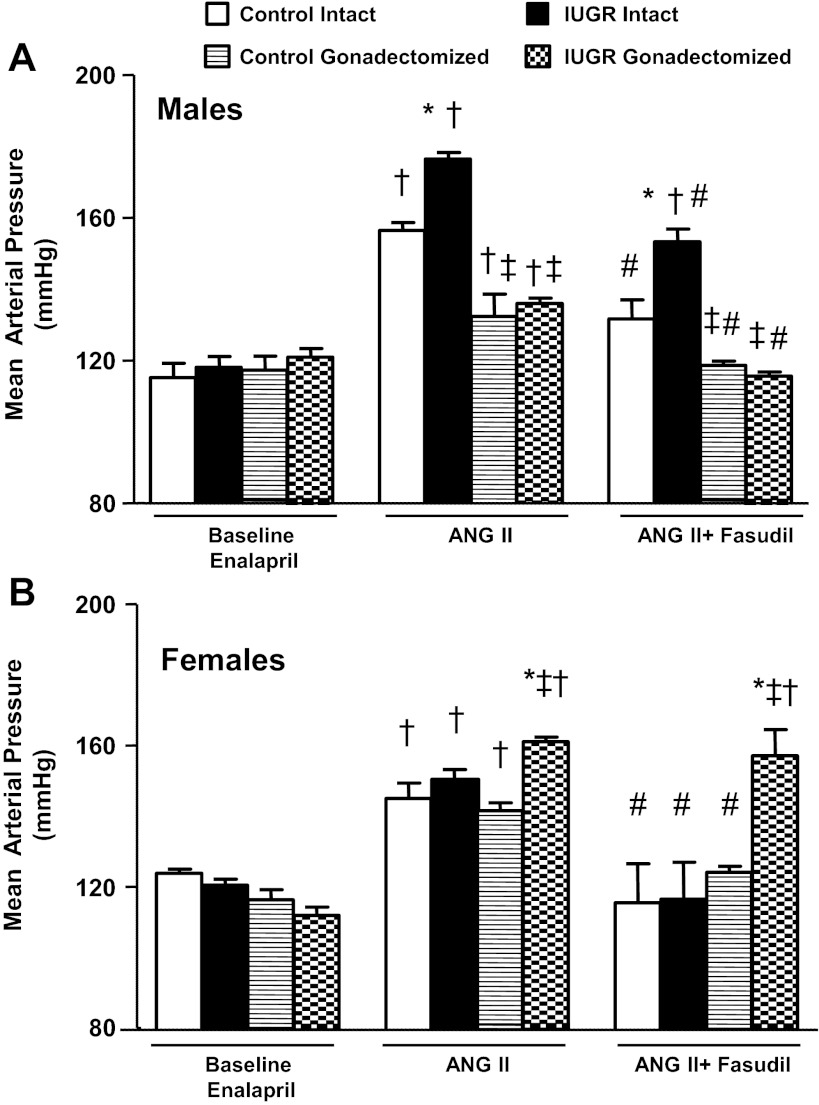
Under baseline conditions following chronic treatment with the ACE inhibitor enalapril, MAP was similar between same-sex control and growth-restricted rats, intact or gonadectomized (Fig. 1, A and B). Intact male growth-restricted rats exhibited an enhanced pressor response or a significantly greater increase in MAP in response to an acute infusion of Ang II (ΔMAP from baseline: 58 ± 2 mmHg) compared with intact male controls (ΔMAP from baseline: 41 ± 3 mmHg) (Fig. 1A). Gonadectomy significantly attenuated the pressor response to an acute infusion of Ang II in male control (ΔMAP from baseline: 15 ± 2 mmHg) and male growth-restricted rats (ΔMAP from baseline: 15 ± 3 mmHg), abolishing the differential pressor response to acute Ang II observed in intact male rats (Fig. 1A). However, MAP remained significantly elevated relative to the baseline counterpart. Coinfusion of the Rho kinase inhibitor fasudil with acute Ang II attenuated the blood pressure response in intact male control (ΔMAP from baseline: 19 ± 3 mmHg) and intact male growth-restricted rats (ΔMAP from baseline: 35 ± 8 mmHg) (Fig. 1A). Yet the blood pressure response to acute Ang II remained enhanced in intact male growth-restricted rats relative to intact male control rats. Coinfusion of fasudil in gonadectomized males completely abolished the blood pressure response to acute Ang II, returning MAP to baseline levels (Fig. 1A).
Fig. 1.
Mean arterial pressure (MAP). A: male intact and gonadectomized control and intrauterine growth-restricted (IUGR) rats. B: female intact and gonadectomized control and IUGR rats. MAP was measured at 16 wk of age in chronically instrumented, conscious animals pretreated with enalapril (250 mg/l for 1 wk) at 3 time points: baseline, during acute angiotensin II (Ang II; 100 ng·kg−1·min−1), and during acute Ang II plus the Rho kinase antagonist fasudil (33 μg·kg−1·min−1). *P < 0.05 vs. control counterpart; †P < 0.05 vs. baseline counterpart; ‡P < 0.05 vs. intact counterpart; #P < 0.05 vs. Ang II counterpart. Values represent means ± SE.
Intact female control and intact female growth-restricted rats demonstrated similar blood pressure responses to an acute infusion of Ang II (Fig. 1B). However, the blood pressure response to acute Ang II was enhanced or significantly greater in gonadectomized female growth-restricted rats (ΔMAP from baseline: 49 ± 3 mmHg) compared with gonadectomized female control rats (ΔMAP from baseline: 25 ± 3 mmHg) (Fig. 1B). Coinfusion of fasudil with acute Ang II abolished the increase in MAP above baseline in female control, intact or gonadectomized, and intact female growth-restricted rats (Fig. 1B). However, coinfusion of fasudil with acute Ang II had no inhibitory effect on the blood pressure response in gonadectomized female growth-restricted rats (ΔMAP from baseline: 49 ± 10 mmHg) (Fig. 1B).
The general linear model (GLM-IBMR, SPSS) with three-way interaction showed statistically significant effects of birth weight (P < 0.014, F = 6), gonadectomy (P < 0.000, F = 29), and treatment with fasudil (P < 0.000, F = 43) on MAP in male growth-restricted offspring. However, only birth weight (P < 0.015, F = 6) and gonadectomy (P < 0.000, F = 47) showed a significant effect on MAP in female growth-restricted rats. Thus, these data indicate that activation of the Rho kinase signaling pathway contributes to the pressor response to acute Ang II in intact male growth-restricted offspring but not in gonadectomized female growth-restricted offspring. The complete elimination of the pressor response in gonadectomized male growth-restricted offspring suggests a synergistic effect of androgen and Rho kinase in male growth-restricted rats.
Renal hemodynamics: GFR, effective renal plasma flow, RVR, and FF.
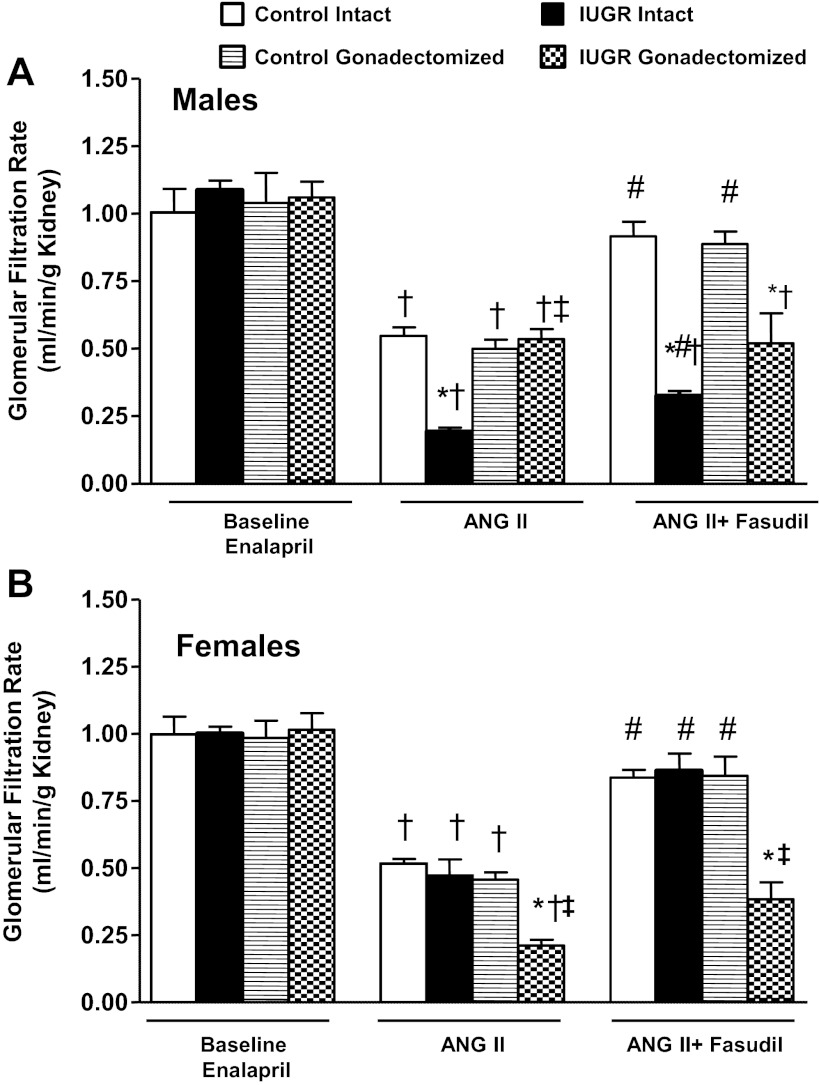
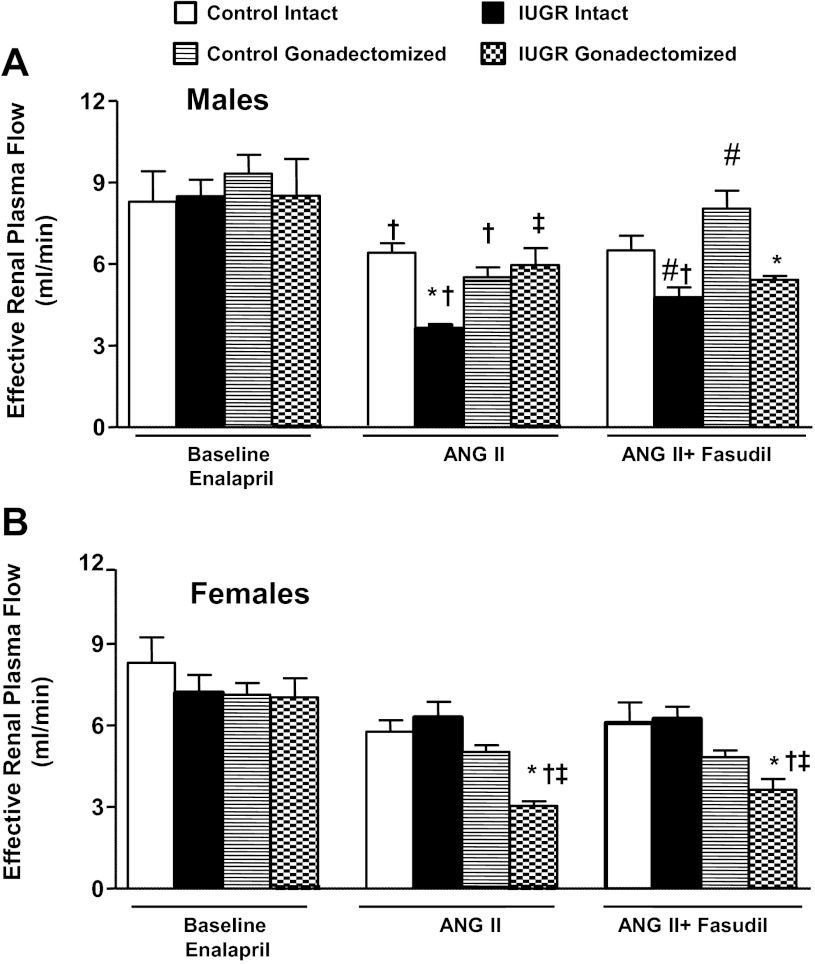
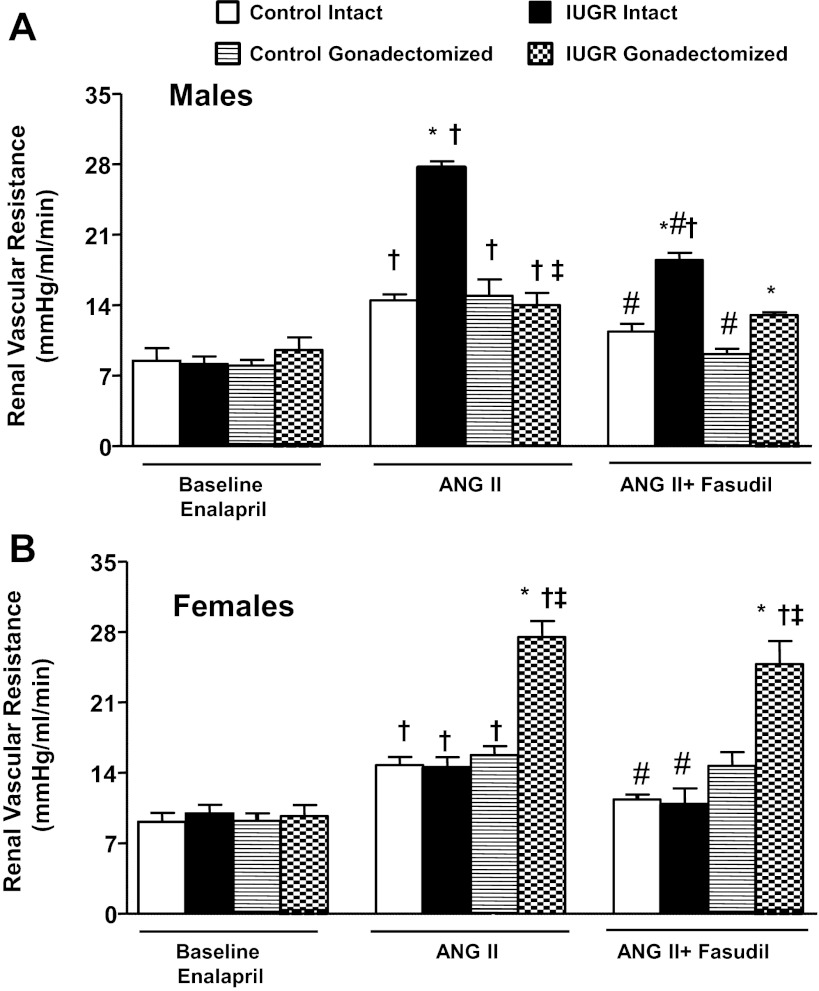
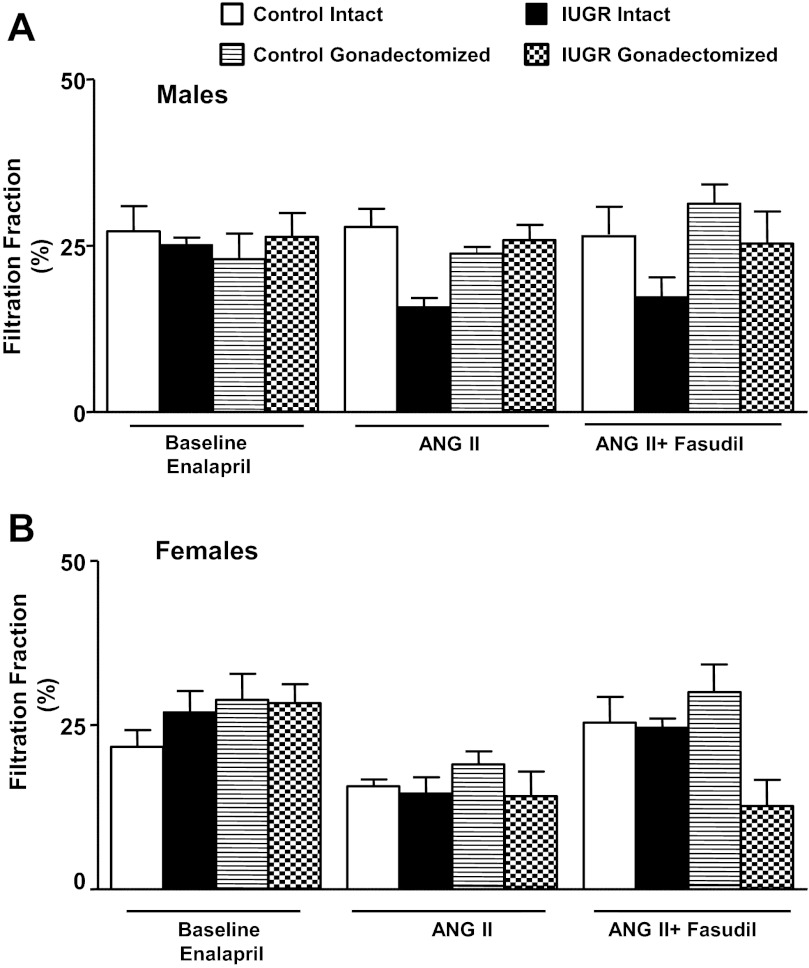
GFR normalized by kidney weight did not differ significantly upon comparison of same-sex groups at baseline (Fig. 2, A and B). In response to an acute infusion of Ang II, the reduction in GFR normalized by kidney weight was significantly greater in intact growth-restricted male rats and gonadectomized female growth-restricted rats compared with their same-sex control counterparts (Fig. 2, A and B). Cotreatment with fasudil did not restore GFR normalized to kidney weight in intact male growth-restricted rats (Fig. 2A) or gonadectomized female growth-restricted rats (Fig. 2B). ERPF was similar under baseline conditions in same-sex groups (Fig. 3, A and B). However, the reduction in ERPF was greater in intact male growth-restricted rats (Fig. 3A) and gonadectomized female growth-restricted rats compared with their same-sex control counterparts (Fig. 3B). ERPF remained reduced relative to its control counterpart in gonadectomized male rats (Fig. 3A) and gonadectomized female growth-restricted rats cotreated with fasudil (Fig. 3B). RVR did not differ upon comparison of same-sex groups at baseline (Fig. 4, A and B). In response to acute Ang II, RVR was increased significantly in all groups relative to their same-sex baseline counterparts (Fig. A and B). However, the increase in RVR was enhanced in intact male growth-restricted rats (Fig. 4A) and gonadectomized female growth-restricted rats (Fig. 4B) relative to their same-sex counterparts. Cotreatment with fasudil attenuated the increase in RVR in intact male growth-restricted rats, but it did not abolish the enhanced response (Fig. 4A). Cotreatment with fasudil in gonadectomized male rats abolished the increase in RVR above baseline, but RVR remained elevated relative to their male control counterparts (Fig. 4A). Cotreatment with fasudil did not alter the marked increase in RVR in gonadectomized female growth-restricted rats relative to other female groups (Fig. 4B). FF did not differ upon comparison of same-sex groups during baseline, acute infusion of Ang II, or cotreatment of Ang II with fasudil (Fig. 5, A and B).
Fig. 2.
Glomerular filtration rate (GFR). A: male intact and gonadectomized control and IUGR rats. B: female intact and gonadectomized control and IUGR rats. GFR was measured at 16 wk of age in chronically instrumented, conscious animals pretreated with enalapril (250 mg/l for 1 wk) at 3 time points: baseline, during acute Ang II (100 ng·kg−1·min−1), and during acute Ang II plus the Rho kinase antagonist fasudil (33 μg·kg−1·min−1). *P < 0.05 vs. control counterpart; †P < 0.05 vs. baseline counterpart; ‡P < 0.05 vs. intact counterpart; #P < 0.05 vs. Ang II counterpart counterpart. Values represent means ± SE.
Fig. 3.
Effective renal plasma flow (ERPF). A: male intact and gonadectomized control and IUGR rats. B: female intact and gonadectomized control and IUGR rats. ERPF was measured at 16 wk of age in chronically instrumented, conscious animals pretreated with enalapril (250 mg/l for 1 wk) at 3 time points: baseline, during acute Ang II (100 ng·kg−1·min−1), and during acute Ang II plus the Rho kinase antagonist fasudil (33 μg·kg−1·min−1). *P < 0.05 vs. control counterpart; †P < 0.05 vs. baseline counterpart; ‡P < 0.05 vs. intact counterpart; #P < 0.05 vs. Ang II counterpart. Values represent means ± SE.
Fig 4.
Renal vascular resistance (RVR). A: male intact and gonadectomized control and IUGR rats. B: female intact and gonadectomized control and IUGR rats. RVR was measured at 16 wk of age in chronically instrumented, conscious animals pretreated with the angiotensin converter enzyme inhibitor enalapril (250 mg/l for 1 wk) at 3 time points: baseline, during acute Ang II (100 ng·kg−1·min−1), and during acute Ang II plus the Rho kinase antagonist fasudil (33 μg·kg−1·min−1). *P < 0.05 vs. control counterpart; †P < 0.05 vs. baseline counterpart; ‡P < 0.05 vs. intact counterpart; #P < 0.05 vs. Ang II counterpart. Values represent means ± SE.
Fig. 5.
Filtration fraction (FF). A: male intact and gonadectomized control and IUGR rats. B: female intact and gonadectomized control and IUGR rats. FF was measured at 16 wk of age in chronically instrumented, conscious animals pretreated with enalapril (250 mg/l for 1 wk) at 3 time points: baseline, during acute Ang II (100 ng·kg−1·min−1), and during acute Ang II plus the Rho kinase antagonist fasudil (33 μg·kg−1·min−1). Values represent means ± SE.
DISCUSSION
Activation of the RhoA/Rho kinase pathway via the AT1R is indicated to play a critical role in Ang II-induced vasoconstriction (10, 20, 38). Androgens can potentiate Ang II-induced renal responsiveness via upregulation of the Rho kinase signaling pathway (34) through a mechanism that may involve activation of specific factors such as Arhgef1 (10). As reported previously, hypertension in male growth-restricted rats is androgen dependent (24); moreover, male growth-restricted rats exhibit an enhanced responsiveness to acute Ang II (27). Thus, one aim of this study was to determine whether Rho kinase contributes to the potentiated pressor response to acute Ang II in male growth-restricted rats. Novel findings from this aim indicated that inhibition of Rho kinase using the inhibitor fasudil significantly reduced the blood pressure response to an acute infusion of Ang II in intact male growth-restricted rats. However, inhibition of Rho kinase by the inhibitor fasudil did not abolish the differential blood pressure response, indicating that the impact of IUGR on MAP in male rats is independent of Rho kinase. Gonadectomy in combination with inhibition of Rho kinase normalized the enhanced blood pressure response to acute Ang II in male growth-restricted rats relative to control counterparts, and yet enhanced alterations in GFR and RVR to acute Ang II were not abolished. Thus, this study indicates a synergistic effect of testosterone with the actions of fasudil on blood pressure in male growth-restricted rats. However, the significant increase in RVR and the decrease in GFR that persisted in gonadectomized male growth-restricted rats treated with fasudil in the absence of an increase in blood pressure suggest that enhanced renal sensitivity to acute Ang II may contribute to the developmental programming of hypertension in male growth-restricted rats.
The second aim of this study was to determine the involvement of the Rho kinase pathway in mediating the enhanced pressor response and renal sensitivity to acute Ang II in female gonadectomized growth-restricted rats. The findings from our second aim indicated that inhibition of Rho kinase abolished the increase in blood pressure above baseline in response to acute Ang II in intact female growth-restricted rats, a response not observed in intact male growth-restricted rats. Moreover, unlike the reduction in blood pressure observed in intact male growth-restricted rats, blockade of Rho kinase did not alter the blood pressure response to acute Ang II in gonadectomized female growth-restricted rats. Sex differences in blood pressure are reported in many experimental models of hypertension (31), and yet the mechanisms involved remain unknown. Few studies have investigated the role of Rho kinase in mediating sex differences in blood pressure and vascular reactivity (35). Hypertension induced by ovariectomy in the female SHR is associated with central activation of Rho kinase, suggesting that modulation of postreceptor signaling mediated via estrogen in this genetic model of hypertension mediates alterations in blood pressure control (14), and yet the sex-specific contribution of Rho kinase to vascular function is not clear. Vasoprotective actions of estrogen via inhibition of the RhoA pathway are implicated at the vascular level in female mice (7). However, glucose-mediated relaxation responses do not involve Rho kinase in female rats (8), and vascular dysfunction in a model of type 1 diabetes involves a greater contribution via Rho kinase in male vs. female rats despite no change in expression of Rho kinase or RhoA, a critical activator of Rho kinase (21).
Renal vascular hyperresponsiveness to acute Ang II is observed in several models of developmental programming of health and disease (29), and yet the mechanisms involved are multifaceted and differ based on developmental insult. A reduction in endothelial nitric oxide buffering capacity contributes to enhanced Ang II-mediated vasoconstriction in a model of early-life stress (17). However, blockade of endothelial nitric oxide synthase activity does not alter the enhanced vascular reactivity to acute Ang II in male rats exposed to nicotine during fetal life (45). Alterations in expression of the vascular AT1R and postreceptor signaling are indicated to contribute to greater vascular reactivity to acute Ang II in male rats following prenatal nicotine exposure (44). Moreover, enhanced vascular reactivity to acute Ang II following prenatal nicotine is mediated via a NADPH oxidase (Nox2)/gp91-dependent mechanism, which is indicative of increased oxidative stress (44). Reactive oxygen species (ROS) are important regulators of Rho kinase pathway activation (36). Recent studies from our laboratory demonstrate that basal and NADPH oxidase-dependent superoxide production are elevated in male growth-restricted rats, with a role for oxidative stress indicated in the etiology of male IUGR hypertension (25). Whether oxidative stress contributes to the enhanced responsiveness to acute Ang II in male growth-restricted rats is not yet known. However, treatment with fasudil did not restore renal or systemic hemodynamics to baseline values in intact male growth-restricted rats following acute Ang II, suggesting that upregulation of Rho kinase via an increase in ROS per se is not the putative mechanism responsible for enhanced sensitivity to acute Ang II in male growth-restricted rats. Oxidative stress is not elevated in intact female growth-restricted rats (25), indicating a sex-specific role for oxidative stress in the programming of hypertension in IUGR offspring. Whether oxidative stress contributes to hypertension or enhanced sensitivity to acute Ang II induced by gonadectomy in female growth-restricted rats is not yet known. However, numerous animal studies indicate that oxidative stress plays a greater role in mediating hypertension in male rats in experimental models of hypertension relative to their female counterparts (16), suggesting that an increase in oxidative stress may not be an important contributor to the enhanced sensitivity to acute Ang II observed in gonadectomized female growth-restricted rats. In this study, a sex difference in the blood pressure response to acute Ang II in the presence of Rho kinase inhibition was observed in intact growth-restricted rats. Whereas blockade of Rho kinase restored blood pressure and RVR back to baseline in response to acute Ang II in intact female growth-restricted rats, the increase in blood pressure and RVR remained potentiated above baseline in intact male growth-restricted rats. This finding would suggest that intact female growth-restricted offspring were more sensitive to inhibition of Rho kinase compared with their male counterparts, an effect that was abolished by gonadectomy in the female growth-restricted rat. However, gonadectomy did not induce a male growth-restricted phenotype per se in the female growth-restricted rat. Fasudil reduced the blood pressure response to acute Ang II in male rats, intact or gonadectomized, and yet fasudil had no effect on blood pressure in female gonadectomized growth-restricted rats. It is well established that the kidney plays an important role in the long-term control of blood pressure through pressure natriuresis, whereby changes in renal perfusion pressure alter sodium and water homeostasis (11). The pressure natriuresis relationship is sex specific (13), and responsiveness to acute Ang II is greater in the males than the females (32). The mechanisms involved have not yet been elucidated. However, findings from this study suggest that sex-specific sensitivity to Rho kinase may contribute to the sex difference in the blood pressure response to acute Ang II in growth-restricted rats.
Sex differences in Ang II sensitivity are not always associated with a reduction in circulating or renal levels of Ang II in females compared with males (35). Moreover, the mechanisms that mediate the sex difference in the pressor response and renal vascular sensitivity to acute Ang II in growth-restricted rats may not involve only modulation of the pressor components of the renin-angiotensin system (RAS), such as the AT1R. The ACE2/Ang-(1–7) component of the RAS opposes the pressor actions of Ang II mediated via the classic Ang II/AT1R RAS pathway (4). Ang-(1–7) mediates its vasodilator actions via the Mas receptor (masR) (4). Antagonism of the Ang-(1–7)/masR component of the RAS has a greater effect on renal vascular responses to acute Ang II in female vs. male rats (30). Thus, modulation of the ACE2/ANG-(1–7) pathway may be one mechanism that contributes to sex difference in acute Ang II hyperresponsiveness in growth-restricted rats. Previous studies from our laboratory demonstrated that renal ACE2 expression and activity are elevated in intact female growth-restricted rats relative to intact female control rats (23). Additionally, increases in renal ACE2 protein expression and ACE2 activity are abolished by gonadectomy in female growth-restricted rats (23,) indicating a potential role for modulation of ACE2 by ovarian hormone status in female growth-restricted rats. Thus, alterations in the ANG-(1–7)/masR pathway may contribute to the hyperresponsiveness to acute Ang II observed in gonadectomized female growth-restricted rats. The angiotensin type 2 receptor (AT2R) also opposes the pressor actions of the classic Ang II/AT1R (12). Despite similar expression levels of the AT2R in male and female mice, AT2R blockade enhances Ang II-induced renal vasoconstriction in female mice but not in males (37). Thus, the AT2R may also be a potential mediator of the sex difference in acute hyperresponsiveness to acute Ang II in growth-restricted rats.
Numerous studies now indicate that developmental programming of hypertension and adult disease is associated not only with marked changes in structure, as evidenced by reductions in nephron number (1), but also with changes in the regulatory systems involved in the long-term control of blood pressure regulation such as the RAS and the sympathetic nervous system (3, 23, 24, 41). Activation of the Rho kinase pathway contributed to the basic pressor response to acute Ang II in male rats, and yet Rho kinase activation was not the sole mediator of the enhanced pressor response to acute Ang II in male growth-restricted rats. The contribution of Rho kinase to renal vascular sensitivity to acute Ang II was altered by gonadectomy in female growth-restricted rats, indicating the important role for sex hormones and modulation of the RAS in IUGR-induced hypertension.
In conclusion, hypertension is one of the most modifiable risk factors for cardiovascular disease. However, the multifactorial etiology of hypertension remains one of the most challenging components in the treatment of essential hypertension. Recent studies indicate that management of hypertension may vary by the sex of the patient. The Rho kinase pathway is a key mediator in cardiovascular and renal physiology. Therefore, findings from this study indicate that Rho kinase biology is sex specific and is impacted by influences during fetal life, implicating the need for sex-specific approaches for the prevention and treatment of hypertension and cardiovascular disease.
GRANTS
B. T. Alexander is supported by National Heart, Lung, and Blood Institute Grants HL-074927 and HL-51971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.B.O. and B.T.A. contributed to the conception and design of the research; N.B.O. and T.P.R. performed the experiments; N.B.O. and B.T.A. analyzed the data; N.B.O. and B.T.A. interpreted the results of the experiments; N.B.O. and B.T.A. prepared the figures; N.B.O. and B.T.A. drafted the manuscript; N.B.O. and B.T.A. edited and revised the manuscript; N.B.O., T.P.R., and B.T.A. approved the final version of the manuscript.
REFERENCES
- 1.Alexander BT. Intrauterine growth restriction and reduced glomerular number: role of apoptosis. Am J Physiol Regul Integr Comp Physiol 285: R933–R934, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Ferrario CM. ACE2: more of Ang-(1–7) or less Ang II? Curr Opin Nephrol Hypertens 20: 1–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fibbi B, Filippi S, Morelli A, Vignozzi L, Silvestrini E, Chavalmane A, De Vita G, Marini M, Gacci M, Manieri C, Vannelli GB, Maggi M. Estrogens regulate humans and rabbit epididymal contractility through the RhoA/Rho-kinase pathway. J Sex Med 6: 2173–2186, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Franco Mdo C, Dantas AP, Akamine EH, Kawamoto EM, Fortes ZB, Scavone C, Tostes RC, Carvalho MH, Nigro D. Enhanced oxidative stress as a potential mechanism underlying the programming of hypertension in utero. J Cardiovasc Pharmacol 40: 501–509, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Gayard M, Guilluy C, Rousselle A, Viollet B, Henrion D, Pacaud P, Loirand G, Rolli-Derkinderen M. AMPK alpha 1-induced RhoA phosphorylation mediates vasoprotective effect of estradiol. Arterioscler Thromb Vasc Biol 31: 2634–2642, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Goel A, Zhang Y, Anderson L, Rahimian R. Gender difference in rat aorta vasodilation after acute exposure to high glucose: involvement of protein kinase C beta and superoxide but not of Rho kinase. Cardiovasc Res 76: 351–360, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med 16: 183–190, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972 [DOI] [PubMed] [Google Scholar]
- 12.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension 59: 409–414, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the Angiotensin type 2 receptor. Hypertension 57: 275–282, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Hirooka Y, Kimura Y, Sagara Y, Sunagawa K. Ovariectomy augments hypertension through rho-kinase activation in the brain stem in female spontaneously hypertensive rats. Hypertension 48: 651–657, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Lamireau D, Nuyt AM, Hou X, Bernier S, Beauchamp M, Gobeil F, Jr, Lahaie I, Varma DR, Chemtob S. Altered vascular function in fetal programming of hypertension. Stroke 33: 2992–2998, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol 295: H466–H474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M. Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol 588: 1997–2010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton JS, Rueda-Clausen CF, Davidge ST. Mechanisms of endothelium-dependent vasodilation in male and female, young and aged offspring born growth restricted. Am J Physiol Regul Integr Comp Physiol 298: R930–R938, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen Dinh Cat A, Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep 13: 122–128, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Nuno DW, Lamping KG. The role of rho kinase in sex-dependent vascular dysfunction in type 1 diabetes. Exp Diabetes Res 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojeda NB. Low birth weight increases susceptibility to renal injury in a rat model of mild ischemia-reperfusion. Am J Physiol Renal Physiol 301: F420–F426, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50: 679–685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NE, Sartori-Valinotti JC, Reckelhoff JF, Royals TP, Alexander BT. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 60: 114–122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ojeda NB, Intapad S, Royals TP, Black JT, Dasinger JH, Tull FL, Alexander BT. Hypersensitivity to acute ANG II in female growth-restricted offspring is exacerbated by ovariectomy. Am J Physiol Regul Integr Comp Physiol 301: R1199–R1205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 298: R1421–R1427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne JA, Alexander BT, Khalil RA. Decreased endothelium-dependent NO-cGMP vascular relaxation and hypertension in growth-restricted rats on a high-salt diet. Hypertension 43: 420–427, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Rasch R, Skriver E, Woods LL. The role of the RAS in programming of adult hypertension. Acta Physiol Scand 181: 537–542, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the Mas receptor. Acta Physiol (Oxf) 206: 150–156, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 299: R899–R906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Kost CK, Jr, Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R1608–R1615, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res 72: 456–463, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal 7: 1302–1314, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Viegas VU, Liu ZZ, Nikitina T, Perlewitz A, Zavaritskaya O, Schlichting J, Persson PB, Regitz-Zagrosek V, Patzak A, Sendeski MM. Angiotensin II type 2 receptor mediates sex differences in mice renal interlobar arteries response to angiotensin II. J Hypertens 30: 1791–1798, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Williams J, Bogwu J, Oyekan A. The role of the RhoA/Rho-kinase signaling pathway in renal vascular reactivity in endothelial nitric oxide synthase null mice. J Hypertens 24: 1429–1436, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 288: R360–R367, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol 565: 125–135, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods LL. Fetal origins of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens 9: 419–425, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol 164: 1400–1409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension 51: 1239–1247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]